Abstract
Indirect and direct models of sexual selection make different predictions regarding the quantitative genetic relationships between sexual ornaments and fitness. Indirect models predict that ornaments should have a high heritability and that strong positive genetic covariance should exist between fitness and the ornament. Direct models, on the other hand, make no such assumptions about the level of genetic variance in fitness and the ornament, and are therefore likely to be more important when environmental sources of variation are large. Here we test these predictions in a wild population of the blue tit (Parus caeruleus), a species in which plumage coloration has been shown to be under sexual selection. Using 3 years of cross-fostering data from over 250 breeding attempts, we partition the covariance between parental coloration and aspects of nestling fitness into a genetic and environmental component. Contrary to indirect models of sexual selection, but in agreement with direct models, we show that variation in coloration is only weakly heritable , and that two components of offspring fitness—nestling size and fledgling recruitment—are strongly dependent on parental effects, rather than genetic effects. Furthermore, there was no evidence of significant positive genetic covariation between parental colour and offspring traits. Contrary to direct benefit models, however, we find little evidence that variation in colour reliably indicates the level of parental care provided by either males or females. Taken together, these results indicate that the assumptions of indirect models of sexual selection are not supported by the genetic basis of the traits reported on here.
Keywords: sexual selection, genetic variance covariance matrix, blue tit, colour
1. Introduction
Indirect and direct benefits to female choice underlie two major hypotheses regarding the evolution and maintenance of sexually selected traits (Andersson 1994). Indirect benefits to mate choice arise because females can secure genetic benefits for their offspring by mating with the most ornamented males (Fisher 1958; Pomiankowski 1988). Under this scenario, the sexually selected trait indicates genetic variation in attractiveness and/or viability. Consequently, a central assumption of indirect models of sexual selection is that positive genetic covariance between the sexually selected trait and total fitness exists (Kokko et al. 2002). The strength of indirect selection therefore depends on the heritability of the sexually selected trait and the magnitude of genetic variance for total fitness (Kirkpatrick & Barton 1997). In contrast, direct benefits to mate choice arise when females directly increase their own fertility, or chance of survival, by being mated to highly ornamented males (Price et al. 1993). In birds, a potentially important form of direct selection is when sexually selected traits indicate the level of parental care a male will provide (Heywood 1989; Hoelzer 1989), although other forms of parental contribution are also feasible. This form of sexual selection is expected to be more important when the heritability of the sexually selected trait is low, and when variance in offspring fitness is mediated by paternal effects (Kokko 1998).
Although the importance of indirect genetic benefits to mate choice has been demonstrated in several laboratory populations (e.g. Jones et al. 1998; Blows 1999), it remains controversial whether indirect genetic benefits play such a large role in wild populations of vertebrates (e.g. Arnqvist & Kirkpatrick 2005), where the potential for direct effects may be greater through the provision of extensive parental care. A challenge rarely met in wild populations is effectively disentangling genetic from environmental benefits to mate choice (Jones 1987, but see Griffith et al. 1999; Qvarnstrom 1999; Johnsen et al. 2003; Garant et al. 2004). Even in those studies that do attempt to estimate quantitative genetic parameters for sexual traits, the study is usually limited to calculating heritabilities rather than genetic covariances. The overall aim of this study is to use the blue tit (Parus caeruleus) as a model system to test quantitative genetic predictions of indirect and direct genetic models of sexual selection.
The plumage coloration of the blue tit is an extensively studied sexual ornament (Andersson et al. 1998; Hunt et al. 1998; Sheldon et al. 1999; Delhey et al. 2003; Johnsen et al. 2003; Limbourg et al. 2004), yet the benefits females receive from mating with highly ornamented males remain unclear. The ultraviolet (UV)/blue cap of this species is strongly sexually dichromatic (Hunt et al. 1998; Andersson et al. 1998), and its function as a sexual signal was confirmed by the findings that it was under mutual mate choice (Hunt et al. 1999) and that individuals assortatively mate with respect to the trait (Andersson et al. 1998). Support for indirect models of sexual selection comes from two manipulative studies that have shown a causal link between male cap colour and female reproductive investment (Sheldon et al. 1999; Limbourg et al. 2004), suggesting that females can evaluate the fitness of a male's offspring according to the reflectance properties of his crown. In addition to these experimental studies, a correlative study has shown that patterns of within pair and extra-pair paternity are correlated with male cap coloration (Delhey et al. 2003). Generally, benefits derived from extra-pair mating are believed to be primarily genetic (Jennions & Petrie 2000, but see Arnqvist & Kirkpatrick 2005), again supporting the view that males advertise indirect benefits to potential mates. However, in the study by Delhey et al. (2003), it was in fact the less ornamented males that gained extra-pair offspring, so these results were inconsistent with the other studies supporting indirect models, in that the sign of the correlation was in the opposite direction. Evidence of a role for direct benefits in the blue tit comes from studies in which the carotenoid based coloration of foster males was correlated with the body size of their foster chicks (Senar et al. 2002).
