Abstract
This study reports an experimental confirmation of the terminal investment hypothesis, a longstanding theoretical idea that animals should increase their reproductive effort as they age and their prospects for survival and reproduction decline. Previous correlational and experimental attempts to test this hypothesis have yielded contradictory results. In the blue-footed booby, Sula nebouxii, a long-lived bird, after initial increase, male reproductive success declines progressively with age. Before laying, males of two age classes were challenged with lipopolysaccharide to elicit an immune response, which induced symptoms of declining survival prospects. Reproductive success of immune-challenged mature males fell, while that of immune-challenged old males showed a 98% increase. These results demonstrate that senescent males with poor reproductive prospects increase their effort when those prospects are threatened, whereas younger males with good reproductive prospects do not.
Keywords: senescence, life history, immune response, plasticity, reproductive success, lipopolysaccharide
1. Introduction
In organisms that reproduce several times over their lifespan, life-history theory predicts increased reproductive effort when residual reproductive value decreases (Williams 1966; Trivers 1972; Pianka & Parker 1975). Because increased parental effort in current reproduction negatively affects future reproduction, organisms commonly restrict their current effort in order to maximize lifetime success (Curio 1983; Stearns 1992). However, when no offspring are expected to be produced in the future, individuals should invest all their capital in their current progeny (Williams 1966; Clutton-Brock 1984). Although this long-standing hypothesis is a key idea in evolutionary theory (e.g. Hirschfield & Tinkle 1975; Morrow et al. 2003; Fessler et al. 2005), only fragmentary evidence supports it.
Evidence supporting the terminal investment hypothesis is mostly based on correlational data of age-specific reproductive effort found in some studies, but not in others (e.g. Clutton-Brock 1984; Pärt et al. 1992; Poizat et al. 1999, but see Ericsson et al. 2001; Yoccoz et al. 2002; Weladji et al. 2002; Fessler et al. 2005). As they age, many long-lived animals show a decrease in reproductive performance and in the costs of reproduction (Clutton-Brock 1984; Sæther 1990; Bennett & Owens 2002). In these species, the changes in parental investment (parental effort relative to costs) predicted by the terminal investment hypothesis are difficult to identify without an experimental approach in which reproductive prospects of experimental individuals are known. Experimental imposition of adverse conditions on individuals of unknown reproductive prospects has produced contradictory results, with reproductive effort increasing in some studies (e.g. Fox & McCoy 2000; Bonneaud et al. 2004; Javois & Tammaru 2004) and decreasing in others (e.g. Ilmonen et al. 2000; Råberg et al. 2000; Bonneaud et al. 2003).
A better way to test whether reproductive effort is calibrated to residual reproductive value (Williams 1966; Clutton-Brock 1984) is to manipulate the future reproductive prospects of age classes that differ in residual reproductive value. Younger individuals with high prospects should prioritize self-maintenance to reduce the risk of mortality; whereas ageing individuals with low prospects should increase investment in current reproduction. Thus, old senescent animals generally unavailable to field experimentalists are crucial for testing the terminal investment hypothesis.
Future reproductive prospects can be diminished by injecting non-replicating antigens to activate the immune system without inducing the negative effects of pathogens, thus mimicking the expected long-term/life-threatening cost (sensu Reznick 1992) of an illness. There is evidence that mounting an immune response is itself energetically costly because the metabolic requirements of immune cells and the indirect consequences of immune upregulation divert resources from other functions such as reproduction (Sheldon & Verhulst 1996; Ilmonen et al. 2000; Bonneaud et al. 2003) and survival (Hanssen et al. 2004). Furthermore, it has been suggested that in natural populations parasites critically increase the rate of mortality. Hence, activation of the immune system may cue organisms to the risk of infections that reduce lifespan (Bonneaud et al. 2004).
