Abstract
Although laboratory and observational studies suggest that many animals are capable of compensatory growth after periods of food shortage, few field experiments have demonstrated structural growth compensation in the wild. Here, we addressed the hypotheses that (i) food restriction can induce structural compensatory growth in free-living animals, (ii) that compensation is proportional to the level of body size retardation and (iii) that compensation induces mortality costs. To test these, wild brown trout (Salmo trutta) yearlings were brought to the lab, tagged individually, subjected to four levels of food deprivation (including a control), released back into the native stream and recaptured after one, five and ten months. Brown trout fully restored condition and partially restored mass within a month, whereas compensation in structure (i.e. body length) was not evident until after five months, supporting hypothesis 1. As the level of growth compensation was similar among the three deprived groups, hypothesis 2 was not supported. A final recapture after winter revealed delayed mortality, apparently induced by the compensatory response in the deprived groups, which is consistent with hypothesis 3. To our knowledge, this is the first field experiment demonstrating structural compensatory growth and associated costs in a wild animal population.
Keywords: trout, Salmo trutta, food deprivation, delayed cost, field study
1. Introduction
Many animals are able to compensate for food deprivation or nutritional stress by increasing growth rates above the levels of non-deprived individuals (Ali et al. 2003; Jespersen & Toft 2003). Thus, growth rates are often kept below the physiological maximum, indicating that rapid growth can be costly (Arendt 1997). The nature of these costs is attracting increasing interest from both empirical and theoretical biologists (e.g. Metcalfe & Monaghan 2001; Yearsley et al. 2004; Mangel & Munch 2005). Short-term costs may include delayed structural development (Arendt & Wilson 2000), reduced tissue maintenance (Morgan et al. 2000) and increased predation risk associated with elevated foraging activity (Jönsson et al. 1996; Gotthard 2000). Recent studies also suggest long-term costs, including increased adult obesity and risk of coronary heart disease in humans (Forsén et al. 1999, 2004; Waterland & Garza 1999), ageing-related telomere loss in seabirds (Hall et al. 2004), and reduced lifespan in rats (Jennings et al. 1999) and zebra finches, Taeniopygia guttata (Birkhead et al. 1999). In fish, food restricted yearling Atlantic salmon (Salmo salar) responded with compensatory growth when feeding restrictions were lifted. However, several months later compensating fish entered a period of reduced growth performance leading to smaller body size, lower incidence of maturation, and reduced lipid stores (Morgan & Metcalfe 2001). The results of this laboratory study suggest that salmonids trade-off the benefits of short-term restoration of fat stores prior to winter against long-term performance.
The present knowledge of compensatory growth and its associated costs is mainly based on laboratory/hatchery experiments or observational studies in the wild. However, results from artificial environments may be difficult to extrapolate to wild populations, because the induced growth trajectories may not represent natural conditions. Observational studies do indicate that compensatory growth may occur in the wild (Bjorndal et al. 2003; Carlson et al. 2004), but preclude analyses of underlying factors. Therefore, manipulative field experiments are necessary to shed light on the ecological and evolutionary implications of compensatory growth. To date, however, few such experiments have been performed. Chicks of the lesser black-backed gulls Larus fuscus restored wing length when feeding conditions were experimentally improved (Royle 2000), as did nestlings of the Alpine swift Apus melba after reduction of the parasite load (Bize et al. 2003). Álvarez & Nicieza (2005) found that food-deprived resident brown trout (Salmo trutta) restored energy levels in the wild after two months, but there was no compensation for depression in structural growth. Furthermore, in a one-year experiment in a natural stream (Johnsson & Bohlin 2005) food-restricted sea-run brown trout rapidly restored lost body mass and condition whereas structural growth was not affected, and no costs of compensation could be detected. Thus, although compensatory restoration of energy levels and mass has been demonstrated in the wild, there is still limited experimental evidence for structural compensation, and no evidence for any costs associated with such compensation.