In this study, we test three predictions regarding indirect and direct genetic models of sexual selection. First, we estimate the relative importance of genetic and environmental effects in determining variation in plumage coloration. Second, we estimate the relative importance of genetic, parental and environmental effects in determining two components of offspring fitness: probability of recruitment into the population and size prior to fledging. Third, we estimate the genetic and non-genetic sources of covariance between these components of offspring fitness and adult coloration. We evaluate our estimates of these parameters against those used in a model suggested by Kirkpatrick & Barton (1997), which is unusual in that it employed estimable parameters, such as heritability and genetic correlations, to predict the strength of indirect selection.
2. Material and methods
(a) Study population
All work was carried out on a population of blue tits at Silwood Park, a 100 ha deciduous woodland site 40 km west of London, UK (Grid Ref: SU 940690). A nest-box population was set up in 2002 with the introduction of 200 nest-boxes onto the site, and a further 30 nest-boxes were added to a previously unused area of woodland in 2004. In the springs of 2002, 2003 and 2004, 111, 177 and 162 nest-boxes, respectively, were occupied by blue tits that successfully laid eggs. The majority of these nests were subject to a cross-fostering experiment each year. Recruitment of the 2002 cohort into the breeding population was high, with approximately a quarter of the breeding adults in 2003 being born in the population in 2002. Recruitment of the 2003 cohort into the breeding population was much lower with only 3% of breeding individuals in 2004 being born in the population the previous year.
(b) Cross-fostering protocol
Genetic and environmental sources of variation are often confounded in observational studies, because genetically related individuals are distributed non-randomly across environments (Lynch & Walsh 1998). This problem may be particularly severe when the trait of interest is directly influenced by an environment that is created by the parental phenotype, which may well be the case in species that provide parental care such as birds (Merila & Sheldon 1999). Under these circumstances, related individuals that contribute to the estimation of genetic effects are often reared by the same parents, and it is impossible to distinguish the effect of inherited parental genes from the effect of the environment that the parents provide. So that variance components of offspring traits and the covariance they share with parental traits could be estimated accurately, we performed a reciprocal cross-fostering experiment (Riska et al. 1985). This involves pairing nests in which the chicks hatch on the same day, and the day after hatching, swapping an equal number of chicks between the two families, to form a cross-fostering dyad.
During the springs of 2002, 2003 and 2004, 374 broods of blue tits were reciprocally cross-fostered. Fifteen days after hatching the head to bill length and tarsus length of all surviving chicks were measured. Head to bill measurements were taken from the tip of the bill to the back of the cranium, and the tarsus measurements were taken from the posterior aspect of the tibiotarsus to the most distal undivided scute (Dhondt 1982). Measurements were taken on 805, 546 and 890 chicks from 100, 97 and 120 cross-fostered broods in 2002, 2003 and 2004, respectively.
Birds used in the analyses presented were also subject to other experimental treatments, which are not discussed here in detail but which must be considered in the statistical models. In 2003, 55 of the 97 nests were also subject to a carotenoid feeding experiment (Hadfield & Owens in press), whereby certain individuals in each brood were fed a supplementary diet. Three levels of feeding treatment were replicated across genetically related half-broods: carotenoid+mealworm, mealworm and unfed control. Chicks receiving the supplementary food were fed twice daily from 3 to 11 days after hatching.
In 2004, 74 of the 120 nests were subject to an immune challenge experiment in which a number of chicks within each genetically related half-brood either received a challenge or served as controls (Hadfield 2005). The majority of chicks from broods that contained treated individuals were also measured every other day from 2 to 16 days after hatching for several morphological traits.
All experimental treatments are controlled for statistically in subsequent analyses.
(c) Colour measurement and quantification
Adult birds were caught in the nest-box 14–17 days after the chicks had hatched during the springs of 2002, 2003 and 2004. In addition, birds were mist-netted between October and March during the winters of 2002–03 and 2003–04. During this period all birds had completed their post-juvenile moult and expressed adult plumage coloration. Three colour measurements were taken from the carotenoid-based chest plumage of each bird and from the structurally coloured crown feathers. The colour measurements were made using an established protocol (see Andersson 1996 for details). Briefly, an Avantes AVS-USB2000 miniature fibre optic spectrometer coupled to a Xenon pulsed light source (XE-2000, Avantes) was used for measuring all reflectance spectra. The optic fibre was held at 90° to the colour patch and a Teflon white reference tile (WS-2, Avantes) was used to standardize the reflectance of each measurement. The three measurements taken from the same individual were averaged.
Using the SPEC package (http://www.bio.ic.ac.uk/research/iowens/spec), and data on the spectral sensitivity of the blue tit visual system (Hart et al. 2000), we reduced complicated spectral data into four quantal cone catches, denoted VS (Very Short wavelength), S (Short), M (Medium) and L (Long) (Vorobyev et al. 1998). These cone catches were transformed into three log contrasts with the L cone catch as the denominator (Endler & Mielke 2005). Treating these contrasts as a multivariate normal response variable is the statistical analogue of opponency models of colour vision, when the cone catches follow a lognormal distribution (Endler & Mielke 2005; Hadfield 2005).
