Abstract
Receptor tyrosine kinase erbB2, which is activated by neuregulin, is expressed in Schwann and muscle cells in the developing neuromuscular junction (NMJ). In vitro studies have shown that neuregulin promotes the survival and migration of Schwann cells and stimulates acetylcholine receptor gene transcription in cultured muscle cells. These findings suggest an important role for erbB2 in the development of the NMJ. Here we examine erbB2-deficient mice to determine whether erbB2 is required for NMJ development in vivo. Our analysis shows that there are pre- and postsynaptic defects of developing NMJ in erbB2-deficient embryos. The presynaptic defects include defasciculation and degeneration of the motor nerves, and an absence of Schwann cells. The postsynaptic defect features an impairment of junctional folds at the neuromuscular synapse in the mutants. These results demonstrate that erbB2 is essential for in vivo development of the NMJ.
Neuromuscular junction (NMJ) development is regulated by reciprocal interactions between presynaptic (nerve terminal and Schwann cell) and postsynaptic (muscle cell) components. Two of the hallmarks of NMJ development are clustering of acetylcholine receptors (AChRs) on muscle cell membranes and selective transcriptional activation of the AChR genes at synaptic sites. Agrin plays an essential role in mediating the clustering of AChRs through its activation of muscle-specific kinase (MuSK) receptor complexes, as demonstrated by a lack of AChR clustering at normal synaptic sites in mice lacking either agrin or MuSK, although some ectopic AChR clusters are detected in agrin mutant mice (1, 2). Several studies demonstrated that AChR gene expression is induced in culture by acetylcholine receptor-inducing activity in the absence of innervation (3). However, there is no in vivo evidence as to whether acetylcholine receptor-inducing activity affects embryonic AChR gene activation. Acetylcholine receptor-inducing activity belongs to a family of growth factors that are translated from alternatively spliced transcripts of the neuregulin-1 (NRG-1) gene (4, 5). Recently, three additional NRG genes (NRG-2, NRG-3, and NRG-4) have been cloned, but their role in AChR gene activation has not yet been established (6–10). In addition to activation of the AChR gene, NRG-1 has been shown to promote the survival and migration of Schwann cells (11, 12).
The biological effects of NRGs are mediated through erbB receptors, including erbB2, erbB3, and erbB4, all of which are members of the epidermal growth factor receptor family. Expression patterns of NRG isoforms and their receptors are complex at developing NMJ. Motor neurons express sensory and motor neuron-derived factor or cysteine-rich domain-NRG isoforms, which do not contain the Ig-domain (13, 14), whereas muscle cells express the Ig-NRG isoform that lacks the cysteine-rich domain (15). erbB2, erbB3, and erbB4 receptors are expressed in muscle cells and concentrated in the synaptic sites of both the mature and developing NMJ (16–19), whereas only erbB2 and erbB3 receptors are expressed in Schwann cells. These results suggest that erbB receptors and NRGs are involved in mediating the development of NMJ through reciprocal interactions between pre- and postsynaptic components (20). Here we performed detailed developmental analysis on motor axons and synapses in these erbB2-deficient mice. We find that the phrenic nerve is dramatically defasciculated and projects aberrantly across the entire diaphragmatic surface. No Schwann cells are associated with motor axons. At synaptic sites, nerve terminals are not capped with Schwann cells. In addition, junctional folds are rarely observed at the NMJ of the intercostal muscle in the mutants. Our finding suggests that erbB2 plays essential roles in the development of the NMJ.
Materials and Methods
Animals.
erbB2-deficient animals were generated and genotyped as described (21). Embryos at various stages [embryonic day 12 (E12), E12.5, E13.5, E14, E14.5, E15.5, E16.5, and E18.5] were collected, and three to eight embryos of the same genotype from each stage were analyzed.
Immunohistochemistry and Whole-Mount Immunochemistry.
The muscles were dissected, rinsed with PBS, pH 7.3, and incubated with 0.1 M glycine in PBS for 1 h, and then with 0.5% Triton X-100 in PBS. The muscles were blocked in dilution buffer (150 mM NaCl/0.01 M phosphate buffer/3% BSA/5% goat serum/0.01% thimerasol) overnight at 4°C, and then incubated with rabbit antibodies against neurofilament (NF150, 1:500, Chemicon), or synaptophysin (1:1,000, kindly provided by R. Jahn, Yale University) in dilution buffer overnight at 4°C. After washing three times for 1 h each in 0.5% Triton X-100 in PBS, the muscles were then incubated with fluorescein-conjugated goat anti-rabbit IgG (1:400, Cappel) and Texas Red conjugated α-bungarotoxin (α-BTX) (10−8 M, Molecular Probes) overnight at 4°C. The muscles were then washed three times for 1 h each with 0.5% Triton X-100 in PBS, once with PBS, and flat-mounted in 90% glycerol, polyvinyl alcohol, and N-propyl gallate.
