Abstract
The lumbering locomotor behaviours of tuataras and salamanders are the best examples of quadrupedal locomotion of early terrestrial vertebrates. We show they use the same walking (out-of-phase) and running (in-phase) patterns of external mechanical energy fluctuations of the centre-of-mass known in fast moving (cursorial) animals. Thus, walking and running centre-of-mass mechanics have been a feature of tetrapods since quadrupedal locomotion emerged over 400 million years ago. When walking, these sprawling animals save external mechanical energy with the same pendular effectiveness observed in cursorial animals. However, unlike cursorial animals (that change footfall patterns and mechanics with speed), tuataras and salamanders use only diagonal couplet gaits and indifferently change from walking to running mechanics with no significant change in total mechanical energy. Thus, the change from walking to running is not related to speed and the advantage of walking versus running is unclear. Furthermore, lumbering mechanics in primitive tetrapods is reflected in having total mechanical energy driven by potential energy (rather than kinetic energy as in cursorial animals) and relative centre-of-mass displacements an order of magnitude greater than cursorial animals. Thus, large vertical displacements associated with lumbering locomotion in primitive tetrapods may preclude their ability to increase speed.
Keywords: locomotion, mechanics, tetrapod evolution, Sphenodon, Ambystoma
1. Introduction
Cursorial mammals, birds and lizards have been shown to use walking and running centre-of-mass (COM) mechanics during locomotion (Cavagna et al. 1977; Farley & Ko 1997). In walking mechanics, the COM arcs upward towards mid-step vaulting over the limbs. Kinetic energy (KE) and gravitational potential energy (PE) of the COM fluctuate out-of-phase, and energy is exchanged like an inverted pendulum. In running mechanics, the COM arcs downward during the step and KE and PE cycle together (in-phase) with their minima at mid-step. It is generally assumed that animals change from walking mechanics to running mechanics when increasing speed (Cavagna et al. 1977; Dickinson et al. 2000).
Similar COM mechanics have also been demonstrated in non-cursorial animals, such as elephants (Hutchinson et al. 2003), penguins (Griffin & Kram 2000), crocodilians (Willey et al. 2004) and frogs (Ahn et al. 2004). Their presence in frogs suggests that both mechanical energy patterns may be a primitive feature of tetrapods. Although frogs appeared about 200 million years ago, they are morphologically derived for hopping and swimming and do not represent the generalized tetrapod condition (Estes & Rieg 1973).
To test the generality of mechanical energy patterns in quadrupedal vertebrates, we studied whole-body locomotor mechanics in the most generalized surviving models for amniotes (tuataras, Sphenodon punctatus) and tetrapods (tiger salamanders, Ambystoma tigrinum). The tuatara (Sphenodontia; Wilkinson & Benton 1996) has changed little morphologically in approximately 225 million years (Apestequia & Novas 2003; Rest et al. 2003). This ‘living fossil’ has long been considered the best living model of the basic amniote morphotype (Romer 1956; Carroll 1988). The tiger salamander, A. tigrinum, represents a good living model for a generalized tetrapod: Ambystoma retain many plesiomorphic features of basal tetrapods (Carroll & Holmes 1980; Jarvik 1980) with a body plan that has remained essentially unchanged for at least 150 million years (Gao & Shubin 2001).
2. Material and methods
(a) Terminology
We distinguish mechanical energy fluctuation patterns of the COM (whole body mechanics) from the patterns of limb placement on the substrate during locomotion (gait). We follow the convention that emerged in the field of locomotor mechanics in the 1970s (Cavagna et al. 1977), terming out-of-phase COM mechanics walking and in-phase COM mechanics running. We use the term gait to refer to its long-held definition as the sequence and pattern of footfalls (e.g. Goiffon & Vincent 1779; Muybridge 1887; Hildebrand 1976). The term trot has a centuries old meaning: a trot is a footfall pattern where diagonal limbs contact the ground more or less together (limb phase of 50±∼10%). However, in the recent biomechanics literature, the term trot is often used as a synonym for running COM mechanics, which is incorrect because: (i) there are many cases of diagonal couplets footfall patterns (trot gaits) that use walking mechanics (e.g. frogs; Ahn et al. 2004; lizards; Farley & Ko 1997; alligators; Willey et al. 2004; crabs; Blickhan & Full 1987; Sphenodon, salamander; this study) and (ii) there are many cases of running mechanics associated with non-trotting gaits (gallop, bound, hopping; e.g. Cavagna et al. 1977; tolt; Biknevicius et al. 2003; human skip; Minetti 1998). Owing to this confusion we use ‘diagonal couplets’ instead of trot.
