Abstract
Kin-based societies, where families represent the basic social unit, occur in a relatively small number of vertebrate species. In the majority of avian kin societies, families form when offspring prolong their association with the parents on the natal territory. Therefore, the key to understanding the evolution of families in birds is to understand natal philopatry (i.e. the tendency to remain on the natal territory). It has been shown that, within populations, the strength of the association between parents and offspring (i.e. family stability) increases when offspring dispersal is constrained by external environmental factors, but it is unclear whether and how family wealth influences juvenile dispersal decisions. Here, we show that young carrion crows (Corvus corone corone) from territories that were food-supplemented year-round were more philopatric and more likely to help at their family's nest than the unfed ones. The results suggest that offspring philopatry and helping behaviour are influenced by the quality of ‘home’ and that the availability of food resources positively affects the cohesion of the family.
Keywords: philopatry, wealth, cooperative breeding, territory quality, carrion crow
1. Introduction
In most animal species, including birds, families usually form when offspring delay natal dispersal and continue to interact with their parents on the natal territory past the age of independence. As non-dispersing offspring usually postpone reproduction while prolonging their association with their parents, typically to avoid inbreeding, the evolutionary theory of the family (Emlen 1995) predicts that families are inherently unstable, because offspring will tend to leave the family territory to secure their own reproduction. However, family stability may be strengthened when (i) external environmental factors such as lack of suitable breeding vacancies, lack of mates or predation risk constrain juvenile dispersal; (ii) families control high-quality resources and non-dispersing offspring hence gain access to them. The latter prediction is of special interest, as resource abundance (wealth hereafter) is commonly believed to be a crucial factor that drives most aspects of social behaviour and social organization in a large variety of organisms, from invertebrates to humans.
The importance of environmental factors in constraining dispersal is supported by a number of studies on vertebrate species (Koenig & Stacey 1990; Luck 2001; Russell 2001) that include elegant experiments on birds and fishes (Pruett-Jones & Lewis 1990; Komdeur 1992; Walters et al. 1992; Heg et al. 2004). Conversely, the importance of resource abundance has received only correlative support (Zack & Ligon 1985; Stacey & Ligon 1991; Komdeur 1992; Luck 2001; Putland & Goldizen 2001; Funston et al. 2003) and has been questioned both theoretically and empirically, since in some species an increase in territory quality either does not affect philopatry (Cochran & Solomon 2000) or, in contrast to the prediction of the theory of the family (Emlen 1995), results in early offspring dispersal (Leturque & Rousset 2003; Russell 2004).
In the carrion crow Corvus corone, a common bird widely distributed over Eurasia, families are known to occur in northern Spain, where offspring often remain linked to their natal territory typically for 1 or 2 years (exceptionally up to 4 years) and help their parents to rear new young (Baglione et al. 2002). As in most cooperative bird species (Brown 1987), the role of territory in the formation of the family is central, because in the carrion crow, the family territory is the only place where parents and offspring interact socially. Within the territory, parents and offspring form cohesive groups, foraging and sheltering close to each other, and cooperate in rearing a single brood and in defending the territory's boundaries year-round (Baglione et al. 2002; Canestrari et al. 2005). In contrast, family relationships are negligible outside the territory, because (i) parents rarely leave it (Baglione et al. 2005) and (ii) family members do not associate when they leave the territory for prospecting trips or temporary visits to communal feeding areas (see §2a). Therefore, in crows, juvenile philopatry (i.e. the tendency to remain on the natal territory) is the key to the association between parents and offspring. However, the amount of time spent on the natal territory, and therefore interacting with the parents, is highly variable among young crows. In this study, we experimentally increased the quality of a number of territories through long-term food supplementation to test whether offspring from wealthy territories (fed) were more philopatric than controls (unfed), as expected if wealth strengthened family bonds. Furthermore, we investigated how supplementary feeding ultimately influenced offspring contribution to nestling care at the family nest. As offspring must trade off the time spent roaming outside the natal territory against the time spent at ‘home’, which can be allocated to help their parents (e.g. Young et al. 2005), the most philopatric individuals have more opportunity to contribute to chick provisioning at the family nest. By increasing philopatry and by possibly enhancing physical condition (Heinsohn & Legge 1999), food supplementation is therefore expected ultimately to promote helping behaviour.