The blue-footed booby, Sula nebouxii is an iteroparous long-lived tropical seabird with lengthy biparental care of progeny (up to six months; Torres & Drummond 1999; Drummond et al. 2003). In this species, reproductive success is affected by the amount of parental care (incubation, defence and chick feeding) provided by the male (Guerra & Drummond 1995; Velando & Alonso-Alvarez 2003). Using long-term data, we first analysed how reproductive success of individuals changes over their lifespans, to test whether residual reproductive value declines with advancing age. Second, to test whether reproductive effort is calibrated to residual reproductive value, we examined how age (and reproductive prospects) affects adjustment of investment in current reproduction after activation of the immune system with lipopolysaccharide (LPS), a cell wall component of Gram-negative bacteria (Raetz & Whitfield 2002). LPSs can mimic a bacterial infection without producing the negative effects of pathogens, yet elicit physiological responses to overcome infection (Bonneaud et al. 2003). In birds, the acute phase after LPS injection lasts a few days and the physiological responses end within two weeks (Nakamura et al. 1998; Xie et al. 2000). The acute phase of an immune response imposes immediate and unavoidable physiological costs and obligates diversion of resources to meet the costs, producing a perception of health deterioration (Martin et al. 2003; Bonneaud et al. 2003). To study adjustment of investment during breeding, we manipulated the immune system of pair bonded blue-footed booby males before laying. We decided to manipulate males because parental investment decisions occur later in males than in females probably after the start of incubation. Hence, an immune challenge before laying should allow males enough time to recover from the physiological costs of mounting a defence, before adjusting allocation between current and future reproduction. If immune activation is a cue to declining reproductive prospects, we predicted that immune challenged older males, but not mature males, would increase their reproductive effort and success.
2. Material and methods
(a) Long-term study
Longitudinal studies are needed to test individual reproductive senescence because in cross-sectional studies, apparent changes in reproduction with age can be due to differential mortality of individuals that invest heavily in reproduction (Reid et al. 2003). To describe changes in male reproductive performance over the lifetime, we used longitudinal data from two cohorts of fledglings in our long-term study of the blue-footed booby colony on Isla Isabel, Nayarit, Mexico. The study was carried out in two areas of a single colony measuring 20 800 and 6089 m2, respectively and lying roughly 400 m apart (Drummond et al. 2003). In every season from 1988 to 2003, all breeding attempts in the study area were monitored until the end of the fledging period (roughly five months each year). Laying date, clutch size, number of hatchlings and survival of chicks were recorded until most chicks reached age 70 days (close to fledging; Drummond et al. 2003). Chicks were individually marked within 3 days of hatching and ringed at age 70 days. At every nest, parents' ring numbers were recorded. Blue-footed boobies on Isla Isabel are highly philopatric and faithful to their nesting neighbourhoods (Osorio-Beristain & Drummond 1993), so we were able to record reproductive histories of all recruits to the breeding colony. For longitudinal analysis, only ringed fledglings that survived the whole 13 years (from the two available cohorts) were used. All individuals used in the longitudinal analysis bred repeatedly in the two study areas.
The average number of fledglings produced by all nesting pairs in the monitored areas was used as an estimation of the annual reproductive success of the colony. To remove year-effects, individual reproductive success of focal males was calculated as the z-normal standardized residuals from annual reproductive success of the colony (1988–2003) and 1992 was excluded because no chicks fledged, due to an El Niño event. The degree of non-independence due to correlation of female and male identity across the duration of the pair bond was relatively minor; in our sample, the duration of pair bonds was 1.7±0.15 years (mean±s.e., estimated from 261 pairs from our sample where both members were ringed).
Age-dependent variation in individual reproductive success was analysed using PROC MIXED in SAS (SAS Institute 1999) with individual birds within cohorts and areas as repeated measures factors with missing values (for 1992) and the study area as random factor, controlling for heterogeneous cohort variances and using the Satterthwaite approximation for the denominator degrees of freedom (Littell et al. 1996). Statistical significance of the random effects was assessed using changes in the likelihood ratio (−2 log-likelihood) of the model with and without the random effect. This difference is distributed as χ12 (Littell et al. 1996).
(b) Immune activation experiment
In 2004, 50 courting males were captured at the study area and randomly assigned to one of two treatment groups. Males were captured on average 13 days before laying (range 2–31 days). Experimental males had their immune system activated by intraperitoneal injection of 0.1 mg of LPS (LPS of Escherichia coli, serotype 055 : B5) in 1 ml of PBS (phosphate buffered saline solution). To avoid provoking nest desertion (Bonneaud et al. 2003), we chose a concentration lower than concentrations used to activate the immune system of poultry (0.5–5 mg kg−1 of body weight; Nakamura et al. 1998; Xie et al. 2000). Control males were injected with an identical volume of PBS. LPS causes inflammation and sickness, triggering diverse immune cells, which in turn produce several pro- and anti-inflammatory hormones and cytokines that regulate different metabolic responses and cause behavioural changes, fever and cachexia, before eventual recovery (Hadden 1993; Nakamura et al. 1998; Xie et al. 2000; Bonneaud et al. 2003). These responses increase the probability of overcoming infection, but induce important physiological costs and produce in the organism a perception of health deterioration.