We studied locomotor mechanics as animals travelled over a force platform at their full range of speeds. Simultaneous kinematic (120 Hz video) and whole-body three-dimensional ground reaction force data (500 Hz sampling) were recorded from three tuataras (0.732–0.747 kg) and six tiger salamanders (0.29–0.63 kg) moving down a 3 m track over an incorporated 0.5 m long force platform. A sandpaper (320 grit) surface provided traction. Body temperatures were 16–18 °C during data collection: tuataras were housed and run at 16–18 °C; salamanders were housed at 16–18 °C and moved momentarily to 19–20 °C room/track temperature during runs.
Tuataras took one to two steps and salamanders approximately five steps while their whole body was over the entire force platform. Both species only used diagonal couplets footfall patterns; therefore, a ‘step’ was the time from the initial impact of one diagonal couplet until the impact of the opposite couplet. Force data from individual steps were recorded, scaled and converted into mechanical energy data as in previous research (Parchman et al. 2003). Forces were integrated to calculate COM velocities, accelerations and fluctuations in gravitational PE, KE and total mechanical energy (TME) of the COM (details in Parchman et al. 2003). KE was the sum of fore-aft, mediolateral and vertical kinetic energies of the COM. The phase shift between fluctuations in PE and KE was used to determine whether the animal was walking (180±45°) or running (0±45°) during each step (Cavagna et al. 1977; Ahn et al. 2004). Pendular effectiveness was estimated as per cent recovery of external mechanical energy following Willey et al. (2004).
Footfall patterns to quantify gaits and synchronize ground reaction forces were quantified from video recordings (downloaded with Studio DV and digitized with APAS). A camera (JVC GRL-9800) mounted above the centre of the force plate and angled mirrors on either side of the force plate allowed simultaneous filming of dorsal and lateral views (and footfall patterns). Lines at 0.12 m intervals along the platform were used as the frame of reference for calculating velocity of the rostral tip of the animal. We calculated mean velocity over the entire force plate and only trials with all 0.12 m interval velocities ±25% of the mean platform velocity were used. Numerous trials were recorded (i) to sample the fullest range of velocities (and, therefore, mechanical patterns and gaits) that the animals would use and (ii) to maximize the number of steps at more or less constant velocity (±25%). Gaits (footfall patterns) for each step were described by plotting duty factor (percentage of the stride that the reference hindlimb was on the ground) versus limb phase (duration between footfalls of the reference hindlimb and ipsilateral forelimb, relative to stride duration) following Hildebrand (1976). All procedures followed approved animal care and use protocols.
