Abstract
Predation has been recognized as a major selective force in the evolution of behavioural characteristics of mammals. As a consequence of local predator extinction, prey may lose knowledge about natural predators but usually express behavioural adjustments after return of predators. Human harvest may replace natural predation but prey selection may differ from that of natural predators leading to a change in the behavioural response of prey. We show that hunting success (HS) of re-colonizing wolves (Canis lupus) on moose (Alces alces) in Scandinavia was higher than reported in North America, where moose have been continuously exposed to wolves and grizzly bears. We found no evidence that moose expressed behavioural adjustments that lowered the HS of wolves in territories that had been occupied by wolves for up to 21 years. Moose behaviour towards wolves and humans typically differs in Scandinavia compared to North America. We explain the differences found to be caused by variation in predation pressure by large carnivores and the rate, and mode, of human harvest during the twentieth century.
Keywords: behavioural adjustments, hunting success, naive prey, moose, wolves
1. Introduction
Predation has long been recognized as a major selective force in the evolution of behavioural characteristics of mammals (Harvey & Greenwood 1978), i.e. alertness and vigilance (Elgar 1989). Foraging theory predicts that animals may sacrifice feeding time in order to reduce the risk of predation (Lima & Dill 1990; Brown 1999). Conversely, isolation from predators should result in a selection against costly anti-predator behaviour (Magurran 1999). A consequence of human-caused exterminations of large predators may therefore be that prey lose or change their anti-predator behaviour (Bayers 1997). However, several studies in North America have demonstrated that after large predators, like wolf (Canis lupus) and brown bear (Ursus arctos) have been reintroduced, or have re-colonized an area where they have been absent for long periods, their prey swiftly regain their former anti-predatory behaviour (Hunter & Skinner 1998; Berger 1999; Berger et al. 2001; Laundré et al. 2001). Typically the hunting success (HS) of re-colonizing predators in such cases is initially high, but will decrease over time as prey re-adapt to its presence. The re-adaptation of prey behaviour may occur after as short a time as one generation, which for moose (Alces alces) or red deer (Cervus elaphus) is no more than 4–5 years (Berger et al. 2001; Laundré et al. 2001). Thus, predator–prey history is an important factor in shaping the behavioural ecology and interaction of current prey species and their predators (Blumstein 2002, Blumstein & Daniel 2005).
On the Scandinavian peninsula (Sweden and Norway), intensive persecution of wolf (Wabakken et al. 2001) and brown bear (Swenson et al. 1994) during the nineteenth and twentieth century exterminated these predators from most of the peninsula. Both wolves and bears were gone from central Scandinavia by the mid-to-late 1800s, and whereas wolves were functionally extinct from the whole peninsula by late 1960s (Haglund 1968) brown bears survived in a small remnant population in the northern part of the peninsula (Swenson et al. 1994). Recovery and expansion into the south-central parts of Scandinavia started in the 1950s for brown bears (Swenson et al. 1994) and as late as the 1980s for wolves (Wabakken et al. 2001). Contrary to wolves, the Scandinavian moose population has grown tremendously throughout the twentieth century (Markgren 1969; Lavsund & Sandegren 1989), and has been exposed to an intensive management that replaced most natural mortality with human harvest (Cederlund & Sand 1991; Saether et al. 1996; Stubsjoen et al. 2000; Solberg et al. 2003).
We studied wolf predation and HS on moose in the re-colonizing wolf population in central Scandinavia. We tested three predictions from the hypothesis that the history of predation will shape the behaviour of prey. First, Scandinavian moose unfamiliar with wolves will initially lack appropriate behaviour to escape wolf predation after wolves have re-colonized an area, and so are relatively easier to kill, i.e. naive compared to moose in populations that have been continuously exposed to wolf predation (Berger 1999). Second, the success rate of wolves hunting moose should decrease with time since occupancy of an area, as a result of moose re-adapting (i.e. learning) their behaviour to the presence of wolf predation (Berger et al. 2001; Laundré et al. 2001). Third, Scandinavian moose should express a higher level of avoidance and a less aggressive behaviour towards humans as compared to North American moose, which are mainly regulated by natural predators.
We tested the first prediction by comparing HS on moose by wolves in Scandinavia with North American moose populations that have been continuously exposed to wolves and grizzly bears. The second prediction was tested by correlating HS for wolves with time since colonization of a new territory whereas the third prediction was examined by comparing the behavioural response between Scandinavian and North American moose cows towards research personnel during capture and handling of moose calves.
