Abstract
Encounters with parasites and pathogens are often unpredictable in time. However, experience of an infection may provide the host with reliable cues about the future risk of infection for the host itself or for its progeny. If the parental environment predicts the quality of the progeny's environment, then parents may further enhance their net reproductive success by differentially providing their offspring with phenotypes to cope with potential hazards such as pathogen infection. Here, I test for the occurrence of such an adaptive transgenerational phenotypic plasticity in the mealworm beetle, Tenebrio molitor. A pathogenic environment was mimicked by injection of bacterial lipopolysaccharides for two generations of insects. I found that parental challenge enhanced offspring immunity through the inducible production of antimicrobial peptides in the haemolymph.
Keywords: ecological immunology, trans-generational immunity, antimicrobial peptides, phenoloxidase
1. Introduction
Variation in offspring phenotype can result from selection and parental effects (e.g. maternal effects; Mousseau & Fox 1998a). While selection on parents in the form of differential mortality or reproduction will allow certain offspring genotypes to be over-represented, parental effects influence the expression of genes in the progeny. Such parental effects are common among plants and animals and can have remarkable consequences on the life history of the progeny. They may be adaptive under some conditions (Mousseau & Fox 1998b and references therein) when the facultative change in the progeny phenotype, attributable to the parental environment, improves progeny fitness (Mousseau & Fox 1998a). Hence, adaptive trans-generational plasticity would require: (i) that variation in offspring environment could potentially reduce their fitness, (ii) that parents may be able to assess their progeny's environment through reliable cues and (iii) that parents can adaptively adjust the phenotype of their offspring to the anticipated environment.
Pathogens and parasites are strong sources of environmental alterations because of their effects on host fitness (e.g. Loye & Zuk 1991) and encounters with these enemies are often temporally unpredictable (Combes 1995). However, after experiencing a parasitic challenge, a host or its progeny might be subsequently exposed to a higher probability of infection, since parasites that are now present in the environment are likely to increase in frequency because of their reliance on host for transmission (e.g. Lui 2000). Hosts exposed to a parasite infection may therefore benefit from adjusting their offspring phenotype to increase their resistance to prevailing parasites. Parental infections have been reported to affect numerous life-history traits in offspring (Møller 1990; Sorci et al. 1994; Sorci & Clobert 1995; Rolff 1999). For instance, in the damselfly Coenagrion puella, a high ectoparasite load (Acari: Arrenurus cuspidator) on mothers stimulates the production of fewer but larger offspring (Rolff 1999). Offspring size is believed to strongly affect offspring fitness and thus parental fitness, as shown in a variety of studies (Crowley & Hopper 1994; Reznick et al. 1996). This way, parasitized females may compensate the reduced number of offspring produced through offspring of better quality. Parental infection has also been found to enhance offspring immunity. Immune challenged vertebrate females transfer specific antibodies to their offspring (Hanson 1998; Coste et al. 2000). Invertebrates do not possess antibodies (Hoffmann et al. 1996); however, the innate immune system of invertebrates is nevertheless able to provide long-term immunity to the host after a first challenge (Kurtz & Franz 2003; Moret & Siva-Jothy 2003; Jacot et al. 2005) and to its progeny (Moret & Schmid-Hempel 2001; Little et al. 2003; Sadd et al. 2005).
Invertebrate immune defence relies on both constitutive and inducible mechanisms (Hoffmann et al. 1996; Gillespie et al. 1997). Among them the prophenoloxidase (proPO) cascade is a common and generalized response to invasion by a parasite and is non-specifically elicited by critical surface molecules of the microorganism such as lipopolysaccharides (LPS; Söderhäll & Cerenius 1998). The proPO system involves numerous large enzymes constitutively synthesized and located both in the haemolymph and haemocytes (Gillespie et al. 1997; Söderhäll & Cerenius 1998). The activation of the proPO enzyme into its active form, phenoloxidase (PO), produces melanin and a variety of cytotoxic substances that are released into the haemolymph and that help to kill the parasites (Nappi & Vass 1993; Söderhäll & Cerenius 1998). However, these cytotoxic substances are also believed to be toxic to the host (Nappi & Vass 1993; Söderhäll & Cerenius 1998; Sugumaran et al. 2000). Recognition of microorganism cell walls also induces the production by the fat body of antimicrobial peptides, which are then secreted into the haemolymph (Hoffmann et al. 1996). This immune pathway is more specific than the proPO system against microbial infection but has the disadvantage of a relatively long lag-phase during synthesis in the response to infection.
While beneficial against parasitic attacks, immune defence is also costly. When immune defence is expressed at high levels it is often traded off against other fitness parameters (Rothenbuhler & Thompson 1956; Sutter et al. 1968; Kraaijeveld & Godfray 1997) and immune effector systems are costly to maintain and use (Moret & Schmid-Hempel 2000; Jacot et al. 2005). Hence, resource allocation to immune defence should be balanced against these costs to achieve an optimal allocation strategy for limited resources (Frank 1993; Simms & Triplett 1994; Jokela et al. 2000). Consequently, facultative increase of offspring immunity induced by parental parasitic challenge may reap the benefits of immune defence while saving potential costs associated with investments in immunity when it is not needed.