2. Material and methods
(a) Study population and area
We have been studying a population of crow in a 45 km2 rural area close to León (northern Spain, 43° N, 5° W) since 1995. Unlike most European populations, approximately 75% of the territories in this population are held by enlarged families (three to nine individuals) that comprise parents, philopatric offspring and/or immigrants that are closely related to the dominant adult of the same sex (Baglione et al. 2003). Cooperative breeding, where at least one helper assists the breeders in rearing the nestlings, occurs in virtually every territory containing a group with more than two birds, although some group members may refrain from helping at the nest (Canestrari et al. 2005). The carrion crow is a single-brooded species, which breeds between April and July. Brood size varies between one and five nestlings. Re-nesting within the same season occurs frequently when the first breeding attempt fails at the egg stage, resulting in asynchrony of fledging among territories (late May to middle July). For further details on the life history of the species, see Cramp & Perrins (1994).
(b) Group living and parent–offspring association
Within the territory (average maximum diameter±s.e.=494±24.4 m, n=21), families are very cohesive (Baglione et al. 2002). While foraging, group members remain in close proximity to one another, with no difference in the average distance between an offspring and the closest parent (mean±s.e.=20.5±4.1 m), between parents (24.6±6.1 m) and between parents and immigrants (27.4±13.9 m; Kruskal-Wallis non-parametric ANOVA, H=0.24, n1=15, n2=8, n3=6, p=0.89; data collected in 36 h of observation from eight banded groups, scanning distances among individuals every 10 min).
Unlike elsewhere in Europe, territories are occupied and defended year-round in the studied population. Parents spend most of the daylight time in their territories and leave them only occasionally in winter for short visits (typically a few hours) to communal feeding areas. Baglione et al. (2005) found that parents were at home in 85% of 256 surveys of 30 territories throughout the non-breeding season.
Further data collected in 2004 confirmed that the family territory is the ‘arena’ where parents and offspring socially interact and that juvenile philopatry is, therefore, the key to family cohesion. In 11 territories, all offspring (n=17) and one parent (n=11) were equipped with a radio-transmitter (see §2c) and radio-tracked three times a week throughout the non-breeding season. Radio contacts were always followed by search and direct-sighting of birds. Out of the 386 observations of parents and offspring together, 97% occurred in the family territory. When both parent and offspring temporarily left the territory (61 cases), they usually moved independently, reaching different places in 82% of cases. Often the adult remained on the territory while the offspring temporarily left (59 cases), although the reverse also happened (24 cases).
(c) Experimental design
In 2003, we banded all 51 nestlings from 21 territories (table 1) with wing tags (Caffrey 2000) and radio-transmitters just before they fledged. Radio-transmitters (Holohil RI-2B, battery maximum life 18 months) weighted 11 g, corresponding to 2.4% of average body weight of crow, and were attached with a leg harness made of 3 mm silicon tubing. The sex of the fledglings was determined by using P2/P8 molecular sexing method (Griffiths et al. 1998) on DNA samples extracted from blood (Baglione et al. 2003).
Table 1.
Sample sizes (number of territories in brackets) and contribution to chick provisioning by fed and unfed young crows.
| fed | unfed | |
|---|---|---|
| number of radio-tagged offspring | 26 (10) | 25 (11) |
| number of non-radio-tagged offspring | 0 | 16 (7) |
| number of offspring alive in the next breeding season (April 2004) | 8 (5) | 14 (9) |
| number of offspring with an active nest on the natal territory in the next breeding season (April 2004) | 7 (4) | 11 (7) |
| average feedings per hour (±s.e.) | 2.16±0.37; n=7 | 0.27±0.24; n=11 |
Immediately after the young had fledged, we started a long-term food supplementation in 10 randomly chosen territories (26 fledglings). The remaining 11 territories (25 fledglings) were kept unfed and served as controls. In the experimental territories, supplementary food consisted of 400 g of canned dog food (a commercial mixture of meat and vegetables containing all valuable nutrients and very palatable for crows) plus 200 g of corn per each potential group member three times a week. The energetic value of supplementary food was 1105 kJ for a bird per day, corresponding to approximately 135% of daily energetic expenditure (Nagy et al. 1999). After habituation to the supplementary food, which never took more than one week, family members typically arrived together at the feeding spot as soon as we left and they stored the food within the territory in ca 20 min. By defending the territory communally, crows effectively prevent conspecific intrusions onto their territories. By placing the food in the core of the territories, we avoided disputes with adjacent family groups. Video-recorded observations at each feeding spot confirmed that target crows were actually those consuming the food and that all group members had access to it. Parent–offspring aggression was invariably negligible. Food supplementation was continued until a new brood was present in the following breeding season and for the duration of that brood until we estimated the contribution of yearlings to chick provisioning (end of April and early May 2004). The duration of the experiment was considered to be the best compromise between (i) examining juvenile movements during a period of high natural variability in philopatry (Baglione et al. 2002); (ii) recording helping behaviour at the family nest; (iii) not exceeding the timing of highest efficiency of radio-transmitters to fully control for mortality of banded crows and (iv) keeping the logistics of conducting the fieldwork and experiment manageable.
