Abstract
It has been reported that capuchin monkeys reject a less preferred food (LPF) when they see a partner capuchin receive a more preferred food (PF) for performing the same task. This behaviour was taken as evidence of ‘inequity aversion’, but an alternative hypothesis is that capuchins reject the LPF because of the mere presence of the PF. We tested this hypothesis in a paradigm, which consisted of presenting two different foods (one PF and one LPF) on a tray and allowing the capuchin to take only the LPF. Refusals to initiate the trial and refusals to take and eat the LPF were higher when the PF was hidden (hiding condition) and when the PF was accumulated in sight but out of reach of the subject (accumulation condition) compared to when two pieces of LPF were placed on the tray (control condition). Interestingly, the subject behaved as in the control condition when its partner was given and ate the PF (partner condition). We argue that capuchins' refusals were due to the frustration of seeing and not obtaining the PF, and that seeing the partner eating increases the LPF acceptance.
Keywords: frustration, inequity, social facilitation, tufted capuchin monkeys
1. Introduction
People make choices on the basis of the options that are available to them. For example, when a person is offered two similar jobs, one with a high salary and the other with a low salary, they will choose the high-salary job. However, when only low salaries are offered this same person will accept a low-salary job. The social context can affect choices in rather complex ways. In human societies decision making processes can be influenced by perceived fairness: a person is more willing to judge something as fair if they get the same as another individual gets. A person may judge getting a low salary unfair if their colleague (having the same expertise) gets a higher salary for the same work. This difference in salary can be felt as especially unfair if the better paid job requires no effort at all.
Although the sense of unfairness, or inequity aversion, seems an immediate and natural reaction, we know very little about its underlying psychological mechanisms. From a cognitive point of view, inequity aversion implies a comparison between the balance of our own effort and salary, and the balance of the effort and salary of the other person. A mismatch between these balances is perceived as unfair, either because it is advantageous or disadvantageous (Fehr & Schmidt 1999).
According to Brosnan & de Waal (2003), unfairness refusal is not a human peculiarity, since it is also observed in capuchin monkeys (Cebus apella), a socially tolerant primate. In their experiment, capuchins were less likely to perform an exchange for a less preferred food (LPF), i.e. to give back to the experimenter a token for a piece of cucumber, if they witnessed a partner exchanging a token for grape, a more preferred food (PF) (inequality condition). Moreover, capuchins refused more frequently to exchange the token, or to accept the food that they received in exchange for the token, when the partner received a grape without having to perform an exchange (effort control condition). According to the authors, capuchins perceived the above conditions as unfair and showed inequity aversion.
In analogy with what we described above, if the capuchins' behaviour was due to inequity aversion, we would have to assume a cascade of mental processes. A capuchin monkey would have to perceive of a relation between relations, i.e. to compare the relation between its own effort and reward (one token for one cucumber) with the relation between the partner's effort and reward (one token for one grape, or no effort for one grape). Only human beings, chimpanzees and to some extent baboons have to date been found capable of solving tasks in which the ability to perceive relations between relations is necessary (Fagot et al. 2001; for a review of the literature see Tomasello & Call 1997; Thompson & Oden 2000).
Although relations between relations have not been tested in capuchins yet, Spinozzi & Natale (1989) showed that capuchins, in contrast to chimpanzees and 18–24 month old infants, do not perform second-order classifications, the latter being considered as the premise for perceiving relations between relations (Langer 1980, 1986; Spinozzi 1993).
These considerations suggest that simpler cognitive mechanisms might explain the results of Brosnan & de Waal (2003). And indeed, upon closer inspection, their interpretation based on inequity aversion is not well supported by their own data (Wynne 2004). Specifically, the authors did not find a statistical difference between their inequality condition, in which the partner exchanged a token for a more PF, and their food control condition, in which the partner was not present and the more PF was accumulated in view but out of reach of the subject (fig. 1; Brosnan & de Waal 2003). The high number of refusals when there was no partner (i.e. when the subject could not experience that the partner had a better deal than itself) and cognitive complexity of perceiving relations between relations call for an interpretation of capuchins' behaviour alternative to ‘inequity aversion’. In particular, it is possible that capuchins were simply expecting their exchange to be rewarded with the (always in view) more PF.