In this study, I mimicked heavy microbial infection by experimentally challenging larvae of the mealworm beetle, Tenebrio molitor, with LPS for two generations. LPS is a non-pathogenic, non-living surface molecule derived from Escherichia coli. It is highly immunogenic (Rattclife et al. 1985; Jomori et al. 1990) and elicits both the activation of the proPO system and the production of antimicrobial peptides over many hours (Rattclife et al. 1985; Kato et al. 1994; Lemaître et al. 1996). Costs associated with the immune response were estimated in terms of larval survival, larval development time, and adult body size and mass to estimate selection potential generated by the cost of the immune response. Since parental LPS challenge mimics an environment with a high prevalence of microbial infection, adaptive modifications of the offspring phenotype are expected. Based on previous findings (Rolff 1999; Moret & Schmid-Hempel 2001; Little et al. 2003), I tested whether individuals born from parents exposed to LPS challenge show changes in body size and enhanced immunity through increased antimicrobial and/or PO activities in their haemolymph.
2. Material and methods
(a) Mealworm cultures
Beetle larvae were taken at random from a stock culture maintained at the University of Sheffield, Sheffield, UK. Eighteen cultures, each containing 100–110 larvae at the same developmental stage (10–15 mm in length), were initiated for the experiment. These experimental cultures (named here ‘parental’ cultures) were assigned to one of three immune treatments (parental challenge; see below). Almost all the surviving beetles from all cultures had reached their adult stage by eight weeks after immune challenge. From each culture, 20 randomly chosen adult beetles that had emerged four weeks after the immune challenge were used as genitor for the next generation (named here ‘offspring’ cultures). Offspring from each parental culture were then divided into three cultures of 100–110 larvae and assigned to an immune treatment (offspring challenge). Cultures were fed ad libitum and kept at 28±1 °C for the duration of the experiment.
(b) Immune treatments
Parental and offspring cultures were assigned to one of the three immune treatments: ‘naive’, ‘Ringer’ and ‘LPS’. Larvae in the naive group were chilled on ice prior to inclusion in the experiment. Larvae in the Ringer group received a single injection of 5 μl of saline solution (Ringer solution) after being chilled on ice. Larvae in the LPS group received a 0.5 mg ml−1 dose of LPS extracted from E. coli (Sigma: L8274) in 5 μl Ringer solution after being chilled on ice. All injections were done at the same developmental stage through the pleural membrane between the second and the third abdominal segments, using sterilized glass capillaries that had been pulled to a fine point.
(c) Mortality and larval development time
For the two generations of beetles (parental and offspring cultures), mortality was recorded once a week for eight weeks after immune challenge. The proportion of surviving insects reaching the adult stage was recorded in order to produce a relative estimation of the development time from larvae to adult. Since it took ca eight weeks from the immune challenge for all surviving insects to reach the adult stage, I used the proportion of adults found at week 4 (corresponding then to the ‘mid-time proportion of adults’) to test for any difference of development time from larvae to adult between immune treatments. Hence, a lower mid-time proportion of adults compared to ‘control’ suggests a longer development time from larvae to adult.
In the parental cultures, additional comparisons for survival and development time were done within the first four weeks post-challenge to a posteriori check whether adult beetles sampled at week 4 and used for breeding the offspring generation were of similar ‘quality’ (do not result from differential mortality and had emerged at the same time). Indeed, if within this range of time survival and development time differ between groups, beetle sampling may potentially generate selection by taking individuals of different quality across groups.
(d) Body size and mass
The body size and mass of adults were recorded from a random sample of 15–20 individuals per culture from both generations of beetles. Fresh body mass was measured to the nearest 1 mg with a Mettler AE200 balance, and body size was estimated by measuring the length of the left elytra with Mitutoyo digital callipers (precision ±0.1 mm).
(e) Immune parameters
In the second generation, 8–10 larvae were sampled at random from each immune treatment 3 days after immune challenge. Three days post-challenge is the moment at which the magnitude of the PO and antimicrobial immune responses are the largest (Moret & Siva-Jothy 2003). After sampling of their haemolymph, these larvae were not returned into the experimental cultures. Each larva was chilled on ice for 10 min and the pleural membrane between the second and the third abdominal segment was punctured with a sterile hypodermic needle. The droplet of haemolymph that came out of the wound was collected into a sterile, pre-chilled glass capillary. For each insect, 5 μl of haemolymph was collected and flushed into a 1.5 ml micro-centrifuge tube containing 50 μl of cold sodium cacodilate/CaCl2 buffer (0.01 M sodium cacodilate, 0.005 M CaCl2, pH 6.5). A 15 μl sub-sample was kept in a 0.5 ml micro-centrifuge tube and stored at −80 °C until later examination for antibacterial activity using a zone-of-inhibition test. The methods for this test were as described in Moret & Schmid-Hempel (2000) except that the assay was performed using haemolymph diluted 12 times with sodium cacodilate/CaCl2 buffer. The remaining haemolymph solution was diluted with 30 μl of cold sodium cacodilate/CaCl2 buffer and immediately stored at −80 °C for later measurement of PO activity. PO activity was assayed by thawing samples of frozen haemolymph solution (dilution 1/20; haemolymph/sodium cacodilate/CaCl2 buffer) on ice and then adding 20 μl to a microplate well containing 140 μl of distilled water, 20 μl of phosphate buffered saline (8.74 g NaCl; 1.78 g Na2HPO4 2H2O; 1000 ml distilled water; pH. 6.5) and 20 μl of l-Dopa solution (4 mg ml−1 of distilled water). The reaction was allowed to proceed at 30 °C in a microplate reader (Versamax, Molecular Devices) for 40 min. Readings were taken every 10 s at 490 nm and analysed using SOFTmaxPRO v. 4.0 software (Molecular Devices). Enzyme activity was measured as the slope (Vmax value) of the reaction curve during the linear phase of the reaction (Barnes & Siva-Jothy 2000).