(d) Philopatry
In our study population, young crows typically use the natal territory as a base for their movements elsewhere, which vary largely in frequency and duration, and are directed either towards new territories or flocks of non-territorial birds. Juveniles that leave the territory may return even after prolonged periods of absence (up to 31 days for radio-tagged juveniles in 2003, but up to five months in the previously documented cases). Under these circumstances, a dichotomous classification of juveniles into ‘dispersers’ and ‘philopatric’, commonly used in studies on birds, would be arbitrary and biologically questionable. A continuous variable, such as the proportion of time spent on the natal territory, is far more appropriate to grasp the individual variability in philopatry. Most important in the context of this study, this variable represents the most direct measure of the strength of the social relationship between parents and offspring. Parents are indeed found only on the natal territory in crows and kin invariably form cohesive groups when they are inside their territory (see §2b). The time spent in the territory is, therefore, the most precise measure of how much offspring interact with their parents.
From chick fledging (June–July 2003) until April 2004, we visited all the territories three times a week to check whether juveniles were at home or elsewhere. Radio-tracking was carried out during the central hours of the day (from 10.00 to 14.00) to avoid including movements to and from the communal roosts in our analysis.
A combination of radio-tracking and visual searches for banded crows allowed us to circumvent virtually any possible confusion between mortality and dispersal. On each territory, all radio locations were followed by search with binoculars and spotting scopes to check if the birds were alive. Radio-tagged crows that died in their territory were always easily found (n=28). Our radio-tracking equipment allowed the reception of the signal within 3–12 km, depending on the morphology of the landscape. This range largely exceeded the average maximum diameter of the territories (494±24.4 m) so that ‘false absences’ were negligible in our dataset.
When a juvenile was missing from its natal territory, we intensively searched for it within and outside the study area. Again, we always tried to see the birds after radio location. When we failed to locate a bird, we ‘quarantined’ that particular data point until we obtained direct information on the fate of the crow, i.e. until we saw it alive or we found the cadaver (n=6). In all but one case this happened within 1–6 days. The exception was one control crow (unfed) that disappeared from the territory and was never seen again. A few radio-contacts suggested that this could have been a case of long-distance dispersal, but direct confirmation was never achieved. Therefore, we excluded this bird from the dataset from the moment of its last sighting.
In addition to the 11 control territories, another seven territories where fledglings had not been radio-tagged (n=16) were kept unfed (table 1). During the last part of the study period, when mortality became negligible (no losses among radio-tagged birds), six of these birds (three territories) were still present in the study area and were followed intensively with the aim of including them into the dataset. We searched for them within and outside their territories with binoculars and spotting scopes at least three times a week. Although crows are usually spotted easily in their territories in our study area due to the lack of dense vegetation, ‘absence’ from the natal territories was considered only when we could actually observe them elsewhere. Roaming might, therefore, have been underestimated, but this is conservative with respect to the conclusions of this study.
(e) Helping behaviour
Helping behaviour at the family nest of the 22 juveniles hatched in 2003 that survived until spring 2004 was recorded with camouflaged micro video cameras close to the nest (table 1; see Canestrari et al. 2005 for details on video-recording equipment). Four recording bouts (4 h each) were carried out when nestlings were 15–20 days old. Four juveniles had no opportunity to allofeed in their natal territories, due to early nest predation or hatching failure, and were not considered in the analyses. We counted feedings, i.e. each delivery of food to a nestling's open gape, to measure the helping effort. Feedings are always unequivocally visible in the video recordings and feeding frequency is the best estimate of provisioning effort in crows (Canestrari et al. 2005).
