Abstract
The oceanic abyss (depths greater than 3000 m), one of the largest environments on the planet, is characterized by absence of solar light, high pressures and remoteness from surface food supply necessitating special molecular, physiological, behavioural and ecological adaptations of organisms that live there. Sampling by trawl, baited hooks and cameras we show that the Chondrichthyes (sharks, rays and chimaeras) are absent from, or very rare in this region. Analysis of a global data set shows a trend of rapid disappearance of chondrichthyan species with depth when compared with bony fishes. Sharks, apparently well adapted to life at high pressures are conspicuous on slopes down to 2000 m including scavenging at food falls such as dead whales. We propose that they are excluded from the abyss by high-energy demand, including an oil-rich liver for buoyancy, which cannot be sustained in extreme oligotrophic conditions. Sharks are apparently confined to ca 30% of the total ocean and distribution of many species is fragmented around sea mounts, ocean ridges and ocean margins. All populations are therefore within reach of human fisheries, and there is no hidden reserve of chondrichthyan biomass or biodiversity in the deep sea. Sharks may be more vulnerable to over-exploitation than previously thought.
Keywords: Chondrichthyes, sharks, deep-sea fishes, abyss, elasmobranches
1. Introduction
The abyssal regions of the world's seas and oceans (depths greater than 3000 m) have been colonized by fishes during the last 70 Myr, contemporary with appearance of birds and mammals on land. This was enabled and is sustained by oxygenation of deep water by the modern global thermohaline circulation (Merrett & Haedrich 1997). Owing to stagnation events, final invasion of the eastern basins of the Mediterranean occurred only 6000 years ago (Rohling 1994). Deep-sea fishes were first discovered in the 1860s and Günther (1880) reported the deepest bony fish as Gonostoma microdon at 5300 m from the Pacific Ocean whereas the deepest Chondrichthyes were a ray from 1033 m and a shark from 915 m. Deep-sea fishes are not a distinct taxonomic group but are derived from a diversity of shallow water types. Among the Chondrichthyes, including Holocephali (chimaeras) and Elasmobranchii (sharks and rays), many species show anatomical adaptations to deep-sea life including eyes sensitive to low light levels and possession of light organs (e.g. Isistius spp.; Widder 1998). They now form an important component of deep-water fisheries down to 2000 m depth (Gordon et al. 2003) and are conspicuous as scavengers at whale carcasses (Smith & Baco 2003) and baited cameras (Priede & Bagley 2000) suggesting that the deep sea may harbour a hidden diversity of Chondrichthyes.
All the major classes of vertebrates, mammals, birds, reptiles, amphibians, bony fish (Osteichthyes) and Chondrichthyes (sharks, rays and chimaeras), are suffering declines in species number and population sizes owing to habitat changes or exploitation (IUCN 2004; Şekercioğlu et al. 2004). Chondrichthyes are almost entirely marine, whereas bony fishes are found in all aquatic environments from the highest altitude freshwater habitats through to the deep sea. Mammals, birds and reptiles occur in terrestrial, freshwater and marine environments and sperm whales Physeter macrocephalus are capable of diving (Wahlberg 2002) to over 1000 m depth feeding on squid at bathyal depths. In comparison with the diversity of environments occupied by these other classes of vertebrates, the Chondrichthyes are rather restricted in their habitat although mobility and world-wide distribution of many species make this a successful group.
From the earliest discoveries in the nineteenth century to the present day, records have consistently shown that Osteichthyes occur to much greater depths than Chondrichthyes. Nevertheless, it has been argued that biological sampling of the deep ocean (see electronic supplementary material) remains inadequate and many species and populations of animals remain to be discovered. However, if the depth distribution of Chondrichthyes is truncated at the boundary of the abyss, this has important implications for management and conservation of these species, which are being heavily exploited throughout their global distribution (Stevens et al. 2000; Baum et al. 2003).
In this study, we deployed sampling equipment to which both Chondrichthyes and Osteichthyes are susceptible over a depth range from less than 500 m on slopes and over mid-ocean ridges to the abyssal plains at 4800 m in the Atlantic Ocean and 5900 m in the Pacific Ocean. Three different techniques were used: baited cameras, long-lines with baited hooks and demersal trawling. We combine this with an analysis of the cumulative historical record to examine the global depth limits of distribution of Chondrichthyes compared with the Osteichthyes.
2. Material and methods
(a) Trawling
A 45 ft (13.7 m) semi-balloon otter trawl was used. The trawl was shot on twin warps with 120 kg otter boards bridled to a single warp once the doors had spread (Merrett & Marshall 1981). Nominal spread of the mouth of the trawl (width of sea-bed sampled) was 8.6 m. Haul duration was varied between a bottom contact time of 30 min at the shallowest stations to 3 h on the abyssal plain and the tow speed was 2–2.5 knots. Sampling was done over a period of 3 years (2000–2002) during five cruises of the RRS Discovery in the northeast Atlantic in the region of the Porcupine Sea-Bight and Porcupine Abyssal Plain. Cruises were in different seasons to avoid biasing of sampling in relation to any fish migrations that might occur (Priede et al. 2003).
