Abstract
Intracellular Wolbachia bacteria are obligate, maternally inherited endosymbionts found frequently in insects and other invertebrates. The evolutionary success of Wolbachia is due in part to an ability to manipulate reproduction. In mosquitoes and many other insects, Wolbachia causes a form of sterility known as cytoplasmic incompatibility (CI). Wolbachia-induced CI has attracted interest as a potential agent for affecting medically important disease vectors. However, application of the approach has been restricted by an absence of appropriate, naturally occurring Wolbachia infections. Here, we report the interspecific transfer of Wolbachia infection into a medically important mosquito. Using embryonic microinjection, Wolbachia is transferred from Drosophila simulans into the invasive pest and disease vector: Aedes albopictus (Asian tiger mosquito). The resulting infection is stably maintained and displays a unique pattern of bidirectional CI in crosses with naturally infected mosquitoes. Laboratory population cage experiments examine a strategy in which releases of Wolbachia-infected males are used to suppress mosquito egg hatch. We discuss the results in relation to developing appropriate Wolbachia-infected mosquito strains for population replacement and population suppression strategies.
Keywords: Wolbachia, Aedes albopictus, Drosophila, cytoplasmic incompatibility
1. Introduction
Intracellular Wolbachia bacteria are estimated to naturally infect approximately 20% of insect species and up to 28% of surveyed mosquito species (Werren et al. 1995; Kittayapong et al. 2000; Ricci et al. 2002). In mosquitoes and other insects, naturally occurring Wolbachia infections can cause cytoplasmic incompatibility (CI), which results in the developmental arrest of early embryos (O'Neill et al. 1997; Tram & Sullivan 2002). CI occurs when a Wolbachia-infected male mates with an uninfected female or a female with a different Wolbachia type. The ability of Wolbachia to manipulate host reproduction has led to considerable scientific attention directed at better understanding the impact of Wolbachia infections on the evolution and ecology of host populations (Charlat et al. 2003).
In medically important mosquitoes, CI-induced sterility has led to the proposal of applied strategies for the suppression of mosquito populations (Laven 1967; Sinkins & O'Neill 2000; Dobson et al. 2002a) and for the use of Wolbachia as a vehicle for driving desired genotypes into medically important disease vectors (Sinkins & O'Neill 2000; Dobson 2003). However, application of proposed strategies to mosquito populations has been restricted by an inability to identify appropriate naturally occurring Wolbachia infection types. Thus, realization of the proposed strategies will require a technique for generating artificial Wolbachia infections.
A technique for artificially generating incompatible CI types has been available in Drosophila for more than a decade. The artificial transfer of Wolbachia (‘transfection’) is accomplished via microinjection of Wolbachia-infected cytoplasm in early embryos (Boyle et al. 1993; Xi & Dobson 2005). Recently, a transfection technique has been developed for Aedes albopictus and used to segregate the naturally occurring Wolbachia superinfection, resulting in an artificial wAlbB single infection (‘HTB’ strain) (Xi et al. 2005). Although the prior report demonstrates an ability to transfect Ae. albopictus, the HTB strain is not useful for applied strategies, because it will be wiped out by the naturally superinfected Ae. albopictus after release. Thus, in order to generate an infection that is incompatible with target mosquitoes, including Anopheles gambiae, an ability of the interspecific transfer of Wolbachia is required.
Here, we use embryonic microinjection to transfer Wolbachia infection from Drosophila simulans to embryos of Ae. albopictus (Asian tiger mosquito). We selected Ae. albopictus for initial transfection experiments, since it is known to naturally support Wolbachia infection (Sinkins et al. 1995; Zhou et al. 1998; Kittayapong et al. 2002) and due to prior development of a transfection technique (Xi et al. 2005). Furthermore, Ae. albopictus is medically important as an invasive pest species and vector of multiple arboviruses and filaria (Moore & Mitchell 1997). The wRi Wolbachia infection from D. simulans was selected due to its phylogenetic similarity to the wAlbA infection that occurs naturally in Ae. albopictus (Ruang Areerate et al. 2003), and prior research demonstrating it to be bidirectionally incompatible with the Ae. albopictus infection in D. simulans hosts (Braig et al. 1994). Prior transfection experiments suggest that the wRi infection is well suited for adaptation to distantly related hosts (Kang et al. 2003). Furthermore, prior direct experience of the researchers with horizontal transfers of wRi reduces concern with Wolbachia viability during microinjection (Xi & Dobson 2005).