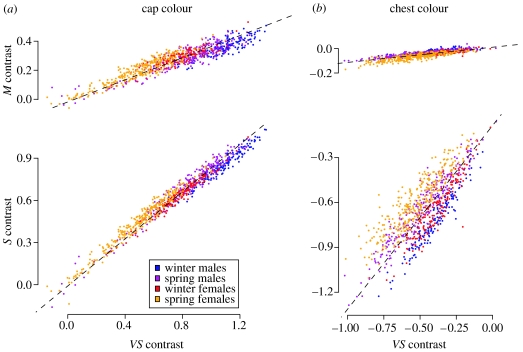
A MANOVA was fitted to the three log contrasts with sex and season (spring or winter), included as fixed effects. Eigendecomposition of the MANOVA residual sum of squares matrix revealed that 98 and 96% of the between-individual variation for cap and chest colour, respectively, was associated with a single axis (figure 1). The mean-centred log contrasts were projected onto this axis prior to analysis so that colour could be treated as a univariate response variable without loss of substantial information. The vectors for cap and chest colour were 0.74, 0.62, 0.28 and 0.63, 0.77, 0.08, respectively, where the first, second and third elements represent VS, S and M log contrasts (figure 1). Positive scores therefore denote colours that reflect strongly in the shorter wavelengths.
Figure 1.
Sexual and seasonal changes in adult cap and chest coloration. (a) Blue tit cap and (b) chest colour plotted in the blue tit's chromatic colour space. All figures are on the same scale and are directly comparable. Upper and lower plots are the same colour solid viewed from above and from the side. The dashed lines are the vectors on to which the data were projected prior to genetic analysis.
So that spring and winter measurements taken from the same bird could be averaged, these principal component (PC) scores were mean and variance standardized with respect to season prior to genetic analysis (Falconer 1983). In both cases, winter birds reflected more short wavelengths (cap: , ; chest: , ) and spring birds were more variable (cap: , ; chest: , ) (Ornborg et al. 2002; figure 1). PC scores were neither standardized nor averaged when testing for covariance between parental colour and chick morphology, as the phenotypic record for each breeding attempt was used.
(d) Genetic basis of colour
Heritability estimates were obtained using an ‘animal model’ (Kruuk 2004), with animal (genetic) and brood as random effects, and sex as a fixed effect. Wild populations are not subject to the strict breeding designs that quantitative geneticists often employ, and consequently many random effects may be confounded and aliased. In this study, 328 individuals from 87 nuclear families contributed to the estimate of additive genetic variance. Because the analyses are based on adult coloration, many individuals were the sole representatives of the broods in which they had hatched and therefore did not contribute to the estimation of brood effects. Brood effects were based on 156 individuals from 53 broods. Because the majority of these birds were part of a large-scale cross-fostering experiment or had a parent–offspring link, genetic and brood effects were never confounded and the inclusion of brood in the model did not reduce the power to detect genetic effects.
(e) Genetic basis of local recruitment
Of the 751 chicks measured prior to fledging in 2002, 183 had been re-caught by the spring of 2004. To ascertain whether genetic or brood effects contributed to variation in the probability of being re-caught after the post-juvenile moult, we fitted a general linear mixed model (Gilmour et al. 1985; Lynch & Walsh 1998) with a logit link function and binomial error structure. The data were treated as binary, with one indicating that the bird was re-caught after the post-juvenile moult and zero otherwise. Recipient and original nest were fitted as random effects, and the original nest variance component was doubled to give additive genetic variance. The reported heritabilities are on the underlying logit scale (Lynch & Walsh 1998). The two morphological traits, sex and hatch date were fitted as fixed effects.
(f) Genetic basis of offspring size
A bivariate ‘animal model’ was fitted with chick head to bill length and tarsus lengths as response variables. Animal (genetic) and brood were fitted as random effects, and year, hatch date, carotenoid treatment and immune treatment were fitted as fixed effects. One thousand nine hundred eighty-five chicks from 283 cross-fostered broods were used in the analysis. In 2004, morphological measurements were missing for 202 chicks from 34 broods. These broods were all from nests that were not used in estimating growth parameters and are therefore a non-random sample. Consequently, year effects in 2004 are unestimable, but estimates of genetic and brood effects should remain unbiased.
(g) Covariation between colour and fitness
To explore the relationship between parental cap and chest colour, and components of offspring fitness, we re-fitted the mixed models used to estimate the variance components of offspring recruitment and size. In these analyses, however, the cap and chest colour of the chick's genetic parents (dam and sire) and foster parents (foster father and nurse) were fitted as fixed effects. Inclusion of both genetic and foster parent's colour as fixed effects allows genetic correlations between adult colour and chick morphology to be separated from correlations that arise from environmental factors (such as parental care). Nests for which one, or both, parents deserted before they were caught on days 14–17 were excluded from the analysis due to missing colour measurements for the deserting parents. Desertion by male parents was much higher than desertion by female parents, and consequently colour measurements often existed for dam and nurse when sire and foster father measurements were absent. Analyses were therefore repeated with dam and nurse colour variables only.