The aim of this study was to investigate whether (i) food restriction can induce structural compensatory growth, (ii) whether the magnitude of the compensation is proportional to the level body size retardation and (iii) if structural compensation induces mortality costs. We captured four groups of yearling brown trout in the wild and subjected them to different durations of food deprivation in the laboratory and compared growth and recapture rates after one, five and ten months in the wild.
2. Material and methods
(a) Population and study area
The experiment was conducted from April 2004 to April 2005 in Norumsån (58° 02′07″ N, 11° 49′20″ E), a small coastal soft-water stream in southwest Sweden (catchment area 19 km2), using 1 year old sea-run brown trout. These generally migrate to sea at age 2 yr (Bohlin et al. 1994), but a varying proportion, mainly males, mature early (from age 1.5 yr) and remain stream-resident throughout their lives (Dellefors & Faremo 1988).
(b) Sampling of fish
Four batches of trout (140–150 individuals in each) were sampled from a 700 m stream section on four occasions, April 26, May 3, May 10 and May 24 2004, using one-pass electric fishing (straight DC). To ensure representative sampling we divided the sampling area (700 m) into seven 100 m main sections of which each was further divided into five 20 m subsections. In the first sampling (April 26), we selected one random subsection from each main section for electric fishing. On the following sampling occasions we followed a similar scheme, but excluded subsections previously sampled.
(c) Lab treatment
The fish were brought to the Department of Zoology and kept in a holding tank overnight. On the following day they were anaesthetized (2-phenoxyethanol, 0.1 ml l−1), measured (fork length to the nearest mm), and weighed (to the nearest 0.1 g). Using body length frequencies we selected 1 year old fish over 60 mm for PIT- tagging (passive integrated transponders, Trovan, ID 100). Each batch was then distributed into two replicate treatment tanks, chosen at random out of eight, where they were kept under food deprivation until release (May 26). The tanks were 50 l flow-through aquaria with a water temperature of 9.5–10 °C, similar to the ambient stream temperature. Natural photoperiod was simulated using electronic timers, and the aquaria were landscaped with gravel, rocks and plastic aquarium plants. No food was provided. On May 25, the fish were again measured and weighed. No mortality occurred during the lab treatment. On the following day, the treatment groups were mixed and released into the study area. According to the number of weeks of food deprivation, the treatment groups were denoted 4w (four weeks), 3w (three weeks), 2w (two weeks) and 0w (controls). The number of individuals released was 114, 123, 128 and 133, respectively.
(d) Recaptures
We made recaptures on June 21 2004, October 19–20 2004 and April 4 2005. The whole study area and 100 m below and above it was sampled using double-pass electric fishing. In the October sampling we checked for early male maturation by gently stroking the body of recaptured individuals for the occurrence of running milt. In the final sampling the sex of 79 individuals was determined by dissecting and checking the gonads.
(e) Statistical analysis
Since the low number of individuals recaptured on all three recaptures did not permit a repeated measurement analysis, separate tests were conducted for each of the three recaptures. We used ANCOVA for the growth analysis to test whether the absolute growth after release differed among treatment groups. The full statistical model used was
where L1 and W1 are body length and body mass at recapture; L0 and W0 are body length and body mass at release, respectively; Tr is treatment with four levels (0w, 2w, 3w, 4w); Repl is replicate tank (random factor, two levels).
Compensation should show up as different elevation among these regressions, that is, a significant effect of Tr with a stronger positive response in deprived fish. As the variance of final body size tended to increase with initial body size, we used log-transformed body size measurements in the growth analyses, which stabilized the variance. We also tested the differences in final mean body size (in mass and body length) among groups for each recapture using the model
with notation as above, which is referred to as size analysis below.
The relative condition C, computed for each individual as the residual from the log (body mass (g))−log (body length (mm)) regression×100, for each recapture, and the change relative in condition ΔC over a time period, was analysed using the same model as for the size analysis.