The results demonstrate that the wRi infection has been successfully transferred from Drosophila into Ae. albopictus and is stably maintained in the transfected line (‘HTR’). The HTR line displays a unique CI pattern and is bidirectionally incompatible with both natural infections and the previously generated artificial infection (Xi et al. 2005). Generation of the HTR strain provides a model system for examining proposed population suppression strategies. As an initial test, releases of HTR males into laboratory cages of naturally infected populations result in reduced egg hatch. We discuss the results in relation to the potential applied use of the HTR strain.
2. Material and methods
(a) Insect strains
Individuals within the Ae. albopictus Houston (Hou) strain are naturally superinfected with both the wAlbA and wAlbB Wolbachia types (Sinkins et al. 1995). The aposymbiotic HT1 strain was generated by tetracycline treatment of the Hou strain (Dobson & Rattanadechakul 2001). The Koh Samui strain (Koh) is naturally single infected with the wAlbA Wolbachia type (Sinkins et al. 1995). The HTB strain is single infected with wAlbB and was artificially generated via microinjection of HT1 with Hou cytoplasm (Xi et al. 2005). Drosophila simulans Riverside (DSR) are naturally infected with the wRi infection (Zhou et al. 1998). Mosquito and Drosophila strains were maintained following standard procedures as described previously (Roberts 1998; Dobson et al. 2001).
(b) Microinjection
Embryo injection was based upon techniques successfully used for mosquito and Drosophila transfection (Xi et al. 2005; Xi & Dobson 2005). Microinjection needles were prepared from quartz microcapillaries (#QF100-70-7.5; Sutter Instrument Co., Novato, CA) using a P2000 micropipette puller (Sutter Instrument Co.; Novato, CA).
DSR embryos were used as the donor of wRi-infected cytoplasm. DSR embryos were collected up to 30 min post-oviposition, using apple juice agar plates with yeast paste (Roberts 1998). Embryos were dechorionated in 50% bleach for 2 min, rinsed, aligned on agar plate and transferred onto a glass slide with double-sided tape (Scotch 665; St Paul, MN), and covered with water-saturated halocarbon 700 oil (Sigma-Aldrich Co.). Donor DSR embryos were not desiccated.
HT1 embryos (recipient embryos) were aligned on wet filter paper, transferred onto a cover-slip with double-sided tape, briefly desiccated, and covered with water-saturated halocarbon 700 oil. Embryos were injected up to 90 min post-oviposition.
Cytoplasm was withdrawn from the posterior of donor DSR embryos and injected into the posterior of recipient HT1 embryos using an IM300 microinjector (Narishige Scientific; Tokyo, Japan) similar to prior descriptions (Xi et al. 2005; Xi & Dobson 2005).
Following injection, HT1 embryos were incubated at 80% relative humidity and 27° C for approximately 40 min. Subsequently, the embryos were removed from oil and transferred onto wet filter paper, where they were allowed to develop for 5 days. The eggs were then submerged in deoxygenated water to hatch. Resulting larvae (G0) were reared using standard conditions as described previously.
(c) Crosses of transfected lines
Eclosing G0 females were isolated as pupae to assure virginity and were subsequently mated with HT1 males. Following blood feeding and oviposition, G0 females were assayed for Wolbachia infection via PCR (described in §2d). G0 males were assayed for Wolbachia infection approximately 2 days post-eclosion. G0 females testing negative for Wolbachia infection were discarded along with their progeny. One wRi-infected line was selected for subsequent experiments and designated as the ‘HTR’ strain: Houston strain, Tetracycline treated, with wRi infection. HTR individuals were sibling mated in the G1 and G2. Beginning in G3, 50 virgin HTR females were outcrossed with 50 HT1 males in every generation.
Crosses were conducted to characterize the pattern of CI and egg hatch rates resulting from crosses between HTR individuals and individuals with differing Wolbachia infection types. In all crosses, 10 virgin females were mated with 10 males. Three replicate cages were conducted for each cross type. All individuals were less than 5 days old when crossed. Subsequently, the female groups were blood fed and provided with oviposition cups.
(d) PCR, fluorescent in situ hybridization staining and maternal transmission assay
For PCR assays, DNA was extracted from adult ovaries or testes via homogenization in 100 μl STE with 0.4 mg ml−1 proteinase K as described previously (O'Neill et al. 1992). Presence of Wolbachia was detected using general Wolbachia primers (81F, 691R) (Zhou et al. 1998). Wolbachia infection type was determined using primers specific for the wAlbA (328F, 691R), wAlbB (183F, 691R) and wRi (169F, 569R) infections (Zhou et al. 1998; Kang et al. 2003). As additional confirmation of infection type, primers specific for a prophage sequence (phgWOf, phgWOr) were used (Masui et al. 2000). The latter primers result in a PCR amplification product with wRi but not with the wAlbA or wAlbB infections in Hou. For mosquitoes failing to amplify with the above primers (e.g. HT1), template quality was confirmed using 12S mitochondrial primers as described previously (O'Neill et al. 1992). For Fluorescent in situ hybridization (FISH) staining, oocytes were dissected from females 4 days after blood feeding and were fixed for 15 min in freshly prepared 4% formaldehyde in PBS and then FISH stained as described previously (Xi et al. 2005). For the maternal transmission assay, 20 HTR G5 females and 11 HTR G6 females were randomly selected and PCR assayed. Progeny (G7) from one infected HTR G6 female were reared to adult and PCR tested. In each generation, the maternal transmission efficiency was estimated using the percentage of PCR positive individuals among those tested.
