Abstract
Species-energy theory indicates that recent climate warming should have driven increases in species richness in cool and species-poor parts of the Northern Hemisphere. We confirm that the average species richness of British butterflies has increased since 1970–82, but much more slowly than predicted from changes of climate: on average, only one-third of the predicted increase has taken place. The resultant species assemblages are increasingly dominated by generalist species that were able to respond quickly. The time lag is confirmed by the successful introduction of many species to climatically suitable areas beyond their ranges. Our results imply that it may be decades or centuries before the species richness and composition of biological communities adjusts to the current climate.
Keywords: biodiversity, butterflies, climate change, species richness
1. Introduction
Most studies of the observed responses of biological assemblages to climate change (Hill et al. 1999; Parmesan et al. 1999; Hughes 2000; McCarty 2001; Parmesan & Yohe 2003; Root et al. 2003; Visser et al. 2003; Genner et al. 2004) have concentrated on changes to the distributions, abundances or phenologies of individual species rather than on more integrated measures of change, such as species richness, community composition and ecosystem properties (Sykes et al. 1996; Lemoine & Böhning-Gaese 2002; Walther et al. 2002). Although species are likely to respond individualistically to climate change, the overall consequences of these changes will be changes in communities and ecosystems. Here, we provide the first assessment, at a geographical scale of how species richness has changed in response to climate change.
Climate change is expected to reduce the number of species globally (Thomas et al. 2004), but the species richness of regional communities might either increase or decrease. For example, cool-temperate regions that experience warming and very dry regions that experience increased moisture availability are both expected to exhibit increases in richness, based on species-energy theory (Hawkins et al. 2003).
Here, we investigate changes in species richness using the well-monitored British butterfly fauna as a model system (54 species, not including continental migrants). Britain is a relatively species-poor region due to its cool-temperate climate and the butterfly fauna shows a species-richness gradient with more species found in hotter areas in the south (Turner et al. 1987; Asher et al. 2001; figure 1, less than 10% of British butterflies have northern and/or montane distributions). We test whether average species richness of resident British butterfly species has increased in recent decades, whether these changes are as great as would be expected given the amount of warming that has taken place (Jones & Hulme 1997; Roy & Sparks 2000), and whether the composition of butterfly communities is changing towards a dominance by generalist species (Warren et al. 2001). We compare patterns of diversity in two periods, 1970–82 and 1995–99, when butterfly distributions were recorded comprehensively (Heath et al. 1984; Asher et al. 2001). A critical test of whether species richness changes are tracking climate would be through artificial releases of individuals: if species are lagging behind climate, many such introductions should be successful (habitat permitting), whereas they should fail if the climate remains unsuitable. We review existing literature on butterfly introductions to test whether success/failure is related to the climate suitability of the site.
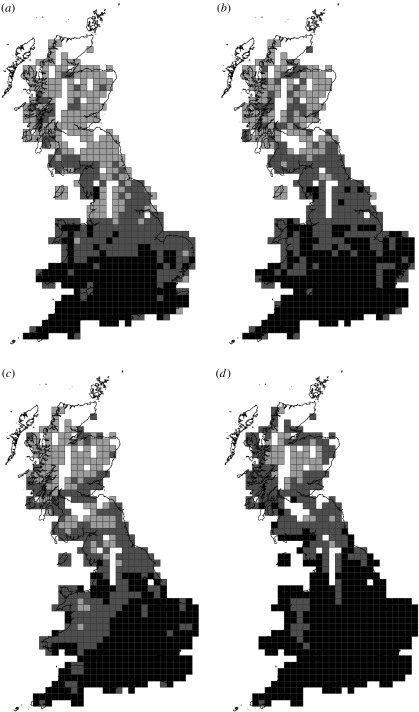
Figure 1.