Frozen sections (12 μm) were prepared from unfixed diaphragm, leg, and intercostal muscle. Immunofluorescence staining was performed according to conventional procedures. The following primary antibodies were used in the analysis: rabbit anti-agrin (1:1,000), rabbit anti-MuSK (1:500), rabbit anti-rapsyn (1:100), and mouse anti-heparan sulfate proteoglycan (1:100, NovoCastra, Newcastle, U.K.).
Whole-Mount in Situ Hybridization.
E18.5 embryos were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer at 4°C overnight. The diaphragm and intercostal muscles were dissected out as described above. Digoxygenin-labeled cRNA probe specific for the α-subunit of mouse AChR (kindly provided by S. Burden, Skirball Institute, New York University, New York) was transcribed in vitro. Hybridization was performed at 70°C overnight in the hybridization buffer containing 50% formamide, 1.3× SSC, 5 mM EDTA, 50 μg/ml yeast tRNA, 0.2% Tween-20, 0.5% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, and 100 μg/ml heparin. After hybridization, the diaphragm and intercostal muscles were washed with Tris-buffered saline containing 1% Tween-20 three times for 1 h each. The muscles were then blocked with 5% goat serum in antibody dilution buffer and incubated with alkaline phosphatase-conjugated anti-digoxygenin (1:1,000) overnight at 4°C. Detection was performed in 100 mM Tris, pH 9.5, containing nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate.
Electron Microscopy.
Pregnant females were sacrificed by cervical dislocation and the embryos (E18) were removed. Embryo tails were collected for PCR analysis, and the remainder of the body was placed in a solution of 4% formaldehyde and 4% gluteraldehyde in 0.1 M sodium cacodylate buffer, pH 7.4. The intercostal muscles were removed and placed in the same solution for a total of 2 h on ice. The tissue was then rinsed with buffer and postfixed in 2% osmium tetroxide in buffer for 1 h on ice. The tissue was then rinsed in H2O, dehydrated in a graded series of ethanol, infiltrated, and polymerized in Durcupan resin (EM Science). Ultrathin sections were stained with Sato lead, and electron micrographs were recorded by using either a JEOL 100CX or 2000FX electron microscope operated at 80 keV.
Results
erbB2-Deficient Mice Die at Birth and Display Defects in Presynaptic Development.
erbB2-deficient mice die at birth (21). Histological analysis of hematoxylin/eosin-stained lung sections showed that the alveoli of mutants were tightly compressed (data not shown), suggesting that the inability for erbB2 mutants to breathe is likely due to a lack of functional NMJs. To study this possibility, we analyzed the innervation of the diaphragm, intercostal, and limb muscles.
Phrenic nerve.
We examined phrenic nerve innervation at various stages of development, ranging from E12 to E16.5. As shown in Fig. 1, the phrenic nerve reached the dorsal portion of the diaphragm at E12 in both control and mutant mice (Fig. 1 A and D, respectively). In the controls, the main nerve trunk remained bundled after reaching the muscle surface (arrow in Fig. 1A). In contrast, the phrenic nerve of the mutants was bundled before reaching the muscle surface (arrow in Fig. 1D) but became widely defasciculated upon reaching the muscle surface (arrowhead in Fig. 1D). At E12.5, the phrenic nerve in the controls extended toward the dorsal and ventral diaphragm, with intramuscular branches emanating perpendicularly from the nerve trunk (Fig. 1B). In contrast, the phrenic nerve of the mutants became markedly defasciculated and diffusely projected to the entire dorsal surface of the muscle (arrowheads in Fig. 1E). As development proceeded, the phrenic nerve of control embryos extended toward the dorsal and ventral diaphragm, and elongated progressively along the central region of the diaphragm from E13.5 to E16.5 (Fig. 1 C and G–I). In contrast, in the mutants, the nerves remained diffusely projected across the dorsal portion of the diaphragm at E13.5 (Fig. 1F), and then migrated across the entire muscle surface by E14 (data not shown). At E14.5, the nerves began to withdraw (Fig. 1J), leaving only a few nerve branches by E15.5 (Fig. 1K). At E16.5, the entire diaphragm was devoid of phrenic innervation (Fig. 1L). Thus, in erbB2-deficient embryos, the phrenic nerves appeared defasciculated and projected diffusely across the diaphragmatic surface for 2 days (E12–E14), and withdrew during the following 2 days (E14.5–E16.5).
Figure 1.