(e) Population suppression
All mosquitoes used in suppression cage tests were isolated as pupae to assure virginity. Fifty Hou females and 10 Hou males were present in all of the cages. The number of HTR males (G5) was varied between cages. Male Hou : HTR ratios were: 10 : 500, 10 : 100, 10 : 20 and 10 : 0. All males were less than one week post-eclosion. One day after adding males to cages, 50 Hou females (less than one week post-eclosion) were added to each cage. Prior to adding females, cages were examined to assure that minimal male mortality had occurred. Mating was observed immediately upon addition of females to cages. Following blood feeding, females were allowed to oviposit for one week. Egg hatch rates were determined as described previously.
(f) Statistics
Statistical comparisons of egg hatch rate were conducted using chi-square. Kruskal–Wallis analysis was used in statistical comparisons of egg mortality (CI levels). All statistical comparisons were performed using SAS v. 8.0 (SAS Institute, Cary, NC).
3. Results
Cytoplasm from wRi-infected DSR embryos was microinjected into aposymbiotic HT1 Ae. albopictus embryos. A total of 695 HT1 eggs were injected in three experiments, resulting in 15 G0 females that survived to adult (table 1). PCR assays were conducted to diagnose Wolbachia infection in G0 adults. As shown in table 1, Wolbachia infection was detected in 33% of surviving G0 females and 40% of surviving G0 males.
Table 1.
Survival of microinjected Ae. albopictus embryos and the resulting Wolbachia infection status in the G0 individuals surviving to adult.
| experiment | per cent survival | G0 infection status (%) (infected/total) | |||
|---|---|---|---|---|---|
| hatch (%) (larvae/injected eggs) | pupation (%) (pupae/larvae) | eclosion (%) (adult/pupae) | female | male | |
| 1 | 3.4 (8/233) | 75.0 (6/8) | 83.3 (5/6) | 40.0 (2/5) | (0/0) |
| 2 | 1.9 (6/316) | 50.0 (3/6) | 100.0 (3/3) | 0 (0/2) | 0 (0/1) |
| 3 | 15.8 (23/146) | 78.3 (18/23) | 66.7 (12/18) | 37.5 (3/8) | 50 (2/4) |
Of the five PCR positive G0 females, only one female produced hatching eggs. The remaining G0 females either failed to oviposit or their eggs failed to hatch. Three daughters (G1) resulting from the PCR positive G0 female were sib-mated, blood fed, isolated and allowed to oviposit. Following oviposition, the G1 females were PCR assayed for Wolbachia infection similar to G0 females. Each of the three G1 females tested positive for Wolbachia infection. One of the latter isofemale lines was randomly selected for subsequent experiments and designated as the ‘HTR’ strain. Subsequently, PCR assays of HTR individuals have consistently detected Wolbachia infection to G11, immediately prior to the submission of this report.
As an initial characterization of infection frequency, HTR females were randomly selected (G5 and G6) and PCR assayed for Wolbachia infection. Wolbachia amplification products were observed in 29/31 (93.5%) females. As a direct test of maternal inheritance rates, the progeny (G7) from an infected G6 female were reared to adult and PCR tested. In the latter test, PCR amplification products were observed in 18/20 (90%) of the assayed G7 females.
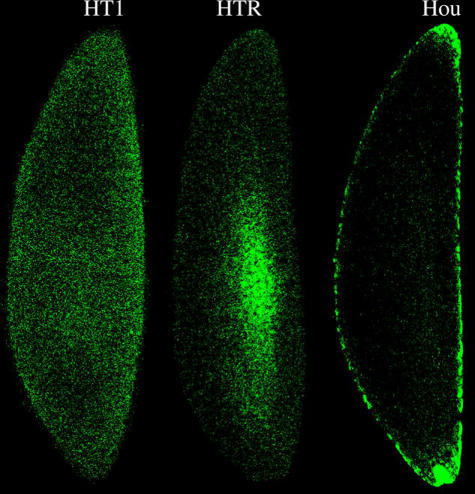
FISH was used to examine the distribution of Wolbachia in transfected HTR oocytes. Wolbachia localization in HTR oocytes was observed to differ from that observed in naturally superinfected Hou oocytes (figure 1). Wolbachia was observed to be concentrated towards the centre of HTR oocytes. In contrast, the anterior and posterior are the focus of infection in naturally infected Hou oocytes and the transfected HTB strain (Xi et al. 2005).