In the model for offspring size, 213 broods had complete records for both foster parents and both genetic parents, and 270 had complete records for dam and nurse. In the model for offspring recruitment, 77 nests had complete records for both foster parents and both genetic parents, and 98 nests had complete records for dam and nurse.
In these analyses, minimum adequate models were obtained by backward stepwise term deletion of least significant colour variables (Crawley 2002). The reciprocal cross-fostering design induces a correlation between colour variables for each parental sex (e.g. cap colour of sire and foster father), so that deletion of single colour variables would result in estimates that may not be unambiguously associated with genetic or environmental effects. To avoid this problem, colour variables were paired so that colour measurements taken from the same colour patch on the same sex were deleted together. Deletion of pairs of colour variables was based on the criterion of the significance of the most significant term of the pair. The robustness of the significance tests were checked by re-analysing the model using forward stepwise term addition. In these tests, pairs of colour variables were added to the model sequentially based on the significance of the most significant term of the colour pair. Terms were added in order of their significance.
We also give approximate estimates of the genetic correlation between parental colour and offspring fitness traits using
where is the estimate slope of the regression between sire or dam coloration and the offspring trait, is the phenotypic variance in parental coloration, is the additive genetic variance in adult coloration and the additive genetic variance in the offspring trait. It should be noted that these values are only approximations, and the result does not hold for binary traits such as offspring recruitment.
(h) Hypothesis testing
In all tests, the significance of the fixed effects was tested using an adjusted F statistic with denominator degrees of freedom equal to the number of broods measured (Gilmour et al. 2002). The significance of each random effect was tested using a log-likelihood ratio test with degrees of freedom equal to the number of parameters removed (Pinheiro & Bates 2000). Standard errors of the variance components are calculated from the inverse of the information matrix, and standard errors of variance component functions (heritabilities and phenotypic variance) are calculated using the Delta method (Lynch & Walsh 1998, appendix 1). The log-likelihood ratio test is inappropriate for testing variance components in the recruitment model since the transformed variable differs between models. Therefore, the significance of the variance components derived from the recruitment model were tested using a one-tailed t-test with a t-statistic equal to the estimate divided by its standard error. The same procedure was used to test the significance of the residual error term and variance component functions. The number of nests was used as the degrees of freedom, except for the residual error term where the number of chicks was used.
All animal models and the threshold model were fitted using ASReml (Gilmour et al. 2002). R (R Development Core Team 2004) was used for all other statistical procedures.
3. Results
(a) Genetic basis of colour
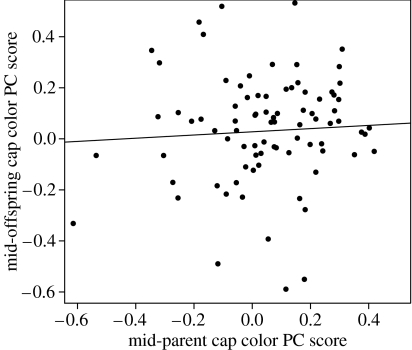
The heritability of cap colour was low (h2=0.10±0.11 s.e.) as was the heritability of chest colour (h2=0.07±0.09 s.e.) (see table 1). Neither of the heritabilities was significantly different from zero , but were significantly less than 0.48 ; the heritability value used in the models of (Kirkpatrick & Barton 1997). Brood effects were absent with estimates of brood variance being fixed at zero during convergence for both colour traits. To illustrate these results, figure 2 presents a mid-parent mid-offspring regression for cap colour ( s.e., , )
Table 1.
Heritability and ratio of common environmental variance to phenotypic variance (Vc/Vp) estimates and standard errors for adult coloration, offspring recruitment and offspring morphology. (Estimated slope of the relationship between offspring traits and parental colour variables with all colour variables included in the animal model. Approximate estimates of the genetic correlations are given in parentheses. *p<0.05; **p<0.01.)
| cap colour | chest colour | recruitment | head length | tarsus length | |
|---|---|---|---|---|---|
| heritability | 0.10±0.11 | 0.07±0.09 | 0.06±0.18 | 0.25±0.05** | 0.41±0.06** |
| Vc/Vp | 0 | 0 | 0.33±0.11** | 0.33±0.03** | 0.18±0.03** |
| parental colour versus offspring traits | |||||
| sire cap colour | — | — | 0.40±0.40 | 0.06±0.11 (0.45) | −0.22±0.10* (−1.19) |
| dam cap colour | — | — | 0.64±0.45 | −0.19±0.09 (−1.35) | −0.17±0.10 (−0.86) |
| foster-father cap colour | — | — | −0.33±0.46 | −0.16±0.11 | −0.01±0.12 |
| nurse cap colour | — | — | 0.19±0.55 | 0.09±0.11 | 0±0.12 |
| sire chest colour | — | — | −0.19±0.52 | 0.09±0.12 (0.58) | 0.08±0.11 (0.38) |
| dam chest colour | — | — | −0.25±0.64 | 0.07±0.14 (0.33) | 0.06±0.13 (0.23) |
| foster-father chest colour | — | — | 0.32±0.61 | −0.06±0.12 | −0.11±0.13 |
| nurse chest colour | — | — | 0.36±0.76 | −0.08±0.13 | −0.14±0.15 |
Figure 2.