Since the factor Repl was non-significant in all following tests (0.458<p<0.865), we pooled the data in order to increase power (Quinn & Keogh 2002). Further, in the growth analysis, we found no significant Tr×body size interactions (0.163<p<0.936), suggesting essentially parallel regressions for final body size on initial body size (figure 1). To increase power we, therefore, did not include the interaction in the final tests.
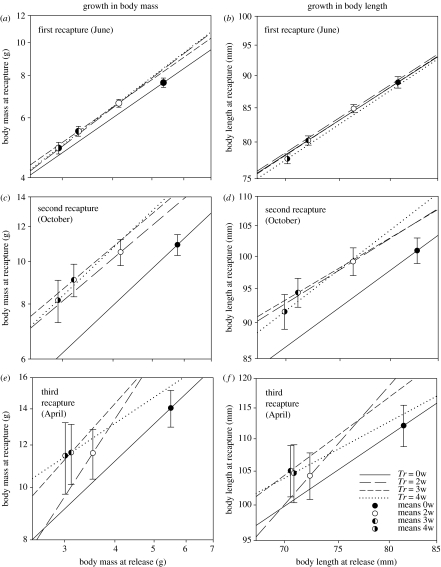
Figure 1.
Growth analysis: estimated regressions for body size at recapture versus body size at release for (a, b) the first (June, after one month), (c, d) second (October, after five months), and (e, f) third (April, after ten months) recaptures. Circles denote the mean values for the recaptured individuals (error bar: 95% C.I.). Compensation is shown by the differences in regression elevation, and is complete if the mean body sizes at recapture are equal among groups.
In the growth analyses, the post hoc pairwise comparisons of marginal means were Sidak adjusted, and in the size analyses we used Tukey's honestly significant difference test (α=0.05 in all cases).
3. Results
(a) Lab treatment
The lab manipulation resulted in the intended body-size discrimination among groups (table 1), with significant heterogeneities among group means in body mass (F(Tr)3,494=2.792, p<0.0001), body length (F(Tr)3,494=71.76, p<0.0001) and condition factor (F(Tr)3,494=162.8, p<0.0001), with all pairwise comparisons significant.
Table 1.
Descriptive statistics of initial and final body length, body mass and relative condition for the lab treatment. Tr=0w, food deprivation for zero weeks; Tr=2w, food deprivation for two weeks; Tr=3w, food deprivation for three weeks; Tr=4w: food deprivation for four weeks.
| Tr | initial state | state at release | |||||
|---|---|---|---|---|---|---|---|
| body length (mm) | body mass (g) | relative condition | body length (mm) | body mass (g) | relative condition | ||
| 0w | mean | 83.23 | 6.098 | 2.76 | 83.23 | 6.098 | 2.76 |
| n | 133 | 133 | 133 | 133 | 133 | 133 | |
| s.d. | 9.76 | 2.33 | 2.64 | 9.76 | 2.33 | 2.64 | |
| 2w | mean | 77.38 | 4.804 | 0.0561 | 77.30 | 4.479 | 0.512 |
| n | 128 | 128 | 128 | 128 | 128 | 128 | |
| s.d. | 8.10 | 1.63 | 1.98 | 8.19 | 1.52 | 2.36 | |
| 3w | mean | 74.24 | 4.241 | 0.1039 | 73.96 | 3.743 | −1.16 |
| n | 123 | 123 | 123 | 123 | 123 | 123 | |
| s.d. | 8.09 | 1.56 | 2.25 | 8.36 | 1.41 | 2.28 | |
| 4w | mean | 71.94 | 3.787 | −0.1751 | 70.98 | 3.142 | −2.54 |
| n | 114 | 114 | 114 | 114 | 114 | 114 | |
| s.d. | 6.98 | 1.26 | 2.44 | 7.20 | 1.08 | 2.61 | |
(b) Field performance
In all ANCOVAs, initial body size had a significant positive effect on final body size (p<0.0001 in all cases; figure 1).