3. Results and discussion
(a) Walking and running mechanics are primitive for tetrapods
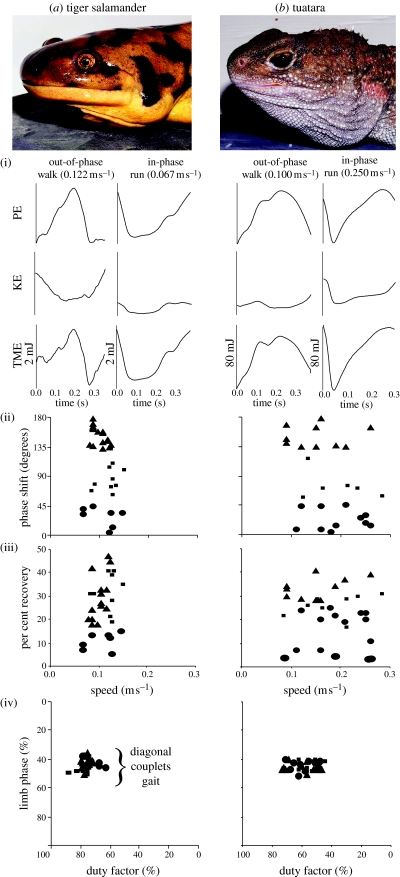
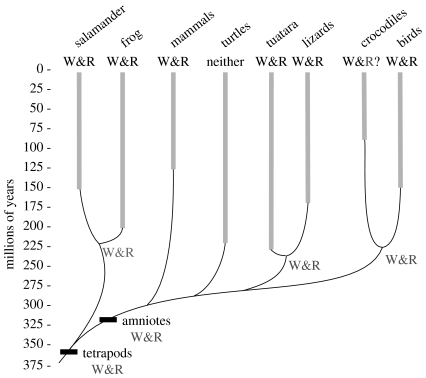
Both tuataras and tiger salamanders utilize walking and running locomotor mechanics (figure 1a(i)(ii),b(i)(ii)). Mapping the occurrence of COM mechanics on a tetrapod phylogeny (Gauthier et al. 1988; figure 2) reveals that both walking and running mechanics are present in every tetrapod clade except turtles. Turtles are unusual in being the only tetrapods that do not use inverted pendular mechanics (Zani et al. 2005). Given that animals virtually identical to our extant tetrapod models have been around since the Late Cretaceous (figure 2), it appears that walking and running mechanics are ancient correlates of quadrupedal locomotion. In fact, the discovery of both modes in salamanders and tuataras suggests that walking and running mechanics have been a correlate of quadrupedal terrestrial locomotion since the first vertebrates moved onto land in the Permian.
Figure 1.
Biomechanics and gaits of primitive tetrapods (a) the tiger salamander and (b) the tuatara. (i) Fluctuations in gravitational potential (PE) and kinetic energy (KE) and total mechanical energy (TME) of the COM showing out-of-phase (walking) and in-phase (running) steps (mJ, millijoules). Each curve illustrates the profile for a single step. (ii) Phase shifts between minima of KE and PE indicating walking (solid triangles, 135–180°), running (solid circles, 0–45°) and intermediate (solid squares, 45–135°) steps. (iii) Inverted pendulum recovery of external mechanical energy (per cent recovery). (iv) Both species use walking and running mechanics and diagonal couplets footfall patterns at all speeds.
Figure 2.
Evolution of walking (W) and running (R) locomotor mechanics in tetrapods. Grey bars indicate the earliest known appearance of animals considered morphologically similar to extant forms (Estes & Rieg 1973; Rest et al. 2003; Gao & Shubin 2001; Parchman et al. 2003; Ji et al. 2002). Nodes indicate estimated divergence times of the major tetrapod groups (Rest et al. 2003). Mechanical patterns of COM movements known for extant forms (Cavagna et al. 1977; Farley & Ko 1997; Ahn et al. 2004; Willey et al. 2004; figure 1) are mapped as walking, W (out-of-phase kinetic and potential energy, and running, R (in-phase kinetic and potential energy), and parsimony was used to infer nodal character states (W&R). R?, running mechanics assumed based on galloping gait in crocodilians (Renous et al. 2002).
Terrestrial locomotion has also evolved in each of the major arthropod groups. Locomotor mechanics, although limited, have been studied in Chelicerata (harvestmen, Sensenig & Shultz in press), Myriapoda (centipedes, Anderson et al. 2000), Hexapoda (cockroaches, Full & Tu 1990) and Crustacea (ghost crabs, Blickhan & Full 1987). All of these use running mechanics, but only the Crustacea use both walking and running mechanics. Arthropods have repeatedly shifted to terrestrial locomotion, but only one group appears to have evolved walking and running mechanics. Therefore, the use of both mechanical energy patterns has evolved independently in the Crustacea and Tetrapoda.