Parent–offspring regression of cap coloration. PC scores averaged over all chicks of a given genetic brood plotted against the average of the dam and sire scores for that brood. PC scores were standardized for sex and broods for which only a single parent had a colour measurement; the mid-parent score was calculated as half the colour score of the single parent since the population mean is zero (Falconer 1983).
(b) Genetic basis of recruitment
Sex was an important predictor of recruitment. The probability of recapture for females was 0.16, whereas that for males was 0.25 (, ), with standard errors on the logit scale (female intercept=−1.63±0.16, male intercept=−1.09±0.18). Variance in recruitment was high at the level of the dyad (the pairs of nests that form cross-fostering units), but inclusion of hatch date as a fixed effect effectively removed this source of variation. The probability of recruitment decreased with hatching date by 0.02 per day , translating to a difference of approximately 40% in the likelihood of recruitment between the first and last nests. No significant relationship was found between recruitment and tarsus length (, ) or head length (, ), and the morphological traits were dropped from the final model. Dyad was also omitted from the final model, but it should be noted that hatch date effects can be viewed as either a genetic or environmental source of variation depending on the heritability of lay date. The heritability of recruitment on the underlying scale was low (, ), but the ratio of brood variance to phenotypic variance was high (, ) (see table 1).
(c) Genetic basis of offspring size
Both tarsus length (slope estimate=−0.03±0.01 mm d−1) and head to bill length (slope estimate=−0.04±0.01 mm d−1) decreased as hatch date increased . Both tarsus length (, ) and head length (, ) had moderate heritabilities, and the genetic correlation between the two morphological traits was 0.56 (see table 1). The ratio of environmental nest variance to phenotypic variance for tarsus length (, ) and head length (, ) were also moderate (see table 1). The correlation between the two morphological traits that arises from a shared natal environment was 0.86.
(d) Covariation between colour and fitness
No parental colour variables were retained in the minimal adequate model for predicting the probability of offspring recruitment (see table 1). All parental colour variables dropped out of the minimum adequate model for predicting offspring size, except sire cap colour (, ), which showed a negative relationship with offspring tarsus length , but not head to bill length () (see table 1).
4. Discussion
Models of indirect sexual selection predict that sexually selected traits should have high heritability, that the magnitude of genetic variance in fitness should be substantial, and that there is significant positive genetic covariation between the sexually selected trait and fitness. In contrast to these predictions, this study has demonstrated that chromatic variation in the cap and chest of the blue tit is only weakly heritable, that variation in chick recruitment is determined to a large degree by environmental, rather than genetic effects, and that the genetic covariation between colour and fitness components is either non-significant or negative. Taken together, these results suggest that neither cap colour nor chest colour are likely to accurately reflect any genetic benefits a female may gain by mating to highly ornamented males (Kirkpatrick & Barton 1997, but see Houle & Kondrashov 2002). Even when taking sampling error into account, our estimates still fall below those values used in the influential model of Kirkpatrick & Barton (1997). In this model, which represents one of the few attempts to use estimable quantitative genetic parameters to estimate the strength of indirect sexual selection, it was assumed that the heritability of the male display trait was 0.48, and that the genetic correlation between the trait and fitness was 1, whereas we estimated the 95% CI for to be between 0 and 0.32 and the range of the genetic correlations between parental coloration and offspring traits to be between −1.35 and 0.45. Although we were not able to measure all components of fitness, and the sampling variance of genetic correlations is notoriously high, the data do suggest that the genetic correlation between fitness and the trait will be less than unity. Together, these results suggest that the ability of a female to accurately assess a male's breeding value for fitness through his plumage colour will be limited. Consequently, indirect selection on female choice is likely to be weak, and easily opposed by direct selection against costly female preferences or overwhelmed by selection arising through direct benefits. Additional work is required to evaluate the cost of female choice (Houle & Kondrashov 2002; Mead & Arnold 2004).
Direct benefit models of sexual selection do not require that the sexually selected trait has high heritability, or that variation in fitness has a large genetic component. However, these models do predict that variance in female fitness is mediated by the direct effect of mating with a given male. Most passerines provide bi-parental care, and the amount of parental care a male provides has been put forward as a likely form of direct benefit (Heywood 1989; Hoelzer 1989). This study has demonstrated that parental effects are an important source of variation in offspring recruitment and growth. However, neither the cap nor chest coloration of foster parents showed any relationship with measures of offspring fitness, and the mechanisms by which direct benefits may be signalled therefore remain unknown in this population.
