Abstract
Mimicry has evolved in a wide range of organisms and encompasses diverse tactics for defence, foraging, pollination and social parasitism. Here, I report an extraordinary case of egg mimicry by a fungus, whereby the fungus gains competitor-free habitat in termite nests. Brown fungal balls, called ‘termite balls’, are frequently found in egg piles of Reticulitermes termites. Phylogenetic analysis illustrated that termite-ball fungi isolated from different hosts (Reticulitermes speratus, Reticulitermes flavipes and Reticulitermes virginicus) were all very similar, with no significant molecular differences among host species or geographical locations. I found no significant effect of termite balls on egg survivorship. The termite-ball fungus rarely kills termite eggs in natural colonies. Even a termite species (Reticulitermes okinawanus) with no natural association with the fungus tended termite balls along with its eggs when it was experimentally provided with termite balls. Dummy-egg bioassays using glass beads showed that both morphological and chemical camouflage were necessary to induce tending by termites. Termites almost exclusively tended termite balls with diameters that exactly matched their egg size. Moreover, scanning electron microscopic observations revealed sophisticated mimicry of the smooth surface texture of eggs. These results provide clear evidence that this interaction is beneficial only for the fungus, i.e. termite balls parasitically mimic termite eggs.
Keywords: egg recognition, mimicry, termites, sclerotia, insect–fungus interaction
1. Introduction
Mimicry has evolved in a wide range of organisms and encompasses diverse tactics for defence (e.g. Lindstrom et al. 2004; Ito et al. 2004; Wiklund & Tullberg 2004), foraging (e.g. Allan et al. 2002; Moland & Jones 2004), pollination (e.g. Singer et al. 2004) and social parasitism (e.g. Kistner 2000). Mimicry has long fascinated evolutionary biologists, because it provides a model system for the study of the ‘evolutionary arms race’ between hosts and parasites (Wickler 1968; Malcolm 1990). Egg protection is an essential behaviour in social animals. In termites, workers recognize the eggs laid by queens and pile the eggs together to take care of them. This egg-protection behaviour is indispensable for egg survivorship (Matsuura et al. 2000). The phenomenon of termites harbouring brown fungal balls (hereafter, termite balls) with their eggs was reported recently in the Japanese termite Reticulitermes speratus (Matsuura et al. 2000). These brown balls were identified as the sclerotia of a corticioid fungus, Fibularhizoctonia sp. nov. (Matsuura et al. 2000). The sclerotium is a tough ball of densely packed filaments that germinate into a fungal colony under favourable conditions. Eggs and termite balls are groomed frequently by workers; their surfaces are smeared with saliva, which contains antibiotic substances, thus providing protection from dryness and pathogens (Matsuura et al. 2000). Although termite balls are inhibited from germination in egg piles, some termite balls that are removed from egg piles germinate and grow on termite excretions in the corners of the nest (Matsuura et al. 2000). Hence, the termite-ball fungus is able to live in termite nests, which represents nearly competitor-free habitat for the fungus.
Termite balls are thought to mimic termite eggs (Matsuura et al. 2000; Matsuura 2003). However, the interaction between the eggs and the fungus may not be simply parasitic, but may include a symbiotic aspect for R. speratus, because termite balls sometimes enhance egg survival under experimental conditions (Matsuura et al. 2000). One possible explanation for the positive effect of termite balls on egg survival may be that the antibiotic substances produced by the termite-ball fungus protect termite eggs from pathogens. Therefore, termites may harbour the termite balls because of their potential benefits to the colony. In this study, I attempted to determine whether this interaction also benefits the termites, i.e. is the relationship mutualistic or parasitic?
Termite balls have been found not only in R. speratus in Japan, but also in Reticulitermes flavipes and Reticulitermes virginicus (figure 1a) in the United States (Matsuura 2005). The relationship between Reticulitermes and the termite-ball fungus is facultative for the termites. Wide-area sampling showed that 89% of R. speratus (n=61), 73% of R. flavipes (n=33) and 88% of R. virginicus (n=8) colonies in the wild contained termite balls (Matsuura 2005). No morphological differences have been detected among termite-ball fungi isolated from different termite species. I conducted a phylogenetic analysis of the termite-ball fungi isolated from the three host termite species. To assess the affect of termite balls on egg survival, I compared egg survivorship with and without termite balls. Termites that do not have a natural association with termite balls may be less likely to carry the fungus without mimicry. I tested whether the Ryukyu termite Reticulitermes okinawanus, which does not naturally interact with termite-ball fungi (Matsuura 2005), tends termite balls if given termite balls experimentally.
