Abstract
Many of the seemingly bizarre animal behaviours can be understood only by acknowledging the power of sex to shape evolution. A case in point is the so-called love-dart that some terrestrial molluscs shoot at their prospective sexual partners. Given that the likelihood of copulation is not different after solid hits than after complete misses, why do these suitors act so violently towards their chosen mates? Previously, it was shown that successful dart shooting enhances paternity. We conducted an experiment to determine whether the dart achieves its effect by a purely mechanical action or by transferring a bioactive substance. We found that injections of mucus from a gland associated with the dart more than doubled paternity relative to injections of saline. These results support the hypothesis that the dart transfers a substance capable of reconfiguring the spermatophore-receiving organs. While dart shooting probably evolved as the result of sperm competition, a role for cryptic female choice cannot be excluded. Our results imply that if cryptic female choice is operating in this system, it is likely to be based on the properties of the mucus and not on properties of the dart itself. Since we also found evidence of early-male sperm precedence, we conclude that snails can optimize their reproductive success by mating with virgins and shooting their darts accurately.
Keywords: sexual selection, sperm competition, sperm precedence, darts, Cantareus aspersus, Helix aspersa
1. Introduction
In animal species that mate promiscuously, store sperm and fertilize internally, the sperm from two or more donors usually compete for access to ova (Wigby & Chapman 2004). The phenomenon of sperm competition has been studied mostly in insects and birds, with much less attention given to other taxa. However, all the prerequisites for sperm competition are present in the mating systems of gastropod molluscs (Baur 1998), and within this group, sperm precedence and dart shooting are well-documented manifestations of sperm competition. Sperm precedence refers to the reproductive advantage enjoyed by a sperm donor solely by virtue of its mating either before or after its competitor (Wigby & Chapman 2004). Dart shooting is a bizarre behavioural component of courtship whereby snails attempt to drive a hard, sharp object into their prospective partners. It occurs in only about eight families of pulmonate gastropods, from a total of about 60 (Koene & Schulenburg 2005; Davison et al. 2005).
This work focuses on dart shooting in the brown garden snail Cantareus aspersus (formerly, Helix aspersa). These snails are hermaphrodites, and both members of a mating pair function simultaneously as both males and females in all encounters (Adamo & Chase 1988; Chase & Vaga 2006). Towards the end of courtship, each animal attempts to strike the other with a sharp and calcareous dart. The behaviour is rapid and forceful, sometimes producing dramatic results (figure 1). However, in about one-half of all shootings, the dart either misses its intended target altogether or it strikes only a glancing blow, after which it falls to the ground. Importantly, the outcomes of the dart shots affect neither the probability that the courtship will culminate in copulation nor the size of the ensuing sperm donation (Adamo & Chase 1988; Chase & Vaga 2006). Instead, previous work has shown that successful dart shooting more than doubles the number of donated sperm that are stored by the recipient (Rogers & Chase 2001), and it significantly increases relative paternity when a successful dart shooter competes with an unsuccessful shooter (Landolfa et al. 2001; Rogers & Chase 2002).
Figure 1.

Two snails, Cantareus aspersus, in reciprocal copulation. During courtship, the snail at the top of the photograph shot a dart through the head of the snail at the bottom (pointed white object at left).
Our goal is to understand how the dart achieves its effect on paternity. We begin with the knowledge that massive sperm digestion occurs in the female tract of C. aspersus. On an average, about 99.98% of transferred sperm are digested in the bursa copulatrix before they reach the spermathecal sacs (Rogers & Chase 2001). Most of the digestion probably occurs at the junction of the bursa tract and the bursa tract diverticulum, because it is here that enzymes from the bursa copulatrix encounter allosperm en route to the spermatheca (Lind 1973). We believe that this junction is the ultimate site of the dart's action. By attenuating sperm digestion at this point, the dart can increase the number of sperm that are safely stored, thus increasing the number of the shooter's sperm that will be selected in a lottery that precedes fertilization.
The specific mechanism by which the dart achieves its effect could depend only on breaking the skin of the recipient. One plausible scenario would have the rupture excite mechanosensory neurons that would, in turn, excite a nervous pathway capable of contracting the radial muscles surrounding the bursa tract. The result would be to constrain the flow of digestive enzymes moving towards the allosperm, thereby allowing more sperm to travel safely to the spermathecal sacs. Comparable sensory–motor systems are common in gastropod molluscs (Chase 2002).
