Abstract
By modifying the behaviour and morphology of hosts, parasites may strongly impact host individuals, populations and communities. We examined the effects of a common trematode parasite on its snail host, Batillaria cumingi (Batillariidae). This widespread snail is usually the most abundant invertebrate in salt marshes and mudflats of the northeastern coast of Asia. More than half (52.6%, n=1360) of the snails in our study were infected. We found that snails living in the lower intertidal zone were markedly larger and exhibited different shell morphology than those in the upper intertidal zone. The large morphotypes in the lower tidal zone were all infected by the trematode, Cercaria batillariae (Heterophyidae). We used a transplant experiment, a mark-and-recapture experiment and stable carbon isotope ratios to reveal that snails infected by the trematode move to the lower intertidal zone, resume growth after maturation and consume different resources. By simultaneously changing the morphology and behaviour of individual hosts, this parasite alters the demographics and potentially modifies resource use of the snail population. Since trematodes are common and often abundant in marine and freshwater habitats throughout the world, their effects potentially alter food webs in many systems.
Keywords: parasite, host, gigantism, behavioural modification, stable carbon isotope ratio
1. Introduction
Flip through the pages of any parasitology textbook (e.g. Roberts & Janovy 2000) or even some popular books (Zimmer 2000), and you will find many spectacular examples of how parasites can modify the behaviour or morphology of hosts. Although these effects on individuals are frequently impressive, it is usually unclear whether and, if so, how they impact host populations (but see Ponton et al. 2005). Pathology caused by parasites can directly affect host population dynamics and demographics (e.g. Lafferty 1993; Hudson et al. 1998 and see review by Hudson et al. 2003). However, while behavioural and morphological modification of individual hosts has the potential to significantly affect host demographics, spatial distribution and habitat use (Lauckner 1987; Thomas et al. 1995; Thomas et al. 1999), reports of this at the population level are rare (but see Thomas et al. 1998; Ponton et al. 2005).
Parasites frequently alter host behaviour (Hindsbo 1972; Curtis 1987; Lafferty & Morris 1996; Berdoy et al. 2000; see review by Moore 2002). Parasite-modified behaviour of hosts often results in increased transmission of parasites to new hosts (see examples in Combes 1991; Moore 2002). Sometimes, such parasite-modified behaviour changes the spatial distribution of the host population (e.g. Helluy 1984; Curtis 1987; Thomas et al. 1998; Kuris 2005). Altering the spatial distribution of hosts can affect intra and interspecific interactions of the host population (Thomas et al. 1998; Ponton et al. 2005), and potentially affect the surrounding community (Thomas et al. 1998, 1999; Mouritsen & Poulin 2005).
Parasites can also alter host morphology (e.g. Hoffman 1956; Seidenberg 1973; Despommier 1990). The level of modification ranges from intracellular to the whole organism. Gigantism of snails is an example of a striking morphological modification induced by infection by trematode parasites: some snail species exhibit growth to abnormally large sizes following infection by trematodes (see reviews in Sousa 1983; Sorensen & Minchella 2001). However, gigantism has only been documented in short-lived (less than 4 years) and primarily fresh water snail species. Studies on long-lived (more than 4 years) marine snail species have found that trematodes have either no effect on growth, or stunt growth (Sousa 1983; Sorensen & Minchella 2001). Parasite-induced gigantism could significantly alter the size-structure, resource-use and intraspecific competitive interactions of the host population.
The mud snail, Batillaria cumingi (Batillaria attramentaria), is usually the most abundant macro-invertebrate in salt marshes and mudflats of the northeastern coast of Asia (Hasegawa 2000). The lifespan of this snail is about 6–10 years, which is moderately long for a marine mollusc (Yamada 1982). This snail is frequently infected with trematode flatworm parasites which castrate their hosts (Ito 1957; Shimura & Ito 1980; Harada 1989; Harada & Suguri 1989; Miura et al. 2005). To determine whether these parasites affect the distribution and morphology of their snail hosts, we first examined the distribution of snail size and infection along vertical gradients in the intertidal zone. We then conducted a mark-and-recapture experiment and a transplant experiment to explore further the effects of parasitism on host growth rates and behaviour. Finally, we used stable carbon isotope ratios to investigate whether food use differed between uninfected and infected snails. Infection by a trematode parasite caused a large portion of the snail population to move lower in the intertidal zone, resume growth after maturation and shift resource use. By shifting host population size structure, as well as habitat and resource use, these non-lethal effects of parasitism can alter energy transfer in littoral food webs.