Figure 1.
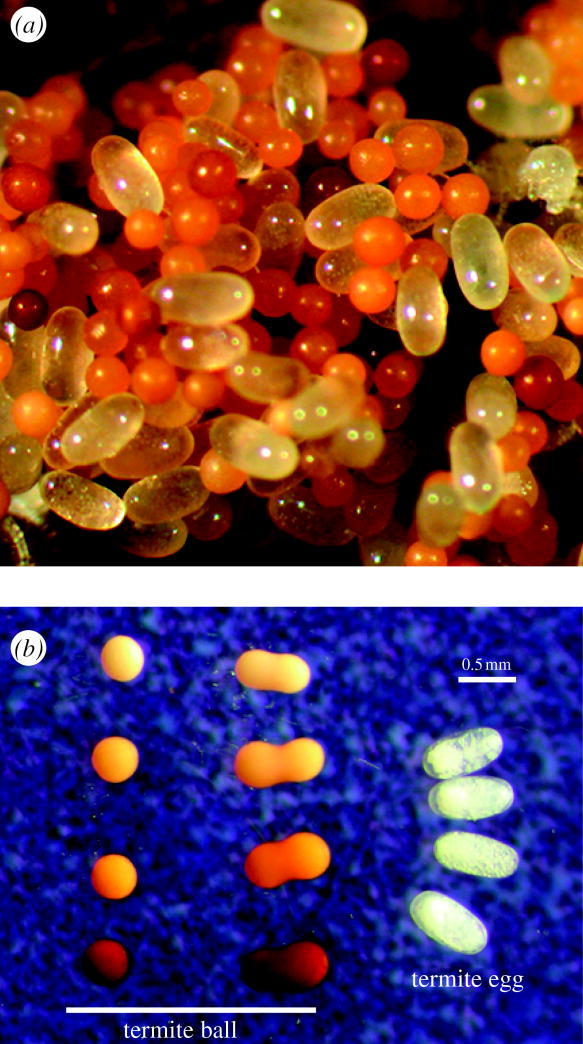
Termite eggs and termite balls. (a) A pile of eggs in the nest of Reticulitermes virginicus. Termite eggs are transparent and oval, whereas termite balls (egg-mimicking fungal sclerotia) are brown and spherical. (b) Comparison of the shapes and colours of termite balls and termite eggs. Most termite balls are spherical, with rare occurrences of oval balls resulting from the fusion of two termite balls.
To understand how termites recognize eggs, I conducted a dummy egg-carrying bioassay using dummy eggs made of glass beads coated with egg-recognition chemicals. To assess whether strict morphological mimicry of eggs is required for termite balls to be carried by termites, I compared the short diameters of termite eggs, termite balls produced by fungal isolates from each colony and termite balls collected from egg piles, i.e. those accepted by termites. In addition, I compared the surface texture of termite eggs, termite balls and the sclerotia of a closely related fungus using scanning electron microscopy (SEM).
2. Material and methods
(a) Termite-ball sampling and phylogeny
Termite balls were obtained from egg piles in the nests of R. speratus in Kyoto, Japan; R. flavipes in Massachusetts, Louisiana and Mississippi, USA; and R. virginicus in Texas and Louisiana, USA. The distribution of R. flavipes and R. virginicus partially overlapped in the southeastern USA. Because of morphological ambiguity, traditional identification of Reticulitermes termites is difficult and unreliable (Szalanski et al. 2003). Therefore, in addition to morphological characters of soldiers (Hostettler et al. 1995), I used PCR–RFLP to separate R. flavipes and R. virginicus (Szalanski et al. 2003; Matsuura 2005). Termite balls extracted from egg piles were inoculated on potato dextrose agar (PDA; Difco, Sparks, MD, USA) and incubated at 27 °C for 20 days. One newly developed sclerotium was re-isolated from each termite ball and cultured on a new PDA plate. In the egg piles of a natural R. flavipes colony, I found several white, marshmallow-like, dead eggs, whose contents were replaced by fungal hyphae. I isolated the egg-pathogenic fungus from the dead eggs and analysed the DNA sequence.