(b) The biological role of locomotor mechanics in early tetrapods
The ancient appearance of walking mechanics may be related to saving energy during locomotion. Pendular exchange during walking reduces the amount of external mechanical energy required to move the COM at slow to moderate speeds (Cavagna et al. 1977; Full 1991). Salamanders and tuataras employed pendular exchange during walking steps (figure 1a(iii),b(iii)). In these species, per cent external mechanical energy recoveries averaged 26.4 and 34.5%, respectively, similar to mean pendular savings in walking frogs (32%; Ahn et al. 2004), lizards (approx. 25%; Farley & Ko 1997), alligators (20%; Willey et al. 2004) and quadrupedal mammals (rams, approx. 30%; monkeys, approx. 30%; Cavagna et al. 1977). Thus, there appears to be an energy saving role to walking mechanics in early tetrapods.
Running mechanics are also associated with energy savings via elastic energy storage in biological spring elements of the limbs and axial column when animals ‘bounce’ during running strides (Alexander 1988; Biewener 2003). However, spring savings are strongly size dependent (Biewener et al. 1981; Pollock & Shadwick 1994) and appear to be limited to larger animals (approx. above 5 kg). Tendons have to be a certain size and shape relative to the loads they transmit before they can store sufficient amounts of elastic energy useful for cyclic quadrupedal locomotion (Biewener 2003). We have no evidence that spring savings are unrealized; however, tuataras and salamanders are in the size range where appreciable elastic energy recovery is not expected (Biewener 2003). Thus, although they clearly use running mechanics we are left to wonder why.
An emerging tenet of locomotor research is that legged animals change to running mechanics as they increase speed (Dickinson et al. 2000). This is true for cursorial mammals that are widely known to transition from footfall patterns (four beat gaits, singlefoot gaits) with walking mechanics, to diagonal couplets gaits with running mechanics, to the galloping gait with a combination of both mechanical patterns (Cavagna et al. 1977; Hoyt & Taylor 1981; Minetti et al. 1999; Biewener 2003). In addition, the ghost crab (Full & Tu 1990) and some lizards (McElroy & Reilly 2005) change both gaits and mechanics with speed.
In contrast, the salamanders and tuataras used only diagonal couplets gaits with both walking (mean limb phase, 42.9 and 45.8%, respectively) and running (44.0 and 44.8%, respectively) mechanics (figure 1a(iv),b(iv)) over the same narrow range of slow speeds (figure 1a(ii),b(ii)). Thus, the salamander and tuatara do not increase speed much or show the speed-related changes in gait or mechanics observed in faster animals. A narrow speed range and a lack of speed effects have also been shown for quadrupedal locomotion in frogs (Ahn et al. 2004). The natural histories of amphibians and tuataras (and by inference early tetrapods) show that these lumbering species use primarily slow locomotion. Accordingly, our data show they take advantage of pendular savings during walking, but have not adapted running mechanics for increasing speed. Therefore, the general tenet that gait and mechanics change with speed appears to be limited to cursorial animals that shift to faster speeds.
(c) Why walk and run at the same speed?
When animals shift to running mechanics with increasing speed there is an increase in TME of the COM (Full 1991; Minetti et al. 1999). However, TME was not significantly different between walking and running in salamanders (mean±s.e.: W, 1.59±0.16 mJ; R, 2.06±0.40 mJ; p=0.215) or tuataras (W, 85.8±3.5 mJ; R, 83.5±7.2 mJ; p=0.814). We believe this is due to the fact that these animals are not really increasing speed. However, part of the reason TME is observed to increase in animals that do increase speed is because they change gaits (Minetti et al. 1999): tuataras and salamanders did not change gaits. This is the first study to show that walking and running mechanics are used indifferently within a gait across the animal's full range of locomotor speeds with little mechanical energetic consequence. If walking and running involves the same TME, we are left with the possibility that both walking and running mechanics have little functional relevance and are simply by-products (mechanical spandrels; sensu Gould & Lewontin 1979) of lumbering locomotion in tetrapods.