An alternative hypothesis for the dart's mechanism of action is based on the fact that all snails that are known to possess a calcareous dart also possess an associated mucus gland (Koene & Schulenburg 2005). The mucus is secreted into the passageway through which the dart passes on its way out of the shooting animal. While it is possible that the sole function of the mucus is to lubricate the passage, the fluted morphology of the dart of C. aspersus, together with structural trends in the dart's inferred evolution (Koene & Schulenburg 2005), suggest that it is adapted to carry a maximal load of mucus. Adamo & Chase (1996) speculated that the dart acts not by a mechanical action, but by injecting mucus into the mating partner. Later, Koene & Chase (1998) found, from experiments performed in vitro, that the dart's mucus does, in fact, contract radial muscles at the critical junction of the bursa tract and the bursa tract diverticulum. In the present study, we test the hypothesis that the dart increases paternity by injecting mucus.
We arranged for an ultimate mother to be successively inseminated by two potential fathers, neither of which shot a dart because the darts and glands had been surgically removed prior to any mating. As a substitute for dart shooting, we stabbed the future mother with a hypodermic needle. A homogenate of the dart gland mucus was injected in association with one of the two sperm donations (providing both mechanical and chemical stimulation), and a saline solution was injected in association with the other sperm donation (providing only mechanical stimulation). After egg laying, we genotyped the mother, the two competing sperm donors and a sample of the offspring to assay paternity.
2. Material and methods
Mature snails were collected in the field. Prior to any mating trials, future mothers were individually isolated for 61.5±17.6 (s.d.) days to prevent the receipt of allosperm; future fathers were isolated for more than 21 days to ensure full autosperm stores. First, the dart sacs and mucus glands were removed by surgery after injecting the snails with an anaesthetic consisting of 2% MgCl2 and 0.02% succinylcholine chloride in saline. The survival rate from the surgeries was 94.4%. The operated animals displayed all normal mating behaviours, including the stereotypic dart-shooting posture (Adamo & Chase 1988).
Each individual in this species possesses a pair of mucus glands. These were removed during surgeries, pooled and homogenized. After centrifuging for 2 min at 5000 g, the supernatant was diluted with a saline solution (Kerkut & Meech 1996), then divided into aliquots that were frozen until use. Each 500 μl injection contained material from approximately 1.33 glands (compared to the two glands in each individual). This dosage was established in preliminary trials, as the highest dosage that did not cause adverse reactions. Thereafter, to estimate the amount of mucus present in each injection, we extruded mucus under pressure from a sample of 20 glands (10 animals). We determined that each injection contained approximately 5.9 mg of mucus, or 2.9 times the amount of mucus that is normally carried on the dart (Chung 1986).
Matings were arranged such that each future mother received sperm from two donors. To optimize the genetic assay of paternity, the two donors were selected from different populations living in distinct geographic locations near Monterey, California. When a snail displayed the dart-shooting posture, its partner (a future mother) was stabbed in the mantle column with a 26 gauge hypodermic needle. In one of its two matings, the future mother received an injection of 500 μl saline and in the other mating the future mother received an injection of 500 μl mucus homogenate. The order of injection types was balanced with respect to mating order.
Seven days following the second mating of a future mother, the snail was induced to lay eggs by provision of a moist and friable substrate. Thirty offspring were indiscriminately selected from each clutch for genotyping. DNA was isolated by phenol extraction as described by Teshima et al. (2003). Paternities were assigned using six highly polymorphic microsatellite markers (Guiller et al. 2000): Ha2, Ha5, Ha6, Ha8, Ha9 and Ha10. Labelled PCR products were analysed at the Genome Québec Innovation Centre using a 3730xl ABI DNA Analyser and GeneMapper 3.7 software. The identities of all 1254 genotypes were coded before assigning paternities to conceal the experimental groups to which the individuals belonged.