(f) Statistics
I used Cox-regressions with a time-dependent covariate to analyse the differences in survival rates with respect to parental challenges (for both parental and offspring cultures) and offspring challenges (for offspring cultures only) during the eight weeks post-challenge. The statistical model used a stepwise procedure and the reference survival function was generated from the control data derived from the ‘parental challenge’ only in parental cultures (parental challenge=naive) and from parental and offspring challenges in offspring cultures (parental challenge=naive and offspring challenge=naive). The challenges were coded as categorical variables. Time (in weeks) was incremented as a covariate in the model as hazard ratios of the survival functions were not constant across time.
The mid-time proportion of adults (four weeks post-challenge), body size, body mass, PO and antimicrobial activities were analysed using analyses of variance (ANOVAs) based on the mean calculated for each culture with parental challenges (for both parental and offspring cultures) and offspring challenges (for offspring cultures only) as factors. When the mid-proportion of adults was similar between parental treatments, the data were further analysed by testing the distribution of adult emergence week by week until the fourth week post-challenge with a two-samples Komolgorov–Smirnov test.
All data were analysed using SPSS v. 10 for Macintosh.
3. Results
(a) Survival
In the parental cultures, a survival cost caused by the injection itself was detected (table 1a). The 5.09- and 4.79-fold survival declines induced by Ringer and the LPS compared to the naive cultures, were not significantly different from each other (table 1a). Within the first four weeks post-challenge, mortality rates caused by the injections of Ringer and LPS were high but not significantly different from each other (table 1b). The survival cost induced by the injection decreased with time (about 25% each week, see T×F0-treatment, table 1a).
Table 1.
Results of the time-dependent Cox regression models for (a) parental cultures along eight weeks post-challenge, (b) parental cultures along four weeks post-challenge and (c) offspring cultures along eight weeks post-challenge. (The tables contain the relevant terms identified by a backward stepwise procedure. See also the electronic supplementary material.)
| factorsa | bb | s.e.c | Waldd | d.f. | pe | oddsf |
|---|---|---|---|---|---|---|
| (a) | ||||||
| F0-treatment | 76.98 | 2 | <0.001 | |||
| F0-Ringer versus F0-naive | 1.62 | 0.20 | 66.98 | 1 | <0.001 | 5.09 |
| F0-LPS versus F0-naive | 1.57 | 0.20 | 61.65 | 1 | <0.001 | 4.79 |
| F0-LPS versus F0-Ringerg | 0.06 | 0.16 | 0.15 | 1 | 0.702 | 1.06 |
| T×F0-treatment | 69.68 | 2 | <0.001 | |||
| T×F0-Ringer | −0.29 | 0.04 | 54.84 | 1 | <0.001 | 0.75 |
| T×F0-LPS | −0.29 | 0.04 | 56.71 | 1 | <0.001 | 0.74 |
| (b) | ||||||
| F0-treatment | 46.86 | 2 | <0.001 | |||
| F0-Ringer versus F0-naive | 2.52 | 0.38 | 43.89 | 1 | <0.001 | 12.46 |
| F0-LPS versus F0-naive | 2.46 | 0.38 | 41.61 | 1 | <0.001 | 11.66 |
| F0-LPS versus F0-Ringerg | −0.07 | 0.22 | 0.09 | 0.764 | 0.93 | |
| T×F0-treatment | 23.94 | 2 | <0.001 | |||
| T×F0-Ringer | −0.56 | 0.12 | 22.04 | 1 | <0.001 | 1.75 |
| T×F0-LPS | −0.53 | 0.12 | 19.97 | 1 | <0.001 | 1.03 |
| (c) | ||||||
| F0-treatment | 11.59 | 2 | 0.003 | |||
| F0-Ringer versus F0-naive | −0.07 | 0.03 | 4.53 | 1 | 0.033 | 0.93 |
| F0-LPS versus F0-naive | −0.11 | 0.03 | 11.37 | 1 | 0.001 | 0.89 |
| F0-LPS versus F0-Ringerg | −0.41 | 0.03 | 1.59 | 1 | 0.207 | 0.96 |
| F1-treatment | 5.97 | 2 | 0.050 | |||
| F1-Ringer versus F1-naive | 0.15 | 0.11 | 1.82 | 1 | 0.18 | 1.16 |
| F1-LPS versus F1-naive | 0.28 | 0.11 | 5.96 | 1 | 0.015 | 1.32 |
| F1-LPS versus F1-Ringerg | 0.12 | 0.11 | 1.21 | 1 | 0.271 | 1.13 |
| F0-treatment×F1-treatment | 10.37 | 4 | 0.035 | |||
| F0-Ringer×F1-Ringer | −0.19 | 0.08 | 5.64 | 1 | 0.018 | 0.83 |
| F0-LPS×F1-Ringer | 0.01 | 0.08 | 0.01 | 1 | 0.941 | 1.01 |
| F0-Ringer×F1-LPS | <0.01 | 0.08 | <0.01 | 1 | 0.958 | 1.00 |
| F0-LPS×F1-LPS | 0.10 | 0.08 | 1.68 | 1 | 0.195 | 1.11 |
| T×F1-treatment | 45.26 | 2 | 0.031 | |||
| T×F1-Ringer | −0.03 | 0.02 | 2.22 | 1 | 0.136 | 0.97 |
| T×F1-LPS | −0.04 | 0.02 | 6.93 | 1 | 0.008 | 0.96 |
Parental (F0) or offspring (F1) immune treatments, Ringer (5 μl); LPS (5 μl, 0.5 mg ml−1); T, time covariate (in weeks).
