Abstract
Assessing a conspecific's potential is often crucial to increase one's fitness, e.g. in female choice, contests with rivals or reproductive conflicts in animal societies. In the latter, helpers benefit from accurately assessing the fertility of the breeder as an indication of inclusive fitness. There is evidence that this can be achieved using chemical correlates of reproductive activity. Here, we show that queen quality can be assessed by directly monitoring her reproductive output. In the paper wasp Polistes dominulus, we mimicked a decrease in queen fertility by regularly removing brood. This triggered ovarian development and egg-laying by many workers, which strongly suggests that brood abundance is a reliable cue of queen quality. Brood abundance can be monitored when workers perform regular brood care in small size societies where each brood element is kept in a separate cell. Our results also show that although the queen was not manipulated, and thus remained healthy and fully fertile, she did not control worker egg-laying. Nevertheless, when workers laid eggs, the queen secured a near reproductive monopoly by selectively destroying these eggs, a mechanism known as ‘queen policing’. By contrast, workers destroyed comparatively few queen-laid eggs, but did destroy each other's eggs.
Keywords: honest signalling, parental manipulation, conflict, Polistes, oophagy, self-restraint
1. Introduction
Intraspecific assessment of each other's potential is important in female choice, contests with rivals and reproductive decisions in animal societies (Alexander et al. 1991; Keller & Nonacs 1993; Bourke & Franks 1995; Bradbury & Vehrencamp 1998; West-Eberhard 2003). In social insects, workers typically do not reproduce in the presence of the queen. Rather, they rear the queen's offspring when they gain sufficient indirect fitness benefits (Hamilton 1964). Inclusive fitness is essentially a function of relatedness to the queen and of queen productivity. Typically, workers benefit from helping when the queen is fertile, but they favour producing their own sons when queen fertility decreases too much to be compensated by relatedness benefits (Bourke & Franks 1995). In many species of social insects, fertility correlates with the pattern of cuticular hydrocarbons (Polistes wasps: Bonavita-Cougourdan et al. 1991; Sledge et al. 2001, 2004; Dapporto et al. 2004; ants: Monnin et al. 1998, 2002; Peeters et al. 1999; Liebig et al. 2000; Cuvillier-Hot et al. 2001, 2004; Hannonen et al. 2002; Heinze et al. 2002; Dietemann et al. 2003). Queen quality may thus be reflected in detectable changes in her cuticular hydrocarbon pattern, which is a reliable cue of fertility. Alternatively, workers may directly assess the fertility of the queen by estimating the abundance of the brood. Fertility is the production of viable brood so that brood abundance is the most direct and reliable measure of fertility. Direct assessment of brood abundance is especially likely in species with small colonies, and where each brood element is located in an individual cell. Workers who continuously check cells to feed the brood and maintain the nest could assess brood abundance at no cost. Additionally, this direct assessment of queen quality is advantageous as it is safe from cheating by the queen who cannot manipulate the presence of viable brood.
We experimentally studied queen quality assessment in the polistine wasp Polistes dominulus. Colonies are small, with a few tens of adults (Turillazzi 1980). They are founded in spring by one or a few single mated foundresses (Queller et al. 2000), i.e. mated females who have hibernated. There is no morphological queen caste in Polistes and all females are capable of mating. However, the daughters of the foundress typically behave like workers. They do not reproduce but care for the brood (Pardi 1948; West-Eberhard 1969; Reeve 1991; Arevalo et al. 1998). Females produced late in the season will hibernate and become next year's foundresses. The nest consists of a single open comb made of paper in which each brood element is kept in a separate cell. This allows for easy manipulation of cell content and easy observation of egg-laying. The foundress starts building new cells where she lays eggs and workers subsequently finish these cells, thereby gradually enlarging the nest. We used colonies founded by a single foundress to standardize relatedness and suppress conflicts between foundresses. Workers are expected to respond to empty cells by egg-laying (Deleurance 1950; Gervet 1964a). We simulated a decline in the quality of the foundress by repeatedly removing brood from cells in order to maintain a large proportion of cells empty. If workers assess queen quality by the presence of brood, and if they are not suppressed by the foundress, they should start laying eggs when many cells are empty.