2. Material and methods
(a) Collection of data
Wolves were immobilized from a helicopter using a CO2-powered dart gun (see Arnemo et al. 2004 for a detailed description of capture and handling of wolves) and were equipped with either a GPS neck collar (Simplex, TVP Positioning AB, Lindesberg, Sweden) or a conventional VHF radio collar (Telonics Mod. 500, Mesa, Arizona). Wolves with VHF-collars were radio tracked 1–5 times per day from the ground during study periods. GPS-collars were programmed for positioning at hourly intervals during study periods and 2–6 positions per day for the rest of the year. Throughout the study periods we downloaded data weekly or every second week from the ground by VHF-coded signals and a VHF receiver with data logger (RX-900, TVP Positioning AB, Lindesberg, Sweden) and a hand-held antenna.
During the winters of 1998–2003, both radio-collared (1831 km) and non-collared (>1600 km) wolves were snow tracked on foot, on skis or occasionally by snowmobile. During snow tracking, all wolf attacks on moose were recorded. A hunting attack was defined as a lengthening of stride patterns for both wolves and moose, indicating bounding gaits (fast running), occurring together, and where local snow conditions indicated that the tracks had been made simultaneously (see also Murray et al. 1995 for a similar definition). An attack was considered successful if a wolf-killed moose was found in connection with an attack, and considered a failure if no carcass was found. Sometimes it was not possible to distinguish the typical attack pattern of tracks in the snow near a moose carcass, due to extensive wolf activity around the carcass. In these cases, presence of fresh bleeding and/or bite marks on the carcass, was also used for classification of a successful wolf attack (Sand et al. in press).
(b) Calculation of hunting success
We used two independent methods and data sets to calculate the rate of HS in different wolf territories. The first method (A) involved estimating kill-rates based on radio-telemetry locations in four territories during seven study periods (Sand et al. in press). We assumed that we found all wolf-killed moose during study periods, but not all failed attacks, because we did not follow the entire paths of wolf movements between kills. Instead, we used average daily travel distance of radio-collared wolves to calculate the time period (number of wolf days) corresponding to the total number of km of wolf tracks followed by research personnel for each wolf territory and year. We then calculated the number of successful attacks during the same time period by using territory-specific estimates of kill rate (Sand et al. in press). Wolf HS for each territory and year was estimated from the calculated number of successful attacks and the actual number of failed attacks recorded, according to the following model
and
where NSmoose is the calculated number of successful attacks on moose, STdist is the snow-tracked distance in km, WTdist is the average daily wolf travelling distance in winter in km, KRinterval is the kill rate calculated as the territory-specific interval in days between moose kills, WHSmoose is the calculated wolf HS on moose and NFmoose is the number of failed attacks on moose registered during snow tracking.
The second method (B) involved data from 16 territories with non-collared wolves, or from territories with radio-collared wolves, collected before or after, study periods of kill rate. In these territories research personnel performed snow tracking whenever fresh wolf tracks were discovered, and tracks were followed without actively searching for prey. Data from these 16 territories may therefore be considered as random samples of wolf attacks on moose by different wolf packs. HS was estimated from B1, the proportion of individual moose killed of the total number of moose attacked, and B2, the proportion of successful attacks in relation to the total number of attacks on moose. Because the exact number of moose encountered during attacks was sometimes difficult to record, this first estimate was based on a smaller sample size.
Data on wolf HS on moose in North America was extracted from five independent studies of wolf–moose interactions covering three geographical regions and summarized by Mech & Peterson (2004).
Capture of newborn (1–10 day old) moose calves from the ground by locating and observing radio collard moose cows shortly after parturition was performed at the Grimsö Wildlife Research Area during 1993–2001. When calf/calves were seen together with the cow, a fast rush towards the moose by the capture crew (usually only one person) almost consistently resulted in flushing the cow while the calves either crouched or tried to outrun the catcher. Captured calves were weighed, ear tagged, and had morphometric measurements taken.
(c) Data analyses
The total dataset was the pool of 122 observations of wolf attacks on moose using data from both method A (n=37) and method B (n=85). Difference in the HS rate between wolf populations in Scandinavia and North America was tested by logistic regression using Scandinavia as a categorical reference group for pair-wise comparison between populations. The effect of time since wolf establishment on HS among wolf territories and years was estimated by including a variable on the number of years since wolf-pair establishment for each territory. This variable was treated both as a continuous, and as a categorical variable, to test for linear and nonlinear effects of time (years of wolf presence) on hunting success. The categorical variable of time since pair establishment was recoded into four classes (1–2 years; 3–5 years; 6–10 years; 11–21 years). Our study included data from wolf territories that have been occupied by wolves more or less continuously for 21 years (mean =4.9, s.d.=5.0), with 11 (9.0%) out of 122 observations from territories where moose had experienced wolves for 10–21 years, and 36 (29.5%) observations from territories that were occupied by wolves for more than 5 years, i.e. equal to approximately one moose generation. Relationships between HS and potential confounding variables were examined by Spearman rank analyses of parameter averages between populations/studies. Variables were considered significant at an alpha level p<0.05. Analyses were performed with SPSS v. 11.5 for Windows.