b=regression coefficient of overall survival function for variable.
Standard error of regression coefficient.
Wald statistic for variable.
Significance level for Wald statistic. Values p≤0.05 are given in bold.
Odds ratio of survival for variable relative to control (=exp(b)).
Result that used the Ringer-treatment as reference category in the model.
In the offspring cultures (table 1c), only the LPS treatment induced a significant survival cost but compared to naive larvae only (table 1c: F1-LPS versus F1-naive). This cost was 3.6 times lower than that observed in parental cultures and this effect decreased with time (4% each week, see T×F1-LPS, table 1c). The Ringer and LPS immune treatments received by the parents increased offspring survival by 7 and 11%, respectively (see F0-treatment, table 1c). There was a significant interaction between parental and offspring immune treatments on offspring survival. This was particularly pronounced for individuals who were both themselves and their parents challenged with Ringer. These individuals had a survival benefit of 17%.
(b) Development time from larvae to adult
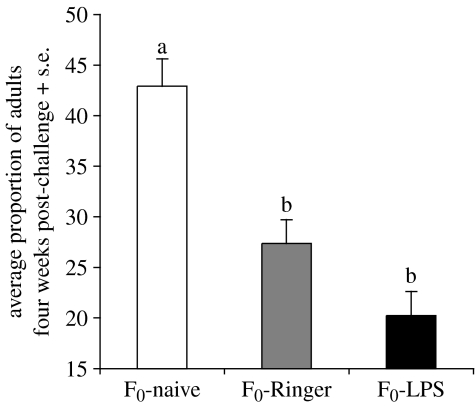
In the parental cultures of beetles, the treatments affected development time to adult (figure 1; ANOVA, F2,15=22.36, p<0.001). The injection itself prolonged development time (Ringer versus naive, Scheffe post hoc tests, mean difference=13.66, p=0.002). The LPS treatment did not have any additional cost on the development time from larvae to adults (LPS versus Ringer, Scheffe post hoc tests, mean difference=7.03, n.s.). Adults started to emerge at week 2 post-challenge and emergence rates of Ringer and LPS parental treatment groups were similar until week 4 post-challenge (Komolgorov–Smirnov test week 2, z=0.58, d.f.=1, n.s.; week 3, z=0.87, d.f.=1, n.s.).
Figure 1.
Tenebrio molitor developmental time from larvae to adult estimated as the proportion of adults at week 4 post-challenge in the parental cultures (n=18 cultures). Bars with different letters are significantly different (see text for statistical results).
In the offspring cultures, development time from larvae to adult was not affected (ANOVA, F4,45=0.46, n.s.).
(c) Body size and mass of the resulting adults
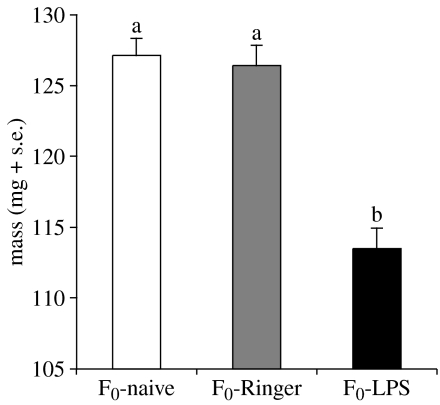
In the parental cultures, the treatments affected body mass (F2,15=14.83, p<0.001, figure 2) but not body size (F2,15=3.22, n.s.) of the resulting adults. While the Ringer treatment did not affect body mass of the resulting adults compared to naive treatment (Scheffe post hoc tests, n.s.), the LPS treatment generated lighter adults (Scheffe post hoc tests, LPS versus naive mean difference=−13.72, p=0.001; LPS versus Ringer mean difference=−13.14, p=0.001).