2. Methods
(a) Study species
Sixteen healthy colonies of P. dominulus with a single foundress and the comb full of brood were collected in Florence in June and July 2002. The foundress was identified by her larger size (workers of the first generation are usually small), worn wings and dominant behaviour. She was marked with enamel paint. Colonies were reared in the laboratory in glass cages (15×15×15 cm) at 25–35 °C, and fed daily with Musca fly larvae, saccharose and water ad libitum.
(b) Brood manipulation
Nests with similar comb size and worker number were paired, and within each pair nests were randomly assigned to either treatment (T) or control (C) (n=8 colonies for each group). T and C colonies had similar size at the beginning of the experiment (56.5 versus 55 cells and 7.5 versus 6.5 workers, median, Mann–Whitney U-tests, U=32 and 30.5, respectively, n.s. for both cells and workers). At the end of the experiment, colony size was still not significantly different, although worker number tended to be larger in controls (T versus C: 62 versus 67 cells and 16.0 versus 22.5 workers, median, Mann–Whitney U-tests, U=18 and 15, respectively, n.s. for both cells and workers). The treatment consisted in removing brood from cells every other day during 22 days. The brood was carefully removed with forceps and special attention was paid not to damage the cells. The first time a cell was emptied the brood was at any stage of development, and it was at the egg stage for subsequent removal as it had no time to develop into a larva. A treatment period consisted of 2 consecutive days: brood was removed the first day, and no manipulation took place the second day. Cells on the edge of the nest were not emptied because preliminary observations had shown that some of these cells remained empty for days and that the foundress preferentially laid in inner cells. Twenty-five per cent of the inner cells were emptied for the first treatment, and 50% of inner cells were emptied for following treatments. Combs were carefully mapped at each manipulation. We always emptied the same cells, so that brood grew and matured in non-manipulated cells, and we carefully recorded the content of all cells. Wasps were removed by cooling down colonies in a fridge at 4–10 °C to allow for the manipulation of the comb. Males were removed and females were returned to the comb immediately after brood removal. Control colonies were treated similarly except that no brood was removed: colonies were cooled down every other day, wasps were removed from the comb, the comb was mapped and cell content was recorded.
(c) Video analysis
The colonies were continuously videotaped throughout the 22 days of the experiment, using video cameras capable of recording in the dark at night, to analyse egg-laying and egg replacement. Half of the colonies (4T, 4C) were monitored with Panasonic AG-TL6720 S-video time lapse VCRs recording at six frames per second. The other half were recorded at normal speed for 4 s every 30 s with Sony DCR-TRV238 Digital 8 camcorders. Although colonies were recorded daily, we only analysed the videos of every second treatment period (i.e. days 1+2, 5+6, 9+10, 13+14, 17+18 and 21+22, with brood being removed the first day of each treatment period). This represents approximately 46 h of video recording per colony per treatment period, and thus 4416 h in total. Egg deposition consisted of a wasp inserting its abdomen deeply into a cell, with its legs and wings spread out, and remaining motionless for more than a minute (usually 2–3 min). This was confirmed by observations of oviposition with the naked eye. For each egg deposition we recorded whether the egg was laid by the foundress or by a worker, the time and duration of oviposition, and the cell where the egg was deposited. When an egg was laid in a cell already containing an egg we assumed that this initial egg had been removed. This is because checks of cell content revealed that very few cells had two eggs, and we observed no eggs laid in cells containing larvae or pupae.
(d) Dissection
All individuals from all colonies were frozen and dissected at the end of the experiment (n=304) to determine their reproductive activity (number of oocytes larger than 0.6 mm, presence of large yellow bodies revealing previous egg-laying typical of the foundress, mating status).