Geographical patterns in Britain at 20×20 km grid resolution of butterfly species richness: (a) observed species richness in 1970–82, (b) observed species richness in 1995–99, (c) predicted species richness in 1970–82 and (d) predicted species richness in 1995–99 (pale grey: less than or equal to 15 species; dark grey: 15–25 species; black: more than 25 species). Predictions are based on mean predictions using 1970–82 GLM models. In all maps, white represents squares excluded from analyses due to sampling effort (see §2).
2. Material and methods
(a) Calculation of species richness
Species richness was calculated as the number of species in 20×20 km Ordnance Survey grid cells (740 squares in the study area), based on distribution data for all resident British butterflies. Butterfly distributions in Britain (England, Wales and Scotland) were recorded at 10×10 km grid resolution in 1970–82 (Heath et al. 1984) and 1995–99 (Asher et al. 2001), allowing the calculation of diversity for each period and the analysis of diversity changes between periods. To ensure adequate recorder effort in both time periods we first grouped data in 20×20 km squares. We then selected those squares containing, in both 1970–82 and 1995–99, at least one spring and one summer indicator species. This identifies squares that had been visited during the main flight periods in Britain (spring: Anthocharis cardamines, Boloria euphrosyne and Callophrys rubi; summer: Aphantopus hyperantus, Erebia aethiops, Maniola jurtina and Pyronia tithonus). The selected squares represent 82% of the area of Britain and include both coastal and internal squares. We included coastal squares because some components of climate are related to distance from the sea and some butterfly species are restricted to, or commoner around, coastal habitats/climates, especially in the north. Moreover, our results were independent of the percentage of land within a square.
We included the 54 resident British butterflies that have regularly been observed in Britain since 1970 and excluded migrants that do not maintain populations in Britain (12 species). Species were classified into groups according to their 1970–82 geographical distributions in Britain (southerly, northerly and ubiquitous distributions) and habitat requirements (habitat specialists: occupying one or a few localized habitats; habitat generalists: occupying widespread and/or many habitats; see Pollard & Yates (1993), Warren et al. (2001) for more details). Twenty-one habitat generalists (one northerly, 12 southerly and eight ubiquitous species) and 33 habitat specialists (four northerly, 25 southerly and four ubiquitous species) were considered. Analyses were carried out only for groups of 12 or more species.
(b) Climate models
For each 20×20 km cell, we computed: Growing Degree Days over 5 °C (GDD5), measured as annual temperature sum above 5 °C, which relates to developmental thresholds for larvae (Bryant et al. 1997); mean temperature of January (TJAN), which relates to over wintering survival (Pullin & Bale 1989); and AET/PET (the ratio of actual to potential evapotranspiration) which relates to moisture availability and hence potentially to host-plant quality. These climate variables have also been used successfully to model species distributions for butterflies and other taxa (Hill et al. 1999; Huntley et al. 2004).
British climate data were obtained from: http://www.metoffice.com/research/hadleycentre/obsdata/ukcip/index.html. GDD5 and TJAN values for each 20×20 km square were calculated as the average of the 16 constituent 5×5 km squares, for each of the two time periods (1970–82 and 1995–99). AET/PET data were available yearly at 10×10 km grid resolution for the mean altitude of each cell. AET/PET values for each 20×20 km square were averages of the four 10×10 km squares, in each of the two time periods.
Generalized linear models (GLMs) were carried out in S-plus (S-plus 2000 professional, MathSoft) for each group of species, using species richness in each grid cell for 1970–82 as the dependent variable and the three climate variables in 1970–82 as independent factors. We assumed a Poisson distribution for the dependent variable and a logarithmic link function. Using the equation obtained from the 1970–82 model and incorporating climate variables from the later period (1995–99), we then calculated the predicted number of species in 1995–99 per grid cell, as well as the lower and upper 95% confidence limits of the mean predictions.