Figure 2.
Body mass of adult T. molitor sampled in the parental cultures (n=18 cultures). Bars with different letters are significantly different (see text for statistical results).
In the offspring cultures, neither adult body mass (F8,45=0.89, n.s.) nor adult body size (F8,45=0.93, n.s.) was affected by parental or offspring challenges.
(d) Immune parameters of the offspring cultures
The PO activity (figure 3a) of the mealworm larvae in the offspring cultures was different across treatments (ANOVA, F8,45=2.93, p=0.010). However, this difference was only due to offspring challenge (F2,45=10.62, p<0.001) in which the Ringer and the LPS treatments increased the enzyme activity (figure 3a). Parental challenge had no effect on offspring PO activity (F2,45=0.29, n.s.) and there was no interaction between parental and offspring challenges (F4,45=0.41, n.s.).
Figure 3.
(a) PO activity and (b) antimicrobial activity in the haemolymph of T. molitor larvae in offspring cultures (n=54 cultures) across parental and offspring challenges.
Antimicrobial activity (figure 3b) of the mealworm larvae in the offspring cultures varied across treatments (ANOVA, F8,45=10.18, p<0.001). As expected, within offspring challenge, the LPS treatment significantly increased the antimicrobial activity of the larvae (F2,45=35.47, p<0.001). Interestingly, the parental challenge also influenced antimicrobial activity of the mealworm larvae (figure 3b). Compared to the other groups of the parental challenge, larvae that had parents challenged with LPS showed higher levels of antimicrobial activity in the haemolymph (parental challenge: F2,45=4.79, p=0.013) and there was no interaction between parental and offspring challenges (F4,45=0.24, n.s.). Despite the absence of significant differences in survival and development time between LPS and Ringer treatments in the parental generation, one can argue that sampling of adults used to generate the next generation could have lead to artificial selection. In fact, about 100% (20/20) of the adults present at week 4 post-challenge in the LPS parental treatment versus about 75% (20/27) of the adults in the Ringer parental treatment were used to generate the next generation. Sampling within the Ringer parental treatment could have lead to artificial selection if 75% of the insects taken among 27 end up to be of the lowest quality. If this scenario has occurred it may explain immunity differences in the offspring across parental treatments. To control for this potential selection effect due to sampling, an additional statistical analysis was performed using the 75% of insects with the largest antimicrobial activity within the naive and Ringer parental treatments against all the individuals in cultures of the LPS parental treatment. Doing so, the results remain unchanged. Offspring antimicrobial activity was still variable across treatments (F8,45=9.52, p<0.001) due to offspring treatment (F2,45=34.11, p<0.001) and parental treatment (F2,45=3.6, p=0.035). There was still no interaction between parental and offspring challenges (F4,45=0.17, n.s.).
4. Discussion
This study presents reasonable evidence for the occurrence of a facultative trans-generational increase in immunity, in the mealworm beetle T. molitor. This experiment demonstrates that induced levels of antimicrobial activity in offspring larvae are higher when their parents received a microbial immune challenge during the larval stage and these results are clearly not the consequences of selection. LPS-challenged T. molitor larvae experienced an environment that mimicked a high risk of microbial infection to which their offspring are likely to be exposed in natural conditions. Parents may then gain a fitness advantage by differentially enhancing their offspring immunity helping them to cope with pathogen infection. This phenomenon of ‘trans-generational immunological priming’ (Little & Kraaijeveld 2004) in invertebrates has been already reported in earlier studies in which parental immune challenge improved offspring resistance to parasitism (Little et al. 2003). The present study directly assays immunity and confirms the recent findings of Sadd et al. (2005) on bumblebees.
In line with the results of Sadd et al. (2005), trans-generational priming in T. molitor involves the offspring's inducible antimicrobial immune pathway instead of the constitutive proPO system. It is possible that the specific action of antimicrobial peptides on microbe infection helps to avoid the cytotoxic use of enzymes belonging to the proPO cascade. Note that in this work and in Sadd et al. (2005), levels of the inactive form of the PO enzyme, the proPO, have not been measured. Levels of proPO can be elevated independently from PO levels following an immune challenge (Jacot et al. 2005). Such a phenomenon may improve insect immunity and avoid the immunopathological cost of the enzyme activity. Trans-generational enhancement of immunity may also potentially be achieved through increased levels of proPO but this remains unknown.
The physiological mechanism through which this trans-generational transfer of immunity is achieved is not yet known. One can argue that some LPS that challenged parents could be passed to the offspring through the eggs and stimulate the progeny's immunity, leading to seemingly parental effects. However, this is pure speculation and further examinations are needed to explore the mechanisms underlying this phenomenon.