Instrumental learning is based on the expectation of a specific outcome following a specific action (Watanabe et al. 2001). Animals expect outcomes and their behaviour changes when an expected outcome changes (Tinklepaugh 1928; Watanabe et al. 2001). It is known that mammals experience an egocentric effect, traditionally called frustration (Papini 2003) in response to the absence, or delay, of the reward that is usually given in the situation associated with its impending presentation (monkeys: Tinklepaugh 1928; Amsel 1958; for review on diverse mammals and birds: Amsel 1992; Papini & Dudley 1997; Papini 2003). Therefore, it is possible that Brosnan and de Waal's capuchins were expecting the more PF and when they did not receive it, they experienced frustration.
The aim of the present study was to investigate whether the mere presence of a more PF affects the acceptance of a LPF. Our paradigm did not require capuchins to exchange tokens for food but consisted of showing a PF and a LPF to the subject, and allowing it to take only the less preferred one.
2. Material and methods
(a) Subjects
We tested six capuchin monkeys (two males and four females). The males were 8 and 7 years old, and the females were 18, 13, 6 and 4 years old. All subjects were laboratory-born except the 13 year old female which was wild-born.
The subjects were housed in three different groups (group size ranges from 2 to 5 individuals) in indoor–outdoor cages, connected by sliding doors (indoor cage: 3.0×3.0 m, 2.5 m high; outdoor cage: 3.0×3.0 m, 2.5 m high). Cages were furnished with perches and slides; a variety of plastic toys and wooden blocks were given on a daily basis. Testing occurred in the indoor cages.
In the morning, capuchins received grains, pumpkin seeds, peanuts, and, three times a week, a spoonful of a mixture of curd cheese, vitamins, eggs, bran, oats and sugar. In the afternoon, they received the main feed consisting of monkey chow (Altromin-A pellets, Rieper standard diet for primates), fresh fruits (apples, oranges, pears, etc.) and vegetables (salad, carrots, onions, etc.). Test sessions were run from 11.00 to 14.00.
(b) Experimental set-up
Foods were presented to the monkeys on a Plexiglas tray (27×40 cm), which was divided in half by a Plexiglas divider, perpendicular to the tray (27×1×9 cm high). This apparatus had a plastic handle on each side, permitting easy handling. The tray had a 0.5 cm deep hollow (1.5 cm diameter) on both sides of the divider, 15 cm from one another. Food items were placed in each hollow during the experiment. The tray was placed on a trolley (104 cm high) and could be moved within reach of the capuchins. Two transparent plastic bowls (21 cm diameter, 11 cm high) were attached to the rear of the trolley and their content could be seen by the subjects. Each bowl was filled up to about one-third of one type of food; according to the condition, either one bowl contained the PF and the other bowl the LPF, or both bowls contained the LPF. Thus, during the trials, the subject saw the two pieces of food placed in the front part of the tray and the two bowls containing the food on the rear of the trolley.
(c) Procedure
(i) Preliminary phase
Food preference test. During a dichotomous choice test, each subject had to choose one of two different food items of similar size. The subject was tested alone in an indoor cage. The food items were placed on the tray and were offered to the capuchin by moving the tray towards the wire mesh of its cage to allow her/him to take one piece of food. Each subject received two 30-trial sessions on 2 days. The location of the food items was counterbalanced between trials. In this dichotomous test, the PF needed to be chosen in each session at least 85% of the trials, and the LPF no more than 15% of the trials. On this basis, we chose the LPF to be apple for all subjects, and the PF to be a raisin for four subjects and a peanut for two subjects.
To present foods of similar size, we presented half a peanut, sliced apple (cubes) and a whole raisin to the capuchins. During the experimental phase, the bowls placed on the trolley in front of the subject were one-third filled with apple cubes, peanuts, or raisins.
Familiarization with the experimental conditions. As capuchins were tested in pairs in one of the experimental conditions, it was necessary to ensure that the subject tolerated a partner being given food and eating it before the subject's turn started, and that the partner was willing to eat despite the close vicinity of the subject. For this purpose, we carried out two sessions to familiarize the capuchins to the experimental conditions and to assess their mutual tolerance. If the presence of one individual prevented the other from taking and eating the food in a relaxed way, the two individuals were not considered as tolerant of one another and the pairing was excluded from the study. On this basis, our six subjects formed three compatible pairs: two pairs were formed by females (two mother–daughter pairs) and one by males (two brothers).