One substantial limitation of this study is using offspring recruitment and size as surrogates for fitness. In particular, an important but unmeasured component of fitness is male mating success, and it remains to be seen whether a strong positive genetic correlation exists between male plumage colour and mating success. However, a recent study found that less ornamented males gained more extra-pair matings, suggesting a strong positive genetic correlation is unlikely (Delhey et al. 2003). This is in direct contrast with two previous studies on blue tits that have provided evidence of indirect selection on female choice (Sheldon et al. 1999; Limbourg et al. 2004). However, one potential problem with these studies is the use of sun block to alter the perceived attractiveness of a mate or rival. Application of sun block to the cap will reduce the VS logcontrast to less than, or close to zero, without a resulting change in the S and M logcontrasts (figure 1). Such a colour would lie far outside the natural variation perceived in these colour traits, and may partly responsible for the discrepancies.
Evidence of direct benefits to mate choice in the blue tit is limited to a single study on the carotenoid-based chest coloration of a Spanish population (Senar et al. 2002). In our study, we found little evidence that cap or chest coloration reliably signalled direct benefits to mate choice. However, analyses of offspring growth (Hadfield 2005) show that a large proportion of parental effect variance can be attributed to the number of parents attending a nest. For these nests, colour measurements were unavailable for the birds (usually males) that provided no parental care. It seems likely that if plumage colours do signal direct benefits, then these birds will be an important sub-sample, and a goal for the future will be to identify and measure such birds. This may be particularly pertinent since these nests may represent the nests of secondary females mated to polygamous males (Alatalo & Lundberg 1986; Kempenaers 1994).
In conclusion, this study provides little support for the quantitative predictions of indirect models of sexual selection. This finding is in broad agreement with several other studies of plumage coloration in birds, which have also identified a substantial environmental component of both chromatic and achromatic aspects of coloration (Griffith et al. 1999; Qvarnstrom 1999; Garant et al. 2004). Our study is unusual in this context, however, because we not only use a cross-foster design but also estimate genetic covariation with fitness as well as heritability. The sample sizes in this study are particularly large for this type of study, suggesting that if indirect benefits to mate choice are present, they must be small. However, although the genetic basis of plumage coloration appears most compatible with direct models of sexual selection, we were unable to find positive evidence that coloration provides a reliable cue for the quality of parental care provided. As an alternative, it may be worth investigating other forms of selection on female preferences, such as pleiotropic effects (Kirkpatrick & Ryan 1991), or the role that cap coloration plays in male contests. The role that the cap plays in agonistic displays is well documented (Cramp & Perrins 1993; Scott & Deag 1998; Ornborg et al. 2002), and it has been demonstrated, again with the use of sun block, that males respond less aggressively to males with reduced UV reflectance (Alonso-Alvarez et al. 2004).
Acknowledgments
We thank N. Hart who kindly provided data on the blue tit visual system; S. Griffith for help with molecular sexing; A. Nutall, F. Eigenbrod, T. Jenkins and J. Burnside for help with fieldwork; J. Metcalf, B. Sheldon and T. Coulson for comments on the manuscript; and D. Osorio, J. Endler, M. Vorobyev, M. Blows and L. Kruuk for discussion. This work was funded by the National Environment Research Council.