Figure 1.
Wolbachia distribution in oocytes of naturally superinfected (Hou), aposymbiotic (HT1) and the wRi transfected HTR line. In HTR oocytes, Wolbachia is concentrated in the centre of oocytes. In contrast, Wolbachia is focused in the anterior and posterior of naturally infected Hou oocytes.
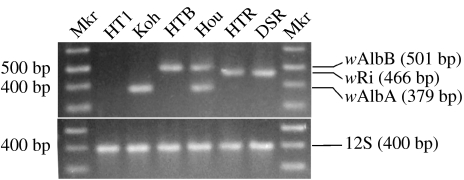
To determine the type of Wolbachia infection present in HTR, diagnostic PCR primers were used (figure 2). Consistent with expectations for host insects infected with the wRi infection only, PCR assays of HTR and DSR individuals result in amplification products of the expected size using the 169F/569R primer set, but not with the 328F/691R or 183F/691R primer sets. The 169F/569R primer set did not amplify any of the other Ae. albopictus strains. As an additional confirmation of Wolbachia type, DNA from each of the Ae. albopictus strains and DSR flies was amplified using the phgWOf/phgWOr primer set. The latter amplification was similar to the 169F/569R primer set, in that amplification products were only obtained from HTR and DSR (data not shown). The Koh and HTB strains amplify with the 328F/691R or 183F/691R primer sets, respectively (figure 2). The Hou strain amplifies with both the 328F/691R and 183F/691R primer sets. The HT1 strain does not amplify with the Wolbachia-specific primers.
Figure 2.
Diagnostic pattern of amplification products resulting with Wolbachia-specific PCR primers. The gel illustrates all of the amplification products that result when each Ae. albopictus strain is amplified with all four primer sets. The mitochondrial 12S primers are used to confirm template DNA quality. For the electrophoresis gel shown in the figure, amplicons resulting from separate PCR amplifications were combined.
Given that wRi causes CI in its natural D. simulans host, crosses between HTR and HT1 were conducted to determine whether the wRi infection causes CI in Ae. albopictus. Additional crosses were conducted to determine the CI pattern relative to the other Ae. albopictus infections (i.e. the wAlbA single infection, wAlbB single infection and the superinfection). As shown in table 2, crosses of HTR individuals with the other infection types resulted in a unique pattern of CI. Specifically, a typical pattern of unidirectional CI was observed in crosses between HTR with uninfected HT1 individuals. Bidirectional CI was observed in crosses of HTR with single-infected (Koh, HTB) and superinfected (Hou) individuals.
Table 2.
Egg hatch resulting from crosses of the transfected HTR line (G3).
| expected CI type | crossa | infection type | per cent egg hatch (%)b | eggs scored | |
|---|---|---|---|---|---|
| female | male | ||||
| bidirectional CI | Koh×HTR | wAlbA | wRi | 5.9±4.8 | 2119 |
| HTR×Koh | wRi | wAlbA | 2.8±1.5 | 2040 | |
| HTB×HTR | wAlbB | wRi | 9.9±5.4 | 1434 | |
| HTR×HTB | wRi | wAlbB | 0.3±0.5 | 2152 | |
| Hou×HTR | wAlbA, wAlbB | wRi | 14.2±6.6 | 2972 | |
| HTR×Hou | wRi | wAlbA, wAlbB | 0.4±0.4 | 3216 | |
| unidirectional CI | HT1×HTR | — | wRi | 3.8±3.3 | 1916 |
| HTR×HT1 | wRi | — | 75.5±9.7 | 2157 | |
| compatible | HTR×HTR | wRi | wRi | 57.1±10.5 | 2136 |
| Koh×Koh | wAlbA | wAlbA | 81.0±4.6 | 635 | |
| HT1×HT1 | — | — | 80.8±0.3 | 1159 | |
| Hou×Hou | wAlbA, wAlbB | wAlbA, wAlbB | 82.6±2.7 | 1883 | |
Female×male.
Average±s.d.; three cages/cross type.