(b) Baited hooks
Long-lining was done using the commercial vessel MS Loran, which is fitted out as an autoliner. During 12 fishing days in July 2004, 61 baited long line sets were done on the Mid-Atlantic Ridge between 42 and 55° N at depths from 450 to 4300 m. A total of 87 500 baited hooks were deployed.
(c) Baited camera
Baited cameras were deployed using a free-fall lander technique (Priede & Bagley 2000). A piece of bait (usually mackerel weighing ca 0.5 kg) was deployed on the sea floor within the field of view of a downward looking video or time-lapse stills (film or digital) camera attached to a lander frame with an onboard computer, data storage, depth and current sensors. The lander was left on the sea floor for up to 12 h during which time images of fish approaching, consuming and departing from the bait were recorded. The system was recovered by acoustic command from the ship and images were downloaded for analysis. Species were identified by reference to standard texts and comparison with voucher specimens captured in trawls. Definitive species names could not necessarily be allocated and cryptic species might not be separable from images alone. Therefore, species counts in photographs can be regarded as minimum estimates. Data from 166 deployments are analysed (see electronic supplementary material).
(d) Archive data
Archive data for depth of occurrence of marine and brackish water fish were abstracted from global data sets available on FishBase (Froese & Pauly 2004). Original references were checked for the deepest species including all occurrences of Chondrichthyes deeper than 2500 m. Dubious records where sampling gear traversed a wide depth range and depth of capture was uncertain were rejected. Records of maximum depth were accepted for 669 species of Chondrichthyes and 8691 species of Actinopterygii.
3. Results
(a) Pelagic species
Chondrichthyes, notably sharks, are found near the surface in the open waters throughout the world's oceans. Filter feeding planktivores clearly must swim near the surface where most of their food is found, but maximum dive depths of 850 m and over 1000 m have been recorded for the basking shark (Cetorhinus maximus; Sims et al. 2003). Graham et al. (2005) recorded a dive of a whale shark (Rhincodon typus) to over 980 m in the Atlantic Ocean off Belize whereas studies around the Seychelles in the Indian Ocean (ESM) logged dives to over 1000 m depth for 6 min but no deeper than 1500 m. Pelagic predatory sharks also spend most time at shallow depths (Sundström et al. 2001) but we cannot exclude the possibility that occasional dives deeper than 1000 m may occur. Discovery of the deeper-living megamouth shark (Megachasma pelagios; Taylor et al. 1983) encouraged speculation on presence of new species of deep-water pelagic sharks, but the depth of capture of the first specimen was 400 m, Nelson et al. (1997) tracked one in coastal waters at depths down to 166 m and putative maximum depth for this species is cited as 1000 m (Froese & Pauly 2004). We can find no evidence of any pelagic chondrichthyan living at depths greater than 1500 m.
(b) Demersal species
The deepest living Chondrichthyes are bottom-living species described as demersal or benthic. We have sampled them in three distinctive ways: trawling, baited hooks on long lines and baited cameras placed on the sea floor.
(i) Trawl capture
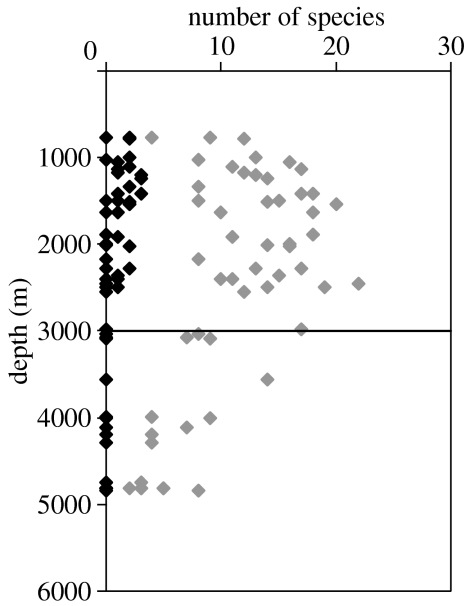
In the northeast Atlantic Ocean, west of Ireland, we carried out 52 bottom trawls at depths from 750 to 4800 m (figure 1) and found no Chondrichthyes in 17 trawls deeper than 2500 m depth. In contrast, the deepest trawl (4835 m) returned eight species of bony fish (Actinopterygii). In a series of 17 trawls on the Mid-Atlantic Ridge at 930–3505 m, we found no Chondrichthyes deeper than 2520 m except for one holocephalan, Harriota spp. at 3010 m.
Figure 1.
North East Atlantic Ocean, numbers of species captured in trawls at different depths. Grey symbols, Actinopterygii; black symbols, Chondrichthyes.