3. Results
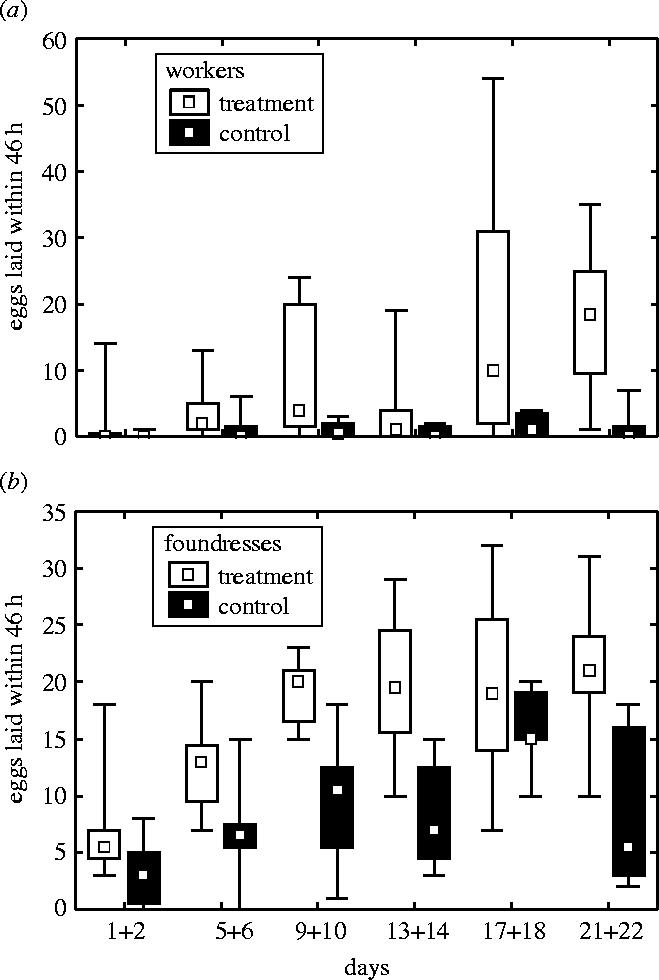
Our manipulation led to a high proportion of empty cells. Before manipulation the mean number of empty inner cells per colony was not different between treatment and control colonies over the course of the experiment (9.1 and 1.6% in T and C, respectively, Mann–Whitney U-test: U=15, p<0.08), whereas it was after manipulation (29.4 and 1.6% in T and C, respectively, Mann–Whitney U-test: U=0, p<0.001). In treatment colonies, the presence of empty cells triggered increased egg-laying by workers, whereas they laid very few eggs in control colonies (figure 1a). This was despite the presence of the foundress who was not manipulated and was free to interact with workers. Some cells in the outer area of the nest remained empty in both controls and treatments (median: 5.6 versus 6.4%, Mann–Whitney U-test, U=29, n.s.) indicating that it is not any empty cells that induce egg-laying. Worker reproduction is further demonstrated by the higher ovarian development of workers in treatments than in controls (figure 2). Workers were virgin. Workers who had emerged in the laboratory could not mate. They only encountered nest-mate males, and these were removed every other day when we manipulated brood. Workers who had emerged in the wild had not mated either, presumably because early males had not emerged or were not active yet when we collected the nests.
Figure 1.
Pattern of egg-laying. Presented are medians, quartiles and ranges. (a) Control workers consistently laid very few eggs throughout the experiment. By contrast, workers gradually increased their egg-laying rates in treatments (Spearman rank correlation, treatment: r=0.51, p<0.01, control: r=0.26, n.s.). Control and treatment did not differ at days 1+2 (Mann–Whitney U-test, U=27.5, n.s.) but treatment laid more eggs at day 21+22 (U=3.5, p<0.005). (b) The foundress gradually increased her egg-laying rate in treatments and in controls (Spearman rank correlation, treatment: r=0.61, control: r=0.42, p<0.01 for both), but the increase was larger in treatments. After the first removal of brood (i.e. at days 1+2), treatment foundresses already tended to lay more eggs than control foundresses (Mann–Whitney U-test, U=14.5, p<0.065). This suggests that foundresses responded to brood removal very fast. The difference in egg-laying rates was significant in all following days (Mann–Whitney U-tests).
Figure 2.
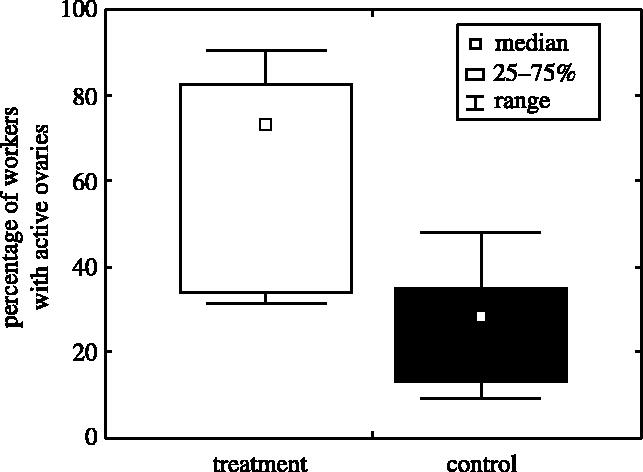
Ovarian development of workers. More workers had ovaries with yolky oocytes in treatments (n=8 colonies) than in controls (n=8 colonies, Mann–Whitney U-test, U=7.5, z=2.57, p<0.02).