The ability of the wRi infection in HTR to induce CI was measured as the level of egg hatch resulting from crosses of HTR males with females differing in their infection type. The strongest CI (less than 4% egg hatch) was observed in crosses with uninfected HT1 females. A similar CI level (ca 6% hatch) was observed in crosses with wAlbA-infected Koh females. In contrast, higher egg hatch levels were observed in crosses with wAlbB-infected HTB females and superinfected Hou females (9.9 and 14.4% hatch rate, respectively). Reciprocal crosses were conducted to examine the ability of wRi to rescue CI caused by the other infection types. Low egg hatch (less than 3%) was observed in crosses of HTR females with males harbouring different Wolbachia types. Consistent with expectations for Wolbachia-induced CI, HTR females are compatible with both HTR males and uninfected males.
The egg hatch resulting from the HTR×HTR cross at G3 was significantly lower than other compatible crosses, including the HTR×HT1 (female×male; chi-square test, p<0.0001) and compatible crosses of Koh, HT1 and Hou (chi-square test, p<0.05). To reduce possible inbreeding depression effects associated with the use of isofemale lines in generating the HTR strain, HTR females were repeatedly outcrossed to HT1 males beginning in G3. The HTR egg hatch was observed to increase from 58% hatch in G3 to 77% in G4, 85% in G6 and 92% in G8. While the increase in hatch rates is consistent with the hypothesized inbreeding effect, the results do not exclude an alternate hypothesis that the low egg hatch resulting in compatible crosses between HTR individuals in early generations was due to variable Wolbachia levels and maternal infection loss.
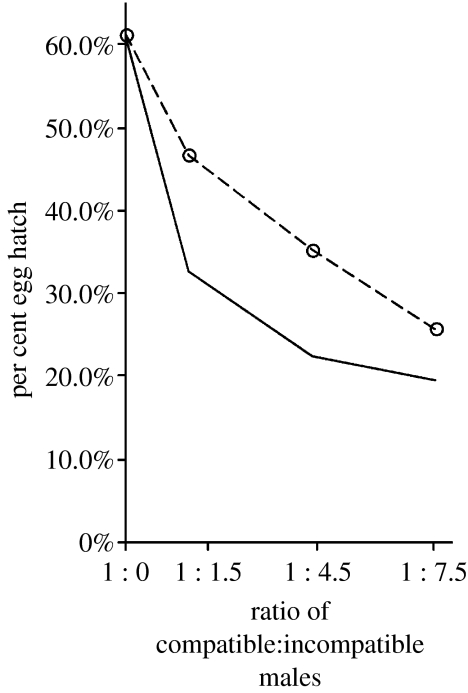
The bidirectional CI observed between HTR and the naturally superinfected Hou strain suggests a strategy for suppressing Ae. albopictus populations via releases of HTR males. As an initial test of this strategy, cages were established with varying ratios of HTR : Hou males. As shown in figure 3, the egg hatch was observed to decrease with an increasing ratio of HTR males in the population.
Figure 3.
Suppression of egg hatch in populations of naturally superinfected Ae. albopictus (Hou) via releases of transfected HTR males. The circles and dashed line illustrate the egg hatch observed in population cage tests. The solid line illustrates the expected egg hatch assuming equal competitiveness of HTR and Hou males (Arunachalam & Curtis 1985). Estimations of the ratio of compatible : incompatible males in the cages (x-axis) assume that 10% of HTR males that are released into cages are uninfected due to incomplete maternal transmission.
4. Discussion
Here, we have demonstrated that embryonic microinjection can be used for interspecific transfer of Wolbachia from D. simulans into Ae. albopictus to generate the HTR strain. FISH staining shows that the wRi infection in Ae. albopictus females is transmitted to offspring during oogenesis. PCR assays demonstrate the HTR infection to be stable, with more than 90% infection frequency throughout the 11 mosquito generations encompassed by this report. The wRi infection is able to induce CI in the transfected HTR strain and displays a unique pattern of CI relative to other infections in Ae. albopictus.
Prior wRi transfection research has yielded a range of CI levels and maternal transmission rates, suggesting that host type can affect Wolbachia dynamics. Specifically, high CI levels are observed in Drosophila mauritiana, Drosophila serrata, the Drosophila yakuba complex and Laodelphax striatellus, similar to that observed in the native D. simulans host (Hoffmann et al. 1986; Giordano et al. 1995; Clancy & Hoffmann 1997; Kang et al. 2003; Zabalou et al. 2004). In contrast, a relatively low CI level (ca 75% hatch) was reported in Drosophila melanogaster transfected with wRi (Boyle et al. 1993). The same studies also demonstrate that maternal transmission of wRi can vary between hosts. High transmission (ca 90% fidelity) is observed in D. simulans and D. serrata, and lower transmission (ca 30% fidelity) is observed in L. striatellus. Thus, the CI levels and maternal transmission rate observed with HTR are equivalent to the highest reported for the wRi infection. A comparison with prior crossing results (Dobson et al. 2002b, 2004; Xi et al. 2005) demonstrates HTR females to be similar to HT1 females in their inability to rescue the Wolbachia-induced sperm modification in Koh, HTB and Hou males. Thus, the wRi infection is unable to rescue the modifications caused by either wAlbA or wAlbB in Ae. albopictus.