3. Results
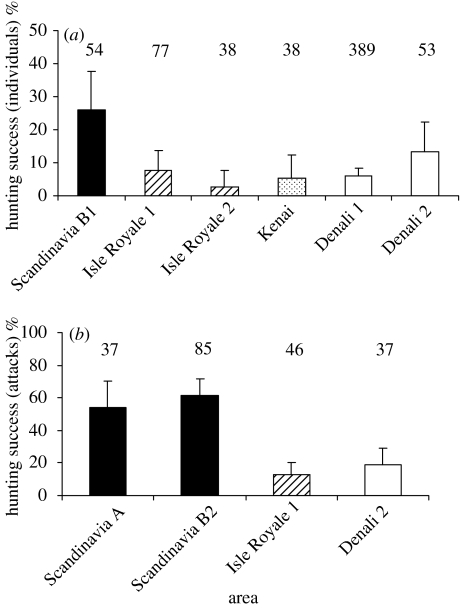
For both methods (B1, B2) of estimating HS the results confirmed our first prediction. HS, based on the proportion of individual moose killed (n=14) of the total number moose (n=54) involved in 32 wolf attacks (B1), was significantly higher in Scandinavia (26%) in four out of five pair-wise comparisons with data from North American moose–wolf populations (figure 1a; χ52=22.1; p<0.02). Only HS in Denali (13%, n=53), Alaska (Mech et al. 1998) did not differ significantly from Scandinavia (d.f.=1; p=0.10). HS based on individual moose killed in Scandinavia was 2.0–9.9 times higher than in North America. A significantly higher HS was also found in Scandinavia using data based on the number of successful hunts (B2), independent of how many moose individuals were involved in each attack, (nmethod1=37; nmethod2=85), compared with two regions in North America (figure 1b; χ32=42.1; p<0.01). Depending on the method used for calculating wolf HS in Scandinavia, the proportion of successful hunts in Scandinavia was 4.2 and 4.7 times higher than the HS found at Isle Royale (Mech 1966), and 2.8 and 3.2 times higher than found in Denali (Mech et al. 1998). Also, during wolf attacks, moose in North America stood at bay, i.e. fronted wolves, significantly more often than in Scandinavia (table 1; Isle Royale: χ12=49.8; p<0.0001 (Mech 1966), Denali: χ12=23.0; p<0.0001, (Peterson et al. 1984)). Contrary to our second prediction, wolves in newly colonized territories in Scandinavia did not enjoy greater HS than wolves in territories that have been occupied up to 21 years (χ12=0.002; p=0.96) and we found no evidence for nonlinear effects of time since wolf establishment on variation in HS (χ32=0.90; p=0.60).
Figure 1.
Wolf hunting success on moose (95% C.I.) based on (a) the number of individual moose attacked and (b) on the number of attacks on moose in Scandinavia and North America; Isle Royale 1 (Mech 1966); Isle Royale 2 (Peterson 1977); Kenai (Peterson et al. 1984); Denali 1 (Haber 1977); Denali 2 (Mech et al. 1998). Sample size (n) for each study is given above bars. Scandinavia A and B refers to the type of method used for calculating hunting success.
Table 1.
Differences in patterns of predation, human harvest, and moose behaviour during the twentieth century between Scandinavia and North America.
| North America | North America | Scandinavia | |
|---|---|---|---|
| Alaska | Isle Royale | south-central | |
| proportion of mortality due to predation from large predators | higha (50–80%) | highb (>58%) | absentc (wolves) or very low <2% (brown bears) |
| annual human harvest in the moose population during the last 50 years | low (<5%) | no harvest (National Park) | highd (25–40%) |
| harvest methods | no use of dogs (use of dogs prohibited for moose harvest) | no harvest (National Park) | dogs commonly used (20–30% of all moose shot by the use of baying dogs) |
| moose fronting wolves during wolf attackse | commonf (20 out of 36=56%) | commong (36 out of 114=32%) | uncommon (4 out of 49=8%) |
| moose cows aggressive towards humans at calf capture events | commonh (helicopters used to keep the cow separated from the calf and capture crew) | no capture of calves (National Park) | rare (2 out of 131=1.5% capture events resulted in aggressive behaviour of moose cows) |
re-calculated data for moose surviving an attack by wolves.