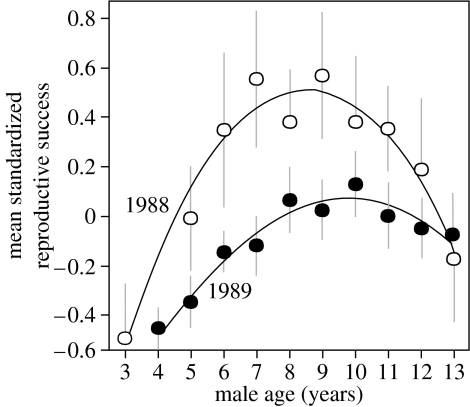
Based on the results of the longitudinal analyses showing that reproductive success decreases around 10 years of age (see figure 1), we classified experimental males in two age classes: 3–9 years (mature males, n=35) or greater than or equal to 10 years (old males, n=15). Although all males in the experiment had been ringed as part of the long-term study (most of them as fledglings), 36% of them were ringed at recruitment, which typically occurs at age 3–7 years (95% of 1180 ringed recruits in the data base). These males could not be aged precisely but each one could be assigned to one of our two age classes: the eight males that were recruited between 1993 and 1997 were considered old and the 10 males that were recruited between 2002 and 2004 were considered mature. Age groups included members of different cohorts, making it unlikely that our results reflect cohort effects rather than age effects. Initial masses of the four treatment groups did not differ (age, F1,46=0.003, p=0.95; treatment, F1,46=0.003, p=0.96; interaction, F1,46=2.69, p=0.11).
Figure 1.
Age-dependent reproductive success of blue-footed booby males from two cohorts. Reproductive success (fledglings produced per pair) was estimated as the standardized residuals from annual reproductive success of the colony. Showing longitudinal data on 13 males from cohort 1988 and 63 males from cohort 1989, which were ringed as chicks and survived 13 years. Individual reproductive performance varied with age (F1,179=21.69, p<0.0001) and age2 (F1,174=18.57, p<0.0001) and this effect was more pronounced in males hatched in 1988, although the interaction was no significant (cohort×age2, F1,174=2.67, p=0.10; cohort×age, F1,179=2.13, p=0.14; cohort, F1,195=0.49, p=0.48; male identity, p<0.01; study area, p>0.5).
The study area was monitored every other day to register laying dates and clutch sizes and then the monitoring programme recorded the numbers of hatchlings and fledglings produced at each male's nest. Breeding experience, measured as the number of previous breeding attempts and previous reproductive output, measured as the number of fledglings previously produced were included in the analyses as covariates. All 50 captured males were included in the analyses and similar results were obtained when only pairs that laid eggs (n=42) were included (data not shown). Data were analysed using a general linear modelling technique (PROC GENMOD in SAS) with Poisson errors and a natural logarithm link for count data (clutch size, number of hatchlings and fledglings and breeding experience) and normal errors and identity link for laying data, with scale correction for over dispersed data (SAS Institute 1999). All main factors and two-way interactions were included in the initial model and final models were obtained using a backward elimination procedure. Similar results were achieved when age was included in the analysis as a continuous variable using the sub-sample of males for which the exact age is known (n=32; data not shown).
3. Results
In the long-term study, individual blue-footed booby males showed a steady increase in reproductive performance until the tenth year, followed by progressive decline (statistical tests in the figure legend; figure 1). On average, cohort 1988 was threefold more successful than cohort 1989 and the effects of age on reproduction were cohort dependent: cohort 1988 showed a steeper increase and decrease in reproductive success than cohort 1989, although this effect was not significant (figure 1).