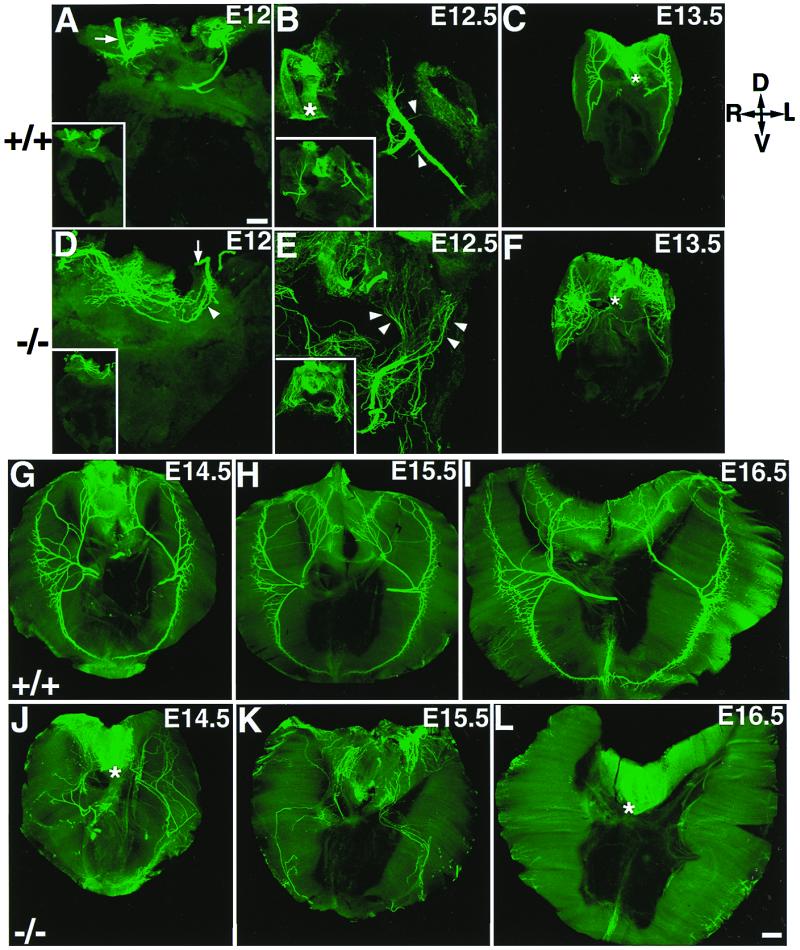
Aberrant innervation of the diaphragm muscle in mutant embryos from E12 to E16.5. All diaphragm images were shown as the top view. The orientation of the diaphragm is indicated in the upper right corner (D, dorsal; V, ventral; R, right; and L, left). In the controls (A), the intramuscular nerve trunk remained as a bundle, reached roughly at the middle of the dorsal diaphragm (arrow); the Inset is the low-power view. In the mutant, the phrenic nerves also reached the dorsal part of the diaphragm (D) but became aberrantly defasciculated (arrowhead). At E12.5, the phrenic nerve in the controls remained as a tight bundle with defined branches emanating perpendicularly from the main nerve trunk (arrows in B); the Inset is the low-power view. In the mutants, the phrenic nerve became more dramatically defasciculated (arrowheads in E) and distributed aberrantly across the entire dorsal surface of the diaphragm; the Inset is the low-power view. At E13.5, the phrenic nerves in the mutant (F) remained grossly defasciculated and aberrant, as compared with that in the control (C). At E14.5, the phrenic nerves in the controls (G) extended more toward dorsal and ventral portions of the diaphragm, innervating a ring of central band on the diaphragm, whereas the phrenic nerves in the mutants (J) were aberrantly distributed throughout the diaphragm and some nerves began to withdraw at this stage. By E15.5, only a small number of nerves remained in the diaphragm in the mutants (K). At E16.5, no phrenic nerve was observed in the entire diaphragm of the mutants [compare (I and L).] Note background staining associated with the esophagus (*) structure in some panels. [Bars = 100 μm (A, B, D, and E) and 200 μm (C, F, and G–L).]
Transient targeting of phrenic nerve terminals to the muscle at E14.
The above observations indicated that the phrenic nerve in the erbB2 mutant projected diffusely across the diaphragm muscle up to E14 and then started to withdraw. To visualize the presynaptic nerve terminals, whole-mount diaphragm was stained with antibodies against synaptophysin. In the control diaphragm, the staining was confined within a narrow band in the middle of the diaphragm muscle (Fig. 2 A and B). In the mutants, nerve terminals were also intensely labeled by synaptophysin antibody, along the middle of the diaphragm muscle (Fig. 2 D and E), whereas the preterminal axons were distributed randomly across the entire diaphragmatic surface (arrowhead in Fig. 2E). Although nerve terminals contacted the central band of muscle, the nerve arborization appeared disorganized in the mutants as compared with controls (compare Fig. 2 B and E, controls and mutants, respectively). Thus, although the phrenic nerve projected diffusely across the diaphragmatic surface, nerve terminals only contacted the muscle in a small region near the center part of muscle, a location similar to that in the wild-type diaphragm. However, the nerve terminals were unstable in the mutants and gradually withdrew. At E15.5, synaptophysin-positive nerve terminals were not detectable in the erbB2 mutants (Fig. 2F). By E16.5, nerve terminals were not observed in the whole diaphragm of the mutants (data not shown).
