Abstract
It is well established that changes to the chemical environment can impair development, physiology and reproductive biology; by contrast, impacts on communication have not been widely reported. This is surprising given that chemical communication is the most widely used sensory modality in nature, and that variation in the chemical composition of the environment is the rule, not the exception. Here, we show that chemically mediated species recognition in a swordtail fish, Xiphophorus birchmanni, can be hindered by anthropogenic disturbance to the signalling environment. Females have a strong preference for conspecific male chemical cues, yet they hybridize in nature with the congener X. malinche. Wild-caught females showed a strong preference for conspecifics when tested in clean water, but failed to show a preference when tested in stream water subject to sewage effluent and agricultural runoff. We hypothesized that this was due to the interaction between chemical communication systems and humic acid (HA), a ubiquitous, natural product elevated to high levels by anthropogenic processes. When exposed to elevated concentrations of HA, female X. birchmanni again lost their preference for conspecific male chemical cues, while visual mating preferences and motivation to mate were retained. Sub-lethal concentrations of seemingly benign substances can thus have a drastic effect on natural populations through their specific impact on communication systems.
Keywords: olfaction, hybridization, environmental disturbance, mate choice, Poeciliidae
1. Introduction
Many animals rely on chemical cues in mate choice and species recognition (Wyatt 2003), and for species that discriminate among potential mates via chemical cues, damage to either chemoreceptors or chemosignals could have drastic evolutionary effects. In the poeciliid fish genus Xiphophorus, females often show mating preferences for the visual cues of other species (Ryan & Wagner 1987; Crapon de Caprona & Ryan 1990; Basolo 1995; Hankison & Morris 2003), yet show robust preferences for the chemical cues produced by conspecific males (Crapon de Caprona & Ryan 1990; McLennan & Ryan 1997; McLennan & Ryan 1999). Given that Xiphophorus often co-occur with closely related congeners, these findings suggest that reproductive isolation may hinge on females' ability to discriminate among male odours of different species.
X. birchmanni and X. malinche are two species of swordtail fish from the Río Panuco Basin in northeastern Mexico. Males of both species exhibit divergent sexually dimorphic visual traits (figure 1a), and hybrids between the two species display easily recognized recombinant phenotypes (Rosenthal et al. 2003; figure 1b). Despite the conspicuousness of hybrids, Rauchenberger et al. (1990) noted ‘no evidence of hybridization’ in 1988 among sympatric populations in the Río Calnali in the state of Hildago, eastern Mexico despite an extensive survey based on morphometrics and isozyme markers. In 1997, Rosenthal et al. (2003) discovered a population of hybrid origin at the same site based on similar morphometrics and the same isozyme markers. Taken together, these studies suggest a recent hybridization event. Continued sampling of the Río Calnali suggests that individuals of hybrid origin are ubiquitous, and pure-species individuals are non-existent or rare (Rosenthal et al. 2003). A survey of mitochondrial haplotypes indicates that most of the hybrids are descended from X. birchmanni females (hybrid individuals sampled from three sites in 2003 from the Río Calnali, X. birchmanni haplotypes, N=62; X. malinche haplotypes, N=18; 435 bp of the control region D-loop) suggesting that disruption of species-recognition mechanisms in X. birchmanni females may have played a role in hybridization. We therefore focused on the ability of female X. birchmanni to discriminate male conspecific cues from those of X. malinche in an altered chemical environment.
Figure 1.
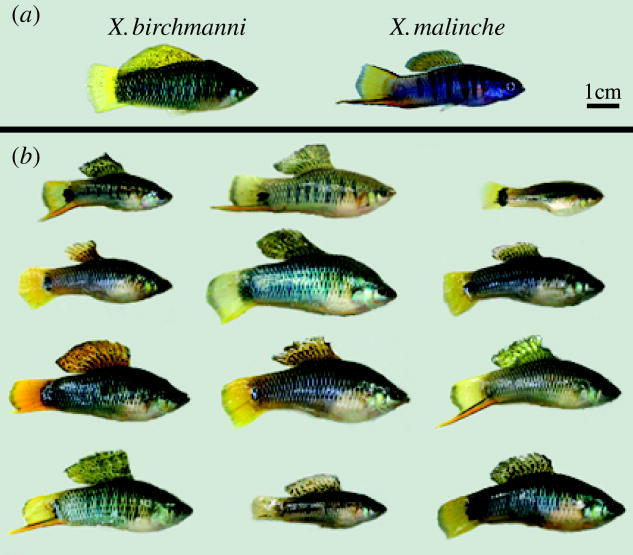
Wild-caught, sexually mature male swordtails. (a) Pure X. birchmanni and X. malinche. (b) X. birchmanni/malinche hybrids collected in the Río Calnali at Chahauco. Hybrids display virtually all possible combinations of male traits and often show traits outside of the range of parental species (Rosenthal et al. 2003).