In the experiment, neither laying date nor clutch size were affected by activation of the immune system of males. Laying date was not affected by treatment, male age, previous male breeding experience or previous reproductive output (treatment, F1,34=0.49, p=0.48; age, F1,37=3.2, p=0.09; treatment×age, F1,33=0.16, p=0.69; breeding experience, F1,36=0.25, p=0.61; previous reproductive output, F1,35=0.67, p=0.41). The number of eggs laid by females increased with male breeding experience up to the fifth breeding attempt and then decreased (breeding experience, β=0.26, p=0.02; breeding experience2, β=−0.034, p=0.006), but was unaffected by treatment, male age or total number of fledglings previously produced (treatment, F1,44=0.00, p=0.99; age, F1,46=0.25, p=0.62; treatment×age, F1,43=1.79, p=0.18; previous reproductive output, F1,45=0.07, p=0.79).
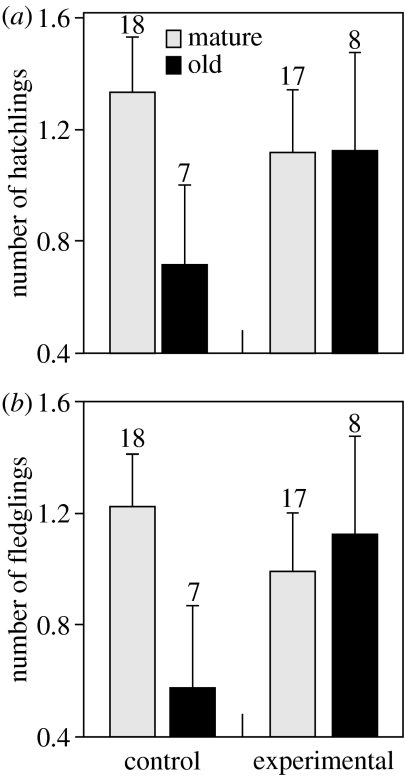
However, hatching success and nest success were substantially affected by activation of the immune system of males. Compared to controls, experimental mature males produced 16% fewer hatchlings, whereas experimental old males produced 59% more and this interaction was significant (figure 2a; statistical tests in figure legend). Production of fledglings showed a similar but more exaggerated pattern. Compared with control males, experimental mature males decreased production by 18% and experimental old males increased production by 98% (figure 2b). Notably, the only treatment group where all hatchlings survived and fledged was that of old males facing an immune challenge. The striking difference in the reproductive output of experimental old males and control old males cannot be attributed to chance differences in their previous reproductive experience, because the two groups did not differ either in previous breeding experience (F1,13=0.64, p=0.44) or total number of fledglings previously produced (F1,13=0.05, p=0.83).
Figure 2.
Reproductive success (mean+s.e.) of control (PBS) and experimental (LPS) mature and old males. Experimental treatment had a different effect according to male age on (a) the number of hatchlings (treatment, F1,44=2.25, p=0.14; age, F1,44=0.90, p=0.34; treatment×age, F1,44=4.28, p=0.044) and (b) the number of fledglings (treatment, F1,44=2.95, p=0.09; age, F1,44=0.25, p=0.62; treatment×age, F1,44=5.35, p=0.025). Male breeding experience, included in the analyses, had a strong quadratic effect on the number of hatchlings (breeding experience, F1,44=14.49, p=0.0004; breeding experience2, F1,44=17.44, p=0.0001) and on the number of fledglings (breeding experience, F1,44=9.31, p=0.004; breeding experience2, F1,44=13.05, p=0.0008). Previous reproductive output did not influence the number of hatchlings (F1,43=1.34, p=0.25) or fledglings (F1,43=0.81, p=0.37). Sample size of each group is indicated.
4. Discussion
This study documents reproductive senescence in the blue-footed booby and reports an experimental confirmation of the terminal investment hypothesis, the key idea of life-history theory that long-lived animals increase reproductive effort when their capacity to reproduce declines with senescence (Williams 1966; Trivers 1972; Pianka & Parker 1975). The longitudinal data indicated that, after initial increase, reproductive success of male boobies declines progressively with age. The decline in male performance with advancing age may result from senescent males being worse parents than younger males, because their condition declines with age or because older males pair with low-quality females or both (Jones et al. 2000). Additionally, older males may be less fertile, suffer from accumulation of deleterious germ-line mutations or suffer from lower genetic quality due to antagonistic pleiotropic effects (Hansen & Price 1995; Brooks & Kemp 2001; Kidd et al. 2001; Radwan 2003). Our data do not allow us to evaluate the proximal causes of the observed decline in reproductive success with age; nonetheless, independently of the causal pathway, the longitudinal analysis clearly indicates that the residual reproductive value of males varies with age and declines steeply after the 10th year of life. In the experimental study, the cross-sectional comparison (between controls) is consistent with results of the longitudinal study, indicating that senescents have only half the reproductive potential of mature animals. Hence, although it has been thought that long-lived birds show negligible reproductive senescence (Holmes et al. 2001; but see Bennett & Owens 2002), when individual data are available ageing is detectable in a wild population of a long-lived bird.