(d) The mechanics of lumbering
In terms of general perceptions, some animals appear to move gracefully (horse) while others lumber (salamander). To quantify this perception, we compared the relative amount of energy used to move the COM forward (KE) versus up and down (PE) during locomotion. Intuitively, a graceful animal will exhibit a smaller fluctuation in PE relative to KE compared to a less graceful animal. Thus, in graceful animals KE should drive the TME. We predicted that lumbering animals should have relatively greater vertical displacements of the COM and thus PE will drive TME. To test this hypothesis, we compared the relative size of PE and KE curves in a variety of terrestrial animals (horses, rams, dogs, monkeys, humans, opossums (Cavagna et al. 1977; Minetti et al. 1999; Parchman et al. 2003; Griffin et al. 2004), cockroaches (Full & Tu 1990), crabs (Blickhan & Full 1987), harvestmen (Sensenig & Shultz in press), alligators (Willey et al. 2004), lizards (Farley & Ko 1997), frogs (Ahn et al. 2004), tuataras and salamanders (this study)). We found that KE is much greater than or, at most, equal to PE (and drives TME) in all species except frogs, alligators, tuataras and salamanders. In these species, PE is greater than KE (and drives TME) in both walking and running. For example, alligators, tuataras, frogs and salamanders exhibit PE 1.6–4.0 times greater than KE in walking, and 1.7–2.0 times greater in running. In addition, vertical displacements in these lumbering species are an order of magnitude greater relative to hip height than in cursorial animals (table 1). Therefore, our perception of lumbering locomotion in these species is manifested in relatively large vertical fluctuations of the COM, in which PE drives the total external mechanical energy. Thus, large vertical displacements in early lumbering tetrapods may impede their ability to increase speed.
Table 1.
Centre-of-mass (COM) displacements in relation to hip height in tetrapod locomotion. (Note that the lumbering species have well over an order of magnitude greater relative displacements of the COM.)
| species | W or R | vertical displacement COM (mm) | hip height (mm) | relative displacement (vertical displacement/hip height) | reference |
|---|---|---|---|---|---|
| Ambystoma | W | 5.4 | 10 | 0.54 | this study |
| R | 3.7 | 10 | 0.37 | this study | |
| tuatara | W | 10.9 | 25 | 0.44 | this study |
| R | 7.9 | 25 | 0.32 | this study | |
| Alligator | W | 21 | 100 | 0.21 | Willey et al. (2004) |
| Monodelphis | R | 3.4 | 51 | 0.07 | Parchman et al. (2003) |
| rat | R | 5 | 65 | 0.08 | Farley et al. (1993) |
| dog | W | 10 | 610 | 0.02 | Griffin et al. (2004) |
| R | 30 | 490 | 0.06 | Jayes & Alexander (1978) | |
| R | 40 | 500 | 0.08 | Farley et al. (1993) | |
| horse | R | 38 | 750 | 0.05 | Farley et al. (1993) |
Activity temperature and metabolism may also influence the ability to increase speed. Amphibians and the tuatara have variable field activity temperatures between 10 and 26 °C (Hutchison & Dupre 1992; Cartland & Grimmond 1994), whereas lizards and mammals maintain high body temperatures when active (30–41 °C; Schmidt-Nielsen 1990). Thus, it appears that temperature effects (on, for example, muscle function and aerobic capacity) may partially explain the lack of speed effects in primitive tetrapods.