For each grid cell, we calculated the expected change in species richness as the predicted number of species in 1970–82 subtracted from the predicted number of species in 1995–99. In addition to mean predicted change (mean predicted value in 1970–82 subtracted from mean predicted value in 1995–99), we also calculated estimates based on the lower 95% confidence limit of the mean predictions (lower 95%CL in 1970–82 subtracted from lower 95%CL in 1995–99) and on the upper 95% confidence limit of the mean predictions (upper 95%CL in 1970–82 subtracted from upper 95%CL in 1995–99). The proportion of expected change that had taken place was calculated as observed change divided by change predicted by the model and was used as an indicator of the response of diversity to climate change.
We used an Artificial Neural Network (ANN) to test the consistency of predictions obtained with GLM (Thuiller 2003). ANN is a non-parametric approach that, in contrast to the GLM, makes no assumptions about the distribution of the dependent variable and the shape of the relation between the dependent and independent variables (see the electronic supplementary material).
(c) Butterfly introductions
We compiled data on releases of butterflies in Britain up to 1990 (Oates & Warren 1990; Fuller 1995; Corke 1997; Asher et al. 2001; Record of Insect Establishment). Introductions were used if the information included: (i) location, (ii) year of introduction (in some cases, less precise dates e.g. ‘Early 1970s’ were used), and (iii) follow-up survey(s) to determine success. An introduction was deemed successful if it lasted more than 10 years, long enough for populations to decline to extinction (even if large numbers were released) but sufficiently short to exclude most extinction due to permanent habitat change. Introductions from 1990 onwards were not used as the long-term success of these introductions was not yet established at the time of the most recent survey.
We allocated each species to one of the ecological categories mentioned above (see the electronic supplementary material). For each introduction, we determined the climate suitability of the introduction site, using ‘climate response surface’ models for that particular species (Huntley et al. 1995; Hill et al. 1999; see electronic supplementary material).
We used a generalized linear mixed model (GLMM) in SAS (SAS/STAT Software v. 8.02, SAS Institute, Inc) to determine the effect of climate on the success of an introduction. The GLIMMIX macro was used for all analyses. Introduction success/failure was the response variable with a binomial error distribution and logit link function. The climate suitability of the introduction site was arcsine-transformed for analysis. Species nested within family/subfamily was entered as a random factor to account for non-independence in the data owing to taxonomic clustering of introductions.
3. Results
(a) Changes in species richness
Average butterfly diversity per grid square increased in Britain during the two study periods (figure 1a,b; table 1). Southerly habitat generalists increased more than southern specialists (Wilcoxon signed ranks test: Z=−9.437, p<0.001, n=609 grid squares). The number of southerly generalists increased in 60% of the study squares, declined in 7% and stayed the same in 33%. In contrast, the number of southerly specialists increased in 42%, declined in 30% and remained unchanged in 28% of squares.
Table 1.
Butterfly species richness in the two study periods. (Values are mean number of species±s.e. for each group of species at 20×20 km grid resolution. Significance for Wilcoxon signed ranks tests, n=609 grid squares.)
| group | 1970–82 | 1995–99 | significance (p) |
|---|---|---|---|
| southerly habitat generalists | 7.24±0.17 | 8.48±0.16 | <0.001 |
| southerly habitat specialists | 4.81±0.20 | 5.10±0.18 | 0.001 |
| all habitat generalists | 15.13±0.18 | 16.37±0.17 | <0.001 |
| all habitat specialists | 6.80±0.23 | 7.22±0.19 | <0.001 |
| all species | 21.92±0.37 | 23.59±0.32 | <0.001 |
(b) Climate model predictions
Species richness for the period 1970–82 was positively associated with energy (GDD5, Growing Degree Days over 5 °C) and moisture availability (AET/PET), with a small negative contribution of winter temperature (TJAN; see electronic supplementary material).
Analyses using GLM and ANN produced similar results. Observed species richness in 1995–99 was well predicted by the 1970–82 model for both GLM and ANN methods (table 2). However, only around 34% (GLM) or 32% (ANN) of the total predicted change had taken place by 1995–99 (table 3). Thus, observed increases in species richness apparently lagged behind changes in climate.
Table 2.