In this study, the cost associated with a standard immune response decreased from one generation of mealworm beetles to the next. Larvae of the parental cultures paid a relatively large survival cost to the injection and it took the survivors longer to reach adulthood. In addition, the immune response to LPS generated lighter adults. In contrast, in the offspring cultures the only cost associated with the immune response was that larvae challenged with LPS had reduced survival, but this cost was lower than that observed in the parental cultures. Interestingly, offspring cultures had a slight survival benefit when their parents had been challenged with either Ringer or LPS while there was no difference between these two parental treatments. This result is difficult to explain but it is nevertheless possible that the trauma resulting from the injection of Ringer and LPS solutions into the parental larvae selected for better quality individuals. However, in that case, it is difficult to deduce whether this parental injection effect results from selection and/or parental effects.
When comparing these results with those of previous studies that used very similar methods or designs, it is interesting to note that survival responses to standard immune challenges are variable. In Moret & Siva-Jothy (2003), challenged mealworm beetles with Ringer or LPS did not show any survival difference with naive beetles in the absence of exposure to an entomopathogenic fungus. With a slightly different method in bumblebee workers (Bombus terrestris), survival rates differed between all the treatments and more importantly survival to the immune challenges was strongly affected by the insects' colony of origin (Moret & Schmid-Hempel 2000). This suggests that the magnitude of the cost associated with the immune response to the same standard challenge (in terms of type of antigens and doses) is highly variable. Such a variable cost of the immune response could potentially be achieved through variable degrees of involvement of the different pathways of the immune response. In insects, the immune response to LPS involves both the ProPO cascade and the antimicrobial immune pathway (Hoffmann et al. 1996; Söderhäll & Cerenius 1998). Each of these mechanisms may carry a different cost when activated and their relative expression may shape the cost of the whole immune response to a standard challenge. While costly, immune defence is also a necessary trait. If variation in degrees of expression between the effector systems involved in the immune response reflects different strategies of resistance to pathogens, then the cost of the immune response could potentially be minimized through optimized patterns of expression between these effector systems (Moret 2003).
From this experiment, the decrease in the cost of the immune response from parental to offspring cultures was not associated with a change in resource allocation between PO and antimicrobial activities. Especially, the base level of the PO activity was not affected by the parental immune treatments. This either suggests that PO activity is not as costly as it is believed to be (Nappi & Vass 1993; Söderhäll & Cerenius 1998; Sugumaran et al. 2000) or it is too important to be counter selected. Indeed, the proPO system is, in addition to providing immunity in invertebrates, important also for pigmentation and sclerotization of many tissues (Cerenius & Söderhäll 2004). These other important functions may constrain the response to negative selection.
To summarize, an important result from this study is the demonstration of a facultative trans-generational increase of immune defence consecutively to a challenge induced to parents as early as in the larval stage. This study failed to demonstrate any selection of optimal pattern of resource allocation between two important pathways of the invertebrate immune system as a consequence of a costly immune response. The optimization of resource allocation between different components of the invertebrate immune system may involve pathways other than the proPO system and the inducible production of antimicrobial peptides (Adamo 2004a,b).
The approaches to life-history evolution, sexual selection and population dynamics of animals often refer to genetically based variation of parasite resistance and immunity. From this study and others (i.e. Joop & Rolff 2004; Jacot et al. 2005), part of the natural variation in immunity can be explained through plastic regulations of immune defences. We may now consider such plastic variation and test how it affects our current theoretical predictions about life-history evolution, sexual selection and population dynamics of hosts and parasites.
Acknowledgments
I would like to thank R. Naylor and B. Sadd for help in the laboratory and M. T. Siva-Jothy, J. Rolff, K. Reinhardt, J. Moreau, C. Biard, T. Rigaud and anonymous referees for comments. This work was supported by a Marie Curie Fellowship.
Supplementary Material
The following figure comes in addition to the results of the survival analyses (see table 1a,c in the paper). For the two generations of beetles (parental and offspring cultures), mortality was recorded once a week for eight weeks after immune challenge and examined according to parental challenges (for both parental and offspring cultures) and offspring challenges (for offspring cultures only).