The pair members were placed in two adjacent indoor cages separated by a concrete wall and by a Plexiglas window (80×60×1 cm) that allowed them to see and hear each other. After about 5 min, the experimenter (D.D. or M.S.G) stood in front of the cages (near to the Plexiglas windows) and placed two pieces of LPF on the tray; both bowls on the trolley were filled with LPF. When the two capuchins were both in front of the experimenter, she handed one of the two pieces of LPF to one of them (the partner) through the wire mesh. While the partner ate it, the experimenter moved the tray close to the cage of the other capuchin (the subject) and offered the remaining LPF. As soon as the subject took the LPF, the experimenter moved backwards and waited at least for 30 s before starting a new trial. This procedure continued for 15–25 presentations, according to the capuchins' level of attention. Each capuchin received two sessions as partner and two sessions as subject.
(ii) Experimental phase
All capuchin monkeys were tested in four experimental conditions. In three conditions, the subject was tested alone, and in one condition the subject was tested while its partner was in the nearby cage. Conditions were presented in a pseudo-random order to each subject. In all four conditions, at least 30 s passed between two consecutive trials. Each subject received two sessions of 25 trials for each experimental condition. Since the experiment required the subjects to be mildly hungry (so that the LPF could be accepted, or refused) and since our experiment was run shortly before their main feed (when capuchins are hungry), the subjects received a snack (a few pieces of carrot, orange, and pellets) 20–30 min before the testing to increase their food selectivity.
In the control condition, at the start of a trial, two pieces of LPF were presented to the subject, one on both sides of the tray. Both bowls were filled with LPF. The experimenter took one of the two pieces of LPF, showed it to the capuchin, and hid it in her pocket. The tray was then moved close to the wire mesh to allow the subject to take the remaining piece of LPF.
In the hiding condition, at the start of a trial, one piece of PF was placed on one side of the tray and one piece of LPF was placed on the other side of the tray. One bowl was filled with PF and the other bowl with LPF. The experimenter took the PF, showed it to the capuchin and hid it in her pocket. The tray was then moved close to the cage to allow the capuchin to take the LPF.
In the accumulation condition, a transparent bowl was placed on the floor of the adjacent cage, a few centimetres in front of the Plexiglas window. At the start of a trial, one piece of PF was placed on one side of the tray and one piece of LPF was placed on the other side of the tray. One bowl was filled with PF and the other bowl with LPF. The experimenter took the PF, showed it to the subject and while it was watching placed it in the transparent bowl in the adjacent cage. The tray was then moved close to the wire mesh to allow the subject to take the LPF. Thus, trial after trial, the PF accumulated in the bowl, out of reach but in view of the subject.
In the partner condition, the partner was in the adjacent cage. At the start of a trial, one piece of PF was placed on one side of the tray and one piece of LPF was placed on the other side of the tray. One bowl was filled with PF and the other bowl with LPF. The experimenter took the PF, showed it to the subject and gave it to the partner while the subject was watching. The tray was then moved close to the wire mesh to allow the subject to take the remaining LPF.
(d) Behaviours scored
All sessions were videotaped using a digital video-camera Canon MV650i. M.S.G scored the data from the tapes. To assess inter- and intra-observer reliability, 12.5% of the sessions were independently scored by M.S.G and D.D. Agreement was 100% for refusals. The latencies taken by the two observers, or by the same observer twice, differed in only 9 and 5% of the trials; the difference between codings was never greater than 1 s.
We scored two types of latencies and two types of refusals. The latency to initiate a trial (LatI) was the time elapsed from the moment the experimenter placed the pieces of food on the tray until when the subject stood in front of the tray, ready to begin the test. As the experimental cages were large, this latency reflects the motivation of the subject to participate in the experiment. Refusal to initiate the test (RI) was scored when the LatI was more than 90 s. The latency to take the LPF (LatT) was the time elapsed from the moment the experimenter moved the tray with the remaining LPF close to the subject's cage until the subject took the LPF. Refusal to take the LPF (RT) was scored when LatT was more than 10 s.
(e) Data analysis
Since the data were not normally distributed, we used non-parametric tests for analyses. For assessing the correlation between LatI or LatT, and the order of trials, we performed Spearman Rank correlations. Both differences in LatI and in LatT across conditions were tested with two Friedman ANOVAs. To assess differences in LatI or in LatT between conditions, we carried out Wilcoxon matched pairs tests. Total refusals were the number of refusals to initiate the trial plus the number of refusals to take and eat the food, scored in each condition for each subject, and were compared using a Chi-square test. Since the analysis showed similar trends for RI and RT, we present them as pooled data.