(ii) Long lines
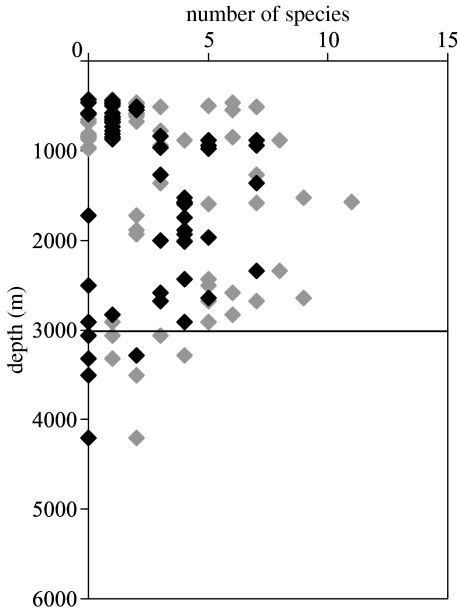
In a survey of the Mid-Atlantic Ridge in 61 baited long line sets at 433–4200 m. The deepest Chondrichthyes below 3000 m, were a ray, Bathyraja pallida and a shark, Centrophorus squamosus both captured at 3280 m. Bathyraja richardsoni, Hydrolagus affinis and Etmopterus princeps were caught at 2909 m and Hydrolagus pallidus at 2650 m and Dipturus batis at 2619 m. Three deeper line sets caught no Chondrichthyes, whereas bony fish were caught at all depths (figure 2). All depths given are the minima for lines which may follow the bottom slope over 100–200 m of depth amplitude.
Figure 2.
Mid-Atlantic Ridge, numbers of species captured on baited lines at different depths. Grey symbols, Actinopterygii; black symbols, Chondrichthyes.
(iii) Baited cameras
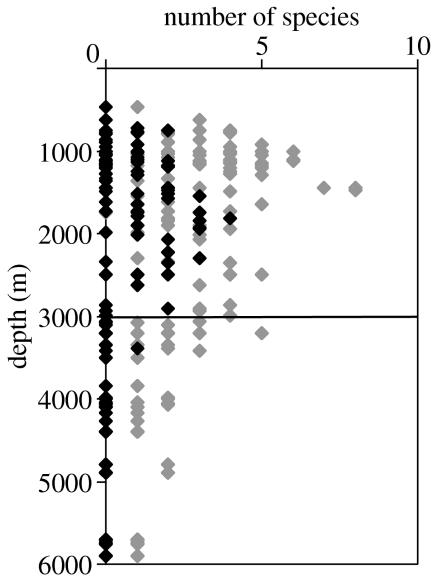
We have collated data from 166 deployments of baited cameras placed on the sea floor at depths from 471 to 5900 m in the Atlantic Ocean at latitudes 53° N–54° S (Armstrong et al. 1992; Priede et al. 1994a,b; Collins et al. 1999a,b; Henriques et al. 2002), the North Pacific Ocean (Priede & Smith 1986; Priede et al. 1990, 1994a,b), Indian Ocean (Witte 1999) and Mediterranean Sea (Jones et al. 2003). Of 84 deployments at depths greater than 2500 m, no Chondrichthyes were found (figure 3) except for observations of rays at 2630 m in the Porcupine Sea-Bight (northeast Atlantic), and at 2908 and 3396 m on the slopes of the Mid-Atlantic Ridge. The deepest sharks were Hexanchus griseus and Etmopterus spinax at 2490 m in the Mediterranean Sea. The deepest chimaera was at 2355 m on the Mid-Atlantic Ridge. Osteichthyes were present at all depths.
Figure 3.
Number of species of fish attracted within view of baited cameras deployed at different depths, Atlantic, Pacific, Indian Oceans and Mediterranean Sea. Grey symbols, Actinopterygii; black symbols, Chondrichthyes. Horizontal line at 3000 m indicates the upper limit of the abyss.
(iv) Archive data
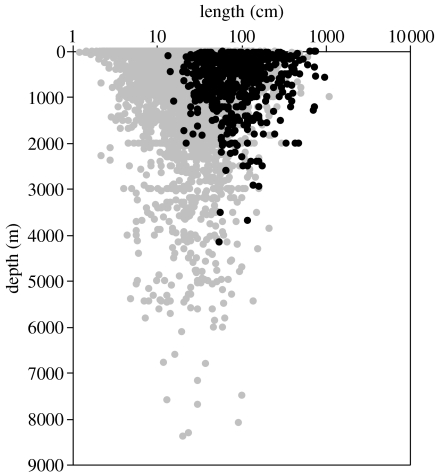
With the exception of seven species, Chondrichthyes were confined to depths less than 2500 m (figure 4). Two Holocephali were reported to occur to 2600 m, the Rhinochimaeridae Harriotta haeckeli and Harriotta raleighana (Last & Stevens 1994). The deepest sharks were Isistius brasiliensis reported from the surface to 3500 m (Compagno et al. 1995) and Centroscymnus coelolepis reported to 3700 m (Forster 1973). The ray Rajella bigelowi is probably the deepest chondrichthyan fish described as ‘benthic on deeper continental slopes and probably abyssal plains between 650 and 4156 m’ (Stehmann 1990). Thus, while Chondrichthyes are largely confined to depths less than 2500 m, 260 species of bony fish from several families were reported from depths greater than 2500 m. The deepest recorded fish was the cuskeel Abyssobrotula galatheae trawled from 8370 m (Nielson 1977).