References
- Adamo S.A. Estimating disease resistance in insects: phenoloxidase and lysozyme-like activity and disease resistance in the cricket Gryllus texensis. J. Insect Physiol. 2004a;50:209–216. doi: 10.1016/j.jinsphys.2003.11.011. 10.1016/j.jinsphys.2003.11.011 [DOI] [PubMed] [Google Scholar]
- Adamo S.A. How should behavioural ecologists interpret measurements of immunity? Anim. Behav. 2004b;68:1443–1449. 10.1016/j.anbehav.2004.05.005 [Google Scholar]
- Barnes A.I, Siva-Jothy M.T. Density-dependent prophylaxis in the mealworm beetle Tenebrio molitor L. (Coleoptera: Tenebrionidae): cuticular melanization is an indicator of investment in immunity. Proc. R. Soc. B. 2000;267:177–182. doi: 10.1098/rspb.2000.0984. 10.1098/rspb.2000.0984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerenius L, Söderhäll K. The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 2004;198:116–126. doi: 10.1111/j.0105-2896.2004.00116.x. 10.1111/j.0105-2896.2004.00116.x [DOI] [PubMed] [Google Scholar]
- Combes C. Masson; Paris, France: 1995. Intéractions durables: écologie et évolution du parasitisme. [Google Scholar]
- Coste A, Sirard J.C, Johansen K, Cohen J, Kraehenbuhl J.P. Nasal immunization of mice with virus-like particles protects offspring against rotavirus diarrhea. J. Virol. 2000;74:8966–8971. doi: 10.1128/jvi.74.19.8966-8971.2000. 10.1128/JVI.74.19.8966-8971.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley H, Hopper K. How to behave around cannibals: a density dependent dynamic game. Am. Nat. 1994;143:117–154. 10.1086/285598 [Google Scholar]
- Frank S.A. A model of inducible defense. Evolution. 1993;47:325–327. doi: 10.1111/j.1558-5646.1993.tb01223.x. [DOI] [PubMed] [Google Scholar]
- Gillespie J, Kanost M.R, Trenczeck T. Biological mediators of insect immunity. Annu. Rev. Entomol. 1997;42:611–643. doi: 10.1146/annurev.ento.42.1.611. 10.1146/annurev.ento.42.1.611 [DOI] [PubMed] [Google Scholar]
- Hanson L.A. Breastfeeding provides passive and likely longlasting active immunity. Ann. Allergy Asthma Immunol. 1998;81:523–537. doi: 10.1016/S1081-1206(10)62704-4. [DOI] [PubMed] [Google Scholar]
- Hoffmann J.A, Reichhart J.M, Hetru C. Innate immunity in higher insects. Curr. Opin. Immunol. 1996;8:8–13. doi: 10.1016/s0952-7915(96)80098-7. 10.1016/S0952-7915(96)80098-7 [DOI] [PubMed] [Google Scholar]
- Jacot A, Scheuber H, Kurtz J, Brinkhof M.W.G. Juvenile immune system activation induces a costly upregulation of adult immunity in field crickets Gryllus campestris. Proc. R. Soc. B. 2005;272:63–69. doi: 10.1098/rspb.2004.2919. 10.1098/rspb.2004.2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokela J, Schmid-Hempel P, Rigby M.C. Dr. Pangloss restrained by the Red Queen—steps towards a unified defence theory. Oikos. 2000;89:267–278. 10.1034/j.1600-0706.2000.890207.x [Google Scholar]
- Jomori T, Kubo T, Natori S. Purification and characterization of lipopolysaccharide-binding protein from hemolymph of American cockroach Periplaneta Americana. Eur. J. Biochem. 1990;190:201–206. doi: 10.1111/j.1432-1033.1990.tb15565.x. 10.1111/j.1432-1033.1990.tb15565.x [DOI] [PubMed] [Google Scholar]
- Joop G, Rolff J. Plasticity of immune defence and condition under risk of predation and parasitism. Evol. Ecol. Res. 2004;6:1051–1062. [Google Scholar]
- Kato Y, Motoi Y, Taniai K, Kadono-Okuda K, Hiramatsu M, Yamakawa M. Clearance of lipopolysaccharide in hemolymph of Bombyx mori: its role in the termination of cecropin mRNA induction. Insect Biochem. Mol. Biol. 1994;24:539–545. 10.1016/0965-1748(94)90089-2 [Google Scholar]
- Kraaijeveld A.R, Godfray H.C.J. Trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. Nature. 1997;389:278–280. doi: 10.1038/38483. 10.1038/38483 [DOI] [PubMed] [Google Scholar]
- Kurtz J, Franz K. Evidence for memory in invertebrate immunity. Nature. 2003;425:37–38. doi: 10.1038/425037a. 10.1038/425037a [DOI] [PubMed] [Google Scholar]
- Lemaître B, Nicolas E, Michaut L, Reichhart J.-M, Hoffman J.A. The dorsoventral regulatory gene cassette spaezle/toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;88:973–983. doi: 10.1016/s0092-8674(00)80172-5. 10.1016/S0092-8674(00)80172-5 [DOI] [PubMed] [Google Scholar]
- Little T.J, Kraaijeveld A.R. Ecological and evolutionary implications of immunological priming in invertebrates. Trends Ecol. Evol. 2004;19:58–60. doi: 10.1016/j.tree.2003.11.011. 10.1016/j.tree.2003.11.011 [DOI] [PubMed] [Google Scholar]
- Little T.J, O'Connor B, Colegrave N, Waat K, Read A.F. Maternal transfer of strain-specific immunity in an invertebrate. Curr. Biol. 2003;13:489–492. doi: 10.1016/s0960-9822(03)00163-5. 10.1016/S0960-9822(03)00163-5 [DOI] [PubMed] [Google Scholar]
- Loye J.E, Zuk M. Oxford University Press; Oxford, UK: 1991. Bird–parasite interactions: ecology, evolution and behaviour. [Google Scholar]