We also analysed possible confounding effects of other factors than geography/history on the variation of HS, i.e. wolf pack size, wolf body size, moose body size, percentage of adult females in kill, percentage old (>10 yr) moose in kill, moose population density, moose population age structure, percentage of wolf-killed calves with depleted (<10%) marrow fat, snow depth, and month of data collection (table 2). We predicted that increased wolf HS would be positively associated with all these factors except moose size, which should be negatively related to HS. Two of these factors could only be qualitatively evaluated in relation to HS, i.e. month of data collection, which was neutral to variation in HS, and population age structure, which had opposite effect on HS than the one predicted. Population age structure was assumed to be skewed towards a higher proportion of older moose in the North American moose populations with none (Isle Royale) or low rate of human harvest (Alaska) as compared to the heavily harvested Scandinavian moose population. Among the remaining factors, four (pack size, snow depth, moose population density, percentage of wolf killed calves with depleted marrow fat) were inversely related to HS, and thus were not likely contributors to the higher HS found in Scandinavia. Four variables (moose size, percentage of calves in kill, percentage of adult females in kill, percentage of old moose in kill) were positively but weakly (d.f.=5, p>0.34) related to HS. One variable (wolf size) gave some support (rs=0.62, d.f.=5, p=0.17) for the prediction that this factor could contribute to the variation found in HS among studies. Adult wolves in Scandinavia and Alaska were equal in body size but on average 25% heavier than Isle Royale wolves. In contrast, adult female moose in Scandinavia and on Isle Royale were of approximately the same size but on average 7–13% smaller than moose in Alaska. Thus, a moose size : wolf size ratio among populations/studies was negatively related to HS but not significantly so (rs=−0.47, d.f. =5, p=0.29).
Table 2.
Population specific estimates of moose, wolf and environmental characteristics from each study and study area used for comparison of wolf hunting success on moose. Data presented are taken from each study unless indicated otherwise.
| area | study | study period | mean pack size | mean size adult moose cows (kg) | mean size adult male wolves (kg) | proportion of cows of adults in wolf kill | mean snow depth during winter (cm) | proportion of calves in wolf kill | proportion >10 years of adults killed | months of data collection | mean moose population density 10 km−2 | mean percentage marrow fat of wolf killed calves | percentage of wolf killed calves with depleted (<10%) marrow fat |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scandinavia | this study | 1998–2003 | 5.2 | 350 | 48 | 0.74 | 30 | 0.71 | 0.39 | Dec–Mar | 12.0 | 56%a | 0% |
| Isle Royale | Mech 1966 | 1959–1961 | 15 | 350b | 39c | 0.57 | 48 | 0.36 | 0.32d | Feb–Mar | 11.0 | — | 16%e |
| Isle Royale | Peterson 1977 | 1970–1974 | 6.9 | 350b | 39c | 0.46 | 60 | 0.42 | 0.32d | Jan–Mar | 15.5 | — | 45% |
| Kenai | Peterson et al. 1984 | 1976–1981 | 11.2 | 375f | 45 | 0.96 | 50 | 0.47 | 0.61 | Dec–Mar | 8.0 | 25–30%g | 48% |
| Denali 1 | Haber 1977 | 1967–1974 | 13.8h | 400f | 47i | 0.55 | 50 | 0.45 | — | — | 1.6j | — | — |
| Denali 2 | Mech et al. 1998 | 1986–1994 | 6.7 | 400f | 47i | 0.64 | 80 | 0.36 | 0.45k | year-roundl | 2.0 | 44%m | 10%n |
Data from two wolf territories (n=24).
Estimated from Mech (1966) and Peterson & Ciucci (2003).
From table 4.1 in Peterson & Ciucci (2003).
Data from Peterson (1977).
Data from 1958–1964 given in table 33 in Peterson (1977).
From Franzman et al. (1978).
estimated mean from fig. 6 in Peterson et al. (1984).
Estimated from data in table 6.4 in Peterson & Ciucci (2003).
From Mech et al. (1998).
From table 6.2 in Peterson & Ciucci (2003).
Re-calculated from fig. 6.5 in Mech et al. (1998).
But most data from the winter period.
Re-calculated from December–April data in table 6.5 in Mech et al. (1998).
Data re-calculated from fig. 6.10 in Mech et al. (1998).