(f) Statistical methods
We used generalized linear mixed models (GLMMs) fitted with a binomial error structure to analyse the records of presence/absence in the natal territory of juvenile crows, where each observation of the banded individuals was entered as data point. GLMMs that allow investigation of repeated measures were performed using GENSTAT 8.0. Initially, we fitted experimental treatment, sex, month and group size as fixed factor, while two random factors (‘individual’ and ‘territory’) controlled for repeated measures. The final minimal model was obtained by sequentially dropping non-significant terms using a backwards-stepwise approach. Probability values of significant terms were those provided by the minimal model, whereas p values of non-significant terms were obtained by fitting individually each non-significant term to the minimal model (Crawley 2002; Russell et al. 2003).
Similarly, GLMMs were used for analysing helping effort (frequency of feeding). To investigate how food supplementation was linked to chick provisioning, we carried out the analysis in three steps, first entering experimental treatment and philopatry during the last month separately into two different models (model a and b), and then together (model c). Sex, group size and number of chicks, which are known to influence helping effort in crow helpers (Canestrari et al. 2005), were entered into the models as fixed factors, while ‘territory’ was fitted as a random factor. However, as the latter was non-significant, we subsequently fitted general lineal models (GLMs) to the data (Pinheiro & Bates 2000; Crawley 2002).
3. Results
(a) Philopatry
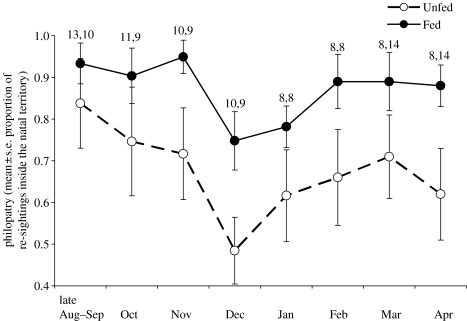
Juveniles hatched in food-supplemented territories showed higher philopatry throughout the whole period of study compared to controls (figure 1). This was reflected in a significant effect of experimental treatment (χ12=5.79, p=0.016; n=1539, 23 individuals from 13 territories) in the final minimal GLMM, which included ‘individual’ as a random factor. Philopatry also varied significantly within the year (effect of month: χ72=10.5, p<0.01) being lowest during the coldest months, December and January, among both experimental and control birds. These results held also when we included data on the six unfed juveniles (three territories), which were not radio-equipped, but that could still be followed closely during the last two months of the experiment. The final minimal GLMM again retained ‘individual’ as a random factor and showed a highly significant effect of food supplementation and month on the philopatry of juvenile crows (χ12=8.74, p<0.01 and χ72=12.0, p<0.01, respectively; n=1588, 29 individuals from 16 territories).
Figure 1.
Philopatry, measured as average proportion of re-sightings ±s.e. inside the natal territories, in fed (filled circles) and unfed (open circles) juvenile crows throughout the study period. Sample sizes are given above bars, for fed and unfed individuals, respectively. The graph includes the data on six non-radio-equipped unfed juveniles that could be followed intensively during the last two months of the experiment.
In contrast to philopatry, food supplementation did not significantly influence survival rate (30.1 and 32% at the end of the study period in fed and unfed birds, respectively; Yates corrected χ12=0.04, n=51, p=0.84). Fed groups were not more likely to receive immigrants than unfed ones (three immigrants joined different control groups, while one immigrant joined a fed group; Fisher Exact test, p=0.48).