In treatments, the presence of many empty cells and/or the occurrence of worker reproduction resulted in a gradual increase of the laying rate of the foundress, which approximately quadrupled after 22 days (figure 1b). In controls, the foundress also laid more eggs with time, but the increase was smaller. Although treatment foundresses laid more eggs than control foundresses, they did not differ in the number of yolky oocytes in their ovaries (Mann–Whitney U-test, U=21.5, p>0.26). This excludes that worker reproduction in treatments stems from egg depletion of the foundress. The higher egg-laying rate of the foundress in treatments cannot be explained by a larger work-force, as treatment colonies were not larger than controls (beginning of the experiment: 7.5 versus 6.5 workers in T and C, respectively, median, Mann–Whitney U-test, U=30.5, p>0.87; end of the experiment: 16 versus 22.5 workers, U=15, p<0.075). On the contrary, controls tended to grow larger than treatments as more new workers emerged in controls where brood was not removed.
Although workers laid eggs in the immediate vicinity of the foundress, we did not see conspicuously increased levels of aggression in the treatments. We can, however, not exclude that there are increased levels of subtle interactions. Nevertheless, if there would have been any aggressions, these did not hinder workers from laying eggs. However, the foundress maintained a near reproductive monopoly by preferentially replacing worker eggs by her own eggs, to which she is more related (figure 3; egg identity was determined through continuous video monitoring of egg-laying and mapping of the comb). Many worker eggs were replaced by the foundress although empty cells were available on the comb. Workers also replaced some eggs by their own, but they preferentially replaced other workers' eggs (30.3%, median) rather than foundress' eggs (2.9%, Wilcoxon paired sample test, T=0, p<0.02).
Figure 3.
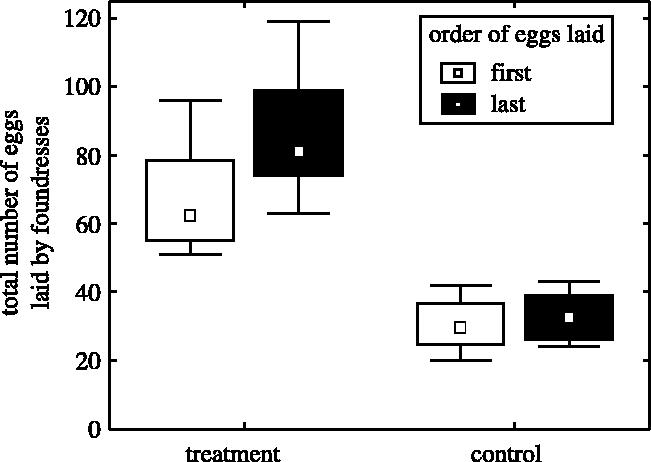
Egg replacement. Up to nine eggs were laid one after the other in the same cell within 46 h. We call first (white boxes) and last eggs (black boxes) those eggs that were deposited first and last in a cell, and intermediate eggs those that were deposited in the meantime. First and intermediate eggs were thus replaced by the last egg. The foundress laid more last than first eggs in both treatments and controls (Wilcoxon paired sample test, treatment: n=8, t=0, z=2.37, p<0.02; control: n=8, t=0, z=2.02, p<0.05), which shows that she increased her share of eggs by preferentially replacing worker-laid eggs by her own. This increase was larger in treatments than in controls (Mann–Whitney U-test: U=8.5, z=2.46, p<0.02). Conversely, workers laid less last than first eggs. The plot reflects medians, quartiles and ranges.