The significance level was set at α=0.05. When multiple comparisons were made, we controlled the family wise error rate by modifying the significance level of α, designated in the text as a α* (α*=α/c, where α=0.05, and c corresponds to the number of comparisons). In particular, we performed four comparisons between conditions: we compared the control condition with each of the other conditions, and since the aim of the present study was to investigate whether the presence of a more PF affects the acceptance of a LPF, we also compared the hiding condition (in which the PF was hidden) with the accumulation condition (in which the PF was accumulated in sight of the subject).
3. Results
(a) Latencies and order of trials
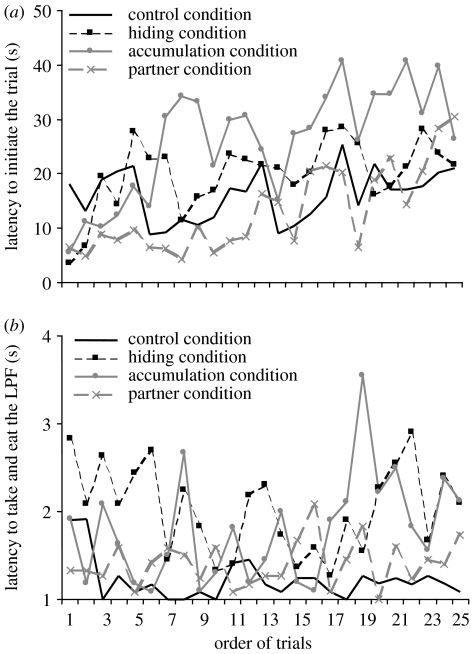
In the hiding, accumulation, and partner conditions, latencies to initiate a trial significantly increased as the session progressed (rs=0.442, n=25, p<0.05; rs=0.69, n=25, p<0.001; rs=0.73, n=25, p<0.001, respectively; figure 1). No such trend was evident in the control condition (rs=0.23, n=25, n.s.). LatT correlated with the order of trials only in the accumulation condition (rs=0.44, n=25, p<0.05). Please see electronic supplementary material file for a comparison of the latencies scored in the two sessions.
Figure 1.
(a) Correlation between the average latency to initiate a trial (LatI) and the order of trials during a session (the first and the second sessions of a condition are analysed together). LatI increased significantly across the session in the hiding, accumulation and partner conditions. No correlation between LatI and the order of trials was observed in the control condition. (b) Correlation between the average latency to take and eat the less preferred food (LatT) and the order of trials during a session (the first and the second sessions of a condition are analysed together). No correlation between LatT and the order of trials was observed in any condition.
(b) Differences among conditions
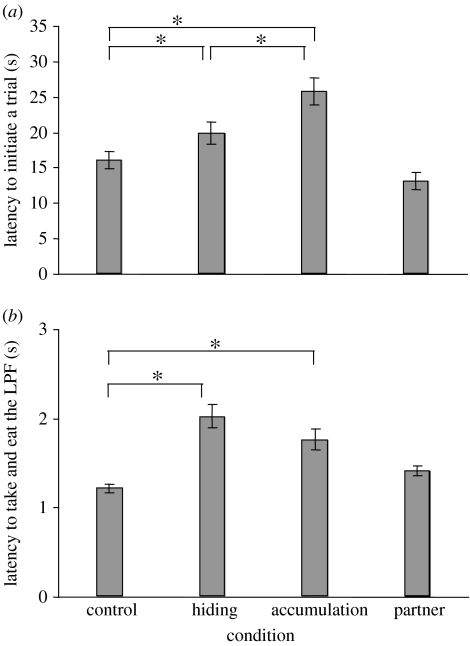
As shown in figure 2, LatI and LatT differed across conditions (Friedman test, LatI: , n=25, p<0.0001; LatT: , n=25, p<0.0001). In particular, capuchins took significantly longer to initiate a trial in the hiding and in the accumulation conditions compared to in the control condition (figure 2a, Z=−2.54, n=25, p=0.011, α*=0.013; Z=−3.54, n=25, p=0.0004, α*=0.013, respectively), whereas the partner condition did not differ from the control condition (Z=−2.018, n=25, n.s.). Moreover, there was a longer LatI in the accumulation condition than in the hiding condition (Z=−2.75, n=25, p<0.006, α*=0.013).
Figure 2.