Reproductive success was measured by estimating the proportions of offspring in each clutch that were sired by each type of sperm donor, i.e. first sperm donors (P1), second sperm donors (P2) and unidentified (‘outside’) sperm donors. General linear modelling (GLM) was run on SPSS v. 11.0 after normalizing distributions using the arcsine square root transformation. Categorical factors were injection type, mating order and geographical origin. Covariates were maternal shell volume and the difference in paternal shell volumes (first donor minus second donor). Shell measurements (in millimetres) were used to estimate shell volumes from the formula, volume (cm3)=(length×width×height) (4.0×10−4)−0.3129 (Chase & Vaga 2006). In addition, the interaction between injection type and mating order was included in some models. Models were run multiple times using all possible combinations of predictor variables to obtain the most parsimonious model. For the overall analysis (first and second donors combined, N=76), the only independent variable present in the most parsimonious model was the injection type. When the first donor was analysed separately (N=38), the most parsimonious model included injection type as well as maternal shell volume. For the analysis of the second donor (N=38), the most parsimonious model included only injection type. Geographical origin was excluded from all models as a non-significant variable. The mean values are reported with standard errors.
3. Results
Over 250 matings were observed, resulting in 55 snails that received injections in association with two successive matings (55 ‘triads’). These 55 snails yielded 43 viable clutches of offspring. Two parental DNA samples were defective, two clutches could not be unambiguously genotyped and one additional triad was discarded after random selection to yield a final sample of 38 triads, of which 19 had mucus injected during the first mating and 19 had saline injected in the first mating.
An interpretable sequence length was obtained at 85% of the probed microsatellite loci, with observed heterozygosities in the range 0.69–0.89. To evaluate the power of the genetic assay, the parental exclusion probability (Jamieson & Taylor 1997) was calculated from the allele frequencies of 112 adult snails in the experimental population. The combined exclusion probability from all of the six loci is 0.9985, indicating a very sensitive assay.
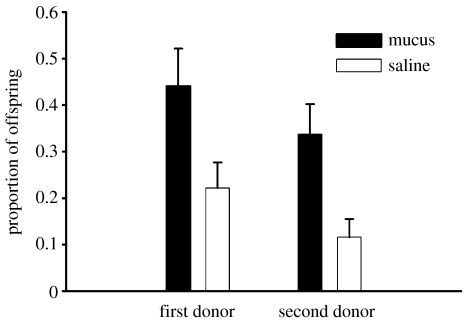
We found that sperm transfers associated with mucus injections resulted in significantly greater paternity than those associated with saline injections (GLM, F1,74=13.756, p<0.0005), indicating that the dart functions not only by mechanical stimulation, but by injecting mucus. The fathers that donated sperm in association with mucus injections sired 38.9±5.2% of the offspring, whereas those that donated sperm in association with saline injections sired only 16.9±3.5% of the offspring. The remaining offspring (44.2±5.5%) were sired by snails that mated with the mother prior to their collection for our study.
To determine whether the positive effect of mucus injections was limited to either one of the two matings, two separate GLM analyses were conducted. The results indicate a significant effect of mucus injection regardless of mating order (figure 2).
Figure 2.
Paternity assignments per egg clutch from an experiment in which needle injections replaced dart shooting. Future mothers were mated twice. In one mating, sperm transfer was accompanied by an injection of mucus from the dart gland and in the other mating sperm transfer was accompanied by an injection of saline. Mean+s.e.m. is shown. First donor: F1,35=5.086, p=0.030; second donor: F1,36=10.652, p=0.002.
Maternal shell volume was found to be a significant covariate of injection type during the first mating (F1,35=4.343, p<0.05), but not during the second mating (F1,35=0.015, p=0.904). However, regression analysis revealed no significant association in either mating between maternal shell volumes and paternities associated with mucus injections (first mating: R2=0.181, F1,18=3.769, p=0.069; second mating: R2=0.013, F1,18=0.225, p=0.641). By contrast, Rogers & Chase (2002) reported that the paternity benefit of a successful dart shot was inversely, and strongly, associated with maternal shell volume, implying that the mucus became less efficient as it was diluted in larger recipients. Here, we injected 2.9 times the amount of mucus that is normally carried on the dart, and we injected it directly into the haemocoel. Thus, it is likely that the influence of recipient size was largely eliminated.