We began our investigation by testing female mating preference in the water collected directly from the stream where hybrids occur. During the last two decades, in the time that the X. birchmanni/malinche hybrid zone is likely to have arisen, the Río Calnali has been subject to increased disturbance, most notably due to the influx of untreated sewage and agricultural waste. It is well established that exogenous substances, including synthetic products, can have damaging effects on chemical communication (Park et al. 2001; Park & Propper 2002). Human impacts, however, may also cause changes in the abundance of naturally occurring substances. We next considered the potential effect of one such substance, humic acid (HA). HA results from degrading organic matter, and thus processes that increase plant and algal growth, such as eutrophication, are likely to be followed by high levels of HA before it is degraded by ultraviolet radiation (Thomas 1997). Natural levels of HA reflect this process and have been observed to fluctuate as much as 5 mg l−1 over a matter of hours, and can range from trace amounts to over 200 mg l−1 (Steinberg 2003). Although its chemical structure is unknown, HA has been shown to produce slight feminization in swordtail fishes (Steinberg et al. 2004), and to affect a variety of biological processes (Sato et al. 1987; Maggioni et al. 1992; Lovley et al. 1996; Qiao & Farrell 2002). In particular, HA has an affinity for two teleost steroidal reproductive pheromones (Mesquita et al. 2003) and causes a decrease in olfactory response to those pheromones in goldfish (Carassius auratus; Hubbard et al. 2002). All known fish sexual pheromones are either steroids or prostaglandins, both of which possess hydrophobic properties (Stacey et al. 2003). In water, HA forms microvesicles containing a hydrophobic core (Simpson et al. 2002), which may bind to pheromones and leave them undetectable to chemoreceptors (Hubbard et al. 2002). Furthermore, HA has been shown to inhibit membrane-bound receptors (Yang et al. 2002), suggesting that HA may also block or damage chemoreceptors.
We tested female X. birchmanni preferences for conspecific and heterospecific male chemical cues in water from a hybrid zone, and in water containing supplemental HA. We then performed visual preference tests to control for the effects of HA on motivation to mate, and food preference tests to investigate effects of HA on chemosensory behaviour.
2. Material and methods
All subjects were wild-caught adults from allopatric populations in Mexico: X. birchmanni were obtained from the Río Garces, Hidalgo (20°57′22 N, 098°16′48 W) and X. malinche from the type locality (Rauchenberger et al. 1990) in the Río Claro at Tlatzintla (20°50′56 N, 098°43′06 W).
(a) Chemical preference tests
We closely followed the published methods of McLennan & Ryan (1997 and 1999) for preparing the male stimulus water, cleaning the testing tanks, delivering the stimuli to the testing tanks and controlling the stimuli; any alterations to these methods are stated below. Male odour stimuli were prepared by placing five conspecific males, into a single 40 l collection aquarium for 24 h adjacent to a tank containing five conspecific females to provide them visual stimulation. Female chemical preference tests were conducted in an aquarium (length×width×height=75×30×30 cm3) divided lengthwise into three equal sections by lines drawn on the sides of the tank, and filled with 40 l of water. Each test tank had two stimulus delivery systems located at either end of the tank containing stimulus water from male conspecifics (X. birchmanni) and heterospecifics (X. malinche).
Each female was acclimatized for 30 min in the test tank containing testing water; stimulus flow was then initiated. A preference test began once the female had passed into both side compartments and continued for 300 s. If the female did not visit both compartments within 300 s, the trial was ended. Variables scored were (i) latency—time from initiation of stimulus flow until female comes within 40 cm of each stimulus outflow, (ii) association time—total time spent within 40 cm of stimulus outflow and (iii) proximity—frequency of female within 10 cm of the stimulus outflow, recorded using one-zero sampling (Martin & Bateson 1993) taken every 30 s during preference test. In cases in which the female failed to respond to both stimuli within 300 s, latency was scored as 300 s for the stimulus not visited, and association time and proximity were scored as zero.
In order to test female X. birchmanni preference in stream water from the Río Calnali, water was collected daily for testing. Water was taken from three sources: (i) stream water at Calnali-low (Rosenthal et al. 2003), (ii) water from a nearby natural spring at Ahuimol and (iii) Calnali municipal tap water, which is obtained directly from the Río Calnali and chlorinated. We treated the tap water with SeaChem Prime water conditioner for aquarium systems to remove the chlorine. Twenty-five wild-caught females were collected and housed in the laboratory for one week to acclimatize them before experimentation began. In order to be sure a female came into contact with both chemical cues, and a choice between the two stimuli was made, only females that visited both tank sides were tested and used in the analysis. To control for order effects, treatments were randomized.