In salamanders, the cost of transport (J kg−1 m−1) is the lowest recorded among animals from insects to lizards (Gatten et al. 1992) and tuataras exhibit maximum O2 consumption rates during activity less than half that of tiger salamanders (Bennett 1982). Low aerobic capacities are believed to be adaptations for activity at low body temperatures (Bennett 1982). However, they appear to constrain the locomotor speed and speed ranges used in these cold adapted species, and thus may limit their ability to take full advantage of energy saving mechanics. The evolution of higher activity temperature may go hand in hand with higher metabolic rate, size (thus springs), posture (thus hip height) and relative COM fluctuations (a switch to KE driven TME) in the evolution of cursoriality to provide ecologically relevant energy savings via walking and running mechanics. Clearly, a broader perspective in terms of locomotor data (reporting hip height and gaits, speed ranges), model species and data on the contributions of springs in small animals is needed to begin to understand how COM mechanics have evolved with the need for speed in tetrapods.
Acknowledgments
The support of the US National Science Foundation, the Ohio University Research Challenge Program and the Department of Herpetology of the Toledo Zoological Society is greatly appreciated.
References
- Ahn A.N, Furrow E, Biewener A.A. Walking and running in the red-legged running frog, Kassina maculata. J. Exp. Biol. 2004;207:399–410. doi: 10.1242/jeb.00761. doi:10.1242/jeb.00761 [DOI] [PubMed] [Google Scholar]
- Alexander R.M. Cambridge University Press; Cambridge, UK: 1988. Elastic mechanisms in animal movement. [Google Scholar]
- Anderson B.D, Full R.J, Garcia M. A spring-mass model of centipede locomotion. Am. Zool. 2000;40:928. [Google Scholar]
- Apestequia S, Novas F.E. Large Cretaceous sphenodontian from Patagonia provides insight into lepidosaur evolution in Gondwana. Nature. 2003;425:609–612. doi: 10.1038/nature01995. [DOI] [PubMed] [Google Scholar]
- Bennett A.F. The energetics of reptilian activity. In: Gans C, Pough F.H, editors. Biology of the reptilia. vol. 13. Academic Press; New York, NY: 1982. pp. 155–199. [Google Scholar]
- Biewener A.A. Oxford University Press; Oxford, UK: 2003. Animal locomotion. [Google Scholar]
- Biewener A, Alexander R.McN, Heglund N.C. Elastic energy storage in the hopping of kangaroos (Macropodidae) J. Zool. Lond. 1981;195:369–383. [Google Scholar]
- Biknevicius A.R, Mullineaux D.R, Clayton H.M. Taking the walk for a run: locomotor mechanics of lateral sequence singlefoot gaits. Intergr. Comp. Biol. 2003;43:988. [Google Scholar]
- Blickhan R, Full R.J. Locomotion energetics of the Ghost Crab II. Mechanics of centre of mass during walking and running. J. Exp. Biol. 1987;130:155–174. [Google Scholar]
- Carroll R.L. W.H. Freeman; New York, NY: 1988. Vertebrate paleontology and evolution. [Google Scholar]
- Carroll R.L, Holmes R. The skull and jaw musculature as guides to the ancestry of salamanders. J. Linn. Soc. 1980;68:1–40. [Google Scholar]
- Cartland L.K, Grimmond N.M. The effect of temperature on the metabolism of juvenile tuatara, Sphenodon punctatus. N. Z. J. Zool. 1994;21:373–378. [Google Scholar]