4. Discussion
Our data unambiguously show that workers respond to brood depletion by reproducing, which strongly suggests that brood abundance is a cue of foundress fertility in P. dominulus. A decline in the productivity of the foundress reduces the workers' indirect fitness. If the productivity of the foundress decreases below the capacity of the colony, which is the case in our experiment, workers increase their inclusive fitness by direct reproduction as long as the costs associated with worker reproduction are sufficiently small. Assessing brood presence in cells is a direct way of assessing the fertility of the queen, compared with indirect ways using correlates of ovarian activity such as cuticular hydrocarbons (see references in Introduction).
Under natural conditions, a failing foundress would not only be detected by a decrease in brood abundance but, potentially, also by changes in physiological correlates such as her cuticular hydrocarbons profile and a decrease in activity. The use of several redundant cues allows workers to reliably make the right decision. In our experiment, workers were exposed to contradictory information. The scarcity of brood revealed that the foundress was unhealthy, whereas the behaviour and the condition of the foundress advertised that she was healthy. This suggests that there is a hierarchy of cues, with empty cells being the most important for assessing queen quality in Polistes. Additionally, the experiment uncoupled the apparent quality of the foundress (failing) from her power to suppress worker reproduction (unaltered). This allows investigating experimentally the queen control and queen signalling hypotheses (Seeley 1985; Keller & Nonacs 1993).
In queen control, the queen suppresses worker reproduction, against workers' interest (Michener & Brothers 1974; Breed & Gamboa 1977). This is clearly not the case in our experiment, where the foundress failed to control workers although she was not manipulated. She did not show overt aggression, even towards workers laying eggs in her immediate vicinity. Our results are consistent with the general idea of the queen signalling hypothesis, that workers use the information about queen fertility to decide to refrain from reproducing and help the queen (Seeley 1985; Keller & Nonacs 1993; Monnin et al. 2002; Endler et al. 2004). Workers did not reproduce in controls, but they did in treatments where they shifted from helping the apparently failing foundress to reproducing. Thus, the lack of worker reproduction in natural colonies with a fully fertile and healthy queen is not due to queen control but to self-restraint by workers.
There was no obvious aggressions between workers, and workers did not attack the apparently failing foundress. In a comparable experiment in foundress associations of P. fuscatus, all eggs were removed only once, and this did not increase the relative frequency of aggressive behaviours either (Nonacs et al. 2004). In fact, the pattern of aggressions indicates that aggressions are not related to reproductive manipulation in this species; at least once a clear dominance hierarchy has been established. Instead, aggressions may stimulate nest-mate activities (e.g. foraging; Reeve & Gamboa 1987; Nonacs et al. 2004; Sumana & Starks 2004). Behavioural signals may thus be less important than fertility cues once a hierarchy has been established. This is further supported by an experiment where P. dominulus foundresses were ovariectomized and consequently became infertile. Yet, they remained behaviourally dominant and were not attacked by subordinates, although some subordinates started egg-laying (Röseler & Röseler 1989).
Our experiment shows that workers respond to brood depletion. We suggest that they respond to a high frequency of empty cells that indicates the presence of a ‘weak’ queen (Strassmann 1993) rather than to a low level of brood-produced pheromones (brood pheromones occur in honeybees; Mohammedi et al. 1998). The assessment of cell content is not costly because regular brood care implies checking cells. Additionally, it is highly reliable. Considering a cell with brood as empty is very unlikely, and cheating by the foundress seems impossible.
The high reliability of the information conveyed by cell status, i.e. with or without brood, may be the reason why workers did not respond to other potential cues or signals of queen quality. Neither the increased egg-laying rate of the foundress—that may be perceived via the large amount of fresh queen-laid eggs—nor the chemical cues that are associated with fertility in P. dominulus (Bonavita-Cougourdan et al. 1991; Sledge et al. 2001, 2004) prevented workers from laying eggs. Similarly, the unaltered fighting ability of the foundress and other potential behavioural signals (Pardi 1948; West-Eberhard 1977) did not prevent worker reproduction either. This suggests that workers consider the level of reliability of the information available when making their reproductive decisions. If there is a discrepancy between various types of information they chose the most reliable one. This may often be a cue that is available from the environment and cannot be faked, such as brood abundance. The latter is less likely to be an important cue in species with large colonies, where workers may not be capable of making a survey of all combs. Workers may also be unable to assess brood quantity in species without brood cells, where brood is stacked in piles.