For the assays investigating effects of HA, X. birchmanni females were arbitrarily assigned as ‘experimental’ (N=15) and ‘control’ (N=13) fish, and we conducted four preference trials on each female. On day 0, all experimental and control fish were tested in water without supplemental HA. On day 2, experimental fish were tested in 200 mg l−1 HA (Sigma-Aldrich 53680), and control fish were again tested without HA. On days 4 and 12, both groups were tested without supplemental HA.
Although 200 mg l−1 HA is consistent with similar studies investigating its effects of fish sensory behaviour (Hubbard et al. 2002; Mesquita et al. 2003), we repeated the experiment at 20 and 2 mg l−1 with a new set of females to investigate the behavioural response over a range of conditions. All females (experimental, N=15; control, N=15) were tested for their preference in water without supplemental HA to establish a baseline, and with each concentration.
An additional 12 females were tested for their preference for water containing 0.1 g l−1 TetraMin fish food over water lacking food odour in water containing 0, 20 and 200 mg l−1 HA as an additional control. Testing methods closely followed those of the mate recognition test, except that females were not fed for 24 h prior to testing. Based on the mate-recognition results, we waited at least 10 days before retesting a female, and treatment order was randomized to control for order effects.
Testing water was buffered and the effects of HA on pH were minimal: pH without supplemental HA was 7.8±0.2, pH with 200 mg l−1 HA was 7.4±0.1; both are well within the range of natural conditions. We alternated the side at which conspecific and heterospecific stimuli (or food versus no food) were presented to control for side biases.
(b) Visual preference tests
We tested females using the same synthetic animated stimuli shown in Wong & Rosenthal (2006) to elicit a strong conspecific preference for X. birchmanni males. X. birchmanni and X. malinche male stimuli were generated using mean trait values for each species in standard length and depth, sword length and dorsal fin height; a representative male from each species was used to generate body texture and vertical bars. Quantitative analysis of X. birchmanni and X. malinche courtship interactions revealed that both species perform a simple, conserved courtship display. Animations showed males repeatedly swimming onscreen, performing this courtship behaviour, and then swimming off-screen.
The testing procedure was identical to that used in a previous study of X. birchmanni mating preferences (Wong & Rosenthal 2006). Experiments were conducted in an aquarium identical to the ones used in the chemical preference tests (see above). We used two 35 cm IBM CRT monitors, each positioned on opposite ends of the aquarium and connected to a central computer (Dell OptiPlex GX260) to deliver the animated sequences. Females were acclimatized for 10 min, then presented with 10 min of monochromatic screen, followed by simultaneous presentation of the 300 s test stimulus. Subjects were then presented with another 10 min of monochromatic screen followed by the same set of 300 s test stimuli, now switched between monitors to control for any side biases. The side from which a given stimulus was first displayed was also alternated between trials. Female preferences were estimated by measuring association time. Females that spent more than 90% of the total time in any one section of the aquarium were defined as unresponsive and were excluded from analysis (Wong & Rosenthal 2006). Females were tested in water either with or without 200 mg l−1 supplemental HA as described above.
(c) Statistical analysis
To assess female preference in each trial, we analysed the association time and proximity data using paired, two-tailed t-tests. To compare the chemical preference data (latency, association time and proximity) over the length of the experiment in each treatment (control and experimental), we used a repeated measures factorial ANOVA. We used a Fisher's exact test to assess female responsiveness in control and experiment treatments. To examine the data from the preference tests conducted in reduced HA concentration, those involving food cues, and the visual preference tests, we analysed females' net association time (time with conspecific cue−time with heterospecific cue) from control versus experimental treatments using two-sample, t-tests.
3. Results
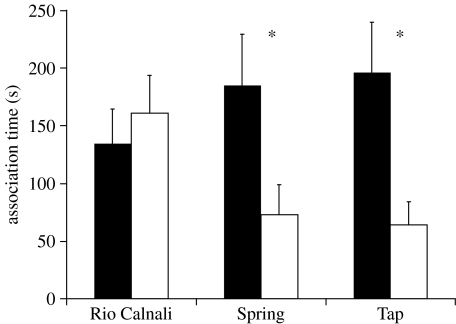
Female X. birchmanni failed to show a significant preference for either male conspecific or heterospecific (X. malinche) chemical stimuli in Río Calnali water (N=12, paired t-test, p=0.59, t=0.54), and preferred conspecific cues in spring (N=15, paired t-test, P=0.05, t=2.15) and tap water trials (N=14, paired t-test, p=0.02, t=2.64; figure 2).