Figure 2.
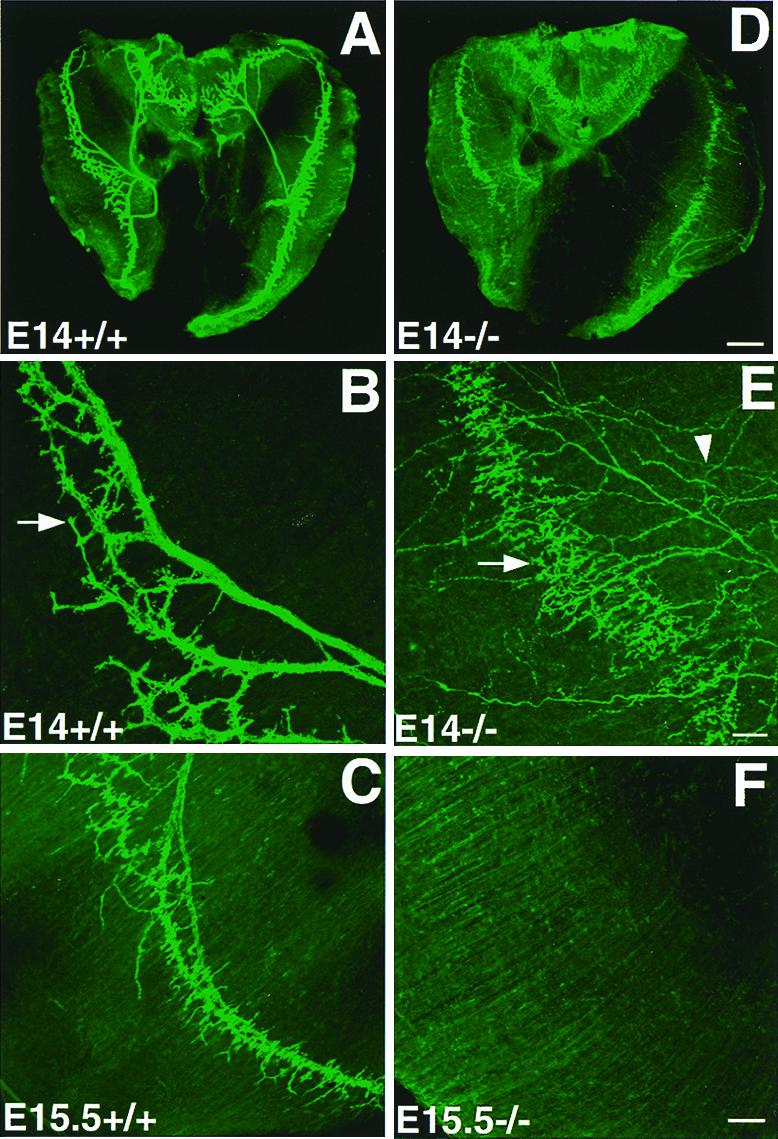
Transient targeting of motor axons and disorganization of nerve terminals in the diaphragm. (A–C) Wild type and (D–F) mutant. At E14, axons terminated at the central endplate band (arrow in B) in the controls (A and B; A, low power; B, higher power). In the mutant embryos, axonal terminals were also located at the central band (arrow in E) of the muscle (D, low-power view; E, high-power view). However, the preterminal nerves (arrowhead in E) and terminals (arrow in E) were disorganized in the mutants, as compared with those in the wild type (B). At E15.5, nerve terminals degenerated in the mutant (F) as compared with the wild-type embryo (C). [Bars = 200 μm (A and B) and 100 μm (C–F).]
Intercostal and limb motor nerves.
Similar to the phrenic nerve, the intercostal nerves were also defasciculated at E12.5–E18.5 (Fig. 3) in the mutants. Terminal arborization was disorganized and diffuse in the mutants (Fig. 3 F and G). In contrast to a complete loss of the phrenic nerve at E16.5 (Fig. 1), the intercostal nerves were still present at E18.5, although they remain defasciculated (Fig. 3G). We have also examined the NMJ of forelimb and hindlimb muscles. Only a small number of motor nerves remained at E18.5 (Fig. 3I).
Figure 3.