- Lui K.J. Confidence intervals of the simple difference between the proportions of a primary infection and a secondary infection, given the primary infection. Biometrical J. 2000;42:59–69. 10.1002/(SICI)1521-4036(200001)42:1%3C59::AID-BIMJ59%3E3.0.CO;2-A [Google Scholar]
- Moret Y. Explaining variable costs of the immune response: selection for specific versus non-specific immunity and facultative life history change. Oikos. 2003;102:213–216. 10.1034/j.1600-0706.2003.12496.x [Google Scholar]
- Moret Y, Schmid-Hempel P. Survival for immunity: the price of immune system activation for bumblebee workers. Science. 2000;290:1166–1168. doi: 10.1126/science.290.5494.1166. 10.1126/science.290.5494.1166 [DOI] [PubMed] [Google Scholar]
- Moret Y, Schmid-Hempel P. Immune defence in bumble-bee offspring. Nature. 2001;414:506. doi: 10.1038/35107138. 10.1038/35107138 [DOI] [PubMed] [Google Scholar]
- Moret Y, Siva-Jothy M.T. Adaptive innate immunity? Responsive-mode prophylaxis in the mealworm beetle, Tenebrio molitor. Proc. R. Soc. B. 2003;270:2475–2480. doi: 10.1098/rspb.2003.2511. 10.1098/rspb.2003.2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousseau T.A, Fox C.W. Oxford University Press; New York, NY: 1998a. Maternal effects as adaptations. [Google Scholar]
- Mousseau T.A, Fox C.W. The adaptive significance of maternal effects. Trends Ecol. Evol. 1998b;13:403–407. doi: 10.1016/s0169-5347(98)01472-4. 10.1016/S0169-5347(98)01472-4 [DOI] [PubMed] [Google Scholar]
- Møller A.P. Effects of a haematophagous mite on the barn swallow (Hirundo rustica): a test of the Hamilton and Zuk hypothesis. Evolution. 1990;44:771–784. doi: 10.1111/j.1558-5646.1990.tb03804.x. [DOI] [PubMed] [Google Scholar]
- Nappi A.J, Vass E. Melanogenesis and the generation of cytotoxic molecules during insect cellular immune reactions. Pigment Cell Res. 1993;6:117–126. doi: 10.1111/j.1600-0749.1993.tb00590.x. [DOI] [PubMed] [Google Scholar]
- Rattclife N.A, Rowley A.F, Fitzgerald S.W, Rhodes C.P. Invertebrate immunity: basic concepts and recent advances. Int. Rev. Cytol. 1985;97:183–350. [Google Scholar]
- Reznick D, Callahan H, Llauredo R. Maternal effects on offspring quality in poecilid fishes. Am. Zool. 1996;36:147–156. [Google Scholar]
- Rolff J. Parasitism increases offspring size in a damselfly: experimental evidence for parasite-mediated maternal effects. Anim. Behav. 1999;58:1105–1108. doi: 10.1006/anbe.1999.1240. 10.1006/anbe.1999.1240 [DOI] [PubMed] [Google Scholar]
- Rothenbuhler W.C, Thompson V.C. Resistance to American foulbrood in honeybees. I. Differential survival of larvae of different genetic lines. J. Econ. Entomol. 1956;49:470–475. [Google Scholar]
- Sadd B.M, Kleinlogel Y, Schmid-Hempel R, Schmid-Hempel P. Trans-generational immune priming in a social insect. Biol. Lett. 2005;1:386–388. doi: 10.1098/rsbl.2005.0369. 10.1098/rsbl.2005.0369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms E.L, Triplett J. Costs and benefits of plant responses to disease: resistance and tolerance. Evolution. 1994;48:1973–1985. doi: 10.1111/j.1558-5646.1994.tb02227.x. [DOI] [PubMed] [Google Scholar]
- Sorci G, Clobert J. Effects of maternal parasite load on offspring life-history traits in the common lizard (Lacerta vivipara) J. Evol. Biol. 1995;8:711–723. 10.1046/j.1420-9101.1995.8060711.x [Google Scholar]
- Sorci G, Massot M, Clobert J. Maternal parasite load increases sprint speed and philopatry in female offspring of the common lizard. Am. Nat. 1994;144:153–164. 10.1086/285666 [Google Scholar]
- Söderhäll K, Cerenius L. Role of the prophenoloxidase-activating system in invertebrate immunity. Curr. Opin. Immunol. 1998;10:23–28. doi: 10.1016/s0952-7915(98)80026-5. 10.1016/S0952-7915(98)80026-5 [DOI] [PubMed] [Google Scholar]
- Sugumaran M, Nellaiappan K, Valivittan K. A new mechanism for the control of phenoloxidase activity: inhibition and complex formation with quinone isomerase. Arch. Biochem. Biophys. 2000;379:252–260. doi: 10.1006/abbi.2000.1884. 10.1006/abbi.2000.1884 [DOI] [PubMed] [Google Scholar]
- Sutter G.R, Rothenbuhler W.C, Raun E.S. Resistance to American foulbrood in honeybees. VII. Growth of resistant and susceptible larvae. J. Invert. Pathol. 1968;12:25–28. 10.1016/0022-2011(68)90239-5 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following figure comes in addition to the results of the survival analyses (see table 1a,c in the paper). For the two generations of beetles (parental and offspring cultures), mortality was recorded once a week for eight weeks after immune challenge and examined according to parental challenges (for both parental and offspring cultures) and offspring challenges (for offspring cultures only).