(a) Average latency to initiate a trial (LatI) in the control, hiding, accumulation and partner conditions. LatI was significantly longer in the hiding and accumulation conditions than in the control condition, whereas LatI in the partner condition was not significantly different from LatI in the control condition. LatI in the accumulation condition was significantly longer than LatI in the hiding condition. (b) Average latency to take and eat the less preferred food (LatT) in the control, hiding, accumulation and partner conditions. LatT was significantly longer in the hiding and accumulation conditions than in the control condition, whereas LatT in the partner condition was not significantly different from LatT in the control condition. The longest average latency was observed in the hiding condition. *p<0.013.
Capuchins took significantly longer to take the LPF in the hiding and accumulation conditions than in the control condition (figure 2b, Z=4.35, n=25, p<0.0001, α*=0.013; Z=−3.43, n=25, p=0.0006, α*=0.013, respectively), whereas the partner condition did not differ from the control condition (Z=−2.34, n=25, n.s.). LatT was not significantly different between the hiding and accumulation conditions (Z=−1.94, n=25, n.s.).
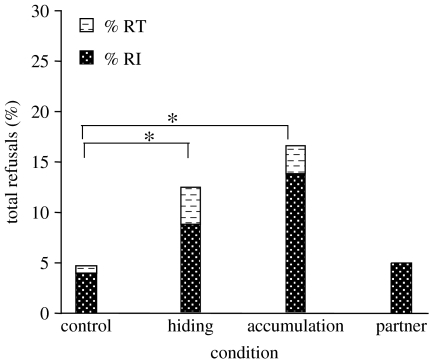
Refusals (figure 3) were significantly higher in the hiding and in the accumulation conditions compared to the control condition (, n=51, p=0.001, α*=0.013; , n=62, p<0.0001, α*=0.013, respectively), whereas the partner condition did not differ from the control condition (, n=29, n.s.). No significant difference was found between the hiding and the accumulation conditions (, n=85, n.s.).
Figure 3.
Percentage of total refusals: total refusals to initiate a trial (RI, black with white dots) and to take and eat the less preferred food (RT, white with black dashes) in each condition. The percentage of total refusals in the hiding and accumulation conditions differed significantly from that of the control condition. *p<0.001.
4. Discussion
(a) Decrease of motivation due to the mere presence of a more preferred food
In all conditions, apart from the control condition, capuchins took longer to initiate a trial as the session progressed, which indicates that they became less motivated over time. Satiety cannot account for the reduction of motivation since in the control condition the LatI did not change as the session progressed. This finding suggests that capuchins perceived the hiding, the accumulation and the partner conditions as frustrating. The common feature across these conditions was the presence of the preferred but out of reach food (on the tray and inside the bowls). In addition, the finding that the accumulation condition was the most frustrating (as shown by the highest average LatI) suggests that seeing an additional bowl in which the PF is accumulated in view of the subject increases its frustration. It is possible that the monkey perceived the food in the bowls as ‘belonging’ to the experimenter (Kummer & Cords 1991), and the food in the nearby cage as something available.
There is evidence that the omission of an expected reward elicits frustration. Rats and pigeons show an ‘escape-from-frustration phenomenon’ when an appetitive reinforcer is not given (or is reduced in magnitude or quality) while the signals for its impending presentation are present (Papini & Dudley 1997). In the laboratory, monkeys are generally used to receiving the food that experimenters handle in front of them. Such situations occur for experimental purposes such as food preference tests, and during the routine provisioning of food. Therefore, seeing the PF can well lead to the expectation that it can be obtained. Consequently, we argue that our subjects faced a quality impairment of the reward when they only received the LPF, which was a cause of frustration. In support of this view, we occasionally observed distress vocalizations and stereotyped pacing, especially in the hiding and accumulation conditions. Experiments aimed to induce frustration by omitting the expected reward (Lyons et al. 2000; Papini 2003) similarly report stereotyped behaviours in rats and monkeys. Thus, it seems plausible that the presence of a PF when a LPF is given is enough to elicit frustration and to induce unwillingness to accept the latter.
(b) Socially facilitated acceptance of a less preferred food
The comparison of the partner condition with the control condition allows an assessment of the impact of the social context on the acceptance of the LPF. The presence of a partner eating a PF appears to make the LPF more acceptable despite the presence of the PF. In fact, no refusals of the LPF occurred in the partner condition: whenever capuchins initiated a trial, they also ate the LPF. Therefore, the partner strongly affects the subject's response towards the LPF. Why is this so?