In the experiment, reproductive success of mature males fell, while that of old males showed a strong increase when they were challenged with LPS to elicit an immune response and induce symptoms of declining survival prospects. Although female boobies may compensate for changes in male effort, this compensation is limited and when male contribution decreases females preferentially allocate resources to maintenance of their body condition at the expense of investment in current reproduction (Velando & Alonso-Alvarez 2003). Thus, the striking increase in the reproductive output of old males injected with LPS could be attributed to changes in male reproductive effort. We did not measure male effort directly, but the treatment groups did not differ in their clutch sizes, suggesting that the critical effect emerged during incubation and brood care. Importantly, increased incubation by male blue-footed boobies reduces the probability of breeding failure (García-Peña 2005) and growth of chicks is highly dependent on male investment, especially in the first two weeks of life (Guerra & Drummond 1995; Velando & Alonso-Alvarez 2003; Velando et al. 2005).
Our results suggest that reproductive decision rules of individuals depend on their residual reproductive value. Experimental manipulation of short-term physiological costs and perceived health risk (Bonneaud et al. 2004) elicited different allocation strategies according to the expected future production of offspring. Consistent with life-history theory, males between 3 and 9 years old appeared to reduce their present reproductive effort when future reproductive prospects were threatened. Similarly, in previous experiments blue-footed booby males of unknown age allocated resources to the maintenance of body condition at the expense of investment in current reproduction when the effort demanded was increased by brood enlargement (Velando & Alonso-Alvarez 2003). By contrast, when the immune system of our older males was activated, those males followed the reverse strategy, allocating substantially more resources to current progeny.
In other studies, experimental imposition of adverse conditions on individuals of unknown age has yielded different results, with reproductive effort increasing in some cases (e.g. Fox & McCoy 2000; Bonneaud et al. 2004; Javois & Tammaru 2004) and decreasing in others (e.g. Ilmonen et al. 2000; Råberg et al. 2000; Bonneaud et al. 2003). These apparently contradictory findings may be partly due to lack of control of residual reproductive value of treated individuals. Our study shows that analysis of adaptive responses in life-history strategies often requires controlling for age and testing animals of different ages and reproductive prospects, as predicted by the terminal investment hypothesis.
Among controls, old males produced fewer offspring than mature males, consistent with results of the longitudinal study. Interestingly, by provoking old males to boost their effort during incubation and brood care, we also revealed that old males normally restrict their current reproductive effort, presumably in favour of future reproduction. Although the reproductive prospects of older males are poor, in unpredictable environments it may ordinarily be a good strategy to allocate investment across several reproductive events rather than a single, terminal event (Goodman 1979; Stearns 1992; Monson et al. 2000). Nevertheless, when an important health risk arises and further diminishes an older male's future prospects, his lifetime reproductive success may be better served by investing maximally in his current progeny.
Although the force of natural selection probably declines with adult age (Charlesworth 1994), our results indicate that old males show phenotypic plasticity in parental investment in response to perceived risk of extrinsic mortality. Parasitism is likely to be one of the major extrinsic causes of mortality among wild animals (Grenfell & Dobson 1995) and the immune system is the most important physiological mechanism of anti-parasite defence (Pastoret et al. 1998). Our study highlights the role played by the immune system in mediating facultative adjustment of reproductive investment. In addition, our data show that immune-mediated flexibility of reproductive decisions is apparently adjusted according to residual reproductive value. Plasticity is expected to be favoured when the environment of a genotype is unpredictable on a time scale that is short relative to generation time (de Jong 1995), as occurs in the blue footed-booby. In long-lived animals, individual variation in life-history traits probably reflects facultative responses to complex interactions between perceived risks and residual reproductive value.