Moose cow behaviour towards capture personnel was recorded during capture of newborn moose calves on 131 occasions in Scandinavia. On only three (2.3%) of these occasions did the female moose remain in the close vicinity (10–20 m) of the captured calf, and aggressive behaviour was recorded on only two (1.5%) of these occasions, including one full attack.
4. Discussion
We show that moose behaviour towards both wolves and humans is different in Scandinavia compared to North America and we explain this pattern as have been caused by a difference in the main mortality factor during the last 100 years. The methods and extent of human harvest as a mortality factor compared to natural predators during the twentieth century is likely to have resulted in a relaxed, or even lost, behavioural response to re-colonizing wolves in the Scandinavian moose population.
Although a number of other population specific parameters of wolves and moose were considered for any confounding effects on the results, only two (wolf and moose size) gave some support to the pattern found. However, although these parameters (and their ratio) correlated as would be predicted with variation in HS, the relationship was not significant among populations/studies. Therefore, we argue that this factor is not likely to be the ultimate cause to the pattern found in HS among populations.
Instead, we interpret our results as support for our first prediction that moose in Scandinavia are currently naive to re-colonizing wolves compared to populations where wolves have occurred continuously (Denali, Alaska) or been absent for a relatively short period of time, i.e. 40–50 years, (Isle Royale, Kenai Alaska).
In contrast to our first prediction, the second prediction failed, i.e. that HS will decrease with time since wolf occupancy of an area. Thus the hypothesis that moose swiftly will adjust behaviour in response to the new presence of wolves was not supported. Our results thus seem to contrast with some other studies where behavioural adjustments in ungulates to reduce predation from wolves and grizzly bears have been documented to occur within a single prey generation (Hunter & Skinner 1998; Berger 1999; Berger et al. 2001; Laundré et al. 2001). Our results were unexpected since a high predation pressure on calves by wolves (70% of all moose killed), as generally found in Scandinavia (Sand et al. in press), would favour a vertically transmitted learning process, which would be the most effective way to achieve a widespread behavioural change in the population, and ultimately reduce the HS of wolves. Surprisingly, our data also seem to contrast with a study of moose response to an expanding brown bear population in Scandinavia (Berger et al. 2001). Bears at the expansion front had a higher HS than bears at the core of the population, suggesting an adaptive change in moose behaviour. One explanation for the difference between moose response to bears and wolves may be that the bear population in Scandinavia has a longer history of sympatric distribution with moose in bear core areas (30–50 years) compared with the relatively recent establishment of the wolf population (10–25 years; Wabakken et al. 2001). This indicates that adaptive change in anti-predator behaviour in Scandinavian moose may take longer than the period covered by our data (up to 21 years). Alternatively, more subtle changes in the behaviour of Scandinavian moose, not reflected in the HS of wolves, may still have occurred in some wolf territories in this study.
Our third prediction however was supported by the fact that Scandinavian moose seem to be much more timid towards humans than North American moose. Aggressive behaviour towards humans has frequently been reported for North American calf-rearing female moose (Geist 1963; Mech 1966, 1970; Peterson 1977; Franzman 1998; Mech et al. 1998), which is why helicopters are normally required to separate calf-rearing females from the capture crew during capture of moose calves (Ballard et al. 1979; Gasaway et al. 1992; Franzman 1998). In Scandinavia, aggressive behaviour by calf-rearing females towards humans is extremely rare (Ekman et al. 1992) as was also corroborated by the behaviour of female moose towards human personnel during capture of newborn moose calves within the Grimsö Wildlife Research Area.
We argue that there are two important factors that either alone, or in combination, constitute the ultimate cause to the behavioural differences found in moose between continents and why Scandinavian, but not North American, moose so far have failed to re-adapt to wolf predation. First, moose in south-central Scandinavia have not experienced wolf (and brown bear) predation for 120–150 years whereas most moose populations in Alaska and Canada have a history of continuous exposure to wolf and bear predation during past centuries (Orians et al. 1997; Mech et al. 1998; Franzman & Schwartz 1998). The main mortality factor in Alaska is predation by large predators such as wolves, black bears (Ursus americanus), and grizzly bears (Orians et al. 1997; Ballard & Van Ballenberge 1998) with an annual harvest rate of <5% of the winter population (Van Ballenberge & Ballard 1998). In contrast, moose on Isle Royale arrived on the island in the early twentieth century and have been protected from human harvest ever since (Peterson 1977). In 1949, wolves colonized the island and are currently the sole moose predator there. Also, moose on the Kenai Peninsula in Alaska escaped wolf predation (but not black bear predation) due to extermination of wolves for a period (1915–1960) during the twentieth century (Peterson et al. 1984). Thus, the period moose were released from wolf predation was significantly shorter on Kenai and Isle Royale (40–50 years) than in Scandinavia (120–150 years).