Relative to the other CI crosses of HTR males, higher egg hatch is observed when wRi-infected males are mated with females infected with wAlbB (chi-square test, p<0.01), including both HTB and Hou females. Interestingly, a similar pattern was observed in a prior transfection study examining the interaction between Ae. albopictus infections and wRi in D. simulans (Braig et al. 1994). In the prior study, egg hatch resulting from crosses of females infected with Ae. albopictus Wolbachia and wRi males was approximately 10% higher than the reciprocal cross. However, the prior study did not determine the specific infection type(s) transfected from Ae. albopictus (Hou strain) into D. simulans (Braig et al. 1994). Thus, additional experiments are required to specifically test the hypothesis that the wAlbB infection is able to partially rescue the modification caused by wRi.
The observed pattern of wRi distribution in Ae. albopictus oocytes is distinct from that of natural infections described in Aedes and Culex. The wAlbA, wAlbB and wPip infections are concentrated at the oocyte poles of Ae. albopictus and Culex pipiens (Rasgon & Scott 2003; Xi et al. 2005). In contrast, the wRi infection is concentrated in the oocyte centre (figure 1). The wRi distribution in HTR also differs from that reported in natural and transfected Drosophila hosts, where it concentrates in the cortical region of oocytes (Veneti et al. 2004). The unusual distribution of wRi in HTR oocytes is consistent with a prior report showing that Wolbachia embryonic distribution can be affected by host type (Pintureau et al. 2000).
The bidirectional CI observed between HTR and the naturally superinfected Hou strain suggests a strategy for suppressing Ae. albopictus populations via releases of HTR males. As an initial test of this strategy, cages were established with varying ratios of HTR : Hou males. As shown in figure 3, egg hatch was observed to decrease with an increasing ratio of HTR males in the population.
Although cage experiments demonstrate that HTR male releases can suppress egg hatch in naturally infected Ae. albopictus populations, the results suggest that HTR is not suitable for an eradication strategy similar to prior Culex work (Laven 1967). Imperfect CI and maternal transmission failure is observed with HTR, resulting in ca 14% egg hatch in crosses of Hou females and HTR males. Thus, continued releases of HTR males into a naturally superinfected population would be expected to reduce, but not eliminate egg hatch. Furthermore, population cage tests suggest that HTR and Hou males are not equally competitive. As shown in figure 3, comparison of the observed egg hatch with theoretical expectations for equally competitive HTR and Hou males (Arunachalam & Curtis 1985) suggests that the HTR males suffer a reduced mating competitiveness relative to Hou males. Further studies are required to test the hypothesis that the observed difference results from persistent inbreeding effects that were not eliminated by two generations of outcrosses.
Demonstrating that microinjection can be used to successfully transfer Wolbachia across taxonomic families into a medically important mosquito emphasizes a need to repeat this work with additional disease vectors (e.g. Anopheles). Additional experiments should also include transfection of the wRi infection into superinfected Ae. albopictus, with the goal of generating a triple-infected strain capable of invading Ae. albopictus field populations. The latter could be used as a vehicle to drive desired transgenes (e.g. genes reducing vector competency) into naturally occurring Ae. albopictus populations.
Acknowledgments
We thank Cecilia Ng for her help with microinjections and Jeffry L. Dean for his assistance in crosses. We are grateful to Craig Coates, K. J. Maragatha Vally and Yuqing Fu for their advice on the injection of mosquito eggs. This work was supported by NIH grant (NIH-AI-51533). This is publication 05-08-047 of the University of Kentucky Agricultural Experiment Station.
Footnotes
Present address: Department of Molecular Microbiology and Immunology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD 21212, USA.