Figure 2.
Association time (s; mean±s.e.) of female X. birchmanni with chemical cues of conspecific (black bars) and heterospecific X. malinche (white bars) males. ‘Río Calnali’ indicates trials conducted in water from the stream; ‘Spring’ represents trials conducted in natural spring water; ‘Tap’ indicated trials conducted in municipal tap water. Asterisks indicate a significant preference for conspecific cues over heterospecific: *p<0.05.
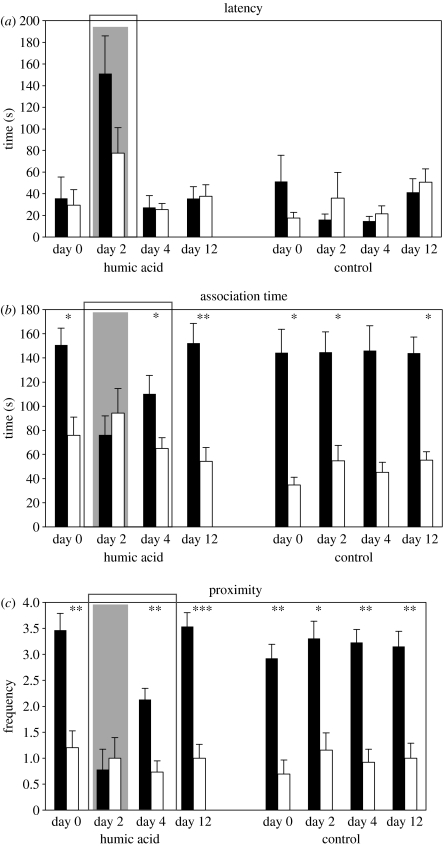
In the experiments conducted on HA, 15 females were exposed to water containing supplemental HA, and 13 control females were tested in water without HA. All females responded to both stimuli in all clear-water trials (N=15), but significantly fewer subjects tested in HA responded to male chemical cues at all (N=9, Fisher's exact test: p=0.018). Female latency to conspecific cues did not differ significantly across trials in the control group (repeated measures ANOVA, F=1.891, p=0.149, d.f.=3, 12); however, latency to conspecific cues in the trial conducted in HA was significantly greater than in all other trials (repeated measures ANOVA, F=11.098, p<0.001, d.f.=3, 14; Bonferroni-corrected post hoc tests, p<0.05), females responded more slowly only when in water treated with HA (figure 3a). In the control group, there were no significant differences in female association time to the conspecific cue among trials (repeated measures ANOVA, F=0.755, p=0.526, d.f.=3, 12), but in the HA experimental group, females responded to conspecific odour cues significantly less during the trial conducted in HA and the trial conducted 2 days following exposure, than in the other trials (repeated measures ANOVA, F=16.913, p<0.001, d.f.=3, 14, Bonferroni-corrected post hoc tests, p<0.05; figure 3b). The results measuring the frequency that females spent within one body length of conspecific versus heterospecific stimulus outflows closely mirror the association time data: in the control group, there was no significant difference in female response to the conspecific cue among trials (repeated measures ANOVA, F=0.108, p=0.955, d.f.=3, 12), but in the HA experimental group females responded to conspecific odour cues significantly less during the trial conducted in HA and the trial conducted 2 days following exposure, than in the other trials (repeated measures ANOVA, F=17.324, p<0.001, d.f.=3, 14, Bonferroni-corrected post hoc tests, p<0.05; figure 3c).
Figure 3.
Responses (mean±s.e.) of female X. birchmanni to chemical conspecific (black bars) and heterospecific X. malinche (white bars) male cues. ‘Humic acid’ represents experimental fish that were subjected to a preference trial conducted in 200 mg l−1 HA; hatched-shaded area depicts the one trial conducted in HA. ‘Control’ indicates fish that were consistently tested in water not containing supplemental HA. Bars surrounded by a black box indicate a significant difference (p<0.001) in females' response to conspecific male cues relative to other trials (bars not included in boxed area). Asterisks indicate a significant preference for conspecific cues over heterospecific: *p<0.05; **p<0.01, ***p<0.001. (a) Latency to respond to conspecific and heterospecific odour cues. (b) Females' association time with each cue. (c) The frequency that females were within one body length of conspecific and heterospecific stimuli outflow.