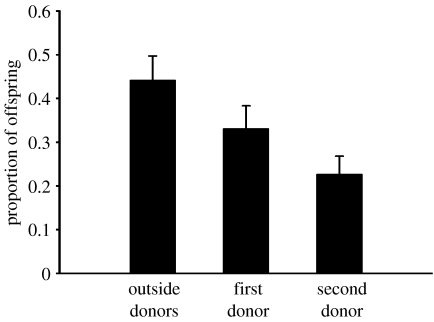
Our results indicate an additional determinant of paternity in land snails, namely mating order. As shown in figure 3, paternities were strongly skewed in favour of early-sperm donors (unidentified snails that mated in the field before this study began, 44.2±5.5%; first donors, 33.1±5.2%; second donors, 22.7±4.2%). The difference between P1 values and P2 values was not statistically significant (t=0.829, d.f.=72, p=0.410).
Figure 3.
Early-male sperm precedence. Outside fathers mated with a mother prior to the collection of snails for this study. Subsequently, the same mother mated with two genotyped sperm donors as part of our experiment. Because the snails were held in isolation prior to the start of the experiment, the sperm of outside donors had already been stored for at least 61.5±17.6 days at the time of the first experimental mating. Mean+s.e.m. is shown.
4. Discussion
In their review of sperm competition, Wigby & Chapman (2004) emphasize the importance of understanding the mechanisms of sexual selection as a prerequisite for understanding their evolution. In the case of the snail's dart, our results clearly show that the mechanism by which it increases paternity is by injecting mucus, not by mechanical stimulation alone. A potential explanation is suggested by previous in vitro experiments done in our lab in which applications of dart gland mucus caused contractions in the snail's spermatophore-receiving organs (Koene & Chase 1998). These contractions reconfigured the organs in such a manner as to allow allosperm to escape enzymatic digestion and precede to the spermathecal storage sacs.
The bioactive substance in the dart gland mucus is likely to be a peptide since peptides are common neural and hormonal messengers in gastropod molluscs (Chase 2002), and peptides are present in the mucus gland secretion (Chung 1986; R. Chase & G. Nagle 2006, unpublished). Regardless of whether the bioactive substance is a peptide or a different type of compound, its identification in a broad sample of dart-shooting species should allow molecular data to be added to behavioural and morphological data to gain a better understanding of how darts evolved in pulmonate molluscs (Koene & Schulenburg 2005; Schilthuizen 2005).
Seen in the light of the present results, dart-shooting merits attention as an unusual product of sexual selection. At the present time, it is the most widely known and perhaps the best-documented molluscan example of a trait evolved through sperm competition (Baur 1998; Schilthuizen 2005). Unfortunately, nearly all studies of its mechanism have been made in C. aspersus. It is imperative to learn whether the dart has the same ultimate function and the same reliance on mucus transfer in other species. In addition, it may prove fruitful to consider the conditions that favoured its evolution. Substances such as the dart gland mucus that are transferred directly into the body of a conspecific, and induce a direct physiological response, are known as allohormones (Koene & ter Maat 2001). Besides their use in Cantareus, allohormones have recently been implicated in two additional hermaphrodites, the earthworm Lumbricus terrestris (Koene et al. 2005) and slugs of the genus Derocerus (Reise in press). These three examples have in common the putative manipulation of sperm uptake and, unusually, the delivery of allohormones by peripheral routes rather than through the seminal fluid (body piercing by darts in Cantareus; body piercing by setae in Lumbricus; body stroking by a digitiform penial gland in Derocerus). This manner of delivery may allow the allohormones to access critical anatomical structures that lie distant from structures contacted by the seminal fluid. Theory suggests that the need to increase paternity may force hermaphrodites, more so than species with separate sexes, to accept and develop intrusive mating mechanisms (Michiels & Koene 2004, unpublished).
The design of our experiment eliminates the possibility that successful dart shooting may be only a correlate of enhanced paternity, not the cause. Since there was no dart shooting in our experiment, and the injections of saline and mucus were arbitrarily assigned, the observed effects were independent of variations in ejaculate quality or indeed any traits other than those directly related to the dart gland mucus.
Each individual snail possesses separate male and female functions. The male function clearly benefits by injecting mucus, but does the female function also benefit from receiving mucus? This would be the case if the ability to shoot successfully were heritable and consistently expressed, or if successful dart shooting were correlated with high-quality traits. One can, therefore, speculate that the female function might preferentially select sperm from successful shooters to fertilize its eggs (Landolfa 2002). However, our results indicate that any such female selection would have to be based on properties of the mucus, not on the properties of the dart itself.