Pearson correlation coefficients (r) between observed and predicted species richness in 1995–99. (Predictions are based on GLM and ANN models for the 1970–82 period. 1995–99 climate variables were input into the 1970–82 models to predict 1995–99 species richness. All correlations are significant at p<0.001 (two-tailed).)
| group | GLM | ANN |
|---|---|---|
| southerly habitat generalists | 0.832 | 0.890 |
| southerly habitat specialists | 0.698 | 0.685 |
| all habitat generalists | 0.862 | 0.883 |
| all habitat specialists | 0.568 | 0.512 |
| all species | 0.795 | 0.789 |
Table 3.
Proportion of expected diversity change (observed diversity change/predicted diversity change) that has taken place between 1970–82 and 1995–99, for each group of species (means±s.e.s, n=609 squares). (Predicted change based on predictions using 1970–82 ANN models and 1970–82 GLM models (mean predictions and the lower and upper 95% confidence limits of the mean predictions).)
| GLM | ||||
|---|---|---|---|---|
| ANN | mean | lower 95% | upper 95% | |
| southerly habitat generalists | 0.49±0.03 | 0.45±0.03 | 0.47±0.03 | 0.44±0.03 |
| southerly habitat specialists | 0.14±0.05 | 0.13±0.03 | 0.14±0.03 | 0.12±0.03 |
| all habitat generalists | 0.47±0.02 | 0.42±0.03 | 0.43±0.03 | 0.41±0.03 |
| all habitat specialists | 0.25±0.05 | 0.26±0.04 | 0.27±0.05 | 0.25±0.04 |
| all species | 0.32±0.06 | 0.34±0.03 | 0.35±0.03 | 0.34±0.03 |
We repeated these analyses separately for each of the two groups of southerly species (specialists and generalists) to identify the possible origin of the lag. For generalists (n=12 species), again GDD5 and AET/PET had positive effects on species richness and TJAN a small negative effect (see the electronic supplementary material). Substituting 1995–99 climate data into the 1970–82 model again successfully predicted 1995–99 species richness (table 2). For this group of species, around 45% of the predicted increase in species richness was estimated to have taken place using GLM and 49% using ANN (table 3).
This analysis of southerly generalists assumed that the number of species could potentially increase above the current species pool of Britain, but for some grid squares this would require colonization by species from continental Europe. However, Britain has not been colonized successfully from continental Europe by any non-migratory butterfly species in over 200 years of butterfly recording (Asher et al. 2001). Thus, we re-analysed the data considering only those squares that had the potential to show substantial species-richness increases without colonization from continental Europe (squares with less than seven species in 1970–82). For these squares, the proportion of expected increase in species richness that had taken place by 1995–99 was 87% (rather than 45% across all squares). Thus, two-thirds of the lag in the response of southerly habitat generalists can apparently be attributed to the failure of new species to colonize Britain from the continent. Continental colonization did not affect estimates for any other group of species for which species richness in the early period (and predicted for the later period) was always far below the species pool already in Britain.
For southerly habitat specialists (n=25 species), the same climatic variables used previously were again significantly related to diversity (see the electronic supplementary material), but the model explained a lower percentage (38%) of variation in species richness than did the models for total richness (54%) or for southerly habitat generalists (67%). Substituting 1995–99 climate data into the 1970–82 model for southerly specialists again successfully predicted 1995–99 species richness (table 2). For specialists, only 13% of the predicted increase in species richness was estimated to have taken place using GLM and 14% using ANN (table 3).
(c) Butterfly introductions
We analysed 176 instances where individuals of either habitat specialists (24 species) or generalists (nine species) were released in areas where the species was not present previously (see electronic supplementary material). The climate suitability of the introduction site had a positive significant effect on the success of an introduction (0.340±0.043 and 0.183±0.018; mean±s.e. climate suitability of successful and unsuccessful introductions, respectively; F1,141=9.83; p=0.002). The effect of climate was stronger for southerly habitat generalists (0.707±0.112 and 0.085±0.023; mean±s.e. climate suitability of successful and unsuccessful introductions, respectively; F1,10=22.00; p=0.0009) than for southerly habitat specialists (0.282±0.041 and 0.169±0.016; mean±s.e. climatic suitability of successful and unsuccessful introductions, respectively; F1,122=6.36; p=0.013).