204 fish were recaptured at the first recapture (0w: 32, 2w: 62, 3w: 56, 4w: 54), about one month after release (June 21). The growth analysis showed a significant treatment effect on body mass (F(Tr)3,199=4.347, p=0.005, figure 1a), with all starved groups growing slightly faster than the 0w group, but with no pairwise differences among the deprived groups (figure 1a,b). In the size analysis there was, however, still significant differences in mean final body mass (F(Tr)3,200=18.302, p<0.0001), with all pairwise comparisons significant except between 0w and 2w. We found no corresponding compensation in body length (F(Tr)3,199=1.574, p=0.197, observed power 0.411; figure 1b). Accordingly, the starved groups regained in relative condition (ΔC: 0w: −3.47, 2w: −0.116, 3w: 1.18, 4w: 1.76; F(Tr)3,200=35.52, p<0.0001, all pairwise comparisons significant). The final relative condition did not differ significantly among groups (F(Tr)3,200=2.312, p=0.07, observed power 0.578), although there was a tendency for a lower condition in 4w fish.
During the second sampling in October, 109 individuals were recaptured (0w: 33, 2w: 26, 3w: 29, 4w: 21). In this period, however, the growth analysis gave significant effects of Tr on both body mass (F(Tr)3,104=10.916, p<0.0001) and length (F(Tr)3,104=5.190, p=0.002), showing growth compensation also in structure for the starved groups (figure 1c,d). In terms of both mass and length, all deprived groups grew significantly faster than controls, but we found no significant pairwise differences among the deprived groups. The size analyses showed significant differences among groups both in final body mass (F(Tr)3,105=4.326, p=0.006) and final body length (F(Tr)3,105=4.720, p=0.004), with a significant pairwise difference between 0w and 4w fish. For the 2w group the compensation appears almost complete, while the 4w group was still lagging behind (figure 1c,d). There were no significant differences in relative condition among groups (F(Tr)3,105=0.320, p=0.811, observed power 0.110).
Eight of the 109 recaptured individuals in the October sampling were sexually mature males (0w: 2, 2w: 2, 3w: 3, 4w: 1). The incidence of maturation did not vary among groups (Fisher Exact Test, p=0.922).
The effect of treatment on the October recapture rates was tested using a logistic regression with initial body length as covariate. None of the factors were significant (initial body length: χ12=1.72, p=0.184; Tr: χ32=3.138, p=0.371), suggesting similar summer survival among groups.
In the third sampling, ten months after release, the number of recaptures was 0w: 30, 2w: 22, 3w: 20 and 4w: 14 individuals. In the growth analysis, Tr had a significant effect on final body mass (F(Tr)3,81=3.572, p=0.017), with pairwise differences between controls and 3 and 4w fish (figure 1e,f). We found no corresponding effect on final body length (F(Tr)3,81=1.652, p=0.184, observed power 0.418). Final mean body mass did not vary significantly among groups (size analysis, F(Tr)3,82=2.520, p=0.064, observed power 0.604), but there was still a tendency for the starved groups to lag behind (figure 1e). In terms of final body length (figure 1f) there was significant heterogeneity among groups (F(Tr)3,82=2.874, p=0.041), with the starved groups smaller than the control. The final relative condition did not vary among groups (F(Tr)3,82=1.029, p=0.384, observed power 0.270).
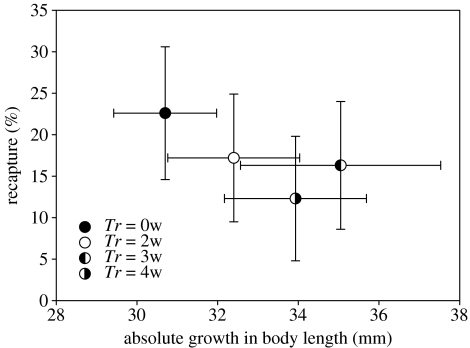
The recapture rates in the final sampling were 22.6% in group 0w, 17.2% in group 2w, 16.3% in group 3w and 12.3% in group 4w (figure 2). A logistic regression analysis showed significant effects of initial body length (negative effect, χ12=9.309, p=0.0029) and treatment (χ32=11.21, p=0.010), with lower recapture rate for the starved groups.