Acknowledgments
We are grateful to H. Kokko, P. Monaghan & N. Metcalfe for useful comments on the manuscript and to René Beamonte for helping during field work. We are also grateful to José Luis Osorno and Cristina Rodríguez who made essential contributions to the long-term fieldwork and database and to the many people who collected data during all these years. The long-term study received financial support from UNAM (PAPIIT, IN230603, IN211491, IN2007023), CONACYT (34500-V, 4722-N9407, D112-903581, PCCNCNA-031528, 31973H), the National Geographic Society (3065–85, 4535–91) and the Conservation and Research Foundation. Permissions were provided by SEMARNAT and logistical support by the Armada de México, the staff of the Parque Nacional Isla Isabel and the fisherman of San Blas and Camichin. A.V. was supported by a ‘Ramón y Cajal’ fellowship from the Spanish Ministerio de Ciencia y Tecnología and a travel grant from the Universidade de Vigo.
References
- Bennett P.M, Owens I.P.F. Oxford University Press; Oxford, UK: 2002. Evolutionary ecology of birds. [Google Scholar]
- Bonneaud C, Mazuc J, Gonzalez G, Haussy C, Chastel O, Faivre B, Sorci G. Assessing the cost of mounting an immune response. Am. Nat. 2003;161:367–379. doi: 10.1086/346134. doi:10.1086/346134 [DOI] [PubMed] [Google Scholar]
- Bonneaud C, Mazuc J, Chastel O, Westerdahl H, Sorci G. Terminal investment induced by immune challenge and fitness traits associated with major histocompatibility complex in the house sparrow. Evolution. 2004;58:2823–2830. doi: 10.1111/j.0014-3820.2004.tb01633.x. [DOI] [PubMed] [Google Scholar]
- Brooks R, Kemp D.J. Can older males deliver the good genes? Trends Ecol. Evol. 2001;16:308–313. doi: 10.1016/s0169-5347(01)02147-4. doi:10.1016/S0169-5347(01)02147-4 [DOI] [PubMed] [Google Scholar]
- Charlesworth B. Cambridge University Press; Cambridge, UK: 1994. Evolution in age-structured populations. [Google Scholar]
- Clutton-Brock T.H. Reproductive effort and terminal investment in iteroparous animals. Am. Nat. 1984;123:212–229. doi:10.1086/284198 [Google Scholar]
- Curio E. Why do young birds reproduce less well? Ibis. 1983;125:400–404. [Google Scholar]
- de Jong G. Phenotypic plasticity as a product of selection in a variable environment. Am. Nat. 1995;145:493–512. doi: 10.1086/285542. doi:10.1086/285752 [DOI] [PubMed] [Google Scholar]
- Drummond H, Torres R, Krishnan V.V. Buffered development: resilience after aggressive subordination in infancy. Am. Nat. 2003;161:794–807. doi: 10.1086/375170. doi:10.1086/375170 [DOI] [PubMed] [Google Scholar]
- Ericsson G, Wallin K, Ball J.P, Broberg M. Age-related reproductive effort and senescence in free-ranging moose, Alces alces. Ecology. 2001;82:1613–1620. [Google Scholar]
- Fessler D.M.T, Navarrete C.D, Hopkins W, Izard M.K. Examining the terminal investment hypothesis in humans and chimpanzees: associations among maternal age, parity, and birth. Am. J. Phys. Anthropol. 2005;127:95–104. doi: 10.1002/ajpa.20039. doi:10.1002/ajpa.20039 [DOI] [PubMed] [Google Scholar]
- Fox S.F, McCoy J.K. The effects of tail loss on survival, growth, reproduction, and sex ratio of offspring in the lizard Uta stansburiana in the field. Oecologia. 2000;122:327–334. doi: 10.1007/s004420050038. doi:10.1007/s004420050038 [DOI] [PubMed] [Google Scholar]
- García-Peña, G. 2005 Efectos y costos de la termorregulación durante la incubación del ave marina Sula nebouxii Master's Degree thesis, Universidad Nacional Autónoma de México.