2. Material and methods
(a) Snail size, vertical distribution and parasitism
These studies were conducted during July 2003 at three mudflat sites near Matsushima Bay in Miyagi Prefecture, Japan. One site was at Ohtakamori (OT; 38°21′ N, 141°9′ E), one just inside the mouth of Katsugigaura Bay (MK; 38°21′ N, 141°10′ E), and another in the inner part of Katsugigaura Bay (IK), 1 km from the mouth. At each site, we randomly collected 20 snails from 21–35 sampling stations along elevational gradients perpendicular to the shoreline. To ensure interspersion of stations along elevational isoclines, we used 15 transects spaced more than 10 m apart. At most stations we found densities of snails of over 50 m−2. However, snails were present at only one of 12 stations more than 120 cm below the extreme high water spring tide-line (EHWS). There were two areas where snails were present: one in the high-tide zone (0–80 cm below EHWS) and the other in the low-tide zone (100–140 cm below EHWS). We measured shell length (from the outer margin of the aperture to the apex of the shell) and width (widest point) of all 1360 snails collected, and dissected them using a stereo-microscope to identify trematode infections (Ito 1957; Shimura & Ito 1980; Harada 1989; Harada & Suguri 1989). We used general linear models (GLMs) to examine the influence of infection and elevation on snail size (initially including an interaction term) and to compare snail size in different tidal zones, and a χ2 test to compare proportion of infected and uninfected snails in the upper and lower zones.
(b) Mark-and-recapture experiment and growth rate estimation
At OT, we collected 10 670 snails and marked the margin of the shell aperture with synthetic resin paint. We released the snails on 7 June 2004 and recaptured 1716 of them between 12 and 14 September 2004. We measured shell width and growth rate (as the angle between the margin of the aperture when the snail was released and the new margin when it was recaptured), and dissected them for parasites. Dead snails, snails infected by parasites other than Cercaria batillariae and snails for which we could not measure growth due to worn off paint, were excluded from the analysis, such that 1019 individuals were analysed. We compared the regression slopes of infected and uninfected snails using analysis of covariance (ANCOVA) on the raw data.
(c) Transplant experiment
At OT, we collected 100 snails from both the upper (0–20 cm below EHWS) and lower shore (100–120 cm below EHWS). The 200 snails were marked individually with paint and transplanted to the boundary between the upper and lower zones (90 cm below EHWS, the position is shown in figure 4) on 3 June 2004. We recaptured 60% of the marked snails on 18 June 2004. The vertical and horizontal distances from the release point were recorded for each snail and all snails were dissected for parasites. We compared movements of infected and uninfected snails using the Mann–Whitney U-test.
Figure 4.
Locations of recaptured snails. Open circles indicate uninfected snails, and filled circles indicate snails infected by C. batillariae. The area of each circle is proportional to the number of the snails found at each location. The intersection of dotted lines indicates the release location of marked snails.
(d) Measurements of stable carbon isotope ratios
Stable carbon isotope ratios were examined to test for differences in resource utilization since carbon isotopic composition reflects the source of primary production (Rau et al. 1992). Using a hammer, we cracked the shells of ten infected and ten uninfected snails and dried the snail tissues in glass vials at 60° C for 24 h. To avoid contamination with parasite tissues, we used only the foot tissue of the snails. We then used a 2 : 1 chloroform–methanol mixture to extract lipids for 24 h. Samples in the vials were filtered on glass filter paper (Whatman GF/F) using a vacuum pump to remove lipids and the chloroform–methanol mixture. The samples on the filter paper were placed in vials and dried for 24 h. Stable carbon isotope ratios (δ13C) were measured using an elemental analyser and a mass spectrometer (Finnigan MAT 252). Isotope ratios were expressed as relative values (‰), as defined by the following equation: δ13C=(Rsample−Rstandard)/Rstandard×1000 where R=13C/12C. Peedee belemnite was used as the carbon isotope standard. Isotope ratios between infected and uninfected snails were compared using ANOVA.