Figure 2.
Recapture rate at last recapture (April 2005) and the absolute growth in body length from release to second recapture. Error bars denote 95% C.I.
The sex ratio in the final sample was even (39 males, 40 females, χ12=0.013, p=0.910), and did not differ among treatment groups (χ32=0.428, p=0.934).
4. Discussion
After one month, mass was restored in the two week group, showing that wild brown trout are able to restore energy status following moderate food deprivation, whereas the more severely deprived fish in the 3 and 4w groups were still lagging behind controls. This is consistent with previous experiments in the laboratory and the field (Ali et al. 2003; Álvarez & Nicieza 2005; Johnsson & Bohlin 2005). Furthermore, we provide the first experimental demonstration of structural compensatory growth for fish in the wild. After five months, trout in all deprived groups showed elevated growth in body length. Structural compensation was complete in the two week group, whereas the three and four week groups had restored length only partially. Our results contradict Álvarez & Nicieza (2005) who did not find compensation for depression in structural growth in stream-resident brown trout, and therefore rejected the hypothesis that structural growth is a general response to growth depression. However, this conclusion seems premature since the growth period in the field in their study was limited to two months, whereas we did not detect structural compensation until after five months. Previous laboratory studies indicate that the mobile energy stores probably need to be restored before energetic resources can be allocated to structure (Broekhuizen et al. 1994; Jobling 2002). Structural compensation may also be delayed by initial effects of food deprivation on the endocrine growth regulation system (Johnsson et al. 1996; Björnsson 1997). As body condition was restored prior to the onset of structural compensation in our study, it seems unlikely that lipostatic cues were used to regulate structural compensation.
A novel hypothesis addressed in our study is whether the compensatory growth is proportional to the degree of body size retardation, which may be expected if selection favours compensation to reach a critical threshold size, for example to ensure over-winter survival (Bull et al. 1996). However, after five months the absolute growth of the two, three and four week groups was similarly elevated, indicating an upper limit to the compensatory response. This could be explained by a trade-off between the benefits and costs (i.e. mortality) of growth (Sibly & Calow 1986), or by physiological constraints: compensating fish are simply growing at their maximal physiological capacity. In addition, the lack of interactions in the growth analyses suggests that the compensation is independent of initial body size, indicating that individuals tend to restore an innate growth trajectory rather than to reach an absolute body size threshold. This view is supported also by the fact that the slope of linear body-length regressions for the October sampling did not differ significantly from unity in any treatment group, which implies that the absolute growth in length over summer is similar for small and large individuals.
Álvarez & Nicieza (2005) suggested that structural compensatory growth is restricted to migratory salmonids and other species where threshold sizes for ontogenetic niche shifts need to be reached within narrow time windows (Ludwig & Rowe 1990). Our results are not inconsistent with this view, but do not support the threshold hypothesis. Further studies are clearly needed to delimit the conditions under which structural compensation occurs, since the relative fitness costs and benefits of mass and structural compensation may vary considerably among individuals, populations and taxa. For example, food restricted larval damselfly (Ischnura verticalis) showed structural compensatory growth whereas adult body mass was not restored, suggesting that structural compensation occurs at the cost of mass gain in this species (Dmitriew & Locke Rowe 2005). Also bird nestlings appear to give priority to structural compensation (Nilsson & Svensson 1996; Royle 2000; Bize et al. 2003).