Second, in most parts of Scandinavia, human harvest completely replaced natural predation on moose by wolves and brown bears during the twentieth century. During the last 40–50 years the annual moose harvest rate has been 25–40% of the total winter population (Markgren 1969; Lavsund & Sandegren 1989; Solberg et al. 2003), and constituted approximately 95% of the total mortality (Cederlund & Sand 1991; Saether et al. 1996; Stubsjoen et al. 2000). This harvest pressure has continued over most of the general wolf range after the return of wolves, while mortality due to wolf predation inside this range today is still generally low compared with human harvest (<5%). Even within present wolf territories moose mortality due to wolf predation is still only 25–50% of human harvest (Solberg et al. 2003).
Also the mode of moose harvest differs between continents. Throughout North America use of dogs for hunting moose is prohibited. In Scandinavia, the use of baying dogs to hunt moose is widespread and has a long (>100 years) tradition (Ekman et al. 1992). Certain dog breeds have been artificially selected for this type of hunting behaviour, which show close similarities to the hunting behaviour of wolves. Contrary to a wolf attack on moose, however, the hunting dogs never press the attack in order to kill the moose, but just try to keep the moose at bay, thereby distracting it and disclosing its location to the hunter, who can then stalk the moose. Approximately, 20–30% of all moose harvested annually within the current core of the wolf range are shot using this method (Sand et al. unpublished data). This means that the most successful anti-predator behaviour moose can use against wolves, i.e. being aggressive and taking a stand against the wolves, as demonstrated e.g. on Isle Royale (Mech 1966; 1970; Peterson 1977), is exactly the behaviour that has been selected against for more than 100 years in Scandinavia. This behavioural difference may also be reflected in the relatively low proportion of moose that stood their ground when attacked by wolves in Scandinavia as compared to moose in North America.
Because maintaining anti-predator behaviour (for example aggressiveness) in the absence of predators is assumed to be costly (Magurran 1999) we expect that it would be eliminated, or at least relaxed, by selection if it had no other advantage. If in addition a new predator appeared, against which the previous anti-predatory behaviour was even more costly, like moose aggressive behaviour towards human hunters, we would expect a strong direct selection against it. Human harvest has replaced natural predators for several centuries in large parts of the world and is currently the major cause of mortality for many large mammalian prey species. Thus, human harvest is likely to have been an important evolutionary factor shaping life-history strategies and behaviour of these species (Festa-Bianchet 2003), but our data support the notion by Berger (2005) that it is not likely to have been functionally equivalent to that by large mammalian carnivores.
We conclude that Scandinavian moose have not regained an efficient anti-predator behaviour towards re-colonizing wolves as swiftly as has been seen in North America. We argue that the reason for this difference is the longer period of separation between the two species in Scandinavia, the longer and much more intensive human harvest of moose compared with any North American area, and the use of baying dogs in Scandinavia. Whether this will only cause a delay in readjustment, or whether selection and/or drift have eliminated some of the genetic basis for this behaviour in the Scandinavian moose population remains to be seen.
Acknowledgments
We are in debt to Jon Swenson and Erling Solberg for constructive comments and discussions on the manuscript. The study was supported by the Swedish Environmental Protection Agency, the Association for Hunting and Wildlife Management, World Wilde Fund for Nature (WWF, Sweden), the Swedish University of Agricultural Sciences, the Norwegian Directorate for Nature Management, the Norwegian Research Council, the Norwegian Institute for Nature Research, Hedmark University College, the County Governor of Hedmark, Borregaard Skoger AS, Glommen Skogeierforening, Olle and Signhild Engkvists Stiftelser, Carl Tryggers Stiftelse, the Swedish Carnivore Association, and Stor-Elvdal, Åmot and Trysil municipalities.
