Abstract
The theory of inbreeding and outbreeding suggests that there is a hump-shaped relationship between the genetic similarity of sexually reproducing parents and the performance of their offspring. Inbreeding depression occurs when genetic similarity is high, whereas hybrid breakdown is expected when genetic similarity is low. Between these extremes, the effect of genetic similarity on fitness is unclear. We studied the shape of this relationship by crossing 65 target genotypes of the clonal, self-incompatible Ranunculus reptans with partner genotypes spanning a broad scale of genetic similarity, ranging from crosses within populations to between-population crosses and hybridisation with a closely related species. Offspring were raised in outdoor tubs. Results revealed a quadratic relationship between parental genetic distance and offspring performance, with the clonal component of fitness more strongly hump-shaped than the sexual component. Optimal genetic similarity encompassed a broad range of within-population and between-population crosses. This pattern of genomic compatibility has important implications for the evolution of mating systems and mate choice.
Keywords: fitness optimum, genomic divergence, outcrossing experiment, self-incompatibility SI system
1. Introduction
Evolutionary biologists have long suggested that there is a hump-shaped relationship between the genetic similarity of sexually reproducing parents and the performance of their offspring (Knowlton & Jackson 1993). Inbreeding depression occurs on one end of the genetic similarity axis. The decrease in offspring performance due to breeding between close relatives arises from increased homozygosity of deleterious recessive alleles and loss of heterosis (Charlesworth & Charlesworth 1987). Hybrid breakdown is expected on the other end of the genetic similarity axis due to disruption of co-adapted gene complexes (Lynch 1991) and reassortment of selfish genes that arose from coevolutionary genomic conflict (Hurst & Pomiankowski 1991). Therefore, we expect a fitness optimum at intermediate levels of parental genetic similarity where offspring benefit from heterosis, the masking of deleterious alleles and sufficient similarity in genetic architecture.
Theory makes few predictions about the width of the optimum and the exact shape of the relationship. Assuming decreasing genetic similarity with increasing geographical distance, Waddington (1983) argued that gene flow influenced the zone of optimal outbreeding by determining both the spatial scale of inbreeding depression and the spatial scale over which individuals are co-adapted to the genotypes with which they co-occur and mate. The breeding system may affect the range of optimal genetic similarity in plants. Frequent selfing may lead to purging of deleterious mutations and a decrease in inbreeding depression (Lande & Schemske 1985) as well as enhancement of genetic co-adaptation (Jain 1976). Hence, species that frequently self may have maximal reproductive output when mating happens among genetically more similar individuals and the range of the optimum may be smaller, whereas outcrossing species should have low fitness over a wider range of close genetic similarity but the optimum thereafter may be wider.
Optimal genetic similarity was first suggested for animals in the context of sexual imprinting (Bateson 1978) and has been tested in populations of tunicates, ungulates and fish (Grosberg 1987; Marshall & Spalton 2000; Neff 2004). Empirical work on plants has mainly addressed the question of whether there is an optimal geographical outcrossing distance (reviewed in Waser 1993). These studies assumed that geographically distant individuals were also more divergent genetically yet geographical distance does not necessarily reflect genetic distance, especially at local scales and in obligately outcrossing plants (Heywood 1991; Waser 1993; Hamrick & Godt 1996). The focus of all these studies was on intrapopulation crossing and they probably only covered a small range of genetic similarity.
We investigated the effect of parental genetic similarity on F1 offspring performance by crossing target genotypes from several populations over a broad scale of genetic similarity, including crosses between partners of the same population, different populations and members of a related species. Our focus was on genetic compatibility of crosses, independent of any possible adaptation to local conditions. The study organism was a clonal and gametophytically self-incompatible plant, Ranunculus reptans, for which we expected a broad outcrossing optimum.
2. Methods
(a) Study species and plant material
Ranunculus reptans (Ranunculaceae) has a circumpolar distribution, mainly in the temperate to boreal-subarctic zones of Europe, Asia and North America (Prati & Peintinger 2000). The species reaches its southern limit in central Europe, where it usually occurs in relatively distinct populations at the shores of pre-alpine lakes. The persistence of these populations is correlated with the regular occurrence of floods. The plant grows clonally and nodes may develop roots. Clonal reproduction is very important for this species because the active period remaining after summer inundation is too short in many years to allow successful seed production and seedling establishment. Thus, the fitness of a genotype can be estimated by the number of rooted rosettes and seeds produced by its offspring; these are the two (clonal and sexual) fitness components examined in this study.
To perform interspecific hybrid crosses, R. reptans was crossed with the closely related Ranunculus flammula. R. flammula has a western Palaearctic distribution, occurs in ditches, swamps and bogs in central Europe (Hess et al. 1980) and can hybridise with R. reptans where their habitats are adjacent (Prati & Peintinger 2000).
In spring 2002, we collected 187 plants from 15 populations of R. reptans; 13 populations from Lake Constance, one on the Bernina Pass and one south of the Alps on Lago Maggiore, Switzerland. At each site, 14 individuals were collected along two parallel transects that were 5 m apart at 5 m intervals. In six populations, the band of R. reptans occurring along the shoreline was so short that we could only sample 8–12 individuals. After collection, plants were held in separate tubs in a growth room. Five out of 187 field-collected plants died during propagation. R. flammula plants were collected from three Swiss populations in July 2002 following the same methods.