Defasciculation of intercostal nerves and abnormal axonal arborization in the intercostal and limb muscles. (A–E) controls and (F–J) mutants. In the control (A), the intercostal nerve trunk formed bundles, with many collateral branches emanating from the main nerve trunk (arrowheads in A); in the mutants (F), the intercostal nerves were defasciculated and nerve branches were disorganized (arrowheads). Note the tip of intercostal nerves terminated aberrantly in the mutants (arrow in F), as compared with the controls (arrow in A). B, C, G, and H show E18.5 intercostal muscles double-labeled with neurofilament antibodies (B and G) and Texas Red-conjugated α-BTX (C and H). In the controls, nerve terminals extended from the nerve bundle (arrow in B) and terminated at a central band labeled by α-BTX (C). In the mutants, the intercostal nerves were defasciculated (arrow in G), and nerve terminals aberrantly projected to the center of muscle fibers (G). As in control embryos, an AChR clustering band was present at the center of muscle fibers in the mutant (H). D, E, I, and J show sections of leg muscle, double-labeled with neurofilament antibodies and α-BTX. In the controls (D and E), neurofilament antibody-labeled nerve terminals were colocalized with α-BTX-labeled AChR clusters (arrowheads in D and E); in the mutants (I and J), only a small number of nerve terminals remained (arrowheads in I and J). [Bars = 100 μm (A and F), 50 μm (B, C, G, and H), and 50 μm (D, E, I, and J).]
The absence of Schwann cells in motor nerves.
Schwann cells have been shown to affect the development of axons in culture, including myelination and axon diameter (22). Immunological examination of developing embryos (E13.5, E14.5, and E15.5) with antibodies against S100 showed that Schwann cells were absent in both the phrenic and intercostal nerves (Fig. 4 B, D, and F). The absence of Schwann cells was then further confirmed by electron microscopy. As shown in Fig. 4G, every single axon of control intercostal nerves was wrapped by Schwann cell processes. Axons were separated by extracellular matrix (Fig. 4G). In contrast, axons of the mutant intercostal nerves were tightly packed together. There were no Schwann cells or extracellular matrix in the mutant (Fig. 4H). Interestingly, the overall size of axons in the mutants was markedly reduced as compared with those in the controls (controls, 1.9 ± 0.3 μm, n = 17; mutants, 1.2 ± 0.2 μm, n = 25; P < 0.05). Taken together, these results demonstrate that erbB2 is essential for the development of the presynaptic component in NMJ, including Schwann cells and nerve terminals.
Figure 4.

Absence of Schwann cells in erbB2-deficient embryos. (A–F) Whole-mount preparations from controls (A, C, and E) and erbB2 mutants (B, D, and F) were labeled with antibodies against S100. In the control preparations, S-100-labeled Schwann cells delineated the entire phrenic nerves as early as E13.5 (A), and were readily detectable at E15.5 (C). S100-positive cells were also found in the intercostal nerve in the controls (arrowheads in E). In contrast, there were no Schwann cells detectable in the mutants in the phrenic nerves (B and D), or in the intercostal nerves (F). Ribs (r) also stained positively for S100. (G and H) Electron micrographs of the intercostal nerves at E18.5. In the control (G), every single axon (ax) was wrapped by Schwann cell processes (SC). Axons were separated apart by extracellular matrix (ec). In the mutant (H), axons were tightly packed together. There were no Schwann cells or extracellular matrix. Note that the density of axons in the mutants is higher, and overall axon diameters are smaller. [Bars = 50 μm (A, B, E, and F), 100 μm (C and D), and 1 μm (G and H).]
Impairment of Postsynaptic Development.
Impairment of postsynaptic junctional folds.
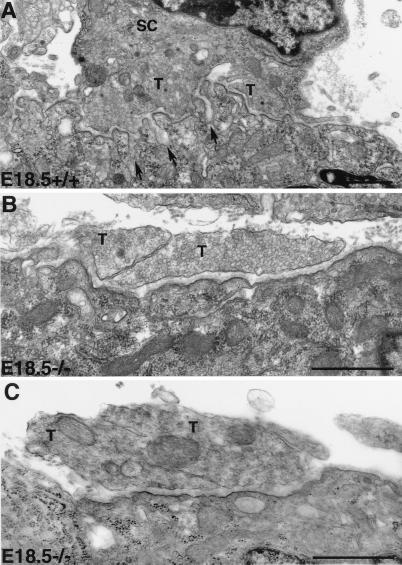
The ultrastructure of the NMJ synapse was examined with electron microscopy. As shown in Fig. 5, nerve terminals in control embryos were capped with Schwann cell processes (Fig. 5A), whereas mutant embryo nerve terminals were devoid of Schwann cells. (Fig. 5 B and C). Furthermore, postsynaptic junctional folds developed at synaptic sites in controls (Fig. 5A, arrows), whereas the postsynaptic membrane in the mutants was lacking junctional folds (Fig. 5 B and C). We analyzed more than 10 neuromuscular synapses in the mutants and we rarely observed any junctional folds. The basal lamina, however, was present and normally arranged at the synaptic site in the mutants, similar to those in the controls. We also labeled muscle sections with several markers that associate with the basal lamina or postsynaptic membrane, such as acetylcholinesterase, β-dystroglycan, agrin, heparan sulfate proteoglycan, rapsyn, and MuSK. These markers were localized at the neuromuscular junction in the mutants, similar to those observed from the controls (data not shown).
Figure 5.