Figure 4.
Maximum depth of occurrence of Chondrichthyes and Actinopterygii. Global data set for maximum adult total body length and depths of 669 species of Chondrichthyes (black symbols) and 8691 species of Actinopterygii (grey symbols).
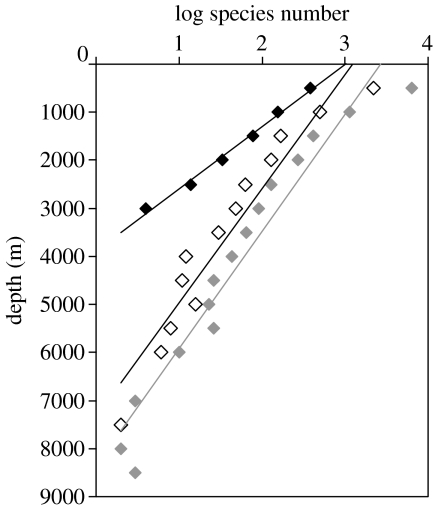
Grouping the species into 500 m depth bins, we have plotted the number of species as a function of their maximum depth (figure 5). For Chondrichthyes, the slope of decrease in species number with depth was 0.8 log units (630%) per 1000 m compared with only 0.4 log units (250%) per 1000 m for Actinopterygii. This indicated a much more rapid disappearance of Chondrichthyes with depth than the bony fish.
Figure 5.
Rates of decrease in species numbers with depth. Log of number of species depth maxima per 500 m stratum for the global data set. Black symbols and line, Chondrichthyes (log N=−0.0008x+2.9969, r2=0.992); grey symbols and line, Actinopterygii (log N=−0.0004x+3.3227, r2=0.9375); open symbols and thin line, large Actinopterygii corresponding to the size range of the Chondrichthyes (log N=−0.0004x+2.9733, r2 =0.9176).
4. Discussion
Recent data from a number of studies using depth sensing devices attached to sharks indicate that pelagic species are found no deeper than 1500 m in the open ocean. On continental slopes, around islands, seamounts and on the mid ocean ridges bottom-living or demersal Chondrichthyes were found down to 3000 m and with rare occurrences down to just over 4000 m. Numerous trawl and baited camera samples on the abyssal plains never revealed the presence of any Chondrichthyes. The Beebe project using deep-diving manned submersibles concluded that sharks may not normally inhabit waters deeper than 2128 m but found rays and chimaeras at greater depths and indicated that more research is needed to reveal to true depth ranges (Clark & Kristof 1990, 1991). In the present study, except for one recorded capture, sharks were absent from all samples deeper than 3000 m, whereas bony fish were found at all depths. We believe the case is already clear that Chondrichthyes have generally failed to colonize the oceans deeper than 3000 m and it is very unlikely that major new populations will be discovered in abyssal regions.
The data presented in figures 4 and 5 are the maximum recorded depths for each species and do not represent typical depth of distribution. We conclude that the cumulative sampling effort over almost 150 years since the first discovery of the deep-sea ichthyofauna has now accurately delineated the maximum depth limits for the Chondricthyes and has found that they are essentially absent from the abyss at depths greater than 3000 m. Extrapolation of the lines of maximum depth in figure 5 gives a theoretical maximum depth for the deepest chondrichthyan of 3893 m. For bony fish, the corresponding depth of deepest occurrence is 8350 m; very close to the actual depth of capture of the A. galatheae.
(a) Volume of ocean occupied by Chondrichthyes
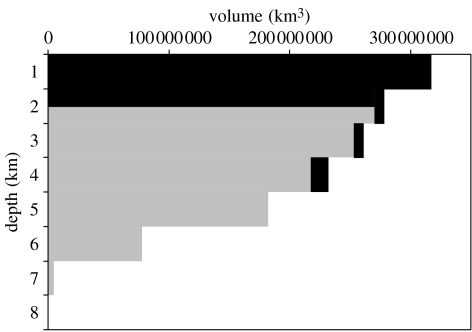
The total volume of the oceans is 1.37×109 km3. Assuming that pelagic Chondrichthyes occur everywhere to a depth of 1500 m, and demersal species down to 4000 m and up to 250 m off the bottom, the ocean volume used by Chondrichthyes is 0.395×109 km3 (figure 6). Hence, over 70% of the global ocean volume is devoid of Chondrichthyes, which are confined to the surface layers, ocean margin regions, around ocean islands, mid-ocean ridges and sea mounts. These areas are all intensively fished and there is no evidence of a deep refuge of chondrichthyan biodiversity or biomass in the abyss. Defining the abyss as depths of 3–6 km (Herring 2002), Chondrichthyes are essentially absent from this and the hadal zone more than 6 km deep. Furthermore, we hypothesize that the abyssal plains may represent barriers to migration and dispersal (especially given the lack of planktonic stages in Chondrichthyes) although such movement cannot be excluded on the basis of existing evidence.
Figure 6.
The volume of ocean occupied by Chondrichthyes. A hypsographic diagram showing the ‘chondrichthyan’ volume in black. Apart from possible absence from the extreme depths of the hadal ocean trenches (greater than 9000 m depth), the Actinopterygii can be assumed to occur throughout the ocean volume (grey).