References
- Arunachalam N, Curtis C.F. Integration of radiation with cytoplasmic incompatibility for genetic control in the Culex pipiens complex (Diptera: Culcidae) J. Med. Entomol. 1985;22:648–653. doi: 10.1093/jmedent/22.6.648. [DOI] [PubMed] [Google Scholar]
- Boyle L, O'Neill S.L, Robertson H.M, Karr T.L. Interspecific and intraspecific horizontal transfer of Wolbachia in Drosophila. Science. 1993;260:1796–1799. doi: 10.1126/science.8511587. [DOI] [PubMed] [Google Scholar]
- Braig H.R, Guzman H, Tesh R.B, O'Neill S.L. Replacement of the natural Wolbachia symbiont of Drosophila simulans with a mosquito counterpart. Nature. 1994;367:453–455. doi: 10.1038/367453a0. 10.1038/367453a0 [DOI] [PubMed] [Google Scholar]
- Charlat S, Hurst G.D.D, Mercot H. Evolutionary consequences of Wolbachia infections. Trends Genet. 2003;19:217–223. doi: 10.1016/S0168-9525(03)00024-6. 10.1016/S0168-9525(03)00024-6 [DOI] [PubMed] [Google Scholar]
- Clancy D.J, Hoffmann A.A. Behavior of Wolbachia endosymbionts from Drosophila simulans in Drosophila serrata, a novel host. Am. Nat. 1997;149:975–988. doi: 10.1086/286033. 10.1086/286033 [DOI] [PubMed] [Google Scholar]
- Dobson S.L. Reversing Wolbachia-based population replacement. Trends Parasitol. 2003;19:128–133. doi: 10.1016/s1471-4922(03)00002-3. 10.1016/S1471-4922(03)00002-3 [DOI] [PubMed] [Google Scholar]
- Dobson S.L, Rattanadechakul W. A novel technique for removing Wolbachia infections from Aedes albopictus (Diptera: Culicidae) J. Med. Entomol. 2001;38:844–849. doi: 10.1603/0022-2585-38.6.844. [DOI] [PubMed] [Google Scholar]
- Dobson S.L, Marsland E.J, Rattanadechakul W. Wolbachia-induced cytoplasmic incompatibility in single- and superinfected Aedes albopictus (Diptera: Culicidae) J. Med. Entomol. 2001;38:382–387. doi: 10.1603/0022-2585-38.3.382. [DOI] [PubMed] [Google Scholar]
- Dobson S.L, Fox C.W, Jiggins F.M. The effect of Wolbachia-induced cytoplasmic incompatibility on host population size in natural and manipulated systems. Proc. R. Soc. B. 2002a;269:437–445. doi: 10.1098/rspb.2001.1876. 10.1098/rspb.2001.1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson S.L, Marsland E.J, Rattanadechakul W. Mutualistic Wolbachia infection in Aedes albopictus: accelerating cytoplasmic drive. Genetics. 2002b;160:1087–1094. doi: 10.1093/genetics/160.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson S.L, Rattanadechakul W, Marsland E.J. Fitness advantage and cytoplasmic incompatibility in Wolbachia single- and superinfected Aedes albopictus. Heredity. 2004:1–8. doi: 10.1038/sj.hdy.6800458. [DOI] [PubMed] [Google Scholar]
- Giordano R, O'Neill S.L, Robertson H.M. Wolbachia infections and the expression of cytoplasmic incompatibility in Drosophila sechellia and D. mauritiana. Genetics. 1995;140:1307–1317. doi: 10.1093/genetics/140.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A.A, Turelli M, Simmons G.M. Unidirectional incompatibility between populations of Drosophila simulans. Evolution. 1986;40:692–701. doi: 10.1111/j.1558-5646.1986.tb00531.x. [DOI] [PubMed] [Google Scholar]
- Kang L, et al. Superinfection of Laodelphax striatellus with Wolbachia from Drosophila simulans. Heredity. 2003;90:71–76. doi: 10.1038/sj.hdy.6800180. 10.1038/sj.hdy.6800180 [DOI] [PubMed] [Google Scholar]
- Kittayapong P, Baisley K.J, Baimai V, O'Neill S.L. Distribution and diversity of Wolbachia infections in southeast Asian mosquitoes (Diptera: Culicidae) J. Med. Entomol. 2000;37:340–345. doi: 10.1093/jmedent/37.3.340. [DOI] [PubMed] [Google Scholar]
- Kittayapong P, Baimai V, O'Neill S.L. Field prevalence of Wolbachia in the mosquito vector Aedes albopictus. Am. J. Trop. Med. Hyg. 2002;66:108–111. doi: 10.4269/ajtmh.2002.66.108. [DOI] [PubMed] [Google Scholar]
- Laven H. Eradication of Culex pipiens fatigans through cytoplasmic incompatibility. Nature. 1967;216:383–384. doi: 10.1038/216383a0. [DOI] [PubMed] [Google Scholar]
- Masui S, Kamoda S, Sasaki T, Ishikawa H. Distribution and evolution of bacteriophage WO in Wolbachia, the endosymbiont causing sexual alterations in arthropods. J. Mol. Evol. 2000;51:491–497. doi: 10.1007/s002390010112. [DOI] [PubMed] [Google Scholar]