(b) Helping at the family nest
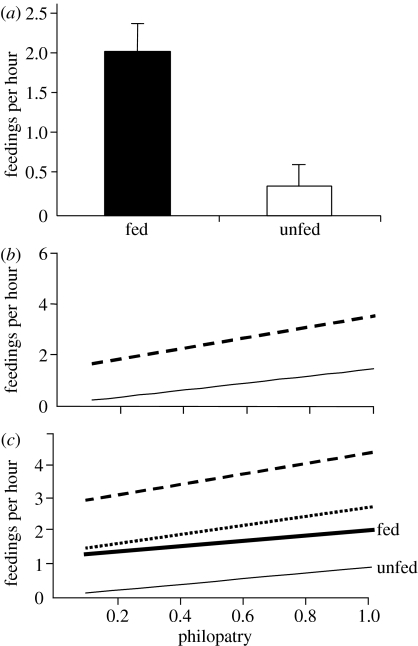
Besides favouring juvenile philopatry, food supplementation seemed to make the offspring more inclined to allofeed at the family nest. Among the 18 juveniles that had a brood in their natal territory (table 1), fed ones made a significant larger contribution to chick provisioning (results of model a: experimental treatment F1,15=13.51, p<0.01; figure 2a). Helping effort was also higher in larger broods (F1,15=9.64, p<0.01), while sex and group size showed no significant effect (F1,14=0.24, p=0.63 and F1,14=1.35, p=0.26, respectively). The effect of food supplementation on helping effort may have been merely a consequence of the higher philopatry of fed young. Offspring can assist their parents in raising younger siblings only as long as they remain in the natal territory, and young that are more philopatric have more time to help their parents compared to those more frequently engaged in roaming outside the territory. Philopatric offspring may as a corollary make a larger contribution to feeding the nestlings. Indeed, offspring that were more philopatric in the last month of the study also provided more food to the chicks (model b: philopatry F1,15=5.41, p=0.03; sex and group size not significant; figure 2b). However, after controlling for the effect of philopatry on individual provisioning rate (model c: F1,14=7.91, p=0.01), young from food-supplemented territories still showed a higher contribution than controls (experiment: F1,14=7.92, p=0.01; sex and group size not significant; figure 2c) suggesting a further direct effect of food supplementation on helping behaviour (see §4).
Figure 2.
Results of GLMs on the effect of food supplementation and philopatry on helping effort, measured as frequency of feedings of young crows. (a) Predicted average helping effort (+s.e.) of fed and unfed young (model a); (b) Predicted helping effort (+s.e., broken line) in relationship with the degree of philopatry (model b); Predicted helping effort of fed (bold line) and unfed (thin line) young in relationship with the degree of philopatry (model c; regression line of predicted values +s.e. represented by broken and dotted line, respectively). In all models brood size was set to its mean value.
4. Discussion
In this study, we have shown that offspring of wealthy parents increase their philopatry and therefore strengthen their family bonds. This fulfils a central prediction of the evolutionary theory of the family (Emlen 1995), which so far has proven to be difficult to test, namely that family stability is influenced by the amount of resources available to the offspring.
The search for ecological correlates of family living in vertebrate species has failed to produce a predictive model for the occurrence of natal philopatry and cooperative breeding across species (Cockburn 1996; Koenig & Dickinson 2004). A more successful approach has been to examine the variation in the timing of dispersal within populations (Russell 2004). Evidence that relaxation of constraints on independent breeding promotes juvenile dispersal, e.g. by experimental provisioning of nest sites (Walters et al. 1992), vacancies (Komdeur 1992) and/or mates (Pruett-Jones & Lewis 1990), has supported the idea that constraints are important, suggesting that natal philopatry is a best-of-a-bad-job tactic, especially in birds (Russell 2004). However, constraints on independent breeding alone cannot explain why juveniles should stay on their natal territory. Philopatry represents only one of several strategies that can be adopted when breeding vacancies are in short supply and not necessarily the best one. Young may disperse and settle elsewhere or use a floating tactic, either alone, in pairs, in coalition with relatives or in groups of unrelated individuals (Ekman et al. 2004). Floating, which allows searching of larger areas, might be theoretically more efficient than philopatry in detecting vacancies and should often be preferred when the habitat is saturated (Koenig et al. 1992; Kokko & Ekman 2002). Therefore, philopatry must entail intrinsic benefits and, as a consequence, young should respond to the variability of such benefits by adjusting their link with the natal territory. Our study supports this view, showing that juveniles respond to an experimental increase of the quality of home by strengthening their bond with it. Dickinson & McGowan (2005) have recently shown that an experimental reduction of the critical winter resource (mistletoe berries) in territories of western bluebirds Sialia mexicana induced offspring to disperse, disrupting family structure. Their work and our study, carried out independently on phylogenetically distant avian systems applying opposite experimental treatments (reducing versus increasing food resources), complement each other and suggest a general role of the variability of territory quality outside the breeding season in shaping the social organization within populations of birds.