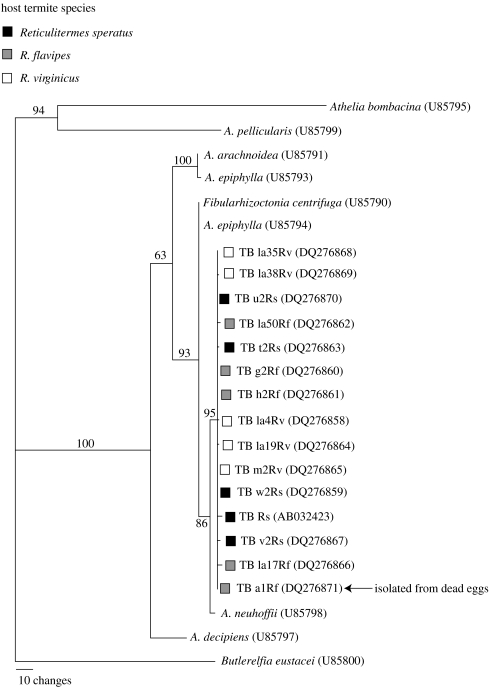
DNA was extracted from each fungal isolate using CTAB extraction methods (Matsuura 2005). The entire region of ITS1–5.8S–ITS2 was amplified by PCR using the primer set ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′; White et al. 1990). PCR consisted of 35 cycles of denaturing at 95 °C for 30 s, annealing at 53 °C for 60 s and extension at 72 °C for 90 s, resulting in an amplification product of 647 bp. This was sequenced in both directions (Big Dye Terminator cycle sequencing, electrophoresis on ABI 3100, Applied Biosystems, Foster City, CA, USA). Sequences were edited with Sequencher v. 4.1 (Gene Codes Corporation, Ann Arbor, MI, USA). The internal transcribed spacer (ITS) region of 15 termite-ball fungus isolates and nine other species of Corticiaceae from GenBank were used for phylogenetic analysis. The alignment was performed using the ClustalX package (Thompson et al. 1997) and was completed by the ‘Slow/Accurate’ method with a gap open penalty of 15.00 and gap extension penalty of 4. Aligned sequences were corrected manually, focusing on gap positions. Phylogenetic analysis was performed with PAUP* v. 4.0b10 (Swofford 2001). Butlerelfia eustacei (U85800) was used as the outgroup. Maximum parsimony analysis was conducted using a heuristic search with equal weighting of all characters (tree bisection–reconnection branch swapping; MULTREES option in effect). Ten replicates were performed within each heuristic search using random stepwise addition. A strict consensus was constructed to reconcile equally parsimonious topologies. To assess the robustness of the relationship, 500 bootstrap replicates were performed with 10 replications of random addition sequence. All nucleotide sequence data of the termite-ball fungus isolates were deposited in the DDBJ/EMBL/GenBank nucleotide sequence database. Accession numbers are given in figure 2.
Figure 2.
Phylogenetic position of the termite-ball fungi (TB) isolated from Reticulitermes speratus in Japan and from R. flavipes and R. virginicus in the USA based on nucleotide sequences of the ITS regions. Strict consensus of the 10 most parsimonious trees is shown. Bootstrap values are indicated for nodes having more than 50% support (500 replicates). GenBank accession numbers are shown in parentheses. The DNA sequence of the egg-pathogenic fungus isolated from dead eggs was identical to that of the termite-ball fungus isolated from the same colony (TB a1Rf).
(b) Egg survivorship with and without termite balls
I compared the survival rates of eggs maintained with and without termite balls using five colonies of R. flavipes (colonies A–E) and four colonies of R. virginicus (colonies F–I) collected in Raleigh, NC, USA, in August 2005. Fifty eggs, 20 termite balls and 20 workers obtained from each colony were placed on moist, unwoven cloth in a 35 mm Petri dish (Corning; Corning, NY, USA). To simulate natural conditions, a piece of nest wood (15×15×3 mm) coated with plaster made of faecal matter, which was obtained from the original nest of each colony, was placed in each Petri dish. This nest material is thought to be important for egg survivorship, because termite faeces contain antibiotic substances (e.g. Rosengaus et al. 1998).
Petri dishes were maintained at 25 °C for one month. The egg survival rate was determined by counting live eggs and newly hatched larvae at one month as a proportion of the initial number of eggs. The control consisted of 50 eggs and 20 workers with no termite balls. Both treatments were replicated five times for each colony, except F and G, for which each treatment was replicated three times because I was unable to obtain enough eggs. I analysed the effects of colony and presence/absence of termite balls on egg survivorship using analysis of covariance (ANCOVA), with worker survival rate as the covariate. Survival rates were arcsine-transformed prior to analysis. Additionally, I prepared Petri dishes without nest material to examine the effect of nest material on egg survival rates. This additional test was performed only for colony A because of limited numbers of eggs in the other colonies.