Females tested at 20 mg l−1 HA also showed a loss of preference for conspecific cues (paired t-test N=15, t=0.273 p=0.788) and their net preference (time with conspecific cue minus time with heterospecific cue) was significantly different from clear-water trials (two-sample t-test N=15, t=2.24 p=0.041). Females showed a significant preference for conspecific cues in water containing only 2 mg l−1 HA (paired t-test N=15, t=2.36, p=0.033) and the net preference did not differ from clear-water trials (two-sample t-test N=15, t=0.805, p=0.434).
Females showed a strong preference for food odour cues in clear-water trials (paired t-test, N=12, t=4.83, p=0.0003), and no preference for food cues in water containing 20 mg l−1 HA (paired t-test, N=12, t=0.422, p=0.679) or 200 mg l−1 HA (paired t-test, N=12, t=1.14, p=0.883). The net preference of females in trials conducted with HA differed significantly from clear-water trials (two-sample t-tests, 20 mg l−1 HA N=12, t=3.71, p=0.002; 200 mg l−1 HA N=12, t=3.35, p=0.005).
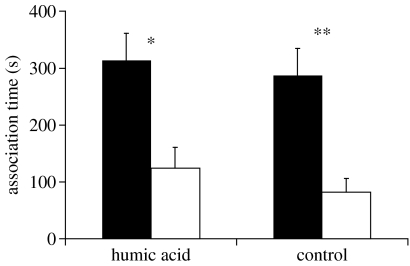
Female X. birchmanni showed a significant preference for conspecific male visual stimuli in water with and without supplemental HA (paired two-tailed t-test; HA tests: N=11, t=2.84, p=0.017; control: N=11, t=3.86, p=0.003), and the net preferences of females in both groups are not significantly different from one another (two-sample t-test, t=0.191, p=0.85).
4. Discussion
Female preference assays showed that disturbance to the chemical environment can, indeed, affect chemical communication and mechanisms of species recognition, suggesting that disturbance may facilitate hybridization. We found that in laboratory experiments conducted in water obtained directly from the Río Calnali, female X. birchmanni, from a population allopatric to the hybrid zone, showed no preference when presented with chemical stimuli from conspecific and heterospecific males (figure 2). However, when the same females were tested in water obtained from a natural spring and in tap water, they showed a significant preference for conspecific cues over heterospecific (figure 2). The stream water is likely to contain much higher concentrations of contaminants than spring or tap water. The primary input of contaminants upstream of the sample point is human and animal waste, including sewage discharge from the small community of Ahuacatlán. Pesticides and other synthetic contaminants are rarely employed by the subsistence farmers and ranchers in the area. Our findings suggest that water within the Río Calnali is facilitating hybridization between the two species, and that the sub-lethal contamination influencing the system can have a significant effect on communication.
In laboratory tests without supplemental HA, X. birchmanni females strongly preferred conspecific male chemical cues over heterospecific cues (figure 3). When we measured female preferences in water containing 200 mg l−1 HA, we found that they responded to both conspecific and heterospecific stimuli more slowly than in tests without HA (figure 3a). All females responded to both stimuli in all clear-water trials, but significantly fewer subjects tested in HA responded to male chemical cues at all. Furthermore, the females that did respond in HA trials, did not exhibit a difference in preference between conspecific and heterospecific male odour cues (figure 3b,c). Females tested at a markedly lower HA concentration, 20 mg l−1, also showed a loss of preference for conspecific cues; female preference, however, was retained in water containing only 2 mg l−1 HA.
In order to determine whether the loss of female preference was due to specific effects on chemical communication, rather than on females' overall motivation to mate, we tested females with computer animations of courting X. birchmanni and X. malinche males, in water with and without 200 mg l−1 of supplemental HA. Females showed nearly identical, significant preferences for conspecific visual cues in both treatments (figure 4). HA therefore inhibited females' species recognition based on chemical, but not visual cues. We also found that while females showed a strong preference for food odour cues in clear-water trials, they showed no preference for food cues in water containing 20 and 200 mg l−1 HA. These results further support the notion that HA disrupts detection of chemical cues in aquatic animals.
Figure 4.
Association time (mean±s.e.) of female X. birchmanni with computer animations of conspecific (black bars) and heterospecific X. malinche (white bars) males. ‘Humic acid’ indicates trials conducted in 200 mg l−1 HA; ‘Control’ represents trials conducted without supplemental humic acid. Asterisks indicate a significant preference for conspecific cues over heterospecific: *p<0.05; **p<0.01.