References
- Arnemo J.M, Ahlqvist P, Segerström P. Norwegian School of Veterinary Science; Norway: 2004. Biomedical protocol for free-ranging wolves (Canis lupus) in Scandinavia: capture, anesthesia, surgery, tagging, sampling and necropsy procedures.http://skandulv.nina.no/skandulv%20new/Publikasjoner/publikasjoner.htm [Google Scholar]
- Ballard W.B, Ballenberge V.V. Predator/prey relationships. In: Franzman A.W, Schwartz C.C, editors. Ecology and Management of the North American Moose. Smithsonian Institutional Press; London, UK: 1998. pp. 247–274. [Google Scholar]
- Ballard W.B, Franzman A.W, Taylor K.P, Spraker T, Schwartz C.C, Peterson R.O. Proc. N. Am. Moose Conf. Workshop. 1979;15:362–387. [Google Scholar]
- Ballenberge V.V, Ballard W.B. Population dynamics. In: Franzman A.W, Schwartz C.C, editors. Ecology and management of the North American moose. Smithsonian Institutional Press; London, UK: 1998. pp. 223–246. [Google Scholar]
- Bayers J.A. University of Chicago Press; Chicago, IL: 1997. American pronghorn: social adaptations and ghost of predators past. [Google Scholar]
- Berger J. Anthropogenic extinction of top carnivores and interspecific animal behaviour: implications of the rapid decoupling of a web involving wolves, bears, moose and ravens. Proc. R. Soc. B. 1999;266:2261–2267. doi: 10.1098/rspb.1999.0917. 10.1098/rspb.1999.0917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J. Hunting by carnivores and humans: does functional redundancy occur and does it matter? In: Ray J, Redford K, Steneck R, Berger J, editors. Large carnivores and the conservation of biodiversity. Island Press; Washington, DC: 2005. pp. 315–341. [Google Scholar]
- Berger J, Swenson J.E, Persson I. Re-colonising carnivores and naive prey: conservation lessons from Pleistocene extinctions. Science. 2001;291:1036–1039. doi: 10.1126/science.1056466. 10.1126/science.1056466 [DOI] [PubMed] [Google Scholar]
- Blumstein D.T. Moving to suburbia: ontogenetic and evolutionary consequences of life on predator-free islands. J. Biogeogr. 2002;29:685–692. 10.1046/j.1365-2699.2002.00717.x [Google Scholar]
- Blumstein D.T, Daniel J.C. The loss of anti-predator behaviour following isolation on islands. Proc. R. Soc. B. 2005;272:1663–1668. doi: 10.1098/rspb.2005.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.S. Vigilance, patch use, and habitat selection: foraging under predation risk. Evol. Ecol. Res. 1999;1:49–71. [Google Scholar]
- Cederlund G.N, Sand H.K.G. Population dynamics and yield of a moose population without predators. Alces. 1991;27:31–40. [Google Scholar]
- Ekman H, Hermansson N, Pettersson J.O, Rulker J, Steen M, Stålfält F. Svenska Jägareförbundet; Stockholm, Sweden: 1992. Älgen — djuret, skötseln och jakten. [Google Scholar]
- Elgar M.A. Predator vigilance and group size in mammals and birds: a critical review of the empirical evidence. Biol. Rev. Camb. Phil. Soc. 1989;64:13–33. doi: 10.1111/j.1469-185x.1989.tb00636.x. [DOI] [PubMed] [Google Scholar]
- Festa-Bianchet M. Exploitative wildlife management as a selective pressure for life-history evolution of large mammals. In: Festa-Bianchet M, Appollonio M, editors. Animal behaviour and wildlife conservation. Island Press; Washington, DC: 2003. pp. 191–207. [Google Scholar]
- Franzman A.W. Restraint, relocation and husbandry. In: Franzman A.W, Schwartz C.C, editors. Ecology and management of the North American moose. Smithsonian Institutional Press; London, UK: 1998. pp. 519–558. [Google Scholar]
- Franzmann A.W, Schwartz C.C. Smithsonian Institutional Press; London, UK: 1998. Ecology and management of the North American moose. [Google Scholar]
- Franzman A.W, LeResche R.E, Rausch R.A, Oldemeyer L.L. Alaskan moose measurements and weights and measurement–weight relationships. Can. J. Zool. 1978;56:298–306. [Google Scholar]
- Gasaway W.C, Boertje R.D, Grangaard D.V, Kelleyhouse D.G, Stephenson R.O, Larsen D.G. The role of predation in limiting moose at low densities in Alaska and Yukon and implications for conservation. Wildl. Monogr. 1992;120:1–59. [Google Scholar]
- Geist V. On the behaviour of North American moose (Alces alces Andersoni Peterson, 1950) in British Columbia. Behaviour. 1963;20:377–416. [Google Scholar]
- Haber, G. C. 1977 Socio-ecological dynamics of wolves and prey in a subarctic ecosystem. Ph.D. thesis, University of British Columbia, Vancouver.