Our results provide the first field experimental support of costs associated with compensatory responses (Metcalfe & Monaghan 2001). Whereas recapture rate in October was independent of treatment, indicating similar summer survival, the deprived groups had significantly lower recapture rates in April than controls. This suggests an increased over-winter mortality resulting from the compensatory growth response the preceding year (figure 2). This difference cannot be explained by the body size differences induced by the feeding treatments, since initial body length was included as a covariate in the analysis. Differential movement patterns are also unlikely, as previous field studies in the experimental stream indicate that young trout are stationary and very rarely move more than 100 m (Bohlin et al. 2002). Moreover, since the final recapture was made in early April it is unlikely that uncaught trout had migrated to sea, as migration in early April is rare in this stream (Bohlin et al. 1993a). In addition, initially smaller individuals tend to migrate later in the season, not earlier, refuting the hypothesis that the lower recapture rate of deprived individuals is caused by size-dependent migration timing (Bohlin et al. 1993b).
Several interacting mechanisms may contribute to the increased winter mortality observed in the present study (Metcalfe & Monaghan 2001). For example, reduced investment in the muscular development of compensating individuals may result in impaired locomotor performance (Álvarez & Metcalfe 2005), in turn increasing maintenance costs and reducing foraging efficiency, predator avoidance and competitive ability (Royle et al. 2005). In addition, compensatory growth may occur at the cost of reduced immunocompetence and disease resistance (Arendt 1997).
To summarize, young wild brown trout are capable of compensating losses in structure and mass. However, over the one-year time span of the study, restoration was only partial, suggesting that compensatory growth in the wild has a limit, which may result from constraints in physiological capacity, as well as trade-offs between rapid growth and mortality. The latter explanation is supported by our demonstration of increased over-winter mortality rates in the compensating groups.
Acknowledgments
J.I.J. was financed by the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning. The experiment was approved by the Ethical Committee for Animal Research in Göteborg (license 199/2002), and comply with current laws in Sweden. We thank David Dalliah for excellent technical assistance.
References
- Ali M, Nicieza A, Wootton R.J. Compensatory growth in fishes: a response to growth depression. Fish Fish. 2003;4:147–190. [Google Scholar]
- Álvarez D, Metcalfe N.B. Catch-up growth and swimming performance in threespine sticklebacks (Gasterosteus aculeatus): seasonal changes in the cost of compensation. Can. J. Fish. Aquat. Sci. 2005;62:2169–2176. 10.1139/f05-130 [Google Scholar]
- Álvarez D, Nicieza A.G. Compensatory response “defends” energy levels but not growth trajectories in brown trout, Salmo trutta L. Proc. R. Soc. B. 2005;272:601–607. doi: 10.1098/rspb.2004.2991. 10.1098/rspb.2004.2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt J.D. Adaptive intrinsic growth rates: an integration across taxa. Q. Rev. Biol. 1997;72:149–177. 10.1086/419764 [Google Scholar]
- Arendt J.D, Wilson D.S. Population differences in the onset of cranial ossification in pumpkinseed (Lepomis gibbosus), a potential cost of rapid growth. Can. J. Fish. Aquat. Sci. 2000;57:351–356. 10.1139/cjfas-57-2-351 [Google Scholar]