In our experiment, the foundress maintained her reproductive monopoly despite the occurrence of worker reproduction. First, she quadrupled her egg production, thereby ensuring that she produced a high proportion of the eggs. The foundress did not show signs of exhaustion at the end of the experiment, after three weeks of increased egg-laying (egg-laying did not decrease and her ovaries were fully developed). This suggests that in the wild, the fertility of the foundress is not limited by her physiology, but by resource availability. Second, the foundress increased further her share of reproduction by selectively destroying worker-laid eggs, a behaviour known as queen policing (Ratnieks 1988; Monnin & Ratnieks 2001). Although empty cells were available, the foundress preferentially laid eggs in cells where workers had laid eggs previously. This strongly suggests that increased egg-laying by the foundress was not only to compensate for the loss of eggs, but also to compete for reproductive rights with workers, and that egg replacement was not a side effect of competition for space but an active means of controlling worker reproduction. Selective destruction of other female's egg is well known at the founding stage, when several foundresses cooperatively start a nest (e.g. Gervet 1964b). There is good evidence that eggs produced by individuals of different social status can be recognized because their odours correlate with that of the egg layer (Monnin & Peeters 1997; D'Etorre et al. 2004; Endler et al. 2004).
In contrast to the foundress, workers did not preferentially replace foundress-laid eggs. This is because workers would only benefit from replacing the foundress' male eggs, to which they are less related than to their own offspring, but they would not benefit from replacing female eggs to which they are highly related (Hamilton 1964). There is no evidence that workers could recognize the sex of queen-laid eggs and, besides, early in the season the foundress mostly produces female eggs. Workers mutually replaced their eggs, indicating that they competed for direct reproduction. This is distinct from mutual control of egg-laying (worker policing; Ratnieks 1988) where policing workers do not reproduce themselves.
This study shows that Polistes workers respond to brood depletion by reproducing and that brood abundance, possibly estimated through the frequency of empty cells in the nest, provides direct and highly reliable information on foundress fertility. Workers thus use the most reliable source of information to decide whether to reproduce or not. The queen signalling hypothesis should thus include all potential cues of queen quality as a source of information. Alternatively, it may be more appropriate to refer to a general worker assessment of queen quality, with workers using any relevant cue or signal to make their reproductive decisions. This highlights that information reliability is a key factor in the evolution of animal communication, and this is not only restricted to honest signalling (Bradbury & Vehrencamp 1998; Maynard Smith & Harper 2003).
Acknowledgments
We thank Andrew Bourke, Abraham Hefetz, Christian Peeters, Peter Nonacs and an anonymous referee for their valuable comments on an earlier version of the manuscript, and Katja Tschirner for technical assistance. While in Florence, T. M. was funded by the European Community's Improving Human Potential Programme under contract HPRN-CT-2000-00052, ‘INSECTS’. J. L.'s visits to Florence were also funded by ‘INSECTS’.
Footnotes
The first two authors contributed in equal part to this work.
References
- Alexander R.D, Noonan K.M, Crespi J. The evolution of eusociality. In: Sherman P.W, Jarvis J.U.M, Alexander R.D, editors. The biology of the naked mole-rat. Princeton University Press; Princeton, NJ: 1991. pp. 3–44. [Google Scholar]
- Arevalo E, Strassmann J.E, Queller D.C. Conflicts of interest in social insects: male production in 2 species of Polistes. Evolution. 1998;52:797–805. doi: 10.1111/j.1558-5646.1998.tb03703.x. [DOI] [PubMed] [Google Scholar]