(b) Explaining the absence of Chondrichthyes from the abyss
Any hypothesis explaining the absence of Chondrichthyes from the abyss should take into account the fact that many species are well adapted to the deep-sea conditions at 2–3000 m. For example, chimaeras are generally cold-water deep-sea fish absent from shallow waters less than 80 m depth, abundant in the 1000–2000 m depth range but never found in the abyss.
(i) Species number
In both bony fish and Chondrichthyes, species number decreases with depth (figure 5). Simple probability implies that the bony fish are more likely to have produced representatives capable of surviving in deep environments by virtue of much higher species numbers in shallow water, rather than any underlying physical, physiological or ecological cause. However, it is evident that species number declines much more rapidly in Chondrichthyes and the lower species reservoir in shallow water does not explain lack of representation in the abyss. If Chondrichthyes had the same species extinction rate with depth as the Actinopterygii, we would expect their maximum depth to be 7500 m permitting survival throughout the abyssal regions of the world and into the margins of the hadal trench systems.
(ii) Body size
There is considerable debate regarding body size trends in fish across depth strata in the oceans. A study of trends in bony fish size between 500 and 5000 m depth in the northeast Atlantic shows that scavenging species are bigger at greater depths whereas there is no significant size trend in non-scavengers. Metabolic modelling of foraging strategies shows a clear advantage of increased size of scavengers in the oligotrophic environment of the abyss (Collins et al. 2005). It is evident from figure 4 that Chondrichthyes are predominantly bigger fish than Actinopterygii. Since many Chondrichthyes can function as scavengers and are attracted to bait, this would suggest a possible advantage for their survival in the abyss. It is paradoxical therefore to observe that it is the Chondrichthyes with their larger size that show a higher extinction rate with depth. Abstracting large Actinopterygii (more than 29 cm max. length, spanning the same size range as the Chondrichthyes) from the global data base, the number of species decreases at a rate of 0.4 log units per 1000 m; the same as for all Actinopterygii (figure 5). Compared with Actinopterygii, Chondrichthyes are clearly deficient in their ability to survive at increasing depth and this has precluded their colonization of the abyss.
(iii) Water temperature
The deep sea is cold with typical temperatures of 2–4 °C, but it is very unlikely that such temperatures exclude Chondrichthyes from the abyss. There is no sharp temperature discontinuity at the upper boundary of the abyss and many species of fish are capable of living within this temperature range. This does not exclude the possibility that the presence of intermediate water masses such as the Mediterranean intermediate water in the northeast Atlantic might act as an environmental cue enabling deep slope-dwelling species to orientate to an optimum depth. The influence of temperature as a barrier to colonization of the abyss is, however, further excluded by the observation that Chondrichthyes are also absent from the abyssal Mediterranean Sea, where we have observed sharks attracted to baits at depths down to 2490 m and only Actinopterygii present at greater depths (Jones et al. 2003). The Mediterranean Sea is warm all the way to the bottom (ESM). It is evident that Chondrichthyes are excluded from abyssal regions of the seas independently of the prevailing temperature regime.
(iv) Hydrostatic pressure
To preserve cellular function at high pressure, deep-sea organisms require homeoviscous adaptation of membranes and structural adaptation of proteins (Macdonald 2001). These effects are evident in bony fish species living deeper than 1000 m and while there are no data for Chondrichthyes, we presume that pressure tolerance is well developed in this group. Furthermore, Chondrichthyes generally have high concentrations of trimethylamine-N-oxide (TMAO) in their body fluids. In addition to acting as an osmolyte, TMAO has been shown to act as a universal stabilizer of protein structure. The muscles of deep-sea shrimps, Agnatha, Chondrichthyes and Osteichthyes all have increased concentrations of TMAO compared with shallow water species apparently conferring pressure tolerance on structural and enzyme proteins (Yancey et al. 2002). With generally high TMAO concentrations it seems that Chondrichthyes are pre-adapted to life at high pressures. Since many species of Chondrichthyes can survive at 2000–3000 m, depth, and there are occasional records to over 4000 m it is unlikely that there is a fundamental physiological barrier to this group extending its distribution to 6000 m, a modest proportional change in pressure.
(v) Buoyancy
A characteristic of the deep-water sharks is large livers, rich in lipids that enable them to attain almost neutral buoyancy. Probably, the most successful demersal scavenging and predatory fish in the abyss is Coryphaenoides (Nematonurus) armatus (Actinopterygii, Macrouridae), which uses a gas-filled bladder for buoyancy. For 1 kg of buoyancy using squalene (specific gravity 0.86) requires 7.14 kg of oil with an energy content of 264 MJ. The same buoyancy using a gas-filled bladder entails a theoretical pumping cost at 4000 m depth of 90 kJ, calculated for oxygen (ESM). Even assuming efficiency of 5–10%, the energy cost of buoyancy using an air bladder is trivial compared with a lipid-based system, making incremental increases in buoyancy during growth much less expensive, assuming the swim bladder wall is gas impermeable. For shallow-water fish adjusting the quantity of gas in the swim bladder during vertical movements can be energetically costly but for abyssal fish such movements, since they are a small proportion of total water depth, result in negligible volume changes and there is no requirement to adjust quantity of gas in the bladder. Once the bladder is inflated, given low permeability of the swim bladder wall, maintenance cost of the swim bladder in abyssal fish is lower than for shallower living species making the same absolute vertical movements.