- Haglund B. De stora rovdjurens vintervanor II. Swedish Wildl. 1968;5:213–361. [Google Scholar]
- Harvey P.H, Greenwood P.J. Anti-predator defence strategies: some evolutionary problems. In: Krebs J.R, Davies N.B, editors. Behavioral ecology: an evolutionary approach. Sinauer Associates; Sunderland, MA: 1978. pp. 129–151. [Google Scholar]
- Hunter L.T.B, Skinner J.D. Vigilance behaviour in African ungulates: the role of predation pressure. Behaviour. 1998;135:195–211. [Google Scholar]
- Laundré J.W, Hernández L, Altendorf K.B. Wolves, elk, and bison: re-establishing the “landscape of fear” in Yellowstone National Park, USA. Can. J. Zool. 2001;79:1401–1409. [Google Scholar]
- Lavsund S, Sandegren F. Swedish moose management and harvest during the period 1964–1989. Alces. 1989;25:58–62. [Google Scholar]
- Lima S.L, Dill L.M. Behavioural decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 1990;68:619–640. [Google Scholar]
- Magurran A.E. The causes and consequences of geographic variation in anti-predator behaviour: perspectives from fish populations. In: Foster S.A, Endler J.A, editors. Geographic variation in behaviour: perspectives on evolutionary mechanisms. Oxford University Press; New York, NY: 1999. pp. 139–163. [Google Scholar]
- Markgren G. Reproduction of moose in Sweden. Swedish Wildl. 1969;6:127–299. [Google Scholar]
- Mech, L. D. 1966 The wolves of Isle Royale. In Fauna of the National Parks of the US Fauna series no. 7. Washington, DC: US Govt. Print. Off.
- Mech L.D. Doubleday/Natural History Press; New York, NY: 1970. The wolf: the ecology and behaviour of an endangered species. [Google Scholar]
- Mech L.D, Peterson R.O. Wolf prey relations. In: Mech L.D, Boitani L, editors. Wolves: behaviour, ecology and conservation. The University of Chicago Press; Chicago, IL: 2004. pp. 131–160. [Google Scholar]
- Mech L.D, Adams L.G, Meier T.J, Burch J.W, Dale B.W. University of Minnesota Press; Minneapolis, MN: 1998. The wolves of Denali. [Google Scholar]
- Murray D, Boutin S, O'Donoghue M, Nams V.-O. Hunting behaviour of a sympatric felid and canid in relation to vegetative cover. Anim. Behav. 1995;50:1203–1210. 10.1016/0003-3472(95)80037-9 [Google Scholar]
- Orians G.H, et al. National Research Council; Washington, DC: 1997. Wolves, bears, and their prey in Alaska: biological and social challenges in wildlife management. [Google Scholar]
- Peterson R.O. Wolf ecology and prey relationships on Isle Royale. US Natl Park Service Sci. Monogr. Ser. No. 11. 1977 [Google Scholar]
- Peterson R.O, Ciucci P. The wolf as a carnivore. In: Mech L.D, Boitani L, editors. Wolves: behaviour, ecology and conservation. The University of Chicago Press; Chicago, IL: 2003. pp. 104–130. [Google Scholar]
- Peterson R.O, Woolington J.D, Bailey T.N. Wolves of the Kenai Peninsula Alaska. Wildl. Monogr. 1984;88:1–52. [Google Scholar]
- Saether B.-E, Engen S, Lande R. Density-dependence and optimal harvesting of fluctuating populations. Oikos. 1996;76:40–46. [Google Scholar]
- Sand, H., Zimmermann, B. Wabakken, P., & Andrén, H. In press. Using GPS-technology and GIS-cluster analyses to estimate kill rates in wolf-ungulate ecosystems. Wildl. Soc. Bull.33
- Stubsjoen T, Saether B.-E, Solberg E.J, Helm M, Rolandsen C.M. Moose (Alces alces) survival in three populations in northern Norway. Can. J. Zool. 2000;78:1822–1830. [Google Scholar]
- Solberg, E. J. et al 2003. The effects of large carnivores on wild ungulates in Norway: implications for ecological processes, harvest and hunting methods. (In Norwegian). NINA Fagrapport 63, Trondheim, Norway.
- Swenson J.E, Sandegren F, Bjärvall A, Soderberg A, Wabakken P, Franzen R. Size, trend, distribution and conservation of the brown bear, Ursus arctos population in Sweden. Biol. Conserv. 1994;70:9–17. 10.1016/0006-3207(94)90293-3 [Google Scholar]
- Wabakken P, Sand H, Liberg O, Bjärvall A. The recovery, distribution, and population dynamics of wolves on the Scandinavian peninsula, 1978–1998. Can. J. Zool. 2001;79:710–725. 10.1139/cjz-79-4-710 [Google Scholar]