- Cavagna G.A, Heglund N.C, Taylor C.R. Mechanical work in terrestrial locomotion: two basic mechanisms for minimizing energy expenditure. Am. J. Physiol. 1977;233:243–261. doi: 10.1152/ajpregu.1977.233.5.R243. [DOI] [PubMed] [Google Scholar]
- Dickinson M.H, Farley C.T, Full R.J, Koehl M.A.R, Kram R, Lehman S. How animals move: an integrative view. Science. 2000;288:100–106. doi: 10.1126/science.288.5463.100. doi:10.1126/science.288.5463.100 [DOI] [PubMed] [Google Scholar]
- Estes R, Rieg O. The early fossil record of frogs: a review of the evidence. In: Vial J, editor. Evolutionary biology of the anurans. University of Missouri Press; Columbia, MO: 1973. pp. 11–63. [Google Scholar]
- Farley C.T, Ko T.C. Mechanics of locomotion in lizards. J. Exp. Biol. 1997;200:2177–2188. doi: 10.1242/jeb.200.16.2177. [DOI] [PubMed] [Google Scholar]
- Farley C.T, Glasheen J, McMahon T.A. Running springs: speed and animal size. J. Exp. Biol. 1993;185:71–86. doi: 10.1242/jeb.185.1.71. [DOI] [PubMed] [Google Scholar]
- Full R.J. The concepts of efficiency and economy in land locomotion. In: Blake R.W, editor. Efficiency and economy in animal physiology. Cambridge University Press; Cambridge, UK: 1991. pp. 97–131. [Google Scholar]
- Full R.J, Tu M.S. Mechanics of six-legged runners. J. Exp. Biol. 1990;148:129–146. doi: 10.1242/jeb.148.1.129. [DOI] [PubMed] [Google Scholar]
- Gao K, Shubin N.H. Late Jurassic salamanders from northern China. Nature. 2001;410:574–577. doi: 10.1038/35069051. doi:10.1038/35069051 [DOI] [PubMed] [Google Scholar]
- Gatten R.E, Jr, Miller K, Full R.J. Energetics at rest and during locomotion. In: Feder M.E, Burggren W.W, editors. Environmental physiology of the amphibians. University of Chicago Press; Chicago, IL: 1992. pp. 314–377. [Google Scholar]
- Gauthier J.A, Kluge G, Rowe T. Amniote phylogeny and the importance of fossils. Cladistics. 1988;4:105–209. doi: 10.1111/j.1096-0031.1988.tb00514.x. [DOI] [PubMed] [Google Scholar]
- Goiffon T, Vincent G. Alfort; Paris, France: 1779. Memoire artificeille des preincipes realtifs a la fide le representation des animaux tant en peiture, qu'en sculpture: I. Partie concernant le cheval Alfort. [Google Scholar]
- Gould S.J, Lewontin R.C. The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proc. R. Soc. B. 1979;205:581–598. doi: 10.1098/rspb.1979.0086. [DOI] [PubMed] [Google Scholar]
- Griffin T.M, Kram R. Penguin waddling is not wasteful. Nature. 2000;408:929–930. doi: 10.1038/35050167. doi:10.1038/35050167 [DOI] [PubMed] [Google Scholar]
- Griffin T.M, Main R.P, Farley C.T. Biomechanics of quadrupedal walking: how do four-legged animals achieve inverted pendulum-like movements. J. Exp. Biol. 2004;207:3545–3558. doi: 10.1242/jeb.01177. doi:10.1242/jeb.01177 [DOI] [PubMed] [Google Scholar]
- Hildebrand M. Analysis of tetrapod gaits: general considerations and symmetrical gaits. In: Herman R.M, Grillner S, Stuart D.G, editors. Neural control of locomotion. vol. 18. Plenum Press; New York, NY: 1976. pp. 203–236. [Google Scholar]
- Hoyt D.L, Taylor C.R. Gait and the energetics of locomotion in horses. Science. 1981;292:239–240. [Google Scholar]
- Hutchison V.H, Dupré R.K. Thermoregulation. In: Feder M.E, Burrgren W.W, editors. Environmental biology of the amphibians. University of Chicago Press; Chicago, IL: 1992. pp. 206–249. [Google Scholar]