Comparing our results with those from several other studies (Baur 1994; Landolfa et al. 2001; Rogers & Chase 2002), the superiority of microsatellites for discriminating paternity, and thus for identifying precedence, is striking. For example, 44.2±5.5% of the offspring in our study were sired by sperm donors that mated with the mother 61.5±17.6 days prior to the first of the two experimental matings (figure 3), whereas Rogers & Chase (2002), using allozymes for paternity determinations in an experiment of similar design, found that only 11±2.5% of the offspring were sired by donors that mated with the mother 45.5 days prior to the first experimental mating. Our inability to detect significant precedence in respect to P1 and P2 is probably explained by the fact that, on an average, 13 of the 30 genotyped offspring had to be excluded from the t-test because they were sired by outside fathers. This increased the sampling error and precluded a significant statistical result. Evanno et al. (2005) avoided this problem by using twice-mated virgins, and they reported a highly significant mean P2 of 0.243 using microsatellites (mean inter-mating interval, 18.3 days).
The dynamics of sperm competition and sperm precedence in snails are not yet completely understood, although first-male sperm precedence is consistently reported in experiments involving only two donors (Baur 1994; Landolfa et al. 2001; Rogers & Chase 2002; Evanno et al. 2005). Since we do not know how many individuals mated with our mothers prior to their coming into the laboratory, the offspring attributed to outside fathers (44.2±5.5%) may be distributed among many sperm donors, some of which may have sired fewer offspring than our first and second donors. It should be a goal of future studies to determine exact paternity ratios for multiple sperm donors (more than 2) all of which have mated with the same mother. In the meanwhile, it seems reasonable to assume that early-arriving sperm have an advantage over late-arriving sperm, possibly because gametes that have already embedded their heads in the spermathecal epithelium continuously beat their tails against incoming sperm (Rogers & Chase 2002).
We conclude that snails have two ways in which to optimize their reproductive success: by shooting darts accurately and by mating with virgins. It is unknown, however, whether snails are able to either control the accuracy of their dart shots or identify virgin partners.
Acknowledgments
This work was supported by a grant from NSERC Canada awarded to R.C. Kris Vaga performed very skilful surgeries on very many snails. We also thank Frédérick Robidoux, Andrei Verner and Amèlie Villeneuve of Genome Québec for their expert assistance. For their comments on an earlier version of this paper we thank Zanna Chase, Joris Koene, Bob Montgomerie, Laura Nilson and Peter Strutton.
References
- Adamo A, Chase R. Courtship and copulation in the terrestrial snail Helix aspersa. Can. J. Zool. 1988;66:1446–1453. [Google Scholar]
- Adamo A, Chase R. Dart shooting in helicid snails: an ‘honest’ signal or an instrument of manipulation? J. Theor. Biol. 1996;180:77–80. doi:10.1006/jtbi.1996.0080 [Google Scholar]
- Baur B. Multiple paternity and individual variation in sperm precedence in the simultaneously hermaphroditic land snail Arianta arbustorum. Behav. Ecol. Sociobiol. 1994;35:413–421. doi:10.1007/s002650050114 [Google Scholar]
- Baur B. Sperm competition in molluscs. In: Birkhead T.R, Møller A.P, editors. Sperm competition and sexual selection. Academic Press; London, UK: 1998. pp. 255–305. [Google Scholar]
- Chase R. Oxford University Press; New York, NY: 2002. Behavior and its neural control in gastropod molluscs. [Google Scholar]
- Chase, R. & Vaga, K. 2006 Independence, not conflict, characterizes dart shooting and sperm exchange in a hermaphroditic snail. Behav. Ecol. Sociobiol. (doi:10.1007/s00265-005-0103-y)