- Goodman D. Regulating reproductive effort in a changing environment. Am. Nat. 1979;113:735–748. doi:10.1086/283429 [Google Scholar]
- Grenfell B.T, Dobson A.P. Cambridge University Press; Cambridge, UK: 1995. Ecology of infectious diseases in natural populations. [Google Scholar]
- Guerra M, Drummond H. Reversed sexual size dimorphism and parental care: minimal division of labour in the blue-footed booby. Behaviour. 1995;132:479–496. [Google Scholar]
- Hadden J.W. Immunostimulants. Immunol. Today. 1993;14:275–280. doi: 10.1016/0167-5699(93)90045-M. doi:10.1016/0167-5699(93)90045-M [DOI] [PubMed] [Google Scholar]
- Hansen T.F, Price D.K. Good genes and old age: do old mates provide superior genes? J. Evol. Biol. 1995;8:759–778. doi:10.1046/j.1420-9101.1995.8060759.x [Google Scholar]
- Hanssen S.A, Hasselquist D, Folstad I, Erikstad K.E. Costs of immunity: immune responsiveness reduces survival in a vertebrate. Proc. R. Soc. B. 2004;271:925–930. doi: 10.1098/rspb.2004.2678. doi:10.1098/rspb.2004.2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfield M.F, Tinkle D.W. Natural selection and the evolution of reproductive effort. Proc. Natl Acad. Sci. USA. 1975;72:2227–2231. doi: 10.1073/pnas.72.6.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D.J, Flückiger R, Austad S.N. Comparative biology of aging in birds: an update. Exp. Gerontol. 2001;36:869–883. doi: 10.1016/s0531-5565(00)00247-3. doi:10.1016/S0531-5565(00)00247-3 [DOI] [PubMed] [Google Scholar]
- Ilmonen P, Taarna T, Hasselquist D. Experimentally activated immune defense in female pied flycatchers results in reduced breeding success. Proc. R. Soc. B. 2000;267:665–670. doi: 10.1098/rspb.2000.1053. doi:10.1098/rspb.2000.1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javois J, Tammaru T. Reproductive decisions are sensitive to cues of life expectancy: the case of a moth. Anim. Behav. 2004;68:249–255. doi:10.1016/j.anbehav.2003.10.022 [Google Scholar]
- Jones T.M, Balmford A, Quinell R. Adaptive female choice for middle-aged mates in a lekking sandfly. Proc. R. Soc. B. 2000;267:681–686. doi: 10.1098/rspb.2000.1056. doi:10.1098/rspb.2000.1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd S.A, Eskeazi B, Wyrobek A.J. Effects of male age on semen quality and fertility: a review of the literature. Fertil. Steril. 2001;75:237–248. doi: 10.1016/s0015-0282(00)01679-4. doi:10.1016/S0015-0282(00)01679-4 [DOI] [PubMed] [Google Scholar]
- Littell R.C, Milliken G.A, Stroup W.W, Wolfinger R.D. SAS Institute; Cary, NC: 1996. SAS system for mixed models. [Google Scholar]
- Martin L.B, II, Scheuerlein A, Wikelski M. Immune activity elevates energy expenditure of house sparrows: a link between direct and indirect costs? Proc. R. Soc. B. 2003;270:153–158. doi: 10.1098/rspb.2002.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson D.H, Estes J.A, Bodkin J.L, Siniff D.B. Life history plasticity and population regulation in sea otters. Oikos. 2000;90:457–468. doi:10.1034/j.1600-0706.2000.900304.x [Google Scholar]
- Morrow E.H, Arnqvist G, Pitnick S. Adaptation versus pleiotropy: why do males harm their mates? Behav. Ecol. 2003;14:802–806. doi:10.1093/beheco/arg073 [Google Scholar]
- Nakamura K, Mitarai Y, Yoshioka M, Koizumi N, Shibahara T, Nakajima Y.S. Serum levels of interleukin-6, α1-acid glycoprotein, and corticosterone in two-week-old chickens inoculated with Escherichia coli lipopolysaccharide. Poult. Sci. 1998;77:908–911. doi: 10.1093/ps/77.6.908. [DOI] [PubMed] [Google Scholar]
- Osorio-Beristain M, Drummond H. Natal dispersal and deferred breeding in the blue-footed booby. Auk. 1993;110:234–239. [Google Scholar]
- Pastoret P, Gabriel P, Bazin H, Govaerts A. Academic Press; San Diego, CA: 1998. Handbook of vertebrate immunology. [Google Scholar]