All experimental females were exposed to a single, brief (approximately 45 min) bout of elevated HA concentration during a preference trial. In order to evaluate the long-term effects of this brief exposure, we retested the females in water without supplemental HA 2 days after the 200 mg l−1 HA treatment. While response latency returned to prior levels (figure 3a) and females again showed a preference for conspecific male odour (figure 3b,c), the strength of the response was significantly weakened: females continued to associate significantly less with conspecific odour when compared to their responses before exposure and 10 days after exposure, and to the responses of control females never exposed to HA (figure 3b). These results suggest that the effects of exposure to high levels of HA can persist even after a pulse of disturbance, likely due to a damaging effect of HA on the binding properties of chemoreceptor cells. In goldfish, HA reduces the response of olfactory epithelium and olfactory bulb to sexual pheromones (Hubbard et al. 2002). Furthermore, HA is known to inhibit binding and interfere with the function of mammalian receptor cells (Yang et al. 2002). Ten days following exposure to 200 mg l−1 HA, we found that females once again showed a strong preference for conspecific male odour (figure 3b,c). These results suggest that if HA did inflict chemoreceptive damage, full functional recovery was obtained after 10 days. Chemoreceptor regeneration time is unknown in Xiphophorus, but full regeneration may not even be necessary for certain olfactory discrimination tasks. In goldfish, behavioural responses and amino-acid discrimination ability persisted even after the surface of olfactory epithelium was reduced by 85% (Zippel 2000). Control females that were tested in water without supplemental HA throughout the series of trials consistently showed a strong significant preference for conspecific male odour, suggesting that the response profile of experimental females was due to the effects of HA rather than to habituation to the stimuli.
In addition to affecting chemoreception, HA may also be interfering with chemical cues themselves. Females' latency to respond to both conspecific and heterospecific cues was significantly greater in the trials conducted with supplemental HA than in any of those without, and latency did not differ across trials without HA (figure 3a). Humic substances are known to bind to hydrophobic chemicals and thus reduce their aqueous concentrations. Available concentrations of a steroid pheromone in goldfish were strongly reduced by the similar concentrations of HA we used in our study (Mesquita et al. 2003). We therefore cannot exclude the possibility that HA may interfere with the concentration of available pheromone in the swordtail signalling environment.
Regardless of the mechanisms whereby HA interferes with the female reproductive behaviour in X. birchmanni, the consequences are clear: elevated levels of HA, a natural and ubiquitous substance, compromises females' ability to discriminate between conspecific and heterospecific male odour cues without disrupting their motivation to mate (as shown in the visual preference tests). And although we cannot exclude the hypothesis that other compounds in Río Calnali may have influenced swordtail hybridization, our laboratory experiments directly test the effects of HA of species recognition of X. birchmanni. Despite the benefits that HA may provide (Kullberg et al. 1993), our results suggest that its impact could also be detrimental for some species. HA concentrations vary broadly in nature (Steinberg 2003). Particular environments such as the Río Negro in the Amazon River Basin, however, contain consistently high natural HA levels (Wood et al. 2003), and are home to diverse assemblages of animals that rely on chemical cues in social communication (Weitzman & Fink 1985). Furthermore, other animals seem unaffected in environments contaminated with sub-lethal amounts wastewater and agricultural effluent. Such animals may have evolved mechanisms for coping with their environments.
The results presented here are particularly important and biologically relevant to the X. birchmanni system given the recent hybridization with X. malinche. Rosenthal et al. (2003) found that most individuals sampled in the hybrid zone are F2 or greater, suggesting that adults of hybrid origin largely contribute to the breeding pool. Therefore, even if the disturbance to the signalling environment is not continual or wide ranging, hybrid offspring will likely mate and result in pervasive hybridization. As exploitation of natural water resources proceeds worldwide, we are gaining an increasingly clear picture of the impact of environmental disturbance on biological communities. While chemical and physical measures reflect conditions when the sample is taken, biological monitoring gives an indication of past, as well as current, conditions. Although lethal consequences are more easily recognized, subtle changes may also inflict substantial ecological disruption (Seehausen et al. 1997, Hayes et al. 2002). Much of the current research on the environmental causes of reproductive distress has focused on exogenous sources of contamination (Zala & Penn 2004). Our results illustrate how even a rare disturbance event may facilitate hybridization through a breakdown in species recognition mechanisms.
Acknowledgments
We thank Francisco García de León, Zuned Khalifa, and Juventino Tovar Ortiz for their assistance, as well as Russell Fernald, Chris Schneider, Matt Wachowiak, Karen Warkentin and two anonymous reviewers for their comments and discussion. We are also indebted to the Mexican federal government for collection permits. Research was supported by NSF grant IOB-0447665 to GGR. H.S.F. was supported by a Palmer-McLeod Fellowship and BBMW by a Sir Keith Murdoch Fellowship. This research adheres to the Association for the Study of Animal Behavior and the Animal Behavior Society Guidelines for the Use of Animals in Research.