(e) Statistical platforms and assumptions
All analyses were performed using JMP v. 5.1 (SAS Institute, Carey, NC) and SYSTAT v. 10 (SPSS Inc. Chicago, IL). For parametric tests, we ensured approximate normality of the residuals and homogeneity of variance by inspecting plots of residuals versus predicted values and normal quantile plots with Lilliefor's curves.
3. Results
(a) Vertical distribution, snail size and parasitism
At all three sites, average shell length significantly increased with depth in the intertidal zone. At our principal study site (OT), the average shell size of the snails dramatically changed between the high-tide zone and the low-tide zone (OT, ANOVA, F1,458=219.38, p<0.001; figure 1a). Results were similar at our other two sites (MK, upper intertidal 22.07±0.16 mm, lower intertidal 27.26±0.23 mm (mean±s.e.), F1,558=381.78, p<0.001; IK, upper intertidal 21.91±0.18 mm, lower intertidal 25.99±0.34 mm, F1,338=133.65, p<0.001).
Figure 1.
Body size and habitat differentiation between uninfected and infected snails in OT. (a) Mean shell length along the vertical gradients. (b) Prevalence of C. batillariae and other trematode species along the vertical gradients. Error bars represent±1 s.e.
We identified two morphotypes of B. cumingi, which we refer to as the large form and the small form. Large forms exhibited shell growth beyond the varix (a thickening of the outer lip of the shell aperture generally associated with maturation and cessation of growth in snails; Vermeji 1993).
We found seven trematode species infecting B. cumingi from these study sites. Cercaria batillariae (Heterophyidae) was the most prevalent species, comprising 87% of the total parasites observed (n=716). The prevalence of parasitism of C. batillariae was much higher in the lower tidal zone than in the upper tidal zone at all 3 sites (n=460, χ2=261.58, p<0.001 for OT, figure 1b; n=560, χ2=264.01, p<0.001 for MK; n=340, χ2=185.51, p<0.001 for IK).
Infected snails were 20–30% longer than uninfected snails at all three sites, even in the small area of distributional overlap (OT, figure 2a, infected 31.49±0.16 mm (mean±s.e.), uninfected 26.52±0.13 mm, analysis of variance (ANOVA), F1,461=566.06, p<0.0001; MK, figure 2b, infected 26.69±0.16 mm, uninfected 20.46±0.20 mm, ANOVA, F1,474=583.97, p<0.0001; IK, figure 2c, infected 26.76±0.23 mm (mean±s.e.), uninfected 21.36±0.20 mm, ANOVA, F1,324=305.21, p<0.0001).
Figure 2.
Mean shell length of uninfected and infected snails along the vertical gradients in all three sites (a) OT (b) MK (c) IK. Error bars represent±1 s.e. Samples of fewer than 5 individuals are not shown.
At all three sites, infected snails were larger than uninfected snails, even after controlling for any effects of elevation on snail size (GLM: OT, F1,460=223.42, p<0.0001; MK, F1,472=81.32, p<0.0001; IK, F1,322=31.39, p<0.0001). The effect of elevation on snail size, after controlling for parasitism, varied between sites and sometimes between infection category. At OT, elevation did not have an effect on snail size after accounting for the effect of parasitism (F1,460=0.0004, p=0.98; figure 2a). At MK, snails increased in size with decreasing elevation (F1,472=56.39, p<0.0001; figure 2b), although uninfected snails increased less than infected snails (effect of interaction, F1,472=11.38, p=0.0008). At IK, although overall snail size increased at lower elevations (F1,322=8.21, p<0.0044; figure 2c), uninfected snails actually decreased in size, while infected snails increased in size (interaction effect, F1,322=19.60, p<0.0001).