(c) Egg-recognition bioassay
Dummy-egg bioassays were performed in 35 mm Petri dishes (Corning), using workers from four R. flavipes colonies (two colonies collected in Boston, MA and two colonies collected in Raleigh, NC, USA). I used dummy eggs made of 0.2, 0.4 or 0.6 mm diameter glass beads that were either coated or not coated with egg chemicals. Termite eggs (450 mg) were homogenized using a pestle in a 2 ml tube; I then added 800 μl of methanol, and the tube was vortexed for 2 min. After centrifugation at 10 000 r.p.m. for 5 min, the precipitate was removed, and the supernatant was dried using a speed vacuum. The extract was re-suspended in methanol to a final concentration of 0.05 mg μl−1. Five microlitres were then added to 100 glass beads and the methanol was evaporated. Twenty eggs and 20 dummy eggs were randomly arranged on moist unwoven cloth in a 30 mm Petri dish, and 30 workers were released in the dish. Dishes were maintained at 25 °C in the dark. After 24 h, the acceptance rates were determined by counting the number of dummy eggs carried onto egg piles. The acceptance rates of termite balls collected from egg piles of each colony were also determined. This bioassay was replicated four times for each treatment; data for the two colonies were pooled because there was no significant difference between the colonies. Arcsine-square-root-transformed data were analysed using two-way ANOVA followed by post hoc Scheffé's tests (Sokal & Rohlf 1995).
Reticulitermes okinawanus, a closely related species to R. speratus, does not interact with termite-ball fungi (Matsuura 2005). An egg-carrying experiment was conducted for workers of an R. okinawanus colony collected in Hetona, Okinawa, Japan, to determine whether the workers would tend termite balls. Twenty eggs from the colony and 20 termite balls collected from R. speratus egg piles were arranged on a Petri dish, and the acceptance rate was examined after 24 h. This experiment was replicated four times.
(d) Size and surface texture
Size measurements of termite eggs and termite balls were made for six R. flavipes colonies collected in Boston, MA, USA. The short diameters of 40 eggs and 100 termite balls randomly extracted from egg piles were measured for each colony. I also examined the sizes of termite balls produced by the termite-ball fungus isolated from each colony. A 5 mm diameter mycelial plug was taken from the margin of a 20-day-old colony of each isolate, placed on a new PDA agar plate and incubated at 27 °C for 20 days. The diameters of 100 termite balls randomly chosen from the plate were examined under a stereomicroscope. The size data were analysed using a two-way ANOVA with colony and object category (eggs, balls produced and balls accepted) as independent variables. Post hoc Scheffé's tests were conducted to compare average sizes among object categories.
I compared the micro-structure of the surface of an egg of R. flavipes, a termite ball, and a sclerotium of the corticioid fungus Athelia epiphylla (IFO 5253) using a variable-pressure SEM (S-3200N, Hitachi). Athelia epiphylla is a closely related congeneric species of the termite-ball fungus (Fibularhizoctonia sp.; figure 2). Fibularhizoctonia is an anamorphic name for Athelia. Termite balls and sclerotia of A. epiphylla were obtained from 20-day-old PDA culture plates.
3. Results
(a) Termite-ball phylogeny and effects on egg survivorship
The phylogenetic tree based on nucleotide sequences of the ITS region illustrated that termite-ball fungi isolated from different hosts (R. speratus, R. flavipes and R. virginicus) were all very similar, with no significant molecular differences among host species or geographical locations (figure 2). The fungus isolated from the white, marshmallow-like, dead eggs grew on an agar plate and formed sclerotia, which appeared identical to termite balls. DNA sequence analysis showed that the ITS sequence of the egg-pathogenic fungus was identical to that of the termite-ball fungus isolated from the same colony (figure 2), indicating that the fungus sometimes consumed termite eggs, even when they were under the care of workers. However, the occurrence of such dead eggs was rare. I only found four dead eggs among ca 7300 eggs (383 mg total weight) in the R. flavipes colony.