References
- Alatalo R.V, Lundberg A. Heritability and selection on tarsus length in the pied flycatcher (Ficedula hypoleuca) Evolution. 1986;40:574–583. doi: 10.1111/j.1558-5646.1986.tb00508.x. [DOI] [PubMed] [Google Scholar]
- Alonso-Alvarez C, Doutrelant C, Sorci G. Ultraviolet reflectance affects male–male interactions in the blue tit (Parus caeruleus ultramarinus) Behav. Ecol. 2004;15:805–809. 10.1093/beheco/arh083 [Google Scholar]
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Andersson S. Bright ultraviolet colouration in the Asian whistling-thrushes (Myiophonus spp.) Proc. R. Soc. B. 1996;263:843–848. [Google Scholar]
- Andersson S, Ornborg J, Andersson M. Ultraviolet sexual dimorphism and assortative mating in blue tits. Proc. R. Soc. B. 1998;265:445–450. 10.1098/rspb.1998.0315 [Google Scholar]
- Arnqvist R, Kirkpatrick M. The evolution of infidelity in socially monogamous passerines: the strength of direct and indirect selection on extrapair copulation behavior in females. Am. Nat. 2005;165:S26–S37. doi: 10.1086/429350. 10.1086/429350 [DOI] [PubMed] [Google Scholar]
- Blows M.W. Evolution of the genetic covariance between male and female components of mate recognition: an experimental test. Proc. R. Soc. B. 1999;266:2169–2174. doi: 10.1098/rspb.1999.0904. 10.1098/rspb.1999.0904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramp S, Perrins C.M. The birds of the Western Palearctic. vol. 7. Oxford University Press; Oxford, UK: 1993. Handbook of the birds of Europe, Middle East and North Africa. [Google Scholar]
- Crawley M. Wiley; New York, NY: 2002. Statistical computing. An introduction to data analysis using S-Plus. [Google Scholar]
- Delhey K, Johnsen A, Peters A, Andersson S, Kempenaers B. Paternity analysis reveals opposing selection pressures on crown coloration in the blue tit (Parus caeruleus) Proc. R. Soc. B. 2003;270:2057–2063. doi: 10.1098/rspb.2003.2460. 10.1098/rspb.2003.2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhondt A.A. Heritability of blue tit tarsus length from normal and cross- fostered broods. Evolution. 1982;36:418–419. doi: 10.1111/j.1558-5646.1982.tb05061.x. [DOI] [PubMed] [Google Scholar]
- Endler J.A, Mielke P.W. Comparing entire colour patterns as birds see them. Biol. J. Linn. Soc. 2005;86:405–431. [Google Scholar]
- Falconer D. Longman; London, UK: 1983. Introduction to quantitative genetics. [Google Scholar]
- Fisher R.A. 2nd edn. Dover Books; New York, NY: 1958. The genetical theory of natural selection. [Google Scholar]
- Garant D, Sheldon B.C, Gustafsson L. Climatic and temporal effects on the expression of secondary sexual characters: genetic and environmental components. Evolution. 2004;58:634–644. [PubMed] [Google Scholar]
- Gilmour A.R, Anderson R.D, Rae A.L. The analysis of binomial data by a generalized linear mixed model. Biometrika. 1985;72:593–599. [Google Scholar]
- Gilmour A.R, Gogel B.J, Cullis B.R, Welham S.J, Thompson R. VSN International Ltd; Hemel Hempstead, UK: 2002. ASReml User Guide Release 1.0. [Google Scholar]
- Griffith S.C, Owens I.P.F, Burke T. Environmental determination of a sexually selected trait. Nature. 1999;400:358–360. 10.1038/22536 [Google Scholar]
- Hadfield, J. D. 2005 The quantitative genetics of plumage colour in the blue tit (Parus caeruleus). Ph.D. thesis, Imperial College, London, UK.
- Hadfield, J. D. & Owens, I. P. F. In press. Strong environmental determination of a carotenoid-based plumage trait is not mediated by carotenoid availability. J. Evol. Biol [DOI] [PubMed]
- Hart N.S, Partridge J.C, Cuthill I.C, Bennett A.T.D. Visual pigments, oil droplets, ocular media and cone photoreceptor distribution in two species of passerine bird: the blue tit (Parus caeruleus L.) and the blackbird (Turdus merula L.) J. Comp. Physiol. A. 2000;186:375–387. doi: 10.1007/s003590050437. 10.1007/s003590050437 [DOI] [PubMed] [Google Scholar]
- Heywood J.S. Sexual selection by the handicap mechanism. Evolution. 1989;43:1387–1397. doi: 10.1111/j.1558-5646.1989.tb02590.x. [DOI] [PubMed] [Google Scholar]
- Hoelzer G.A. The good parent process of sexual selection. Anim. Behav. 1989;38:1067–1078. [Google Scholar]
- Houle D, Kondrashov A.S. Coevolution of costly mate choice and condition-dependent display of good genes. Proc. R. Soc. B. 2002;269:97–104. doi: 10.1098/rspb.2001.1823. 10.1098/rspb.2001.1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt S, Bennett A.T.D, Cuthill I.C, Griffiths R. Blue tits are ultraviolet tits. Proc. R. Soc. B. 1998;265:451–455. 10.1098/rspb.1998.0316 [Google Scholar]
- Hunt S, Cuthill I.C, Bennett A.T.D, Griffiths R. Preferences for ultraviolet partners in the blue tit. Anim. Behav. 1999;58:809–815. doi: 10.1006/anbe.1999.1214. 10.1006/anbe.1999.1214 [DOI] [PubMed] [Google Scholar]