- Moore C.G, Mitchell C.J. Aedes albopictus in the United States: ten-year presence and public health implications. Emerg. Infect. Dis. 1997;3:329–334. doi: 10.3201/eid0303.970309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill S.L, Giordano R, Colbert A.M, Karr T.L, Robertson H.M. 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc. Natl Acad. Sci. USA. 1992;89:2699–2702. doi: 10.1073/pnas.89.7.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill S.L, Hoffmann A.A, Werren J.H. Oxford University Press; Oxford, UK: 1997. Influential passengers: inherited microorganisms and arthropod reproduction. [Google Scholar]
- Pintureau B, Grenier S, Boleat B, Lassabliere F, Heddi A, Khatchadourian C. Dynamics of Wolbachia populations in transfected lines of Trichogramma. J. Invert. Path. 2000;76:20–25. doi: 10.1006/jipa.2000.4953. 10.1006/jipa.2000.4953 [DOI] [PubMed] [Google Scholar]
- Rasgon J.L, Scott T.W. Wolbachia and cytoplasmic incompatibility in the California Culex pipiens mosquito species complex: parameter estimates and infection dynamics in natural populations. Genetics. 2003;165:2029–2038. doi: 10.1093/genetics/165.4.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci I, Cancrini G, Gabrielli S, D'Amelio S, Favi G. Searching for Wolbachia (Rickettsiales: Rickettsiaceae) in mosquitoes (Diptera: Culicidae): large polymerase chain reaction survey and new identifications. J. Med. Entomol. 2002;39:562–567. doi: 10.1603/0022-2585-39.4.562. [DOI] [PubMed] [Google Scholar]
- Roberts D.B. Practical approach series. IRL Press at Oxford University Press; Oxford, UK: 1998. Drosophila: a practical approach. [Google Scholar]
- Ruang Areerate T, Kittayapong P, Baimai V, O'Neill S.L. Molecular phylogeny of Wolbachia endosymbionts in Southeast Asian mosquitoes (Diptera: Culicidae) based on wsp gene sequences. J. Med. Entomol. 2003;40:1–5. doi: 10.1603/0022-2585-40.1.1. [DOI] [PubMed] [Google Scholar]
- Sinkins S.P, O'Neill S.L. Wolbachia as a vehicle to modify insect populations. In: Handler A.M, James A.A, editors. Insect transgenesis: methods and applications. CRC Press; Boca Raton, FL: 2000. pp. 271–287. [Google Scholar]
- Sinkins S.P, Braig H.R, O'Neill S.L. Wolbachia superinfections and the expression of cytoplasmic incompatibility. Proc. R. Soc. B. 1995;261:325–330. doi: 10.1098/rspb.1995.0154. [DOI] [PubMed] [Google Scholar]
- Tram U, Sullivan W. Role of delayed nuclear envelope breakdown and mitosis in Wolbachia-induced cytoplasmic incompatibility. Science. 2002;296:1124–1126. doi: 10.1126/science.1070536. 10.1126/science.1070536 [DOI] [PubMed] [Google Scholar]
- Veneti Z, Clark M.E, Karr T.L, Savakis C, Bourtzis K. Heads or tails: host–parasite interactions in the Drosophila-Wolbachia system. Appl. Environ. Microbiol. 2004;70:5366–5372. doi: 10.1128/AEM.70.9.5366-5372.2004. 10.1128/AEM.70.9.5366-5372.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren J.H, Windsor D, Guo L.R. Distribution of Wolbachia among neotropical arthropods. Proc. R. Soc. B. 1995;262:197–204. [Google Scholar]
- Xi Z, Dobson S.L. Characterization of Wolbachia transfection efficiency by using microinjection of embryonic cytoplasm and embryo homogenate. Appl. Environ. Microbiol. 2005;71:3199–3204. doi: 10.1128/AEM.71.6.3199-3204.2005. 10.1128/AEM.71.6.3199-3204.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z, Dean J.L, Khoo C, Dobson S.L. Generation of a novel Wolbachia infection in Aedes albopictus (Asian tiger mosquito) via embryonic microinjection. Insect Biochem. Mol. Biol. 2005;35:903–910. doi: 10.1016/j.ibmb.2005.03.015. 10.1016/j.ibmb.2005.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabalou S, Charlat S, Nirgianaki A, Lachaise D, Mercot H, Bourtzis K. Natural Wolbachia infections in the Drosophila yakuba species complex do not induce cytoplasmic incompatibility but fully rescue the wRi modification. Genetics. 2004;167:827–834. doi: 10.1534/genetics.103.015990. 10.1534/genetics.103.015990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Rousset F, O'Neill S.L. Phylogeny and PCR based classification of Wolbachia strains using wsp gene sequences. Proc. R. Soc. B. 1998;265:509–515. doi: 10.1098/rspb.1998.0324. 10.1098/rspb.1998.0324 [DOI] [PMC free article] [PubMed] [Google Scholar]