Workers began to gather eggs soon after the start of the egg survivorship experiment. Egg piles were formed within 24 h in all Petri dishes. Interestingly, workers always brought eggs to the pieces of nest material and maintained egg piles on the nest material, but never on the unwoven cloth. Egg survival rates were significantly different among colonies in both R. flavipes and R. virginicus (table 1), and I found a significant correlation between worker survival rates and egg survival rates (table 1). However, no significant effect of termite balls on egg survivorship was found in either R. flavipes or R. virginicus (table 1). In colony A, nest material had a significant effect on egg survival rates (ANOVA, F1,16=6.54, p<0.05). The survival rate of eggs kept with nest material was significantly greater than that of eggs without nest material (0.55±0.08s.d. versus 0.34±0.19s.d., respectively; Scheffé's test, p<0.05).
Table 1.
Statistical results from ANCOVA examining the effects of the colony and termite ball treatment (with or without termite ball) on egg survivorship in R. flavipes and R. virginicus with worker survival rate as covariate. (Arcsine-root transformed survival rates were used in the statistical analysis.)
| effect | d.f. | MS | F | p | |
|---|---|---|---|---|---|
| R. flavipes | worker | 1 | 0.869 | 52.62 | <0.0001 |
| colony | 4 | 0.134 | 8.10 | <0.0001 | |
| termite ball | 1 | 0.003 | 0.15 | 0.697 | |
| colony×termite ball | 4 | 0.012 | 0.72 | 0.582 | |
| error | 39 | 0.017 | |||
| R. virginicus | worker | 1 | 1.424 | 172.68 | <0.0001 |
| colony | 3 | 0.136 | 16.56 | <0.0001 | |
| termite ball | 1 | 0.003 | 0.32 | 0.574 | |
| colony×termite ball | 3 | 0.006 | 0.71 | 0.554 | |
| error | 33 | 0.008 |
(b) Egg-recognition bioassay
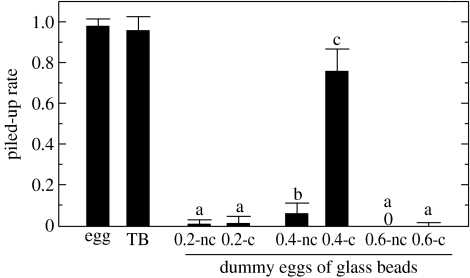
Both termite eggs and termite balls collected from egg piles were carried by workers (Mann–Whitney U-test, Z=0.72, p=0.382; figure 3). The glass bead bioassay showed highly significant effects of size and chemical condition on egg recognition (size: F2,90=364.96, p<0.0001; chemical: F1,90=189.36, p<0.0001; size×chemical: F2,90=181.74, p<0.0001). The 0.4 mm glass beads, whose diameter was similar to that of eggs, were carried by termites when they were coated with egg chemicals, whereas slightly larger (0.6 mm) and slightly smaller (0.2 mm) glass beads were rejected by termites, even when they were densely coated with egg chemicals (figure 3). The 0.4 mm glass beads (113.4±2.7s.d. μg) were much heavier than termite eggs (52.7±4.4s.d. μg), which may explain why the piling rate of these beads coated with egg-recognition chemicals was still lower than that of termite eggs (Mann–Whitney U-test, Z=4.60, p<0.0001) and termite balls (Mann–Whitney U-test, Z=4.20, p<0.0001).
Figure 3.
Comparison of the piling rates of termite eggs, termite balls and dummy eggs (glass beads). Glass beads (0.2, 0.4 or 0.6 mm in diameter) that were coated (c) or not coated (nc) with egg chemicals were used as dummy eggs. For example, 0.2-c and 0.2-nc indicate 0.2 mm glass beads that were and were not coated with egg chemicals, respectively. Termite balls collected from egg piles were carried at the same rate as were termite eggs (Mann–Whitney U-test, Z=0.72, p=0.382). Different letters indicate significant differences at the 0.05 level, as shown by post hoc Scheffé's tests. Note that 0.4 mm beads are within the range of the short diameter of termite eggs.
Reticulitermes okinawanus workers tended termite balls similarly to their own eggs (piling rate: 0.975±0.05s.d. versus 0.988±0.025s.d., respectively; Mann–Whitney U-test, Z=0.144, p=0.885).