- Hutchinson J.R, Famini D, Lair R, Kram R. Biomechanics: are fast-moving elephants really running? Nature. 2003;422:493–494. doi: 10.1038/422493a. doi:10.1038/422493a [DOI] [PubMed] [Google Scholar]
- Jarvik E. Academic Press; London, UK: 1980. Basic structure and evolution of vertebrates. [Google Scholar]
- Jayes A.S, Alexander R.M. Mechanics of locomotion of dogs (Canis familiaris) and sheep (Ovis aries) J. Zool. Lond. 1978;185:289–308. doi: 10.1111/j.1469-7998.1978.tb03334.x. [DOI] [PubMed] [Google Scholar]
- Ji Q, Luo Z.X, Yuan C.X, Wible J.R, Zhang J.P, Georgi J.A. The earliest known eutherian mammal. Nature. 2002;416:816–822. doi: 10.1038/416816a. doi:10.1038/416816a [DOI] [PubMed] [Google Scholar]
- McElroy E.J, Reilly S.M. Preliminary analyses of locomotor consequences of foraging mode in lizards. Integr. Comp. Biol. 2005;44:600. [Google Scholar]
- Minetti A.E. The biomechanics of skipping gaits: a third locomotion paradigm. Proc. R. Soc. B. 1998;265:1227–1235. doi: 10.1098/rspb.1998.0424. doi:10.1098/rspb.1998.0424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minetti A.E, Ardigo L.P, Reinach E, Saibene F. The relationship between mechanical work and energy expenditure of locomotion in horses. J. Exp. Biol. 1999;202:2329–2338. doi: 10.1242/jeb.202.17.2329. [DOI] [PubMed] [Google Scholar]
- Muybridge E. University of Philadelphia Press; Philadelphia, PA: 1887. Animal locomotion. [Google Scholar]
- Parchman A.J, Reilly S.M, Biknevicius A.R. Whole-body mechanics and gaits in the gray short-tailed opossum, Monodelphis domestica: kinetic and kinematic patterns of locomotion in a semi-erect mammal. J. Exp. Biol. 2003;206:1379–1388. doi: 10.1242/jeb.00267. doi:10.1242/jeb.00267 [DOI] [PubMed] [Google Scholar]
- Pollock C.M, Shadwick R.E. Allometry of muscle, tendon and elastic storage capacity in mammals. Am. J. Physiol. 1994;266:R1022–R1031. doi: 10.1152/ajpregu.1994.266.3.R1022. [DOI] [PubMed] [Google Scholar]
- Renous S, Gasc J.-P, Bels V.L, Wicker R. Asymmetrical gaits of juvenile Crocodylus johnstoni, galloping Australian crocodiles. J. Zool. Lond. 2002;256:311–325. [Google Scholar]
- Rest J.S, Ast J.C, Austin C.C, Waddell P.J, Tibbetts E.A, Hay J.M, Mindell D.P. Molecular systematics of primary reptilian lineages and the tuatara mitochondrial genome. Mol. Phylogenet. Evol. 2003;29:289–297. doi: 10.1016/s1055-7903(03)00108-8. doi:10.1016/S1055-7903(03)00108-8 [DOI] [PubMed] [Google Scholar]
- Romer A.S. University of Chicago Press; Chicago, IL: 1956. Osteology of the reptiles. [Google Scholar]
- Schmidt-Nielsen K. Cambridge University Press; Cambridge, UK: 1990. Animal physiology. [Google Scholar]
- Sensenig A.T, Shultz J.W. Kinematics and inferred kinetics of locomotion in the harvestman Leiobunum vittatum (Opiliones) Am. Arachnol. 2004;68:17. [Google Scholar]
- Wilkinson M, Benton M.J. Sphenodontid phylogeny and the problems of multiple trees. Phil. Trans. R. Soc. B. 1996;351:1–16. [Google Scholar]
- Willey J.S, Biknevicius A.R, Reilly S.M, Earls K.D. The tale of the tail: limb function and locomotor mechanics in Alligator mississippiensis. J. Exp. Biol. 2004;207:553–563. doi: 10.1242/jeb.00774. doi:10.1242/jeb.00774 [DOI] [PubMed] [Google Scholar]
- Zani P.A, Gottschall J.S, Kram R. Giant tortoises walk without inverted pendulum mechanical-energy exchange. J. Exp. Biol. 2005;208:1489–1494. doi: 10.1242/jeb.01554. doi:10.1242/jeb.01554 [DOI] [PubMed] [Google Scholar]