- Jennions M.D, Petrie M. Why do females mate multiply? A review of the genetic benefits. Biol. Rev. 2000;75:21–64. doi: 10.1017/s0006323199005423. 10.1017/S0006323199005423 [DOI] [PubMed] [Google Scholar]
- Johnsen A, Delhey K, Andersson S, Kempenaers B. Plumage colour in nestling blue tits: sexual dichromatism, condition dependence and genetic effects. Proc. R. Soc. B. 2003;270:1263–1270. doi: 10.1098/rspb.2003.2375. 10.1098/rspb.2003.2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J.S. The heritability of fitness—bad-news for good genes. Trends Ecol. Evol. 1987;2:35–38. doi: 10.1016/0169-5347(87)90096-6. 10.1016/0169-5347(87)90096-6 [DOI] [PubMed] [Google Scholar]
- Jones T.M, Quinnell R.J, Balmford A. Fisherian flies: benefits of female choice in a lekking sandfly. Proc. R. Soc. B. 1998;265:1651–1657. 10.1098/rspb.1998.0484 [Google Scholar]
- Kempenaers B. Polygyny in the blue tit—unbalanced sex-ratio and female aggression restrict mate choice. Anim. Behav. 1994;47:943–957. 10.1006/anbe.1994.1126 [Google Scholar]
- Kirkpatrick M, Barton N.H. The strength of indirect selection on female mating preferences. Proc. Natl Acad. Sci. USA. 1997;94:1282–1286. doi: 10.1073/pnas.94.4.1282. 10.1073/pnas.94.4.1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick M, Ryan M.J. The evolution of mating preferences and the paradox of the lek. Nature. 1991;350:33–38. 10.1038/350033a0 [Google Scholar]
- Kokko H. Should advertising parental care be honest? Proc. R. Soc. B. 1998;265:1871–1878. 10.1098/rspb.1998.0515 [Google Scholar]
- Kokko H, Brooks R, McNamara J.M, Houston A.I. The sexual selection continuum. Proc. R. Soc. B. 2002;269:1331–1340. doi: 10.1098/rspb.2002.2020. 10.1098/rspb.2002.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruuk L.E.B. Estimating genetic parameters in natural populations using the ‘animal model’. Phil. Trans. R. Soc. B. 2004;359:873–890. doi: 10.1098/rstb.2003.1437. 10.1098/rstb.2003.1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbourg T, Mateman A.C, Andersson S, Lessels C.M. Female blue tits adjust parental effort to manipulated male UV attractiveness. Proc. R. Soc. B. 2004;271:1903–1908. doi: 10.1098/rspb.2004.2825. 10.1098/rspb.2004.2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M, Walsh B. Sinauer Associates; Sunderland, MA: 1998. Genetics and analysis of quantitative traits. [Google Scholar]
- Mead L.S, Arnold S.J. Quantitative genetic models of sexual selection. Trends Ecol. Evol. 2004;19:264–271. doi: 10.1016/j.tree.2004.03.003. 10.1016/j.tree.2004.03.003 [DOI] [PubMed] [Google Scholar]
- Merila J, Sheldon B.C. Genetic architecture of fitness and nonfitness traits: empirical patterns and development of ideas. Heredity. 1999;83:103–109. doi: 10.1046/j.1365-2540.1999.00585.x. [DOI] [PubMed] [Google Scholar]
- Ornborg J, Andersson S, Griffith S.C, Sheldon B.C. Seasonal changes in a ultraviolet structural colour signal in blue tits, Parus caeruleus. Biol. J. Linn. Soc. 2002;76:237–245. 10.1046/j.1095-8312.2002.00061.x [Google Scholar]
- Pinheiro J.C, Bates D.M. Springer; New York, NY: 2000. Mixed-effects models in S and S-PLUS. [Google Scholar]
- Pomiankowski A. The evolution of female mate preferences for male genetic quality. Oxford Surv. Evol. Biol. 1988;5:136–184. [Google Scholar]
- Price T, Schluter D, Heckman N.E. Sexual selection when the female directly benefits. Biol. J. Linn. Soc. 1993;48:187–211. 10.1006/bijl.1993.1014 [Google Scholar]
- Qvarnstrom A. Genotype-by-environment interactions in the determination of the size of a secondary sexual character in the collared flycatcher (Ficedula albicollis) Evolution. 1999;53:1564–1572. doi: 10.1111/j.1558-5646.1999.tb05419.x. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2004. R: a language and environment for statistical computing. [Google Scholar]
- Riska B, Rutledge J.J, Atchley W.R. Covariance between direct and maternal genetic effects in mice, with a model of persistent environmental influences. Genet. Res. 1985;45:287–297. doi: 10.1017/s0016672300022278. [DOI] [PubMed] [Google Scholar]
- Scott G.W, Deag J.M. Blue tit (Parus caeruleus) agonistic displays: a reappraisal. Behaviour. 1998;135:665–691. [Google Scholar]
- Senar J.C, Figuerola J, Pascual J. Brighter yellow blue tits make better parents. Proc. R. Soc. B. 2002;269:257–261. doi: 10.1098/rspb.2001.1882. 10.1098/rspb.2001.1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon B.C, Andersson S, Griffith S.C, Ornborg J, Sendecka J. Ultraviolet colour variation influences blue tit sex ratios. Nature. 1999;402:874–877. 10.1038/47239 [Google Scholar]
- Vorobyev M, Osorio D, Bennett A.T.D, Marshall N.J, Cuthill I.C. Tetrachromacy, oil droplets and bird plumage colours. J. Comp. Physiol. A. 1998;183:621–633. doi: 10.1007/s003590050286. 10.1007/s003590050286 [DOI] [PubMed] [Google Scholar]