- Bonavita-Cougourdan A, Théraulaz G, Bagnères A.-G, Roux M, Pratte M, Provost E, Clément J.-L. Cuticular hydrocarbons, social organization and ovarian development in a polistine wasp: Polistes dominulus Christ. Comp. Biochem. Physiol. B. 1991;100:667–680. [Google Scholar]
- Bourke A.F.G, Franks N.R. Princeton University Press; Princeton, NJ: 1995. Social evolution in ants. [Google Scholar]
- Bradbury J.W, Vehrencamp S.L. Sinauer; Sunderland, MA: 1998. Principles of animal communication. [Google Scholar]
- Breed M.D, Gamboa G.J. Behavioral control of workers by queens in primitively eusocial bees. Science. 1977;195:694–696. doi: 10.1126/science.195.4279.694. [DOI] [PubMed] [Google Scholar]
- Cuvillier-Hot V, Cobb M, Malosse C, Peeters C. Sex, age and ovarian activity affect cuticular hydrocarbons in Diacamma ceylonense, a queenless ant. J. Insect Physiol. 2001;47:485–493. doi: 10.1016/s0022-1910(00)00137-2. [DOI] [PubMed] [Google Scholar]
- Cuvillier-Hot V, Lenoir A, Peeters C. Reproductive monopoly enforced by sterile police workers in a queenless ant. Behav. Ecol. 2004;15:970–975. [Google Scholar]
- Dapporto L, Theodora P, Spacchini C, Pieraccini G, Turillazzi S. Rank and epicuticular hydrocarbons in different populations of the paper wasp Polistes dominulus (Christ) (Hymenoptera, Vespidae) Insectes Soc. 2004;51:279–286. [Google Scholar]
- Deleurance E.P. Sur le mécanisme de la monogynie fonctionnelle chez les Polistes (Hymenoptères Vespides) C. R. Acad. Sci. Paris. 1950;230:782–784. [Google Scholar]
- D'Etorre P, Heinze J, Ratnieks F.L.W. Worker policing by egg eating in the ponerine ant Pachycondyla inversa. Proc. R. Soc. B. 2004;271:1427–1434. doi: 10.1098/rspb.2004.2742. 10.1098/rspb.2004.2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietemann V, Peeters C, Liebig J, Thivet V, Hölldobler B. Cuticular hydrocarbons mediate recognition of queens and reproductive workers in the ant Myrmecia gulosa. Proc. Natl Acad. Sci. USA. 2003;100:10341–10346. doi: 10.1073/pnas.1834281100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endler A, Liebig J, Schmitt T, Parker J.E, Jones G.R, Schreier P, Hölldobler B. Surface hydrocarbons of queen eggs regulate worker reproduction in a social insect. Proc. Natl Acad. Sci. USA. 2004;101:2945–2950. doi: 10.1073/pnas.0308447101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervet J. La ponte et sa régulation dans la société polygyne de Polistes gallicus (Hyménoptère Vespidae) Ann. Sci. Nat. (Zool.) 1964a;12:601–778. [Google Scholar]
- Gervet J. Le comportement d'oophagie différentielle chez Polistes gallicus L. (Hymen. Vesp.) Insectes Soc. 1964b;11:343–382. [Google Scholar]
- Hamilton W.D. The genetical evolution of social behaviour. I.J. Theor. Biol. 1964;7:1–16. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- Hannonen M, Sledge M.F, Turillazzi S, Sundström L. Queen reproduction, chemical signalling and worker behaviour in polygyne colonies of the ant Formica fusca. Anim. Behav. 2002;64:477–485. [Google Scholar]
- Heinze J, Stengl B, Sledge M.F. Worker rank, reproductive status and cuticular hydrocarbon signature in the ant, Pachycondyla cf. in versa. Behav. Ecol. Sociobiol. 2002;52:59–65. [Google Scholar]
- Keller L, Nonacs P. The role of queen pheromones in social insects: queen control or queen signal? Anim. Behav. 1993;45:787–794. [Google Scholar]
- Liebig J, Peeters C, Oldham N.J, Markstädter C, Hölldobler B. Are variations in cuticular hydrocarbons of queens and workers a reliable signal of fertility in the ant Harpegnathos saltator? Proc. Natl Acad. Sci. USA. 2000;97:4124–4131. doi: 10.1073/pnas.97.8.4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard Smith J, Harper D. Oxford Series in Ecology and Evolution. Oxford University Press; New York: 2003. Animal signals. [Google Scholar]