(vi) Metabolism
Studies of swimming speed (Collins et al. 1999a,b) and metabolism (Bailey et al. 2002) in bathyal (1000–3000 m) and abyssal fish species indicate much lower activity in the latter linked to a decrease in food supply with depth. We believe the proximal reason for absence of Chondrichthyes from the abyss is the absence of truly low energy forms for survival in a deep oligotrophic environment. The penalty of the need for a large lipid-rich liver may be decisive in excluding Chondrichthyes from the abyss. The complete lack of ‘whole-animal’ or tissue metabolic rate studies leave this as an open question, which certainly demands further investigation.
(vii) Reproduction
Chondrichthyes have direct life cycles without larval stages reproducing either through hatching of small adult forms from egg cases (Stehmann & Merrett 2001) or through bearing of live young. Such miniature adults may be vulnerable in the abyss and some species migrate into shallower water to breed. Many deep-water bony fishes, however, produce buoyant eggs and larvae that are presumed to develop remote from the sea floor and benefit from greater food supply at surface layers of the oceans (Merrett & Headrich 1997).
5. Conclusions
Observations or captures of Chondrichthyes at depths over 4000 m are very rare whereas at these depths bony fishes and other fauna can be quite abundant. The deepest confirmed reports of a shark C. coelolepis at 3700 m and the ray R. bigelowi at 4156 m are both species with their main zone of distribution at much shallower depths; minimum depths are 270 and 650 m, respectively. This is in contrast to a number of bony fishes that have minimum depths in excess of 3000 m. The world's deepest fish, A. galatheae has a minimum depth of 3110 m and Coryphaenoides yaquinae, which is an abundant macrourid on the abyssal floor of the Pacific Ocean seen in our baited camera images at 5900 m (figure 3), has a minimum depth of 3400 m (Froese & Pauly 2004). Discovery of a new shark, ray or chimaera at depths greater than 3000 m with its predominant distribution in the abyss is very unlikely. Special expeditions to reveal such a fauna may be difficult to justify but as research expands in the deep sea more of the ocean will be surveyed and gradually certainty regarding faunal composition of the abyss will increase.
There is probably no single simple explanation for the absence of Chondrichthyes from the abyss. More research is needed on the effects of pressure on metabolic enzyme activity, membrane structure and adaptations to pressure in Chondrichthyes. Most information that is available is for bony fishes.
The Chondrichthyes and sharks in particular are threatened world-wide by the intensity of human fishing activity. The finding that they are largely absent from regions of the deep sea beyond the reach of commercial fisheries further emphasizes concern regarding the conservation of this class of vertebrates. Owing to low productivity and slow growth rates in the deep sea, exploitation of deep-water species is generally of doubtful sustainability. However, some of the Actinopterygii that are captured commercially have a depth distribution beyond the maximum depth of economically viable fisheries. For example, the roundnose grenadier Coryphaenoides rupestris with a fishery targeted at shoals 500–1000 m deep, occurs down to 2200 m. There is no such deep ‘protected area’ for Chondrichthyes, and the whole Class may potentially be at risk.
Acknowledgements
This work was supported by Census of Marine Life, NERC grant no. GR3/12789 and NERC studentships to E.G.J and N.K. C.H. was supported by Fundação para a Ciência e Tecnologia, Portugal. Camera work off Angola and on the Mid-Atlantic Ridge was supported by B.P. We thank personnel on board the ships RRS Discovery, RV G.O. Sars and MS Loran.