Euphydryas aurinia had by far the most introduction attempts (nearly 30% of all introductions, see the electronic supplementary material). To check whether this species was having a disproportionate effect on the results, we repeated the GLMM analyses with and without E. aurinia. Exclusion of E. aurinia resulted in slightly less significant effect of climate suitability on both southerly habitat specialists (0.321±0.049 and 0.209±0.026; mean±s.e. climate suitability of successful and unsuccessful introductions, respectively; F1,70=4.61; p=0.035) and all species combined (0.387±0.050 and 0.219±0.025; mean±s.e. climate suitability of successful and unsuccessful introductions, respectively; F1,89=8.37; p=0.005).
4. Discussion
Despite declines of individual species (Asher et al. 2001; Thomas et al. 2004b), average species richness of the British butterfly fauna at 20×20 km grid resolution has increased since 1970–82, during a period when climate warming would lead us to expect increases. Of particular interest are the southerly distributed species, northwards expansions of which would be required to bring about increases in species richness (Warren et al. 2001). Southerly habitat generalists increased more than specialists, so local butterfly assemblages have, on average, become increasingly dominated by species that occupy widespread habitats. Population losses due to habitat destruction (Thomas et al. 2004b), a failure to expand northwards because of the patchy distribution of potential habitats and limited dispersal capacity (Thomas et al. 2001; Warren et al. 2001) have constrained the response of southerly habitat specialists.
However, observed species richness increases are lagging behind those expected on the basis of climate change, particularly in southerly habitat specialists that have achieved only 13% of the predicted response since 1982. Moreover, these lags might be even greater than we have predicted if species richness was already lagging behind climate during the period 1970–82. Comparable analyses are now required for other regions to evaluate whether equally delayed decreases in species richness are taking place in regions with predicted declines in richness.
The success of an introduction was positively related to the climate suitability of the introduction site and the effect of climate was stronger for habitat generalists than for specialists. These results support the hypothesis that species are lagging behind climate and that generalist butterflies are primarily limited by climate, whereas specialists are also limited by other factors (Menéndez et al. submitted). Most dispersing individuals of specialist species would fail to find suitable habitats, either retarding the rate of range expansion or halting it entirely. Successful introductions into areas of suitable climate for southerly habitat generalists confirm that even these species lag behind climate change to some extent in their distributional responses (Hill et al. 1999).
Biodiversity researchers frequently adopt the term ‘extinction debt’ to denote the time delay between environmental change and the extinctions that will eventually take place as a result of those environmental changes (Tilman et al. 1994). Just as important in the context of climate change (and species invasions) are ‘colonization lags’ to denote the time delay between environmental changes and colonization events. In both cases, important questions are (i) what proportion of the remaining ‘lag/debt’ will eventually take place and (ii) what is the time-scale of the delayed response? The 45–49 and 13–14% responses for two groups of butterflies in ca 22 years (between midpoints of the two time periods) suggest time-scales of a few decades for habitat generalists, to centuries for habitat specialists (many specialists may not respond at all, unless habitat restoration takes place). Moreover, plant species (butterfly host plants) may lag behind climate change by centuries, as has been shown by their responses to post-glacial climatic warming (Prentice et al. 1991). The results presented here indicate that the level of climate warming that has already taken place is sufficient to have generated communities that are likely to continue to change for decades or centuries to come (even in the hypothetical situation that no further climate change takes place).
Acknowledgments
We thank the contributors to the Butterfly surveys in Britain; Ralph Ohlemueller for help with statistical analysis; Jan Bissinger for help collating the introduction dataset; Rob Wilson for comments on the manuscript and the Meteorological Office Hadley Centre for access to the climate data. The work was supported by The Leverhulme Trust and NERC.