(b) Mark-and-recapture experiment and growth rate estimation
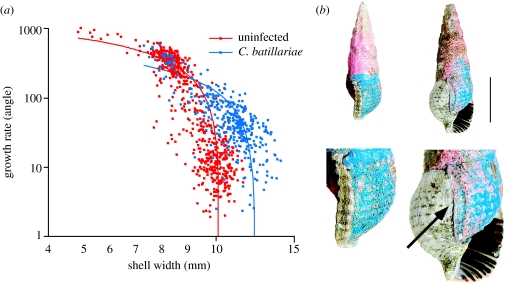
Growth rate of the uninfected snails was relatively high in the juvenile stage, but decreased as the snails grew, eventually ceasing with the formation of a terminal varix at maturity (approximately 10 mm in shell width; figure 3b). However, growth of infected snails continued after formation of the varix and growth rate was significantly higher compared to uninfected snails (ANCOVA, F1,1015=401.43, p<0.001; figure 3a). All examined snails with post-varix growth were infected by C. batillariae (n=233) and uninfected snails never exhibited growth beyond the varix (n=656). The width of the shell of mature uninfected snails was the same as the width of the shell at the varix (i.e. the old maturation point) of infected snails with post-varix growth (10.01±0.06 mm and 9.96±0.06 mm (mean±s.e.), respectively; ANOVA, F1,198=0.31, p=0.58).
Figure 3.
The growth rate of uninfected and infected snails. (a) Relationship between growth rate and shell width in uninfected B. cumingi snails and in those infected by the trematode, C. batillariae. The data are shown as logarithmic dot-plots. The regression lines were calculated with linear regression on raw data for infected and uninfected snails. (b) Examples of marked shells of uninfected (left) and infected (right) snails (scale bar, 1 cm). The snail on the left shows the thickened lip of the aperture (varix) indicating cessation of rapid growth in an uninfected snail. The snail on the right, infected by C. batillariae, exhibits gigantism, rapid growth beyond the varix. The arrow indicates the varix (old maturation point) of the infected snail.
(c) Transplant experiment
After transplanting snails to the boundary between the upper and lower tidal zones, infected snails consistently moved to the lower zone while uninfected snails moved to the upper zone (U-test, n=120, U=25, p<0.001; figure 4).
(d) Measurements of stable carbon isotope ratios
The average stable carbon isotope ratios between uninfected and infected snails (mean±s.e.,−13.84±0.17%, −11.75±0.17%, respectively) were significantly different (ANOVA, F1,18=76.32, p<0.001).
4. Discussion
Our observations and experiments indicate that the larval trematode, C. batillariae, markedly alters both the morphology and the behaviour of individual snails, and consequently affects the demographics and probably the resource use of the snail population.
Our combined findings indicate that parasitism causes gigantism of infected snails, altering the size structure of the snail population. First, each of the 233 snails examined that exhibited growth beyond the varix was infected by C. batillariae. The mean width at the varix of both uninfected snails and infected snails was the same. However, the varix was always terminal in uninfected snails. A terminal varix (i.e. a thickened outer lip of the aperture) indicates that growth of snails ceases when they mature. Only infected snails exhibited growth beyond the varix (figure 3b). Thus, the infected snails were not only parasitically castrated, but it appears that the physiological mechanisms promoting somatic growth were reinstated. Second, our direct measures of growth rates showed that infected snails grew more rapidly at larger sizes than did uninfected snails. An alternate explanation is that the differential growth rates were due to some unmeasured environmental factor that covaried with parasitism and elevation. This is unlikely because further analysis of our data showed that uninfected snails either experienced no increase in size with elevation (OT), scarcely increased with elevation (MK) or actually decreased with elevation (IK). Notably, despite previous study (Sousa 1983; Sorensen & Minchella 2001), to the best of our knowledge gigantism has not previously been reported in long-lived marine prosobranch snails (such as B. cumingi).
Our study also indicated that parasitism shifts the spatial distribution of the snail population lower in the intertidal zone. The transplant experiment demonstrated differential movements of infected and uninfected snails. Parasitized hosts migrated lower, exploiting a habitat not otherwise used by the snail population. Although it is possible that larger snails in the lower zone simply preferred that habitat and were more susceptible or more exposed to infection, we found no uninfected snails in the lower zone at all three sites (figure 1b for OT). The absence of uninfected, susceptible snails suggests that an increased infection rate in the lower zone is an unlikely hypothesis. Additionally, the lack of size variation along the vertical gradients within uninfected or infected snail categories at OT (figure 2a) suggests that age- (size-) dependent migration did not occur. Overall, these results suggest that small uninfected snails inhabited the upper intertidal zone. Only after being parasitized by trematodes did they move lower and resumed growth.