Impairment of junctional folds at the synaptic site of NMJ in erbB2-deficient embryos. In the controls (A), nerve terminals (T) were capped by Schwann cell (SC), and junctional folds (arrows) developed in apposition with axon terminals. In the mutants (B and C), Schwann cells were absent in the nerve terminals. No junctional fold was observed in the NMJ from the mutant (B and C). Note that synaptic vesicles were present in both control and mutant nerve terminals. Multiple nerve terminals (T) were present at the synaptic site in both the wild type and mutant. [Bar = 1 μm (A–C).]
Transcriptional activation of the AChRα gene.
We then determined whether transcriptional activation of the AChR gene is affected in erbB2 mutants. In situ hybridization experiments demonstrated that α-subunit transcripts were concentrated in the central band of the muscle in both the control (Fig. 6A) and erbB2-deficient diaphragm (Fig. 6C). Similar results were observed in the intercostal muscles (Fig. 6B, controls; Fig. 6D, mutants). These results suggest that erbB2 is not essential in AChRα gene activation at synaptic sites.
Figure 6.

AChRα gene transcription in erbB2-deficient embryos. A whole-mount in situ hybridization of E18.5 diaphragm (A and B) and intercostal muscle (C and D) labeled with a digoxygenin-cRNA probe for the AChRα subunit. Labeling was concentrated in a central band of muscle in both the wild type [arrowheads in (A and B)] and mutant [arrowheads in (C and D)].
Discussion
In the present study, we showed that erbB2-deficient mice died at birth, probably due to abnormal NMJ development at the diaphragm. The phrenic nerve was severely defasciculated from E12 to E14, yet nerve terminals transiently contacted the diaphragm muscle at similar locations as those in the controls. Motor nerves degenerated at later stages and completely disappeared after E16.5. In contrast to the phrenic nerve, the intercostal nerve did not degenerate at birth. Interestingly, AChRs were clustered along the central band of muscles. Because the nerves completely degenerated in the diaphragm, no neurotransmitters were released to activate AChRs on the diaphragm, thereby leading to a respiratory failure.
Several lines of evidence indicate that the neural form of agrin, through the activation of the MuSK receptor complex, is necessary for inducing AChR clustering. The muscle isoform of agrin fails to induce clustering (23). Although in erbB2 mutant embryos, the phrenic nerve terminals only transiently contacted the muscle for about 2 days, this may be sufficient for neural agrin to be deposited at the basal lamina at synaptic sites to initiate AChR clustering. Alternatively, in addition to agrin, several other molecules, including laminin, heparin-binding growth-associated molecule, midkine, and basic fibroblast growth factor have been shown to induce AChR clustering in cultured muscle cells (24–28). These results raise the possibility that in the context of the erbB2 mutation, these neural agrin-independent pathways induce clustering of AChRs. Our results showed that α-subunit transcripts were concentrated in a central band of the muscle in both the control and erbB2-deficient diaphragm, suggesting that erbB2 is not essential for embryonic AChR gene activation. Alternatively, other erbB receptors might compensate for the loss of erbB2 in synapse-specific activation of the AChR gene. Indeed, both erbB3 and erbB4 receptors are expressed in the diaphragm and other skeletal muscles of erbB2 mutants. If there are no nerves, where do NRGs come from? Muscle cells have been shown to express the Ig-domain containing the NRG isoform (15, 18). Thus, the muscle form of NRGs may activate erbB receptor via an autocrine mechanism.
Several lines of evidence suggest that ligand-induced erbB receptor heterodimerization is the key step in the activation of NRG-mediated signaling pathways. Our present results demonstrated that Schwann cells are absent in the peripheral nerve in erbB2 mutant embryos. Similarly, Schwann cells in peripheral nerve are also absent in erbB3-deficient embryos (29, 30). Taken together, these studies support the idea that both erbB2 and erbB3 are required for normal development of Schwann cells.
In erbB2 mutant mice, the phrenic nerve became defasciculated and subsequently degenerated. Because erbB2 is expressed in both Schwann and muscle cells, the loss of Schwann cells and/or a defect in the muscle might lead to axonal defasciculation and degeneration. Schwann cells have been shown to promote the growth of motor axons to their muscle targets during development and nerve regeneration. In the absence of Schwann cells in the chick following neural crest cell ablation, motor axons become defasciculated and fail to enter the limb bud to innervate limb muscles (31). These results suggest that glial cells play an essential role in axonal fasciculation and growth during development. In addition, the muscle may also provide important signals for axonal fasciculation and growth. Because erbB2 is expressed in muscle cells, this lack of erbB2 may result in the loss of these important signals.