Kokko & Lundberg (2001) showed theoretically that while the variability in territory quality can explain differences in offspring philopatry within a population, it has no predictive power across species or populations. This contradicts Stacey & Ligon (1991), who had suggested that species that face a high variability of territory quality should exhibit natal philopatry, because the benefits derived from occupying a good territory should lead the offspring to be choosy and to postpone dispersal until a good opportunity arises. The case of the carrion crow, which represents an ideal model to test these two alternatives thanks to the geographic variability of its social organization (Baglione et al. 2005) fits into the Kokko and Lundberg framework. The variability in territory quality, which influenced offspring philopatry in Spain (this study), is similar in cooperative and socially monogamous populations (Baglione et al. 2005). At the population level, family formation seems to be linked instead to year-round territoriality of parents, which by defending a territory and being tolerant towards their kin, create a safe haven for their offspring and hence the conditions for natal philopatry to arise (Baglione et al. 2005). Generalizing conclusions from single population studies to understand the occurrence of family living across species or populations and vice versa has proven to be a very misleading approach to the ecology of cooperative breeding (Cockburn 1996). The case of the carrion crows shows that the evolutionary study of the family needs to address the two levels separately.
The proximate mechanism behind the higher philopatry of wealthy crows in Spain may involve the social interactions within the group. Parents might adjust their tolerance towards the offspring according to available levels of wealth, evicting them when resources are scarce, and/or sibling rivalry may increase in poor-quality territories pushing some young to leave. However, in 51 h of observations (26 bouts) of foraging kin groups (n=10) we observed only one mild aggressive interaction among close relatives (i.e. parent–offspring, siblings), suggesting that intra-group interactions are not a main factor in modulating offspring philopatry. Alternatively, we suggest that young evaluate the quality of their home and behave accordingly. This might be mediated by a physiological response to body condition, as it occurs for example in the eastern screech owl (Otus asio), where hunger triggers dispersal restlessness in young, through an increased level of corticosteroids (Ritchison et al. 1992).
In many animals, juvenile philopatry is the permissive factor for the expression of cooperative breeding. In crows, as well as in most cooperative avian species, helping is a two step process, where offspring must first remain philopatric and then decide whether to allofeed or not (Brown 1987). Although our study mainly focused on the former of the two processes, it sheds some light on the latter. By enhancing philopatry, food supplementation was expected ultimately to affect family cooperation, because extraterritorial roaming is likely to trade off against helping, as offspring need to be at home to help their parents (Young et al. 2005). Indeed, we found that more philopatric young helped more, suggesting an indirect proximate mechanism behind the greater effort invested by wealthy young. However, our data also showed that, when controlling for the degree of philopatry, fed juveniles still showed a larger contribution to nestling provisioning than controls, suggesting that long-term food supplementation may also influence helping directly, probably by increasing body condition and/or reducing the costs of provisioning (Boland et al. 1997). Costs of helping have been often neglected in studies on cooperative behaviour (Heinshon & Legge 1999). Our data indicate that they surely deserve further investigation in the crow.
Acknowledgments
We thank Janis Dickinson, Nick Davies, Andy Radford, Claire Spottiswoode, Vicky Jones and two anonymous referees for helpful comments on the manuscript, Andrea Manica, Suhel Quader and Pedro Olea for statistical advice, Gloria Robles, Rubén Vera and Elisa Chiarati for help in the field. Financial support was provided by the program ‘Ramón y Cajal’ of the Spanish Ministry of Education and Science and the Ax:son Johnsons Stiftelse (to V.B.), the Gates Cambridge Trust (to D.C.) and the Swedish Council (to J.E.). Bird banding was authorized by Junta de Castilla y León.