Since infected snails never recover from parasitic castration, growing large would only be beneficial to the parasite (Baudoin 1975). Gigantism enables the parasite to increase its biomass (the larger snails we studied clearly had more soft tissue mass than smaller snails). Increased biomass is associated with an increase in asexual production of its swimming cercarial larval stages (Zischke 1967; Lim & Lie 1969). Further, behavioural modification of hosts by parasites sometimes facilitates transmission of the parasite to their next host (Curtis 1987; Lafferty & Morris 1996; reviewed by Moore 2002). By moving to the lower intertidal zone, infected B. cumingi increase their submergence time, which should increase access of cercariae to their next host (a fish).
The shift in habitat may also facilitate a shift in resource utilization, as suggested by the differences in the average stable carbon isotope ratios between infected and uninfected snails. However, we cannot exclude the possibility that these differences could be caused by physiological changes following parasite infection. Larger B. cumingi also consume more food resources than do smaller snails (Byers 2000). This implies that the larval trematodes, governing the behaviour of the infected component of the snail population, disproportionately alter the flow of carbon through the system. Interestingly, the movement of infected snails away from uninfected snails may alleviate competition with the remaining reproductive snails. Since infected snails are no longer truly snails (they are castrated and will produce only larval trematodes), this would be interspecific competition between uninfected snails and trematodes using the same (snail) phenotype (O'Brien & Van Wyk 1985; Lafferty 1993).
The simultaneously induced migration and gigantism of infected snails integrate to change several basic elements of the snail population. Without understanding these effects, research evaluating the ecology of this abundant snail will be obscured. Further, since trematodes are common and often abundant components of other communities throughout the world (Pechenik et al. 2001; Poulin & Mouritsen 2003; Huspeni et al. 2005), their potentially widespread effects on the ecology and evolution of their hosts deserve attention.
Acknowledgements
We thank S. Ogihara, Y. Sasaki and N. Takahashi for their assistance in sampling of B. cumingi and H. Doi for his assistance in laboratory experiments. We also thank J. Urabe and two anonymous reviewers for providing useful comments on the manuscript. This study was supported by grants from the Japan Society for the Promotion of Science to S.C. and a grant from the US NIH/NSF Ecology of Infectious Disease Program (DEB-0224565) to A.M.K.
References
- Baudoin M. Host castration as a parasitic strategy. Evolution. 1975;29:335–352. doi: 10.1111/j.1558-5646.1975.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Berdoy M, Webster J.P, Macdonald D.W. Fatal attraction in rats infected with Toxoplasma gondii. Proc. R. Soc. B. 2000;267:1591–1594. doi: 10.1098/rspb.2000.1182. 10.1098/rspb.2000.1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers J.E. Competition between two estuarine snails: implications for invasions of exotic species. Ecology. 2000;81:1225–1239. [Google Scholar]
- Combes C. Ethological aspects of parasite transmission. Am. Nat. 1991;138:867–880. 10.1086/285257 [Google Scholar]
- Curtis L.A. Vertical distribution of an estuarine snail altered by a parasite. Science. 1987;235:1509–1511. doi: 10.1126/science.3823901. [DOI] [PubMed] [Google Scholar]