- Birkhead T.R, Fletcher F, Pellatt E.J. Nestling diet, secondary sexual traits and fitness in the zebra finch. Proc. R. Soc. B. 1999;266:385–390. 10.1098/rspb.1999.0649 [Google Scholar]
- Bize P, Roulin A, Bersier L.F, Pfluger D, Richner H. Parasitism and developmental plasticity in Alpine swift nestlings. J. Anim. Ecol. 2003;72:633–639. doi: 10.1046/j.1365-2656.2003.00734.x. 10.1046/j.1365-2656.2003.00734.x [DOI] [PubMed] [Google Scholar]
- Bjorndal K.A, Bolten A.B, Dellinger T, Delgado C, Martins H.R. Compensatory growth in oceanic loggerhead sea turtles: response to a stochastic environment. Ecology. 2003;84:1237–1249. [Google Scholar]
- Björnsson B.Th. The biology of salmon growth hormone: from daylight to dominance. Fish Physiol. Biochem. 1997;17:9–24. 10.1023/A:1007712413908 [Google Scholar]
- Bohlin T, Dellefors C, Faremo U. Timing of sea-run brown trout (Salmo trutta) smolt migration: effects of climatic variation. Can. J. Fish. Aquat. Sci. 1993a;50:1132–1136. [Google Scholar]
- Bohlin T, Dellefors C, Faremo U. Optimal time and size for smolt migration in wild sea trout (Salmo trutta) Can. J. Fish. Aquat. Sci. 1993b;50:224–232. [Google Scholar]
- Bohlin T, Dellefors C, Faremo U. Probability of first sexual maturation of male parr in wild sea-run brown trout (Salmo trutta) depends on condition factor 1 yr in advance. Can. J. Fish. Aquat. Sci. 1994;51:1920–1926. [Google Scholar]
- Bohlin T, Sundström L.F, Johnsson J.I, Höjesjö J, Pettersson J. Density-dependent growth in brown trout: effects of introducing wild and hatchery fish. J. Anim. Ecol. 2002;71:683–692. 10.1046/j.1365-2656.2002.00631.x [Google Scholar]
- Broekhuizen N, Gurney W.S.C, Jones A, Bryant A.D. Modelling compensatory growth. Funct. Ecol. 1994;8:770–782. [Google Scholar]
- Bull C.D, Metcalfe N.B, Mangel M. Seasonal matching of foraging to anticipated energy requirements in anorexic juvenile salmon. Proc. R. Soc. B. 1996;263:13–18. [Google Scholar]
- Carlson S.M, Hendry A.P, Letcher B. Natural selection acting on body size, growth rate and compensatory growth: an empirical test in a wild trout population. Evol. Ecol. Res. 2004;6:955–973. [Google Scholar]
- Dellefors C, Faremo U. Early sexual maturation in males of wild sea trout, Salmo trutta L:, inhibits smoltification. J. Fish Biol. 1988;33:741–749. [Google Scholar]
- Dmitriew C, Rowe L. Resource limitation, predation risk and compensatory growth in a damselfly. Oecologia. 2005;142:150–154. doi: 10.1007/s00442-004-1712-2. 10.1007/s00442-004-1712-2 [DOI] [PubMed] [Google Scholar]
- Forsén T, Eriksson J.G, Tuomilehto J, Osmond C, Barker D.J.P. Growth in utero and during childhood among women who develop coronary heart disease: longitudinal study. Br. Med. J. 1999;319:1403–1407. doi: 10.1136/bmj.319.7222.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsén T, Osmond C, Eriksson J.G, Barker D.J.P. Growth of girls who later develop coronary heart disease. Heart. 2004;90:20–24. doi: 10.1136/heart.90.1.20. 10.1136/heart.90.1.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotthard K. Increased risk of predation as a cost of high growth rate: an experimental test in a butterfly. J. Anim. Ecol. 2000;69:896–902. doi: 10.1046/j.1365-2656.2000.00432.x. 10.1046/j.1365-2656.2000.00432.x [DOI] [PubMed] [Google Scholar]
- Hall M.E, Nasir L, Daunt F, Gault E.A, Croxall J.P, Wanless S, Monaghan P. Telomere loss in relation to age and early environment in long-lived birds. Proc. R. Soc. B. 2004;271:1571–1576. doi: 10.1098/rspb.2004.2768. 10.1098/rspb.2004.2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings B.J, Ozanne S.E, Dorling M.W, Hales C.N. Early growth determines longevity in male rats and may be related to telomere shortening in the kidney. FEBS Lett. 1999;448:4–8. doi: 10.1016/s0014-5793(99)00336-1. 10.1016/S0014-5793(99)00336-1 [DOI] [PubMed] [Google Scholar]