References
- Basolo A.L. A further examination of a preexisting bias favoring a sword in the genus Xiphophorus. Anim. Behav. 1995;50:365–375. 10.1006/anbe.1995.0252 [Google Scholar]
- Crapon de Caprona M, Ryan M. Conspecific mate recognition in swordtails, Xiphophorus nigrensis and X. pygmaeus: olfactory and visual cues. Anim. Behav. 1990;39:290–296. [Google Scholar]
- Hankison S.J, Morris M.R. Avoiding a compromise between sexual selection and species recognition: female swordtail fish assess multiple species-specific cues. Behav. Ecol. 2003;14:282–287. 10.1093/beheco/14.2.282 [Google Scholar]
- Hayes T, Haston K, Tsui M, Hoang A, Haeffele C, Vonk A. Herbicides: feminization of male frogs in the wild. Nature. 2002;419:895–896. doi: 10.1038/419895a. 10.1038/419895a [DOI] [PubMed] [Google Scholar]
- Hubbard P.C, Barata E.N, Canario A.V.M. Possible disruption of pheromonal communication by humic acid in the goldfish, Carassius auratus. Aquat. Toxicol. 2002;60:169–183. doi: 10.1016/s0166-445x(02)00002-4. 10.1016/S0166-445X(02)00002-4 [DOI] [PubMed] [Google Scholar]
- Kullberg A, Bishop K.H, Hargeby A, Jansson M, Petersen R.C. The ecological significance of dissolved organic-carbon in acidified waters. Ambio. 1993;22:331–337. [Google Scholar]
- Lovley D, Coates J, Blunt-Harris E, Phillips E, Woodward J. Humic substances as election acceptors for microbial respiration. Nature. 1996;382:445–448. 10.1038/382445a0 [Google Scholar]
- Maggioni A, Varanini Z, Pinion R, de Biasi M. Humic substances affect transport properties of root membranes. In: Kubat J, editor. Humus: its structure and role in agriculture and environment. Elsevier Science Publishers; Amsterdam, The Netherlands: 1992. pp. 137–144. [Google Scholar]
- Martin P, Bateson P. 2nd edn. Cambridge University Press; Cambridge, UK: 1993. Measuring behaviour. [Google Scholar]
- McLennan D.A, Ryan M.J. Responses to conspecific and heterospecific olfactory cues in the swordtail Xiphophorus cortezi. Anim. Behav. 1997;54:1077–1088. doi: 10.1006/anbe.1997.0504. 10.1006/anbe.1997.0504 [DOI] [PubMed] [Google Scholar]
- McLennan D, Ryan M. Interspecific recognition and discrimination based upon olfactory cues in northern swordtails. Evolution. 1999;53:880–888. doi: 10.1111/j.1558-5646.1999.tb05382.x. [DOI] [PubMed] [Google Scholar]
- Mesquita R, Canario A.V.M, Melo E. Partition of fish pheromones between water and aggregates of humic acids. Consequences for sexual signaling. Environ. Sci. Technol. 2003;37:742–746. doi: 10.1021/es025987e. 10.1021/es025987e [DOI] [PubMed] [Google Scholar]
- Park D, Hempleman S.C, Propper C.R. Endosulfan exposure disrupts pheromonal systems in the red-spotted newt: a mechanism for subtle effects of environmental chemicals. Environ. Health Perspect. 2001;109:669–673. doi: 10.1289/ehp.01109669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Propper C.R. Endoculfan affects pheromonal detection and glands in the male red-spotted newt, Notophthalmus viridescens. Bull. Environ. Contam. Toxicol. 2002;69:609–616. doi: 10.1007/s00128-002-0104-8. 10.1007/s00128-002-0104-8 [DOI] [PubMed] [Google Scholar]
- Qiao P, Farrell A.P. Influence of dissolved humic acid on hydrophobic chemical uptake in juvenile rainbow trout. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2002;133:575–585. doi: 10.1016/s1532-0456(02)00178-3. 10.1016/S1532-0456(02)00178-3 [DOI] [PubMed] [Google Scholar]
- Rauchenberger M, Kallman K, Morizot D.C. Monophyly and geography of the Rio Panuco Basin swordtails (Genus Xiphophorus) with descriptions of four new species. Am. Mus. Nov. 1990;2975:1–41. [Google Scholar]