- Despommier D.D. Trichinella spiralis—the worm that would be virus. Parasitol. Today. 1990;6:193–196. doi: 10.1016/0169-4758(90)90355-8. 10.1016/0169-4758(90)90355-8 [DOI] [PubMed] [Google Scholar]
- Harada M. A new cercaria, Cercaria shikokuensis n. sp. (Trematoda), from littoral gastropods in Kanagawa prefecture, Shikoku, Japan. Jap. J. Parasitol. 1989;38:135–138. [Google Scholar]
- Harada M, Suguri S. Surveys on cercariae in brackish water snails in Kagawa prefecture, Shikoku, Japan. Jap. J. Parasitol. 1989;38:388–391. [Google Scholar]
- Hasegawa K. Batillariidae. In: Okutani T, editor. Marine mollusks in Japan. Tokai University Press; Tokyo: 2000. pp. 130–133. [Google Scholar]
- Helluy S. [Host–parasite relations of the trematode Microphallus papillorobustus (Rankin 1940). III Factors involved in the behavioural changes of the Gammarus, intermediate hosts and predator tests.] Ann. Parasitol. Hum. Comp. 1984;59:41–56. [In French.] [PubMed] [Google Scholar]
- Hindsbo O. Effect of Polymorphus (Acanthocephala) on colour and behaviour of Gammarus lacustris. Nature. 1972;238:333. 10.1038/238333a0 [Google Scholar]
- Hoffman G.L. The life cycle of Crassiphiala bulboglossa (Trematoda: Strigeida). Development of the metacercaria and cyst, and effect on the fish hosts. J. Parasitol. 1956;42:435–444. [PubMed] [Google Scholar]
- Hudson P.J, Dobson A.P, Newborn D. Prevention of population cycles by parasite removal. Science. 1998;282:2256–2258. doi: 10.1126/science.282.5397.2256. 10.1126/science.282.5397.2256 [DOI] [PubMed] [Google Scholar]
- Hudson P.J, Dobson A.P, Newborn D. Parasitic worms and population cycles of red grouse. In: Berryman A, editor. Population cycles. Oxford University Press; New York, NY: 2003. pp. 109–129. [Google Scholar]
- Huspeni T.C, Hechinger R.F, Lafferty K.D. Trematode parasites as estuarine indicators: opportunities, applications and comparisons with conventional community approaches. In: Bortone S, editor. Estuarine indicators. CRC Press; Boca Raton, FL: 2005. pp. 297–314. [Google Scholar]
- Ito J. Studies on the brackish water cercaria in Japan 3. Three new cercaria in Tokyo Bay, with a list of Japanese Echinostome cercaria (Trematoda) Jap. J. Med. Sci. Biol. 1957;10:439–453. doi: 10.7883/yoken1952.10.439. [DOI] [PubMed] [Google Scholar]
- Kuris A.M. Trophic transmission of parasites and host behavior modification. Behav. Process. 2005;68:215–217. doi: 10.1016/j.beproc.2004.08.012. 10.1016/j.beproc.2004.08.012 [DOI] [PubMed] [Google Scholar]
- Lafferty K.D. Effects of parasitic castration on growth, reproduction and population dynamics of the marine snail Cerithidea californica. Mar. Ecol. Prog. Ser. 1993;96:229–237. [Google Scholar]
- Lafferty K.D, Morris A.K. Altered behavior of parasitized killifish increases susceptibility to predation by bird final hosts. Ecology. 1996;77:1390–1397. [Google Scholar]
- Lauckner G. Ecological effects of larval trematode infestation on littoral marine invertebrate populations. Int. J. Parasitol. 1987;17:391–398. 10.1016/0020-7519(87)90114-7 [Google Scholar]
- Lim H.K, Lie K.J. Redial population of Paryphostomum segregatum (Trematoda: Echinostomatidae) in the snail Biomphalaria glabrata. Zeit. Parasitenkunde. 1969;32:112–119. doi: 10.1007/BF00259973. [DOI] [PubMed] [Google Scholar]
- Miura O, Kuris A.M, Torchin M.E, Hechinger R.F, Dunhum E.J, Chiba S. Molecular-genetic analyses reveal cryptic species of trematodes in the intertidal gastropod, Batillaria cumingi (Crosse) Int. J. Parasitol. 2005;35:793–801. doi: 10.1016/j.ijpara.2005.02.014. 10.1016/j.ijpara.2005.02.014 [DOI] [PubMed] [Google Scholar]