- Michener C.D, Brothers D.J. Were workers of eusocial Hymenoptera initially altruistic or oppressed? Proc. Natl Acad. Sci. USA. 1974;71:671–674. doi: 10.1073/pnas.71.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammedi A, Paris A, Crauser D, Le Conte Y. Effect of aliphatic esters on ovary development of queenless bees (Apis mellifera L) Naturwissenschaften. 1998;85:455–458. [Google Scholar]
- Monnin T, Peeters C. Cannibalism of subordinates' eggs in the monogynous queenless ant Dinoponera quadriceps. Naturwissenschaften. 1997;84:499–502. [Google Scholar]
- Monnin T, Ratnieks F.L.W. Policing in queenless ponerine ants. Behav. Ecol. Sociobiol. 2001;50:97–108. [Google Scholar]
- Monnin T, Malosse C, Peeters C. Solid-phase microextraction and cuticular hydrocarbon differences related to reproductive activity in the queenless ant Dinoponera quadriceps. J. Chem. Ecol. 1998;24:473–490. [Google Scholar]
- Monnin T, Ratnieks F.L.W, Jones G.R, Beard R. Pretender punishment induced by chemical signalling in a queenless ant. Nature. 2002;419:61–65. doi: 10.1038/nature00932. [DOI] [PubMed] [Google Scholar]
- Nonacs P, Reeve H.K, Starks P.T. Optimal reproductive-skew models fail to predict aggression in wasps. Proc. R. Soc. B. 2004;271:811–817. doi: 10.1098/rspb.2003.2668. 10.1098/rspb.2003.2668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi L. Dominance order in Polistes wasps. Physiol. Zool. 1948;21:1–13. doi: 10.1086/physzool.21.1.30151976. [DOI] [PubMed] [Google Scholar]
- Peeters C, Monnin T, Malosse C. Cuticular hydrocarbons correlated with reproductive status in a queenless ant. Proc. R. Soc. B. 1999;266:1323–1327. 10.1098/rspb.1999.0858 [Google Scholar]
- Queller D.C, Zacchi F, Cervo R, Turillazzi S, Henshaw M.T, Santorelli L.A, Strassmann J.E. Unrelated helpers in a social insect. Nature. 2000;405:784–787. doi: 10.1038/35015552. [DOI] [PubMed] [Google Scholar]
- Ratnieks F.L.W. Reproductive harmony via mutual policing by workers in eusocial Hymenoptera. Am. Nat. 1988;132:217–236. [Google Scholar]
- Reeve H.K. Polistes. In: Ross K.G, Matthews R.W, editors. The social biology of wasps. Cornell University Press; Ithaca, NY: 1991. pp. 99–148. [Google Scholar]
- Reeve H.K, Gamboa G.J. Queen regulation of worker foraging in paper wasps: a social feedback control system (Polistes fuscatus, Hymenoptera, Vespidae) Behaviour. 1987;102:147–167. [Google Scholar]
- Röseler P.F, Röseler I. Dominance of ovariectomized foundresses of the paper wasp, Polistes gallicus. Insect Soc. 1989;36:219–234. [Google Scholar]
- Seeley T.D. Princeton University Press; Princeton, NJ: 1985. Honeybee ecology: a study of adaptation in social life. [Google Scholar]
- Sledge M.F, Boscaro F, Turillazzi S. Cuticular hydrocarbons and reproductive status in the social wasp Polistes dominulus. Behav. Ecol. Sociobiol. 2001;49:401–409. [Google Scholar]
- Sledge M.F, Trinca I, Massolo A, Boscaro F, Turillazzi S. Variation in cuticular hydrocarbon signatures, hormonal correlates and establishment of reproductive dominance in a polistine wasp. J. Ins. Physiol. 2004;50:73–83. doi: 10.1016/j.jinsphys.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Strassmann J.E. Weak queen or social contract? Nature. 1993;363:502–503. [Google Scholar]
- Sumana A, Starks P.T. The function of dart behavior in the paper wasp, Polistes fuscatus. Naturwissenschaften. 2004;91:220–223. doi: 10.1007/s00114-004-0527-7. [DOI] [PubMed] [Google Scholar]
- Turillazzi S. Seasonal variations in the size and anatomy of Polistes gallicus (L) (Hymenoptera, Vespidae) Monitore Zoologico Italiano. 1980;14:63–75. [Google Scholar]
- West-Eberhard M.J. The social biology of polistine wasps. Misc. Publ. Mus. Zool. Univ. Michigan. 1969;140:1–101. [Google Scholar]
- West-Eberhard M.J. The establishment of reproductive dominance in social wasp colonies. 1977. Proc. 8th Intl Congress IUSSI. pp. 223–227. Wageningen. [Google Scholar]
- West-Eberhard M.J. Oxford University Press; New York: 2003. Developmental plasticity and evolution. [Google Scholar]