- Rosenthal G.G, de la Rosa Reyna X.F, Kazianis S, Stephens M.J, Morizot D.C, Ryan M.J, García de León F.J. Dissolution of sexual signal complexes in a hybrid zone between the swordtails Xiphophorus birchmanni and X. malinche (Poeciliidae) Copeia. 2003;2:299–307. [Google Scholar]
- Ryan M.J, Wagner W.E.J. Asymmetries in mating preferences between species: female swordtails prefer heterospecific males. Science. 1987;236:595–597. doi: 10.1126/science.236.4801.595. [DOI] [PubMed] [Google Scholar]
- Sato T, Ose Y, Nagase H, Hayase K. Mechanism of the desmutagenic effect of humic acid. Mut. Res. 1987;176:199–204. doi: 10.1016/0027-5107(87)90050-9. [DOI] [PubMed] [Google Scholar]
- Seehausen O, Van Alphen J.J.M, Witte F. Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science. 1997;277:1808–1811. 10.1126/science.277.5333.1808 [Google Scholar]
- Simpson A.J, Kingery W.L, Hayes M.H.B, Spraul M, Humpfer E, Dvortsak P, Kerssebaum R, Godejohann M, Hofmann M. Molecular structures and associations of humic substances in the terrestrial environment. Naturwissenschaften. 2002;89:84–88. doi: 10.1007/s00114-001-0293-8. 10.1007/s00114-001-0293-8 [DOI] [PubMed] [Google Scholar]
- Stacey N.E, Chojnacki A, Narayanan A, Cole T.B, Murphy C.A. Hormonally-derived sex pheromones in fish: exogenous cues and signal from gonad to brain. Can. J. Physiol. Pharmacol. 2003;81:329–341. doi: 10.1139/y03-024. 10.1139/y03-024 [DOI] [PubMed] [Google Scholar]
- Steinberg C.E.W. Springer; New York, NY: 2003. Ecology of humic substances in freshwaters. [Google Scholar]
- Steinberg C.E.W, Hoss S, Kloas W, Lutz I, Meinelt T, Pflugmacher S, Wiegand C. Hormonelike effects of humic substances on fish, amphibians, and invertebrates. Environ. Toxicol. 2004;19:409–411. doi: 10.1002/tox.20019. 10.1002/tox.20019 [DOI] [PubMed] [Google Scholar]
- Thomas J.D. The role of dissolved organic matter, particularly free amino acids and humic substances, in freshwater ecosystems. Freshwater Biol. 1997;38:1–36. 10.1046/j.1365-2427.1997.00206.x [Google Scholar]
- Weitzman S.H, Fink S.V. Xenurobryconin phylogeny and putative pheromone pumps in glandulocaudine fishes (Teleostei: Characidae) Smithson. Contrib. Zool. 1985;421:1–121. [Google Scholar]
- Wong B.B.M, Rosenthal G.G. Female disdain for swords in a swordtail fish. Am. Nat. 2006;167:136–140. doi: 10.1086/498278. [DOI] [PubMed] [Google Scholar]
- Wood C.M, Matsuo A.Y.O, Wilson R.W, Gonzalez R.J, Patrick M.L, Playle R.C, Val A.L. Protection by natural blackwater against disturbances in ion fluxes caused by low pH exposure in freshwater stingrays endemic to the Rio Negro. Physiol. Biochem. Zool. 2003;76:12–27. doi: 10.1086/367946. 10.1086/367946 [DOI] [PubMed] [Google Scholar]
- Wyatt T. Cambridge University Press; Cambridge, UK: 2003. Pheromones and animal behavior. [Google Scholar]
- Yang M.L, Huang T.S, Lee Y.S, Chen T.H, Chen S.Y, Lu F.J. Inhibition of endogenous thyroid hormone receptor-beta and peroxisome proliferator-activated receptor-alpha activities by humic acid in a human-derived liver cell line. Thyroid. 2002;12:361–371. doi: 10.1089/105072502760043422. 10.1089/105072502760043422 [DOI] [PubMed] [Google Scholar]
- Zala S.M, Penn D.J. Abnormal behaviors induced by chemical pollution: a review of the evidence and new challenges. Anim. Behav. 2004;68:649–664. 10.1016/j.anbehav.2004.01.005 [Google Scholar]
- Zippel H.P. In goldfish the discriminative ability for odours persists after reduction of the olfactory epithelium, and rapidly returns after olfactory nerve axotomy and crossing bulbs. Phil. Trans. R. Soc. B. 2000;355:1219–1223. doi: 10.1098/rstb.2000.0671. 10.1098/rstb.2000.0671 [DOI] [PMC free article] [PubMed] [Google Scholar]