- Moore J. Oxford University Press; New York, NY: 2002. Parasites and the behavior of animals. [Google Scholar]
- Mouritsen K.N, Poulin R. Parasites boosts biodiversity and changes animal community structure by trait-mediated indirect effects. Oikos. 2005;108:344–350. 10.1111/j.0030-1299.2005.13507.x [Google Scholar]
- O'Brien J, Van Wyk P. Effects of crustacean parasitic castrators (epicaridean isopods and rhizocephalan barnacles) on growth of crustacean hosts. In: Wenner A, editor. Crustacean Issues: factors in adult growth. A.A. Balkema; Rotterdam, The Netherlands: 1985. pp. 191–218. [Google Scholar]
- Pechenik J.A, Fried B, Simpkins H.L. Crepidula fornicata is not a first intermediate host for trematodes: who is? J. Exp. Mar. Biol. Ecol. 2001;261:211–224. doi: 10.1016/s0022-0981(01)00270-2. 10.1016/S0022-0981(01)00270-2 [DOI] [PubMed] [Google Scholar]
- Ponton F, Biron D.G, Joly C, Duneau D, Thomas F. Ecology of populations parasitically modified: a case study from a gammarid (Gammarus insensibilis)–trematode (Microphallus papillorobustus) system. Mar. Ecol. Prog. Ser. 2005;299:205–215. [Google Scholar]
- Poulin R, Mouritsen K.N. Large-scale determinants of trematode infections in intertidal gastropods. Mar. Ecol. Prog. Ser. 2003;254:187–198. [Google Scholar]
- Rau G.H, Ainley D.G, Bengtson J.L, Torres J.J, Hopkins T.L. 15N/14N and 13C/12C in Weddell Sea birds, seals and fish: implications for diet and trophic structure. Mar. Ecol. Prog. Ser. 1992;84:1–8. [Google Scholar]
- Roberts L.S, Janovy J., Jr . McGraw-Hill Companies, Inc; Boston, MA: 2000. Gerald D. Schmidt & Larry S. Roberts' foundations of parasitology. [Google Scholar]
- Seidenberg A.J. Ecology of the acanthocephalan, Acanthocephalus dirus (Van Cleave, 1931), in its intermediate host, Asellus intermedius Forbes (Crustacea: Isopoda) J. Parasitol. 1973;59:957–962. [Google Scholar]
- Shimura S, Ito J. Two new species of marine cercariae from the Japanese intertidal gastropod, Batillaria cumingi (Crosse) Jap. J. Parasitol. 1980;29:369–375. [Google Scholar]
- Sorensen R.E, Minchella D.J. Snail–trematode life history interactions: past trends and future directions. Parasitology. 2001;123:S3–S18. doi: 10.1017/s0031182001007843. 10.1017/S0031182001007843 [DOI] [PubMed] [Google Scholar]
- Sousa W.P. Host life history and the effect of parasitic castration on growth a field study of Cerithidea californica (Gastropoda: Prosobranchia) and its trematode parasites. J. Exp. Mar. Biol. Ecol. 1983;73:273–296. 10.1016/0022-0981(83)90051-5 [Google Scholar]
- Thomas F, Renaud F, Rousset F, Cézilly F, De Meeüs T. Differential mortality of two closely related host species induced by one parasite. Proc. R. Soc. B. 1995;260:349–352. [Google Scholar]
- Thomas F, Renaud F, De Meeüs T, Poulin R. Manipulation of host behaviour by parasites: ecosystem engineering in the intertidal zone? Proc. R. Soc. B. 1998;265:1091–1096. 10.1098/rspb.1998.0403 [Google Scholar]
- Thomas F, Poulin R, De Meeüs T, Guégan J.F, Renaud F. Parasites and ecosystem engineering: what roles could they play? Oikos. 1999;84:167–171. [Google Scholar]
- Vermeji G.J. Princeton University Press; Princeton, NJ: 1993. A natural history of shells. [Google Scholar]
- Yamada S.B. Growth and longevity of the mud snail Batillaria attramentaria. Mar. Biol. 1982;67:187–192. 10.1007/BF00401284 [Google Scholar]
- Zimmer C. Free Press; New York, NY: 2000. Parasite rex: inside the bizarre world of nature's most dangerous creatures. [Google Scholar]
- Zischke J.A. Redial populations of Echinostoma revolutum developing in snails of different sizes. J. Parasitol. 1967;53:1200–1204. [PubMed] [Google Scholar]