(c) Size and surface texture
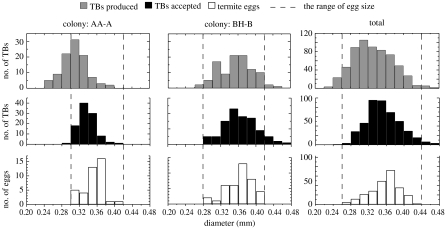
The size analysis showed significant effects of colony and object category (two-way ANOVA, colony: F5,1249=45.32, p<0.0001; object category: F2,1249=86.20, p<0.0001). The mean diameter of produced termite balls was significantly smaller than that of accepted termite balls (Scheffé's test, p<0.0001). Nevertheless, there was no significant difference between the mean diameter of accepted termite balls and the mean short diameter of termite eggs (Scheffé's test, p=0.484). This result clearly shows that termites selectively carry termite balls whose diameters are similar to the eggs, whereas the diameter range of termite balls produced by the fungus did not exactly match the range of the short diameter of termite eggs (figure 4). Termite balls less than 0.24 mm in diameter were never tended by termites, even when densely coated with egg chemicals. There were significant differences among termite colonies in egg size (one-way ANOVA, F5,234=20.74, p<0.0001). The size of termite eggs changes as the embryo develops, and nutritional condition may also affect egg size, which accounts for differences in egg size among colonies.
Figure 4.
Comparison of the frequency distributions of the short diameter of eggs, the diameter of termite balls collected from egg piles of each colony (accepted TBs) and the diameter of termite balls produced by fungi isolated from each colony (produced TBs). The data of two representative colonies (AA-A and BH-B) and the pooled data for all colonies are shown. Termites almost exclusively accepted only termite balls with diameters similar to those of eggs.
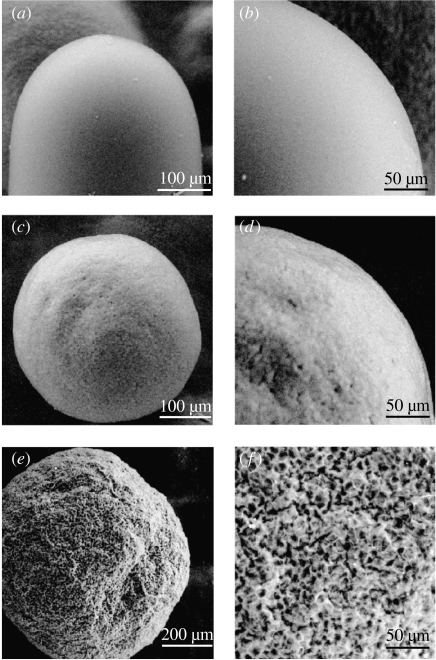
SEM observations revealed extremely smooth surface structure of both termite eggs and termite balls, and termite balls showed marked differences in surface texture from the sclerotia of the closely related fungus A. epiphylla (figure 5). The sclerotia of A. epiphylla were never tended by termites, even if they were densely coated with egg chemicals.
Figure 5.
(a, b) Comparative observations of the micro-structure of the surfaces of an egg of R. flavipes, (c, d) a termite ball and (e, f) a sclerotium of the closely related corticioid fungus Athelia epiphylla, using a variable-pressure scanning electron microscope. Termite balls have remarkably smooth surfaces compared to those of the sclerotia of A. epiphylla. Note that this termite ball was formed on an agar plate without termites; therefore, the smooth surface structure is not the result of grooming by termites.
4. Discussion
Comparison of egg survival rates with and without termite balls showed no significant effect in either R. flavipes or R. virginicus. Termite workers always formed egg piles on pieces of nest material. This may improve egg survivorship because the nest wood was coated with plaster made of termite faecal matter, which contains antibiotic substances (e.g. Rosengaus et al. 1998). Indeed, the survival rates of eggs maintained with nest material were greater than those without nest material. Previously, a positive effect of termite balls on egg survivorship was detected in R. speratus under experimental conditions without nest material (Matsuura et al. 2000). However, such a positive effect seems unlikely under natural conditions and may have only been detectable as an incidental by-product of antagonistic interactions between the termite-ball fungus and other micro-organisms under unnatural conditions. Termites do not consume termite balls, and the termite-ball fungus confers no nutritional benefit to termites (Matsuura 2002). The number of termite balls sometimes exceeds the number of termite eggs in egg piles (Matsuura et al. 2000; Matsuura 2005). Workers need to groom tens of thousands of eggs and termite balls daily, thereby incurring substantial time and energetic costs. Moreover, I found that the fungus sometimes killed the eggs of R. flavipes, even when they were under the care of workers, although the occurrence of egg consumption by the fungus was rare. Therefore, the net outcome of the interaction seems most likely to be negative for termites. In contrast, the termite-ball fungus profits from this interaction by receiving virtually competitor-free habitat in termite nests and protection and transport from termites. Thus, I conclude that the interaction is parasitic, in that it is beneficial only for the fungus but costly for the host termites.