Footnotes
Present address: Departmento Biología Animal y Ecología, Facultad de Ciencias, Universidad de Granada, 18071 Granada, Spain.
Present address: Department of Animal and Plant Sciences, University of Sheffield, Western Bank, Sheffield S10 2TN, UK.
Supplementary Material
Appendix A (GLMs equations used to predict current species richness in the period 1995–99.) Appendix B (Species used in the analyses of butterfly introductions.)
References
- Asher J, Warren M, Fox R, Harding P, Jeffcoate G, Jeffcoate S. Oxford University Press; Oxford, UK: 2001. The millennium atlas of butterflies in Britain and Ireland. [Google Scholar]
- Bryant S.R, Thomas C.D, Bale J.S. Nettle-feeding nymphalid butterflies: temperature, development and distribution. Ecol. Entomol. 1997;22:390–398. doi:10.1046/j.1365-2311.1997.00082.x [Google Scholar]
- Corke D. Lopinga Books; Ipswich, UK: 1997. The butterflies of Essex. [Google Scholar]
- Fuller M. Pisces Publications; Oxford, UK: 1995. The butterflies of Wiltshire: their history, status and distribution 1982–1994. [Google Scholar]
- Genner M.J, Sims D.W, Wearmouth V.J, Southall E.J, Southward A.J, Henderson P.A, Hawkins S.J. Regional climatic warming drives long-term community changes of British marine fish. Proc. R. Soc. B. 2004;271:655–661. doi: 10.1098/rspb.2003.2651. doi:10.1098/rspb.2003.2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins B.A, et al. Energy, water, and broad-scale geographic patterns of species richness. Ecology. 2003;84:3105–3117. [Google Scholar]
- Heath J, Pollard J.E, Thomas J.A. Viking Books; Harmondsworth, UK: 1984. Atlas of butterflies in Britain and Ireland. [Google Scholar]
- Hill J.K, Thomas C.D, Huntley B. Climate and habitat availability determine 20th century changes in a butterfly's range margins. Proc. R. Soc. B. 1999;266:1197–1206. doi:10.1098/rspb.1999.0763 [Google Scholar]
- Hughes L. Biological consequences of global warming: is the signal already apparent. Trends Ecol. Evol. 2000;15:56–61. doi: 10.1016/s0169-5347(99)01764-4. doi:10.1016/S0169-5347(99)01764-4 [DOI] [PubMed] [Google Scholar]
- Huntley B, Berry P.M, Cramer W, McDonald A. Modelling present and potential future ranges of some European higher plants using climate response surfaces. J. Biogeogr. 1995;22:967–1001. [Google Scholar]
- Huntley B, et al. The performance of models relating species geographical distributions to climate is independent of trophic level. Ecol. Lett. 2004;7:417–426. doi:10.1111/j.1461-0248.2004.00598.x [Google Scholar]
- Jones P.D, Hulme M. The changing temperature of central England. In: Hulme M, Barrow E.M, editors. Climates of the British Isles: present, past and future. Routledge; London, UK: 1997. pp. 173–195. [Google Scholar]
- Lemoine N, Böhning-Gaese K. Potential impact of global climate change on species richness of long-distance migrants. Conserv. Biol. 2002;17:577–586. doi:10.1046/j.1523-1739.2003.01389.x [Google Scholar]
- McCarty J.P. Ecological consequences of recent climate change. Conserv. Biol. 2001;15:320–331. doi:10.1046/j.1523-1739.2001.015002320.x [Google Scholar]
- Menéndez, R., González-Megías, A., Collingham, Y., Fox, R., Roy, D. B. & Thomas, C. D. Submitted. Direct and indirect effects of climate and habitat factors on specialist and generalist butterfly diversity in Britain. Ecol. Lett. [DOI] [PubMed]
- Oates, M. R. & Warren, M. S. 1990 A review of butterfly introductions in Britain and Ireland A contract report for the conservation of British insects (JCCBI) funded by the World Wide Fund for nature.
- Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. doi:10.1038/nature01286 [DOI] [PubMed] [Google Scholar]
- Parmesan C, et al. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature. 1999;399:579–583. doi:10.1038/21181 [Google Scholar]
- Pollard E, Yates T.J. Chapman & Hall; London, UK: 1993. Monitoring butterflies for ecology and conservation. [Google Scholar]
- Prentice C, Bartlein P.J, Webb T. Vegetation and climate change in eastern north America since the last glacial maximum. Ecology. 1991;72:2038–2056. [Google Scholar]
- Pullin A.S, Bale J.S. Effects of low temperature on diapausing Aglais urticae and Inachis io (Lepidoptera: Nymphalidae): cold hardiness and overwintering survival. J. Insect Physiol. 1989;35:277–281. doi:10.1016/0022-1910(89)90075-9 [Google Scholar]
- Root T.L, Price J.T, Hall K.R, Schneider S.H, Rosenzweig C, Pounds J.A. Fingerprints of global warming on wild animals and plants. Nature. 2003;421:57–60. doi: 10.1038/nature01333. doi:10.1038/nature01333 [DOI] [PubMed] [Google Scholar]
- Roy D.B, Sparks T.H. Phenology of British butterflies and climate change. Global Change Biol. 2000;6:407–416. doi:10.1046/j.1365-2486.2000.00322.x [Google Scholar]
- Sykes M.T, Prentice I.C, Cramer W. A bioclimatic model for the potential distributions of north European tree species under present and future climates. J. Biogeogr. 1996;23:203–233. [Google Scholar]
- Thomas C.D, Bodsworth E.J, Wilson R.J, Simmons A.D, Davies Z.G, Musche M, Conradt L. Ecological and evolutionary processes at expanding range margins. Nature. 2001;411:577–581. doi: 10.1038/35079066. doi:10.1038/35079066 [DOI] [PubMed] [Google Scholar]
- Thomas C.D, et al. Extinction risk from climate change. Nature. 2004a;427:145–148. doi: 10.1038/nature02121. doi:10.1038/nature02121 [DOI] [PubMed] [Google Scholar]
- Thomas J.A, Telfer M.G, Roy D.B, Preston C.D, Greenwood J.J.D, Asher J, Fox R, Clarke R.T, Lawton J.H. Comparative losses of British butterflies, birds, and plants and the global extinction crisis. Science. 2004b;303:1879–1881. doi: 10.1126/science.1095046. doi:10.1126/science.1095046 [DOI] [PubMed] [Google Scholar]
- Thuiller W. BIOMOD—optimising predictions of species distributions and projecting future shifts under global change. Global Change Biol. 2003;9:1353–1362. doi: 10.1111/gcb.12728. doi:10.1046/j.1365-2486.2003.00666.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilman D, May R.M, Lehman C.L, Nowak M.A. Habitat destruction and the extinction debt. Nature. 1994;371:65–66. doi:10.1038/371065a0 [Google Scholar]
- Turner J.R.G, Gatehouse C.M, Corey C.A. Does solar-energy control organic diversity—butterflies, moths and the British climate. Oikos. 1987;48:195–205. [Google Scholar]
- Visser M.E, et al. Variable responses to large-scale climate change in European Parus populations. Proc. R. Soc. B. 2003;270:367–372. doi: 10.1098/rspb.2002.2244. doi:10.1098/rspb.2002.2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther G.R, Post E, Convey P, Menzel A, Parmesan C, Beebee T.J.C, Fromentin J.M, Hoegh-Guldberg O, Bairlein F. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. doi:10.1038/416389a [DOI] [PubMed] [Google Scholar]
- Warren M.S, et al. Rapid responses of British butterflies to opposing forces of climate and habitat change. Nature. 2001;414:65–69. doi: 10.1038/35102054. doi:10.1038/35102054 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A (GLMs equations used to predict current species richness in the period 1995–99.) Appendix B (Species used in the analyses of butterfly introductions.)