- Jespersen L.B, Toft S. Compensatory growth following early nutritional stress in the Wolf Spider Pardosa prativaga. Funct. Ecol. 2003;17:737–746. 10.1111/j.1365-2435.2003.00788.x [Google Scholar]
- Jobling M. Environmental factors and rates of development and growth. In: Hart P.J.B, Reynolds J.D, editors. Handbook of fish biology and fisheries. Blackwell; Oxford, UK: 2002. pp. 97–122. [Google Scholar]
- Johnsson J.I, Bohlin T. Compensatory growth for free? A field experiment on brown trout (Salmo trutta) Oikos. 2005;111:31–38. 10.1111/j.0030-1299.2005.13972.x [Google Scholar]
- Johnsson J.I, Jönsson E, Björnsson B.Th. Dominance, nutritional state, and growth hormone levels in rainbow trout (Oncorhynchus mykiss) Horm. Behav. 1996;30:13–21. doi: 10.1006/hbeh.1996.0003. 10.1006/hbeh.1996.0003 [DOI] [PubMed] [Google Scholar]
- Jönsson E, Johnsson J.I, Björnsson B.Th. Growth hormone increases predation exposure of rainbow trout. Proc. R. Soc. B. 1996;263:647–651. doi: 10.1098/rspb.1996.0097. [DOI] [PubMed] [Google Scholar]
- Ludwig D, Rowe L. Life-history strategies for energy gain and predator avoidance. Am. Nat. 1990;135:686–707. 10.1086/285069 [Google Scholar]
- Mangel M, Munch S.B. A life-history perspective on short- and long-term consequences of compensatory growth. Am. Nat. 2005;166:E155–E176. doi: 10.1086/444439. 10.1086/444439 [DOI] [PubMed] [Google Scholar]
- Metcalfe N.B, Monaghan P. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 2001;16:54–260. doi: 10.1016/s0169-5347(01)02124-3. 10.1016/S0169-5347(01)02124-3 [DOI] [PubMed] [Google Scholar]
- Morgan I.J, Metcalfe N.B. Deferred costs of compensatory growth after autumnal food shortage in juvenile salmon. Proc. R. Soc. B. 2001;268:295–301. doi: 10.1098/rspb.2000.1365. 10.1098/rspb.2001.1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan I.J, McCarthy I.D, Metcalfe N.B. Life-history strategies and protein metabolism in overwintering juvenile Atlantic salmon: growth is enhanced in early migrants through lower protein turnover. J. Fish Biol. 2000;56:637–647. 10.1111/j.1095-8649.2000.tb00761.x [Google Scholar]
- Nilsson J.A, Svensson M. Sibling competition affects nestling growth strategies in marsh tits. J. Anim. Ecol. 1996;65:825–836. [Google Scholar]
- Quinn G, Keough M.J. Cambridge University Press; Cambridge, UK: 2002. Experimental design and data analysis for biologists. [Google Scholar]
- Royle N.J. Overproduction in the lesser black-backed gull: can marginal chicks overcome the initial handicap of hatching asynchrony? J. Avian Biol. 2000;31:335–344. 10.1111/j.0908-8857.2000.310309.x [Google Scholar]
- Royle N.J, Lindström J, Metcalfe N.B. A poor start in life negatively affects dominance status in adulthood independent of body size in green swordtails Xiphophorus helleri. Proc. R. Soc. B. 2005;272:1917–1922. doi: 10.1098/rspb.2005.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibly R.M, Calow P. Blackwell; Oxford, UK: 1986. Physiological ecology of animals. [Google Scholar]
- Waterland R.A, Garza C. Potential mechanisms of metabolic imprinting that lead to chronic disease. Am. J. Clin. Nutr. 1999;69:179–197. doi: 10.1093/ajcn/69.2.179. [DOI] [PubMed] [Google Scholar]
- Yearsley J.M, Kyriazakis I, Gordon J. Delayed costs of growth and compensatory growth rates. Funct. Ecol. 2004;18:563–570. 10.1111/j.0269-8463.2004.00879.x [Google Scholar]