- Chung D.J.D. Stimulation of genital eversion in the land snail Helix aspersa by extracts of the glands of the dart apparatus. J. Exp. Zool. 1986;238:129–139. doi:10.1002/jez.1402380202 [Google Scholar]
- Davison A, Wade C.M, Mordan P.B, Chiba S. Sex and darts in slugs and snails. J. Zool. 2005;267:329–338. doi:10.1017/S0952836905007648 [Google Scholar]
- Evanno G, Madec L, Arnaud J.-F. Multiple paternity and postcopulatory sexual selection in a hermaphrodite: what influences sperm precedence in the garden snail Helix aspersa? Mol. Ecol. 2005;14:805–812. doi: 10.1111/j.1365-294X.2005.02449.x. doi:10.1111/j.1365-294X.2005.02449.x [DOI] [PubMed] [Google Scholar]
- Guiller A, Arnaud J.F, Vautrin D, Solignac M. Highly polymorphic microsatellite markers in the landsnail Helix aspersa (Mollusca Gastropoda) Mol. Ecol. 2000;9:1191–1103. doi: 10.1046/j.1365-294x.2000.00954-13.x. doi:10.1046/j.1365-294x.2000.00954-13.x [DOI] [PubMed] [Google Scholar]
- Jamieson A, Taylor S.S. Comparisons of three probability formulae for parentage exclusion. Anim. Genet. 1997;28:397–400. doi: 10.1111/j.1365-2052.1997.00186.x. doi:10.1111/j.1365-2052.1997.00186.x [DOI] [PubMed] [Google Scholar]
- Kerkut G.A, Meech R.W. The internal chloride concentration of H and D cells in the snail brain. Comp. Biochem. Physiol. 1996;19:819–832. [Google Scholar]
- Koene J.M, Chase R. Changes in the reproductive system of the snail Helix aspersa caused by mucus from the love dart. J. Exp. Biol. 1998;201:2313–2319. doi: 10.1242/jeb.201.15.2313. [DOI] [PubMed] [Google Scholar]
- Koene J.M, Schulenburg H. Shooting darts: co-evolution and counter-adaptation in hermaphroditic snails. BMC Evol. Biol. 2005;25:5. doi: 10.1186/1471-2148-5-25. doi: 10.1186/1471-2148-5-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koene J.M, ter Maat A. “Allohormones”: a class of bioactive substances favoured by sexual selection. J. Comp. Physiol. A. 2001;187:323–326. doi: 10.1007/s003590100214. doi:10.1007/s003590100214 [DOI] [PubMed] [Google Scholar]
- Koene J.M, Pförtner T, Michiels N.K. Piercing the partner's skin influences sperm uptake in the earthworm Lumbricus terrestris. Behav. Ecol. Sociobiol. 2005;59:243–249. doi:10.1007/s00265-005-0030-y [Google Scholar]
- Landolfa M.A. On the adaptive function of the love dart of Helix aspersa. Veliger. 2002;45:231–249. [Google Scholar]
- Landolfa M.A, Green D.M, Chase R. Dart shooting influences paternal reproductive success in the snail Helix aspersa (Pulmonata Stylommatophora) Behav. Ecol. 2001;12:773–777. doi:10.1093/beheco/12.6.773 [Google Scholar]
- Lind H. The functional significance of the spermatophore and the fate of spermatozoa in the genital tract of Helix pomatia (Gastropoda: Sylommatophora) J. Zool. 1973;169:39–64. [Google Scholar]
- Reise, H. In press. A review of mating behavior in Deroceras slugs (Pulmonata: Agriolimacidae). Am. Malacol. Bull.
- Rogers D, Chase R. Dart receipt promotes sperm storage in the garden snail Helix aspersa. Behav. Ecol. Sociobiol. 2001;50:122–127. doi:10.1007/s002650100345 [Google Scholar]
- Rogers D, Chase R. Determinants of paternity in the garden snail Helix aspersa. Behav. Ecol. Sociobiol. 2002;52:289–295. doi:10.1007/s00265-002-0519-6 [Google Scholar]
- Schilthuizen M. The darting game in snails and slugs. Trends Ecol. Evol. 2005;20:581–584. doi: 10.1016/j.tree.2005.08.014. doi:10.1016/j.tree.2005.08.014 [DOI] [PubMed] [Google Scholar]
- Teshima H, Davison A, Kuwahara Y, Yokoyama J, Chiba S, Fukuda T, Ogimura H, Kawata M. The evolution of extreme shell shape variation in the land snail Ainohelix editha: a phylogeny and hybrid zone analysis. Mol. Ecol. 2003;12:1869–1878. doi: 10.1046/j.1365-294x.2003.01862.x. doi:10.1046/j.1365-294X.2003.01862.x [DOI] [PubMed] [Google Scholar]
- Wigby S, Chapman T. Sperm competition. Curr. Biol. 2004;14:R11–R103. doi:10.1016/S0960-9822(04)00028-4 [PubMed] [Google Scholar]