Supplementary Material
References
- Armstrong J.D, Bagley P.M, Priede I.G. Photographic and acoustic tracking observations of the behaviour of the grenadier Coryphaenoides (Nematonurus) armatus, the eel Synaphobranchus bathybius, and other abyssal demersal fish in the North Atlantic Ocean. Mar. Biol. 1992;112:535–544. 10.1007/BF00346170 [Google Scholar]
- Bailey D.M, Jamieson A.J, Bagley P.M, Collins M.A, Priede I.G. Measurement of in situ oxygen consumption of deep-sea fish using an autonomous lander vehicle. Deep-Sea Res. I. 2002;49:1519–1529. 10.1016/S0967-0637(02)00036-5 [Google Scholar]
- Baum J.K, Myers R.A, Kehler D.G, Worm B, Harley S.J, Doherty P.A. Collapse and conservation of shark populations in the Northwest Atlantic. Science. 2003;299:389–392. doi: 10.1126/science.1079777. 10.1126/science.1079777 [DOI] [PubMed] [Google Scholar]
- Clark E, Kristof E. Deep sea elasmobranchs observed from submersibles in Grand Cayman, Bermuda and Bahamas. In: Pratt H.L, Gruber S.H, Tanuichi T, editors. Elasmobranchs as living resources: advances in the biology, ecology, systematics and status of fisheries. Technical Report 90. NOAA; Washington, DC: 1990. pp. 275–290. [Google Scholar]
- Clark E, Kristof E. How deep do sharks go? Reflections on deep sea sharks. In: Gruber S.H, editor. Discovering sharks. Special Publication 14. American Littoral Society; Highlands, NJ: 1991. pp. 79–84. [Google Scholar]
- Collins M.A, Priede I.G, Bagley P.M. In situ comparison of activity in two deep-sea scavenging fishes occupying different depth zones. Proc. R. Soc. B. 1999a;266:2011–2016. 10.1098/rspb.1999.0924 [Google Scholar]
- Collins M.A, Yau C, Nolan C.P, Bagley P.M, Priede I.G. Behavioural observations on the scavenging fauna of the Patagonian slope. J. Mar. Biol. Assoc. UK. 1999b;79:963–970. 10.1017/S0025315499001198 [Google Scholar]
- Collins M.A, Bailey D.M, Ruxton G.D, Priede I.G. Trends in body size across an environmental gradient: a differential response of scavenging and non-scavenging demersal deep-sea fish. Proc. R. Soc. B. 2005;272:2051–2057. doi: 10.1098/rspb.2005.3189. 10.1098/rspb.2005.3189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagno L.J.V, Krupp F, Schneider W. Tiburones. In: Fischer W, et al., editors. Guia FAO para Identification de Especies para los Fines de la Pesca. Pacifico Centro-Oriental. FAO; Rome: 1995. pp. 647–744. [Google Scholar]
- Forster G.R. Line fishing on the continental slope—the selective effect of different hook patterns. J. Mar. Biol. Assoc. UK. 1973;53:749–751. [Google Scholar]
- Froese, R. & Pauly, D. (eds) 2004 FishBase. World Wide Web electronic publication. (www.fishbase.org)
- Gordon J.D.M, Bergstad O.A, Figueiredo I, Menezes G. The deep-water fisheries of the Northeast Atlantic: I. Description and current trends. J. Northwest Atl. Fish. Sci. 2003;31:137–150. [Google Scholar]
- Graham R.T, Roberts C.M, Smart J.C.R. Diving behaviour of whale sharks in relation to a predictable food pulse. J. R. Soc. Interf. 2005;3:109–116. doi: 10.1098/rsif.2005.0082. 10.1098/rsif.2005.0082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther A.C.L.G. Adam & Charles Black; Edinburgh, UK: 1880. An introduction to the study of fishes. [Google Scholar]
- Henriques C, Priede I.G, Bagley P.M. Baited camera observations of deep-sea demersal fishes of the northeast Atlantic Ocean at 15–28° N off West Africa. Mar. Biol. 2002;141:307–314. 10.1007/s00227-002-0833-6 [Google Scholar]
- Herring P.J. Oxford University Press; Oxford, UK: 2002. The biology of the deep ocean. [Google Scholar]
- International Union for Conservation of Nature and Natural Resources 2004 IUCN Red List of Threatened Species (www.redlist.org)
- Jones E.G, Tselepides A, Bagley P.M, Collins M.A, Priede I.G. Bathymetric distribution of some benthic and benthopelagic species attracted to baited cameras and traps in the eastern Mediteranean. Mar. Ecol. Prog. Ser. 2003;251:75–86. [Google Scholar]
- Last P.R, Stevens J.D. CSIRO; Melbourne, Australia: 1994. Sharks and rays of Australia. [Google Scholar]
- Macdonald A.G. Effects of high pressure on cellular processes. In: Sperelakis N, editor. Cell physiology sourcebook: a molecular approach. Academic Press; San Diego, CA: 2001. pp. 1003–1023. [Google Scholar]
- Merrett N.R, Haedrich R.L. Chapman & Hall; London, UK: 1997. Deep-sea demersal fish and fisheries. [Google Scholar]
- Merrett N.R, Marshall N.B. Observations on the ecology deep-sea bottom-living fishes collected off northwest Africa (08°–27° N) Prog. Oceanogr. 1981;9:185–244. 10.1016/0079-6611(80)90002-6 [Google Scholar]