The phenomenon of social facilitation of eating, i.e. the increased likelihood of eating when somebody else is eating, can account for the above results. Visalberghi & Addessi (2000) demonstrated that capuchins increase their acceptance of a novel food (novel foods are generally eaten with caution and in small amounts) when they see group members eating the same food. This phenomenon occurs also when the subject and the partner eat different foods (Addessi & Visalberghi 2001; Galloway et al. 2005). Moreover, we believe that the fact that the partner is offered a PF induces the subject to come to the tray (i.e. to initiate its trial) but decreases its willingness to immediately take the LPF, given what the partner has received. Nevertheless, as soon as the subject accepts the food, its eating response becomes socially facilitated.
Our partner condition can be compared with the Inequality condition of Brosnan & de Waal's study (2003). The main difference between both studies is that in the latter study the monkeys had to exchange a token in order to receive the food. However, in both studies the subject and the partner did the same thing and received different rewards; therefore, in both cases the subject faced ‘unfairness’. Whereas our subjects did not refuse to eat the food after having initiated the trial, Brosnan & de Waal's capuchins did. In the latter study, the subjects first had to give a token to the experimenter in order to be rewarded; this exchange required time and led to a mismatch in timing between when the partner and the subject ate. Since the partner ate before the food was given to the subject, the eating behaviour of the latter could not be socially facilitated. In contrast, in our study the eating behaviour of the two monkeys were likely to overlap.
(c) Concluding remarks
Do our results suggest that capuchin monkeys refuse inequity? We will briefly discuss which of the following possibilities is more likely. Inequity is prompted: (i) by the food options apparently available and/or (ii) by what the partner receives. Although both these points require proper investigation, our results indicate that the presence of the PF plays a major role in decreasing the acceptance of a LPF (in the control condition, when the LPF was the only food they could see, our capuchins were willing to receive it). We ignored whether in the absence of the experimenter (who causes the inequity by rewarding the subject with a low quality food), capuchins would behave the same. For sure, an adult human facing an experimenter who hands out a low-quality reward when a high-quality one seems available, might think that the experimenter is unfair and feels frustrated. Would a human attribute ‘unfairness’ to a vending machine, too? Probably not, but they would certainly experience frustration, as rats and pigeons do even when tested in the absence of the experimenter (for review Papini 2003).
Finally, Brosnan & de Waal's results (2003) also support our interpretation that seeing the partner exchanging a token for a more PF is not mandatory to induce the subject's willingness to accept the LPF (or, as the authors label it, to experience ‘inequity aversion’). In fact, the number of refusals observed in the inequality condition (when the partner had to exchange the token for a PF) and in the food control condition (when there was no partner and the PF was accumulated in the adjacent cage in sight of the subject) did not differ. Future experiments should control for the possibility that the presence of the PF affects the acceptance of the LPF by devaluing it. This hypothesis is parsimonious and does not require the ability of perceiving a relation between relations, i.e. comparing the relation between its own effort and reward and the relation between the partner's effort and reward, which has not been demonstrated in capuchin monkeys yet.
Acknowledgments
We thank Carlotta Maggio for help with data collection and all animal caretakers for their assistance with the animals. We also thank Francesco Natale and Flavia Chiarotti for statistical advice and useful comments. We thank as well Elsa Addessi and Camillo Padoa-Schioppa and the anonymous referees for their helpful comments. We thank Annika Paukner for revising the English and providing her comments. We are grateful to the Bioparco Foundation for hosting the laboratory, where the experiment was carried out and to the Fondation Fyssen that provided Diane Dubreuil with a post-doctoral fellowship. This study was supported by the grant RBNE01SZB4 from FIRB/MIUR. This experiment was conducted in compliance with all current Italian laws on the ethical standards of the treatment of animals.