Despite the dramatic defasciculation and disorganization, synaptic connections were established transiently at normal locations in the diaphragm of mutant embryos at E14, whereas synaptic connections persisted in the intercostal muscle at least until birth. However, terminal arborization was reduced and less elaborate. These synapses in the diaphragm were not stable and the nerve withdrew shortly afterwards. Although synapses were formed in the intercostal muscle, normal junctional folding was not observed in the mutants. Schwann cells have been shown to affect terminal arborization (32) and axon caliber (22) in vitro. The absence of Schwann cells in erbB2-deficient embryos may contribute to these axonal and synaptic defects. Why are the synapses not stable? Several studies show that Schwann cells produce trophic factors that promote the survival of neurons and the integrity of axons. We cannot rule out the possibility that erbB2-deficient muscle also play a role in this phenotype. Additional trophic factors may be produced in the intercostal muscle but not in the diaphragm muscle, which may explain why intercostal nerve remained in the mutants at birth. In addition, Schwann cells may maintain the tight adhesion between pre- and postsynaptic elements and promote synaptic transmission. Schwann cells express molecules such as N- and E-cadherins, which are implicated in the stability of synapses (33). Schwann cells have been shown to modulate synaptic efficacy and synaptic depression at the NMJ (34). Thus, the synapses may be unstable and may not convey synaptic transmission properly in the absence of Schwann cells, and therefore, result in retraction of the axons because of the lack of activity. Finally, because erbB2 is expressed in both Schwann and muscle cells, the Schwann and synaptic defects could be separate consequences of the erbB2 deficiency.
Acknowledgments
We thank M. Reugg, S. Carbonetto, S. Burden, J. Sanes, Z. Hall, R. Jahn, D. Glass, and G. Yancopoulos for reagents. We thank F. Gage for the use of the confocal microscope. The use of animals is in compliance with the guidelines of the Institute Animal Care and Use Committee of the Salk Institute. J.K.M was supported by a National Institutes of Health National Research Service Award Fellowship HL09756. W.L. was supported by a National Institutes of Health Training Grant CA09370. H.B.S. was supported by a Medical Science Training Program grant from the National Institutes of Health. This work was supported by National Institutes of Health Grants HD34534 and AG10435 (to K.-F.L.) and NS14718 and RR04050 (to M.H.E.) and by the Foundation of the March of Dimes. K.-F.L. is a Pew Scholar.
Abbreviations
- NRG
neuregulin
- NMJ
neuromuscular junction
- AChR
acetylcholine receptor
- MuSK
muscle-specific kinase
- BTX
bungarotoxin
References
- 1.Gautam M, Noakes P G, Moscoso L, Rupp F, Scheller R H, Merlie J P, Sanes J R. Cell. 1996;85:525–535. doi: 10.1016/s0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- 2.DeChiara T M, Bowen D C, Valenzuela D M, Simmons M V, Poueymirou W T, Thomas S, Kinetz E, Compton D L, Rojas E, Park J S, et al. Cell. 1996;85:510–512. doi: 10.1016/s0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- 3.Falls D L, Rosen K M, Corfas G, Lane W S, Fischbach G D. Cell. 1993;72:801–815. doi: 10.1016/0092-8674(93)90407-h. [DOI] [PubMed] [Google Scholar]
- 4.Marchionni M A, Marchionni M A, Goodearl A D, Chen M S, Bermingham-McDonogh O, Kirk C, Hendricks M, Danehy F, Misumi D, Sudhalter J, et al. Nature (London) 1993;362:312–318. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- 5.Fischbach G D, Rosen K M. Annu Rev Neurosci. 1997;20:429–458. doi: 10.1146/annurev.neuro.20.1.429. [DOI] [PubMed] [Google Scholar]
- 6.Busfield S J, Michnick D A, Chickering T W, Revett T L, Ma J, Woolf E A, Comrack C A, Dussault B J, Woolf J, Goodearl A D J, et al. Mol Cell Biol. 1997;17:4007–4014. doi: 10.1128/mcb.17.7.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carraway K L, III, Weber J L, Unger M J, Ledesma J, Yu N, Gassmann M, Lai C. Nature (London) 1997;387:512–516. doi: 10.1038/387512a0. [DOI] [PubMed] [Google Scholar]