- Nelson D.R, McKibben J.N, Strong W.R, Jr, Lowe C.G, Sisneros J.A, Schroeder D.M, Lavenberg R.J. An acoustic tracking of a megamouth shark, Megachasma pelagios: a crepuscular vertical migratory. Environ. Biol. Fish. 1997;49:389–399. 10.1023/A:1007369619576 [Google Scholar]
- Nielson J.G. The deepest living fish Abyssobrotula galathea. A new genus and species of oviparous ophidiids (Pisces, Brotulidae) Galathea Rep. 1977;14:41–48. [Google Scholar]
- Priede I.G, Bagley P.M. In situ studies on deep-sea demersal fishes using autonomous unmanned lander platforms. Oceanogr. Mar. Biol. Annu. Rev. 2000;38:357–392. [Google Scholar]
- Priede I.G, Smith K.L., Jr Behaviour of the abyssal grenadier Coryphaenoides yaquinae, monitored using ingestible acoustic transmitters in the Pacific Ocean. J. Fish Biol. 1986;29:199–206. 10.1111/j.1095-8649.1986.tb05011.x [Google Scholar]
- Priede I.G, Smith K.L, Jr, Armstrong J.D. Foraging behaviour of abyssal grenadier fish: inferences from acoustic tagging and tracking in the North Pacific Ocean. Deep-Sea Res. 1990;37:81–101. 10.1016/0198-0149(90)90030-Y [Google Scholar]
- Priede I.G, Bagley P.M, Smith A, Creasey S, Merrett N.R. Scavenging deep demersal fishes of the Porcupine Seabight, North-east Atlantic: observations by baited camera, trap and trawl. J. Mar. Biol. Assoc. UK. 1994a;74:481–498. [Google Scholar]
- Priede I.G, Bagley P.M, Smith K.L., Jr Seasonal change in activity of abyssal demersal scavenging grenadiers Coryphaenoides (Nematonurus) armatus in the eastern Pacific Ocean. Limnol. Oceanogr. 1994b;39:279–285. [Google Scholar]
- Priede I.G, Deary A.R, Bailey D.M, Smith K.L., Jr Low activity and seasonal change in population size structure of grenadiers in the oligotrophic central North Pacific Ocean. J. Fish Biol. 2003;63:187–196. 10.1046/j.1095-8649.2003.00142.x [Google Scholar]
- Rohling E.J. Review and new aspects concerning the formation of eastern Mediterranean sapropels. Mar. Geol. 1994;122:1–28. 10.1016/0025-3227(94)90202-X [Google Scholar]
- Şekercioğlu Ç.H, Daily G.C, Ehrlich P.R. Ecosystem consequences of bird declines. Proc. Natl Acad. Sci. USA. 2004;101:18 042–18 047. doi: 10.1073/pnas.0408049101. 10.1073/pnas.0408049101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims D.W, Southall E.J, Richardson A.J, Reid P.C, Metcalfe J.D. Seasonal movements and behaviour of basking sharks from archival tagging: no evidence of winter hibernation. Mar. Ecol. Prog. Ser. 2003;248:187–196. [Google Scholar]
- Smith C.R, Baco A.R. Ecology of whale falls at the deep-sea floor. Oceanogr. Mar. Biol. Annu. Rev. 2003;41:311–354. [Google Scholar]
- Stehmann M. Rajidae. In: Quero J.C, et al., editors. Check-list of the fishes of the eastern tropical Atlantic. vol. 1. Junta Nacional de Investigaçao Cientifica e Tecnológica; Lisbon, Portugal: 1990. pp. 29–50. [Google Scholar]
- Stehmann M.F.W, Merrett N.R. First records of advanced embryos and egg capsules of Bathyraja skates from the deep-north-eastern Atlantic. J. Fish Biol. 2001;59:338–349. 10.1111/j.1095-8649.2001.tb00134.x [Google Scholar]
- Stevens J.D, Bonfil R, Dulvy N.K, Walker P.A. The effects of fishing on sharks, rays and chimaeras (chondrichthyans), and the implications for marine systems. ICES J. Mar. Sci. 2000;57:476–494. 10.1006/jmsc.2000.0724 [Google Scholar]
- Sundström L.F, Gruber S.H, Clermont S.M, Correia J.P.S, de Marignac J.R.C, Morrissey J.F, Lowrance C.R, Thomassen L, Oliveira M.T. Review of elasmobranch behavioral studies using ultrasonic telemetry with special reference to the lemon shark, Negaprion Brevirostris, around Bimini Islands, Bahamas. Environ. Biol. Fish. 2001;60:225–250. [Google Scholar]
- Taylor L.R, Jr, Compagno L.J.V, Struhsaker P.J. Megamouth—a new species, genus and family of lamnoid shark (Megachasma pelagios, family Megachasmidae) from the Hawaiian Islands. Proc. Calif. Acad. Sci. 1983;4:87–110. [Google Scholar]
- Wahlberg M. The acoustic behaviour of diving sperm whales observed with a hydrophone array. J. Exp. Mar. Biol. Ecol. 2002;281:53–62. 10.1016/S0022-0981(02)00411-2 [Google Scholar]
- Widder E.A. A predatory use of counterillumination by the squaloid shark, Isistius brasiliensis. Environ. Biol. Fish. 1998;53:267–273. 10.1023/A:1007498915860 [Google Scholar]
- Witte U. Consumption of large carcasses by scavenger assemblages in the deep Arabian Sea: observations by baited camera. Mar. Ecol. Prog. Ser. 1999;183:139–147. [Google Scholar]
- Yancey P.H, Blake W.R, Conley J. Unusual organic osmolytes in deep-sea animals: adaptations to hydrostatic pressure and other perturbants. Comp. Biochem. Physiol. A. 2002;133:667–676. doi: 10.1016/s1095-6433(02)00182-4. 10.1016/S1095-6433(02)00182-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.