- Pärt T, Gustafsson L, Moreno J. ‘Terminal investment’ and a sexual conflict in the collared flycatcher (Ficedula albicollis) Am. Nat. 1992;140:868–882. doi: 10.1086/285445. doi:10.1086/285445 [DOI] [PubMed] [Google Scholar]
- Pianka E.R, Parker W.S. Age-specific reproductive tactics. Am. Nat. 1975;109:453–464. doi:10.1086/283013 [Google Scholar]
- Poizat G, Rossechi E, Crivelli A.J. Empirical evidence of a trade-off between reproductive effort and expectation of future reproduction in female three-spined sticklebacks. Proc. R. Soc. B. 1999;266:1543–1548. doi:10.1098/rspb.1999.0813 [Google Scholar]
- Radwan J. Male age, germline mutations and the benefits of polyandry. Ecol. Lett. 2003;6:581–586. doi:10.1046/j.1461-0248.2003.00484.x [Google Scholar]
- Raetz C.R.H, Whitfield C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. doi:10.1146/annurev.biochem.71.110601.135414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Råberg L, Nilsson J.A, Ilmonen P, Stjernman M, Hasselquist D. The cost of an immune response: vaccination reduces parental effort. Ecol. Lett. 2000;3:382–286. doi:10.1046/j.1461-0248.2000.00154.x [Google Scholar]
- Reid J.M, Bignal E.M, McCracken D.I, Monaghan P. Age-specific reproductive performance in red-billed choughs Pyrrhocorax pyrrhocorax: patterns and processes in a natural population. J. Anim. Ecol. 2003;72:765–776. doi:10.1046/j.1365-2656.2003.00750.x [Google Scholar]
- Reznick D. Measuring the costs of reproduction. Trends Ecol. Evol. 1992;7:42–45. doi: 10.1016/0169-5347(92)90104-J. doi:10.1016/0169-5347(92)90150-A [DOI] [PubMed] [Google Scholar]
- Institute SAS. SAS Institute; Cary, NC: 1999. SAS/STAT Version 8. [Google Scholar]
- Sæther B.E. Age-specific variation in reproductive performance of birds. Curr. Ornithol. 1990;7:251–283. [Google Scholar]
- Sheldon B.C, Verhulst S. Ecological immunology: costly parasites defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. doi:10.1016/0169-5347(96)10039-2 [DOI] [PubMed] [Google Scholar]
- Stearns S.C. Oxford University Press; New York, NY: 1992. The evolution of life histories. [Google Scholar]
- Torres R, Drummond H. Does large size make daughters of the blue-footed booby more expensive than sons? J. Anim. Ecol. 1999;68:1–10. doi:10.1046/j.1365-2656.1999.00357.x [Google Scholar]
- Trivers R.L. Parental investment and sexual selection. In: Campbell B, editor. Sexual selection and the descent of man. Aldine Press; Chicago, IL: 1972. pp. 136–179. [Google Scholar]
- Velando A, Alonso-Alvarez C. Differential body condition regulation by males and females in response to experimental manipulations of brood size and parental effort in the blue-footed booby. J. Anim. Ecol. 2003;72:846–856. doi:10.1046/j.1365-2656.2003.00756.x [Google Scholar]
- Velando A, Torres R, Espinosa I. Male coloration and chick condition in blue-footed booby: a cross-fostering experiment. Behav. Ecol. Sociobiol. 2005;58:175–180. doi:10.1007/s00265-005-0911-0 [Google Scholar]
- Weladji R.B, Mysterud A, Holand O, Lenvik D. Age-related reproductive effort in reindeer (Rangifer tarandus): evidence of senescence. Oecologia. 2002;131:79–82. doi: 10.1007/s00442-001-0864-6. doi:10.1007/s00442-001-0864-6 [DOI] [PubMed] [Google Scholar]
- Williams G.C. Natural selection, the costs of reproduction, and a refinement of Lack's principle. Am. Nat. 1966;100:687–690. doi:10.1086/282461 [Google Scholar]
- Xie H, Rath N.C, Huff G.R, Huff W.E, Balog J.M. Effects of Salmonella typhimurium lipopolysaccharide on broiler chickens. Poult. Sci. 2000;79:33–40. doi: 10.1093/ps/79.1.33. [DOI] [PubMed] [Google Scholar]
- Yoccoz N.G, Mysterud A, Langvatn R, Stenseth N.C. Age- and density-dependent reproductive effort in male red deer. Proc. R. Soc. B. 2002;269:1523–1528. doi: 10.1098/rspb.2002.2047. doi:10.1098/rspb.2002.2047 [DOI] [PMC free article] [PubMed] [Google Scholar]