The cost of parasitism to the host should favour the evolution of egg discrimination by the host, which in turn should lead to the evolution of egg mimicry by the parasite (Brooke & Davies 1988; Lyon & Eadie 2004). The sensory system used for egg recognition and the cognitive constraints of termites should affect the evolution of egg mimicry by the termite-ball fungus. Because termite workers live in the dark and do not have eyes, they cannot visually recognize eggs. Therefore, no selection acts on the colour of termite balls, which is consistent with the observation of various colours of termite balls that are quite different from the colour of termite eggs (figure 1b). This contrasts with egg mimicry in cuckoos, in which the colour and pattern of the eggshell resembles that of the host bird eggs, because host birds recognize their eggs visually (Brooke & Davies 1988; Servedio & Lande 2003).
In termites, precise morphological similarity and chemical mimicry are necessary for the fungus to be recognized as eggs. SEM observations revealed extremely smooth surface structure of both termite eggs and termite balls, with termite balls showing marked differences in surface texture from the sclerotia of a closely related fungus (figure 5). In comparison with sclerotia of other closely related fungi (Matsuura et al. 2000), termite balls are the smoothest and smallest sclerotia, probably resulting from selection for egg mimicry. Interestingly, the size experiment showed that termites selectively carry termite balls with diameters similar to those of eggs (figure 4). Even R. okinawanus workers, which have no natural interaction with termite-ball fungi, tended termite balls. This species may be less likely to carry termite balls without mimicry.
In the laboratory, the termite-ball fungus reproduced only by forming sclerotia, and I have not observed the formation of spores. Phylogenetic analysis showed no significant molecular difference among host species or geographical locations, suggesting long-distance gene flow and horizontal transmission. In addition, most natural termite colonies have termite balls in their egg piles (Matsuura 2005). This seems impossible to explain without spore formation, indicating that information on part of the life cycle of the termite-ball fungus is still missing. This fungus may have a free-living sexual stage independent of termites.
Acknowledgments
I thank Drs N. E. Pierce, E. Vargo, S.-P. Quek, D. S. Hibbett, J. N. Thompson, T. Nishida, C. Tanaka, K. Fujisaki, T. Tamura and F. Nakasuji for helpful advice and discussion. I gratefully acknowledge Y. Hirai for help with SEM observations. I thank Dr A. Nakagiri, Institute for Fermentation, Osaka, for providing IFO strains. This work was funded by the Japan Society for the Promotion of Science (JSPS) and the Program for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN). Experiments (b) and (c) were partially conducted in Japan. Reticulitermes flavipes and R. virginicus colonies were imported to Japan from the USA with the special permission of the Ministry of Agriculture, Forestry and Fisheries of Japan (permission no. 17-514, Kobe plant protection station).
References
- Allan R.A, Capon R.J, Brown W.V, Elgar M.A. Mimicry of host cuticular hydrocarbons by salticid spider Cosmophasis bitaeniata that preys on larvae of tree ants Oecophylla smaragdina. J. Chem. Ecol. 2002;28:835–848. doi: 10.1023/a:1015249012493. 10.1023/A:1015249012493 [DOI] [PubMed] [Google Scholar]
- Brooke M. de L, Davies N.B. Egg mimicry by cuckoos Cuculus canorus in relation to discrimination by hosts. Nature. 1988;335:630–632. 10.1038/335630a0 [Google Scholar]
- Hostettler N.C, Hall D.W, Scheffrahn R.H. Intracolony morphometric variation and labral shape in Florida Reticulitermes (Isoptera: Rhinotermitidae) soldiers: significance for identification. Fla. Entomol. 1995;78:119–129. [Google Scholar]
- Ito F, Hashim R, Huei Y.S, Kaufmann E, Akino T, Billen J. Spectacular Batesian mimicry in ants. Naturwissenschaften. 2004;91:481–484. doi: 10.1007/s00114-004-0559-z. 10.1007/s00114-004-0559-z [DOI] [PubMed] [Google Scholar]
- Kistner D.H. A new genus and species of a queen mimicking termitophile from Brazil (Coleoptera: Staphylinidae) Sociobiology. 2000;35:191–195. [Google Scholar]
- Lindstrom L, Alatalo R.V, Lyytinen A, Mappes J. The effect of alternative prey on the dynamics of imperfect Batesian and Mullerian mimicries. Evolution. 2004;58:1294–1302. doi: 10.1111/j.0014-3820.2004.tb01708.x. [DOI] [PubMed] [Google Scholar]
- Lyon B.E, Eadie J.M. An obligate brood parasite trapped in the intraspecific arms race of its hosts. Nature. 2004;432:390–393. doi: 10.1038/nature03036. 10.1038/nature03036 [DOI] [PubMed] [Google Scholar]
- Malcolm S.B. Mimicry: status of a classical evolutionary paradigm. Trends Ecol. Evol. 1990;5:57–62. doi: 10.1016/0169-5347(90)90049-J. 10.1016/0169-5347(90)90049-J [DOI] [PubMed] [Google Scholar]
- Matsuura, K. 2002 Sociobiology of the termite Reticulitermes speratus [In Japanese.] Doctoral thesis, Kyoto University, Kyoto.