References
- Baglione V, Marcos J.M, Canestrari D. Cooperatively breeding groups of carrion crow (Corvus corone corone) in northern Spain. Auk. 2002;119:790–799. [Google Scholar]
- Baglione V, Canestrari D, Marcos J.M, Ekman J. Kin selection in cooperative alliances of carrion crows. Science. 2003;300:1947–1949. doi: 10.1126/science.1082429. doi:10.1126/science.1082429 [DOI] [PubMed] [Google Scholar]
- Baglione V, Marcos J.M, Canestrari D, Griesser M, Andreotti G, Bardini C, Bogliani G. Does year-round territoriality rather than habitat saturation explain delayed natal dispersal and cooperative breeding in the carrion crow? J. Anim. Ecol. 2005;74:842–851. doi:10.1111/j.1365-2656.2005.00983.x [Google Scholar]
- Boland C.R, Heinsohn R, Cockburn A. Experimental manipulation of brood reduction and parental care in cooperatively breeding white-winged choughs. J. Anim. Ecol. 1997;66:683–691. [Google Scholar]
- Brown J.L. Princeton University Press; Princeton, NJ: 1987. Helping and communal breeding in birds: ecology and evolution. [Google Scholar]
- Caffrey C. Marking crows. N. Am. Bird Band. 2000;26:146–148. [Google Scholar]
- Canestrari D, Marcos J.M, Baglione V. Effect of parentage and relatedness on the individual contribution to cooperative chick care in carrion crows. Behav. Ecol. Sociobiol. 2005;52:422–428. doi:10.1007/s00265-004-0879-1 [Google Scholar]
- Cochran G.R, Solomon N.G. Effects of food supplementation on the social organization of prairie voles (Microtus ochrogaster) J. Mammal. 2000;81:746–757. doi:10.1644/1545-1542(2000)081<0746:EOFSOT>2.3.CO;2 [Google Scholar]
- Cockburn A. Why do so many Australian birds cooperate: social evolution in the Corvida? In: Floyd R.B, Sheppard A.W, de Barro P.J, editors. Frontiers of population ecology. CSIRO Publishing; Melbourne, Australia: 1996. pp. 451–472. [Google Scholar]
- Cramp S, Perrins C.M. Crows to Finches. vol. VIII. Oxford University Press; Oxford, UK: 1994. The birds of the western Paleartic. [Google Scholar]
- Crawley M.J. Wiley; London, UK: 2002. Statistical computing. [Google Scholar]
- Dickinson J.L, Mc Gowan A. Winter resource wealth drives delayed dispersal and family-group living in western bluebirds. Proc. R. Soc. B. 2005;272:2423–2428. doi: 10.1098/rspb.2005.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emlen S.T. An evolutionary theory of the family. Proc. Natl Acad. Sci. USA. 1995;92:8092–8099. doi: 10.1073/pnas.92.18.8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman J, Dickinson J.L, Hatchwell B.J, Griesser M. Delayed dispersal. In: Koenig W.D, Dickinson J.L, editors. Ecology and evolution of cooperative breeding in birds. Cambridge University Press; Cambridge, UK: 2004. pp. 210–227. [Google Scholar]
- Funston P.J, Mills M.G.L, Richardson P.R.K, van Jaarsveld A.S. Reduced dispersal and opportunistic territory acquisition in male lions (Panthera leo) J. Zool. 2003;259:131–142. doi:10.1017/S0952836902003126 [Google Scholar]
- Griffiths R, Double M.C, Orr K, Dawson R.J.G. A DNA test to sex most birds. Mol. Ecol. 1998;7:1071–1075. doi: 10.1046/j.1365-294x.1998.00389.x. doi:10.1046/j.1365-294x.1998.00389.x [DOI] [PubMed] [Google Scholar]
- Heg D, Bachar Z, Brouwer L, Taborsky M. Predation risk is an ecological constraint for helper dispersal in a cooperatively breeding cichlid. Proc. R. Soc. B. 2004;271:2367–2374. doi: 10.1098/rspb.2004.2855. doi:10.1098/rspb.2004.2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinsohn R, Legge S. The cost of helping. Trends. Ecol. Evol. 1999;14:53–57. doi: 10.1016/s0169-5347(98)01545-6. doi:10.1016/S0169-5347(98)01545-6 [DOI] [PubMed] [Google Scholar]
- Koenig W, Dickinson J. Introduction. In: Koenig W, Dickinson J, editors. Ecology and evolution of cooperative breeding in birds. Cambridge University Press; Cambridge, UK: 2004. pp. 1–4. [Google Scholar]