Supplementary Material
Figure 1A, average latency to initiate a trial in each condition split by session
References
- Addessi E, Visalberghi E. Social facilitation of eating novel food in tufted capuchin monkeys (Cebus apella): input provided by group members and responses affected in the observer. Anim. Cogn. 2001;4:297–303. doi: 10.1007/s100710100113. 10.1007/s100710100113 [DOI] [PubMed] [Google Scholar]
- Amsel A. The role of frustrative nonreward in noncontinuous reward situations. Psychol. Bull. 1958;55:102–109. doi: 10.1037/h0043125. [DOI] [PubMed] [Google Scholar]
- Amsel A. Frustration theory: many years later. Psychol. Bull. 1992;112:396–399. doi: 10.1037/0033-2909.112.3.396. 10.1037/0033-2909.112.3.396 [DOI] [PubMed] [Google Scholar]
- Brosnan S.F, de Waal F.B.M. Monkeys reject unequal pay. Nature. 2003;425:297–299. doi: 10.1038/nature01963. 10.1038/nature01963 [DOI] [PubMed] [Google Scholar]
- Fagot J, Wasserman E.A, Young M.E. Discriminating the relation between relations: the role of entropy in abstract conceptualization by baboons (Papio papio) and humans (Homo sapiens) J. Exp. Psychol. Anim. Behav. Process. 2001;27:316–328. 10.1037/0097-7403.27.4.316 [PubMed] [Google Scholar]
- Fehr E, Schmidt K.M. A theory of fairness, competition, and cooperation. Q. J. Econ. 1999;114:817–868. 10.1162/003355399556151 [Google Scholar]
- Galloway A.T, Addessi E, Fragaszy D.M, Visalberghi E. Social facilitation of eating familiar food in tufted capuchin monkeys (Cebus apella): does it involve behavioral coordination? Int. J. Primatol. 2005;26:181–189. 10.1007/s10764-005-0729-7 [Google Scholar]
- Kummer H, Cords M. Cues of ownership in long-tailed macaques, Macaca fascicularis. Anim. Behav. 1991;42:529–549. [Google Scholar]
- Langer J. Academic Press; New York, NY: 1980. The origins of logic: six to twelve months. [Google Scholar]
- Langer J. Academic Press; New York, NY: 1986. The origin of logic: one to two years. [Google Scholar]
- Lyons D.M, Fong K.D, Schrieken N, Levine S. Frustrative nonreward and pituitary-adrenal activity in squirrel monkeys. Physiol. Behav. 2000;71:559–563. doi: 10.1016/s0031-9384(00)00384-x. 10.1016/S0031-9384(00)00384-X [DOI] [PubMed] [Google Scholar]
- Papini M.R. Comparative psychology of surprising nonreward. Brain Behav. Evol. 2003;62:83–95. doi: 10.1159/000072439. 10.1159/000072439 [DOI] [PubMed] [Google Scholar]
- Papini M.R, Dudley R.T. Consequences of surprising reward omissions. Rev. Gen. Psychol. 1997;1:175–197. 10.1037/1089-2680.1.2.175 [Google Scholar]
- Spinozzi G. Development of spontaneous classificatory behavior in chimpanzees (Pan troglodytes) J. Comp. Psychol. 1993;107:193–200. doi: 10.1037/0735-7036.107.2.193. 10.1037/0735-7036.107.2.193 [DOI] [PubMed] [Google Scholar]
- Spinozzi G, Natale F. Classification. In: Antinucci F, editor. Cognitive structure and development in nonhuman primates. Lawrence Erlbaum Associates, Publishers; Hillsdale, NJ: 1989. pp. 163–187. [Google Scholar]
- Thompson R.K.R, Oden D.L. Categorical perception and conceptual judgments by nonhuman primates: the paleological monkey and the analogical ape. Cogn. Sci. 2000;24:363–396. 10.1016/S0364-0213(00)00029-X [Google Scholar]
- Tinklepaugh O.L. An experimental study of representation factors in monkeys. J. Comp. Psychol. 1928;8:197–236. [Google Scholar]
- Tomasello M, Call J. Oxford University Press; New York, NY: 1997. Primate cognition. [Google Scholar]
- Visalberghi E, Addessi E. Seeing group members eating a familiar food enhances the acceptance of novel foods in capuchin monkeys. Anim. Behav. 2000;60:69–76. doi: 10.1006/anbe.2000.1425. 10.1006/anbe.2000.1425 [DOI] [PubMed] [Google Scholar]
- Watanabe M, Cromwell H.C, Tremblay L, Hollerman J.R, Hikosaka K, Schultz W. Behavioral reactions reflecting differential reward expectations in monkeys. Exp. Brain Res. 2001;140:511–518. doi: 10.1007/s002210100856. 10.1007/s002210100856 [DOI] [PubMed] [Google Scholar]
- Wynne C.D.L. Fair refusal by capuchin monkeys. Nature. 2004;428:140. doi: 10.1038/428140a. 10.1038/428140a [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1A, average latency to initiate a trial in each condition split by session