- 8.Chang H, Riese D J, II, Gilbert W, Stern D F, McMahan U J. Nature (London) 1997;387:509–512. doi: 10.1038/387509a0. [DOI] [PubMed] [Google Scholar]
- 9.Harari D, Tzahar E, Romano J, Shelly M, Pierce J H, Andrews G C, Yarden Y. Oncogene. 1999;18:2681–2689. doi: 10.1038/sj.onc.1202631. [DOI] [PubMed] [Google Scholar]
- 10.Zhang D, Sliwkowski M X, Mark M, Frantz G, Akita R, Sun Y, Hillan K, Crowley C, Brush J, Godowski P J. Proc Natl Acad Sci USA. 1997;94:9562–9567. doi: 10.1073/pnas.94.18.9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong Z, Brennan A, Liu N, Yarden Y, Lefkowitz G, Mirsky R, Jessen K. Neuron. 1995;15:589–596. doi: 10.1016/0896-6273(95)90147-7. [DOI] [PubMed] [Google Scholar]
- 12.Mahanthappa N K, Anton E S, Matthew W D. J Neurosci. 1996;16:4673–4683. doi: 10.1523/JNEUROSCI.16-15-04673.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho W-H, Armanini M P, Nuijens A, Phillips H S, Osheroff P L. J Biol Chem. 1995;270:14523–14532. doi: 10.1074/jbc.270.24.14523. [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Kuo Y, Devay P, Yu C, Role L. Neuron. 1998;20:255–270. doi: 10.1016/s0896-6273(00)80454-7. [DOI] [PubMed] [Google Scholar]
- 15.Meier T, Masciulli F, Moore C, Schoumacher F, Eppenberger U, Denzer A J, Jones G, Brenner H R. J Cell Biol. 1998;141:715–726. doi: 10.1083/jcb.141.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altiok N, Bessereau J-L, Changeux J-P. EMBO J. 1995;14:4258–4266. doi: 10.1002/j.1460-2075.1995.tb00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jo S A, Zhu X, Marchionni M A, Burden S J. Nature (London) 1995;373:158–161. doi: 10.1038/373158a0. [DOI] [PubMed] [Google Scholar]
- 18.Moscoso L M, Chu G C, Gautam M, Noakes P G, Merlie J P, Sanes J R. Dev Biol. 1995;172:158–169. doi: 10.1006/dbio.1995.0012. [DOI] [PubMed] [Google Scholar]
- 19.Zhu X, Lai C, Thomas S, Burden S. EMBO J. 1995;14:5842–5848. doi: 10.1002/j.1460-2075.1995.tb00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burden S J. Genes Dev. 1998;12:133–148. doi: 10.1101/gad.12.2.133. [DOI] [PubMed] [Google Scholar]
- 21.Morris J K, Lin W, Hauser C, Marchuk Y, Getman D, Lee K-F. Neuron. 1999;23:1–20. doi: 10.1016/s0896-6273(00)80779-5. [DOI] [PubMed] [Google Scholar]
- 22.Windebank A J, Wood P, Bunge R P, Dyck P J. J Neurosci. 1985;5:1563–1569. doi: 10.1523/JNEUROSCI.05-06-01563.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burgess R, Nguyen Q T, Son Y-J, Lichtman J W, Sanes J R. Neuron. 1999;23:33–44. doi: 10.1016/s0896-6273(00)80751-5. [DOI] [PubMed] [Google Scholar]
- 24.Montanaro F, Gee S H, Jacobson C, Lindenbaum M H, Froehner S C, Carbonetto S. Neuroscience. 1998;18:1250–1260. doi: 10.1523/JNEUROSCI.18-04-01250.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng H B, Baker L P, Chen Q. Neuron. 1991;6:237–246. doi: 10.1016/0896-6273(91)90359-8. [DOI] [PubMed] [Google Scholar]
- 26.Peng H B, Ali A A, Dai Z, Daggett D F, Raulo E, Rauvala H. J Neurosci. 1995;15:3027–3038. doi: 10.1523/JNEUROSCI.15-04-03027.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugiyama J E, Glass D J, Yancopoulos G D, Hall Z W. J Cell Biol. 1997;139:181–191. doi: 10.1083/jcb.139.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou H, Halfter W, Tsim K W, Peng H B. Mol Cell Neurosci. 1997;10:56–70. doi: 10.1006/mcne.1997.0638. [DOI] [PubMed] [Google Scholar]
- 29.Riethmacher D, Sonnenberg-Riethmacher E, Brinkmann V, Yamaai T, Lewin G R, Birchmeier C. Nature (London) 1997;389:725–730. doi: 10.1038/39593. [DOI] [PubMed] [Google Scholar]
- 30.Erickson S L, O'Shea K S, Ghaboosi N, Loverro L, Frantz G, Bauer M, Lu L H, Moore M W. Development (Cambridge, UK) 1997;124:4999–5011. doi: 10.1242/dev.124.24.4999. [DOI] [PubMed] [Google Scholar]
- 31.Noakes P G, Bennett M R, Stratford J. J Comp Neurol. 1988;277:214–233. doi: 10.1002/cne.902770205. [DOI] [PubMed] [Google Scholar]
- 32.Tropea M, Johnson M I, Higgins D. Glia. 1988;1:380–392. doi: 10.1002/glia.440010605. [DOI] [PubMed] [Google Scholar]
- 33.Colman D R. Mol Cell Neurosci. 1997;10:1–6. doi: 10.1006/mcne.1997.0648. [DOI] [PubMed] [Google Scholar]
- 34.Robitaille R. Neuron. 1998;21:847–855. doi: 10.1016/s0896-6273(00)80600-5. [DOI] [PubMed] [Google Scholar]