- Koenig W, Stacey P.B. Acorn woodpeckers: group-living and food storage under contrasting ecological conditions. In: Stacey P.B, Koenig W, editors. Cooperative breeding in birds: long-term studies of ecology and behavior. Cambridge University Press; Cambridge, UK: 1990. pp. 413–453. [Google Scholar]
- Koenig W.D, Pitellea F.A, Carmen W.J, Mumme R.L, Stanback M.T. The evolution of delayed dispersal in cooperative breeders. Q. Rev. Biol. 1992;67:111–150. doi: 10.1086/417552. [DOI] [PubMed] [Google Scholar]
- Kokko H, Ekman J. Delayed dispersal as a route to breeding: territorial inheritance, safe havens, and ecological constraints. Am. Nat. 2002;160:468–484. doi: 10.1086/342074. doi:10.1086/342074 [DOI] [PubMed] [Google Scholar]
- Kokko H, Lundberg P. Dispersal, migration, and offspring retention in saturated habitats. Am. Nat. 2001;157:188–202. doi: 10.1086/318632. doi:10.1086/318632 [DOI] [PubMed] [Google Scholar]
- Komdeur J. Importance of habitat saturation and territory quality for evolution of cooperative breeding in the Seychelles warbler. Nature. 1992;358:493–495. doi:10.1038/358493a0 [Google Scholar]
- Leturque H, Rousset F. Joint evolution of sex ratio and dispersal: conditions for higher dispersal rates from good habitats. Evol. Ecol. 2003;17:67–84. doi:10.1023/A:1022405415375 [Google Scholar]
- Luck G.W. The demography and cooperative breeding behaviour of the rufous treecreeper, Climacteris rufa. Aust. J. Zool. 2001;49:515–537. doi:10.1071/ZO00087 [Google Scholar]
- Nagy K.A, Girard I.A, Brown T.K. Energetic of free-ranging mammals, reptiles and birds. Annu. Rev. Nutr. 1999;19:247–277. doi: 10.1146/annurev.nutr.19.1.247. doi:10.1146/annurev.nutr.19.1.247 [DOI] [PubMed] [Google Scholar]
- Pinheiro J.C, Bates D.M. Springer; New York, NY: 2000. Mixed-effects models in S and S-plus. [Google Scholar]
- Pruett-Jones S.G, Lewis M.J. Sex ratio and habitat limitation promote delayed dispersal in superb fairy-wrens. Nature. 1990;348:541–542. doi:10.1038/348541a0 [Google Scholar]
- Putland D.A, Goldizen A.W. Family dynasties in the Tasmanian native hen (Gallinula mortierii) Behav. Ecol. Sociobiol. 2001;51:26–32. doi:10.1007/s002650100404 [Google Scholar]
- Ritchison G, Belthoff J.R, Sparks E.J. Dispersal restlessness—evidence for innate dispersal by juvenile eastern screech-owls. Anim. Behav. 1992;43:57–65. [Google Scholar]
- Russell A.F. Dispersal costs set the scene for helping in an atypical avian cooperative breeder. Proc. R. Soc. B. 2001;268:95–99. doi: 10.1098/rspb.2000.1335. doi:10.1098/rspb.2000.1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A.F. Mammals; comparisons and contrasts. In: Koenig W.D, Dickinson J.L, editors. Ecology and evolution of cooperative breeding in birds. Cambridge University Press; Cambridge, UK: 2004. pp. 210–227. [Google Scholar]
- Russell A.F, Brotherton P.N.M, McIlrath G.M, Sharpe L.L, Clutton-Brock T. Breeding success in cooperative meerkats: effect of helper number and maternal state. Behav. Ecol. 2003;14:486–492. doi:10.1093/beheco/arg022 [Google Scholar]
- Stacey P.B, Ligon J.D. The benefits-of-philopatry hypothesis for the evolution of cooperative breeding—variation in territory quality and group-size effects. Am. Nat. 1991;137:831–846. doi:10.1086/285196 [Google Scholar]
- Walters J.R, Copeyon C.K, Carter J.H. Test of the ecological basis of cooperative breeding in red-cockaded woodpeckers. Auk. 1992;109:90–97. [Google Scholar]
- Young A.J, Carlson A.A, Clutton-Brock T. Trade-offs between extraterritorial prospecting and helping in a cooperative mammal. Anim. Behav. 2005;70:829–837. doi:10.1016/j.anbehav.2005.01.019 [Google Scholar]
- Zack S, Ligon J.D. Cooperative breeding in lanius shrikes. 1. Habitat and demography of 2 sympatric species. Auk. 1985;102:754–765. [Google Scholar]