- Matsuura K. Symbionts affecting termite behavior. In: Miller T, Bourtzis K, editors. Insect symbiosis. CRC Press; Boca Raton, FL: 2003. pp. 131–143. [Google Scholar]
- Matsuura K. Distribution of termite egg-mimicking fungi (termite balls) in Reticulitermes spp. (Isoptera: Rhinotermitidae) nests in Japan and the United States. Appl. Entomol. Zool. 2005;40:53–61. 10.1303/aez.2005.53 [Google Scholar]
- Matsuura K, Tanaka C, Nishida T. Symbiosis of a termite and a sclerotium-forming fungus: sclerotia mimic termite eggs. Ecol. Res. 2000;15:405–414. 10.1046/j.1440-1703.2000.00361.x [Google Scholar]
- Moland E, Jones G.P. Experimental confirmation of aggressive mimicry by a coral reef fish. Oecologia. 2004;140:676–683. doi: 10.1007/s00442-004-1637-9. 10.1007/s00442-004-1637-9 [DOI] [PubMed] [Google Scholar]
- Rosengaus R.B, Guldin M.R, Traniello J.F.A. Inhibitory effect of termite fecal pellets on fungal spore germination. J. Chem. Ecol. 1998;24:1697–1706. 10.1023/A:1020872729671 [Google Scholar]
- Servedio M.R, Lande R. Coevolution of an avian host and its parasitic cuckoo. Evolution. 2003;57:1164–1175. doi: 10.1111/j.0014-3820.2003.tb00325.x. [DOI] [PubMed] [Google Scholar]
- Singer R.B, Flach A, Koehler S, Marsaioli A.J, Amaral M.D.E. Sexual mimicry in Mormolyca ringens (Lindl.) Schltr. (Orchidaceae: Maxillariinae) Ann. Bot. 2004;93:755–762. doi: 10.1093/aob/mch091. 10.1093/aob/mch091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal R.R, Rohlf F.J. 3rd edn. W.H. Freeman; New York, NY: 1995. Biometry: the principles and practice of statistics in biological research. [Google Scholar]
- Swofford D.L. Smithsonian Institution/Sinauer Associates; Washington, DC/Sunderland, MA: 2001. PAUP*: phylogenetic analysis using parsimony and other methods. Version 4.0b10. [Google Scholar]
- Szalanski A.L, Austin J.W, Owens C.B. Identification of Reticulitermes spp. (Isoptera: Rhinotermitidae) from south central United States by PCR–RFLP. J. Econ. Entomol. 2003;96:1514–1519. doi: 10.1093/jee/96.5.1514. [DOI] [PubMed] [Google Scholar]
- Thompson J.D, Gibson T.J, Plewniak F, Jeanmougin F, Higgins D.G. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. 10.1093/nar/25.24.4876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T.J, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A, Gelfand D.H, Sninsky J.J, White T.J, editors. PCR protocols: a guide to methods and applications. Academic Press; San Diego, CA: 1990. pp. 315–322. [Google Scholar]
- Wickler W. McGraw-Hill; New York, NY: 1968. Mimicry in plants and animals. [Google Scholar]
- Wiklund C, Tullberg B.S. Seasonal polyphenism and leaf mimicry in the comma butterfly. Anim. Behav. 2004;68:621–627. 10.1016/j.anbehav.2003.12.008 [Google Scholar]