(b) Crossing design
Six randomly chosen Lake Constance R. reptans populations provided target genotypes for our study of genetic similarity and offspring performance. All 65 target genotypes were randomly crossed with two other genotypes from the same population, along with one genotype from two or three R. reptans partner populations. Eleven populations were used once as partner populations and two populations were used twice. We chose partner populations to cover a wide range of genetic divergence: for each target population, at least one partner population came from the same lake basin (mean Fst values±s.d.: 0.04±0.04; based on eight allozyme loci as described below) and one came from a different lake basin (0.11±0.08). In two instances, a further partner population came from a different region (0.18±0.05). This crossing design ensured considerable overlap in genetic similarity among crosses within and between populations. Finally, each target genotype was crossed with one genotype of an R. flammula population (Fst between populations of the two species: 0.46±0.05). This procedure gave 375 total crosses, each of which was performed reciprocally.
(c) Measurements of offspring performance
In May 2003, we germinated the seeds, counted seedlings after six weeks and haphazardly chose one seedling per seed family for planting into a tub (10×10×11 cm) with a 1 : 2 mixture of horticulture soil and sand. We distributed tubs in random positions within outdoor beds covered with 50% shade cloth and watered them daily (unless it rained). We checked survival after 3 days, 2 weeks and 4 weeks. We did not calculate survival because mortality was low (24 of the 654 plants died) and its estimate was based on only one representative of a seed family. Instead, dead plants were subtracted from the number of seedlings and another representative of the same seed family was used to estimate growth and reproductive performance.
We assessed fitness by estimating sexual and clonal offspring performance. Two months after the transfer into the garden, from the 11 to 20 August, we counted and measured the numbers of rooted rosettes, flowers, flower buds, infructescences and the average seed set of all infructescences (two classes: 0–50% of ovules successfully developing, or >50%). Sexual performance was defined as seed production of F1 plants per ovule in the parental generation. This was the proportion of maternal ovules that produced seedlings multiplied by the sum of buds, flowers and infructescences produced by the F1 family representative and by a factor representing seed production (1 or 2, for 0–50% seed production or >50%, respectively). Clonal performance was the number of rooted rosettes in the F1 per ovule in the parental generation, calculated as seedling emergence multiplied by the number of rooted rosettes of the family representative. For both measures we calculated relative fitness as the observed value divided by the mean of all crosses.
(d) Allozyme electrophoresis and genetic similarity
We estimated genetic similarity of parents using eight polymorphic allozyme loci at seven enzyme systems, AAT, ACON, GPI, MDH (2 loci), MPI, SKD and 6-PGDH, following standard methods (Hebert & Beaton 1993). Both R. reptans and R. flammula are tetraploid, with 32 chromosomes (Hess et al. 1980; Willi et al. 2005). As a measure of pairwise genetic similarity, we calculated Ps, the proportion of shared alleles averaged over loci (Bowcock et al. 1994). A distance measure between pairs of individuals was calculated as (1-Ps).
(e) Statistical analysis
The relationship between parental genetic similarity and offspring performance was tested with two different models. In the first model, genetic similarity was represented as a continuous variable, (1-Ps), while in the second, genetic similarity was a fixed factor distinguishing the three types of crosses (within population, between population and hybrid crosses between the two Ranunculus species). Cross-type and (1-Ps) could not be tested in a single model because they were highly confounded. The two dependent variables of relative sexual and clonal performance were first tested by multivariate analyses because they were strongly positively correlated (r=0.77, n=732, p<0.0001). The models tested for the effect of target genotype, genetic distance and the square term of genetic distance (or cross-type), the sexual function of the target genotype (pollen donor or pollen acceptor) and interaction terms. We assessed the shape of the outcrossing fitness optimum by fitting a cubic spline using the Tpspline procedure in SAS (SAS Institute Inc. 1999). The smoothing parameters λ were chosen by minimizing the generalized cross validation (GCV) function.
3. Results
There was a curvilinear relationship between parental genetic similarity and the two fitness components of sexual and clonal performance (table 1). Significant linear and quadratic effects in the multivariate analysis indicated that sexual and clonal offspring performance increased with genetic distance and then decreased. This result is consistent with inbreeding depression at short outcrossing distances and reduced hybrid performance at large outcrossing distances. Neither target genotype nor interactions involving target genotypes were significant, suggesting that the relationship was similar for all genotypes. Univariate tests showed that clonal growth was—in comparison to flower and seed production—more strongly affected by parental genetic similarity (table 1). The interaction between target genotype and sex indicates that the fitness difference between male and female function was not the same for all genotypes. Analysis excluding incompatible crosses, which were recognized when both reciprocal crosses developed no seeds, did not change the direction and significance of the results. When genetic similarity was replaced by geographical distance, neither the linear nor the square term was significant (both p>0.4).
Table 1.
MANCOVA examining effects of target genotype, sex of target genotype, genetic distance to the partner plant and interaction terms on two estimates of relative fitness: sexual and clonal performance of offspring.
| source of variation | error term | d.f. | Wilks' F | p | type III sums of squares of univariate tests | ||
|---|---|---|---|---|---|---|---|
| d.f. | sexual performance | clonal performance | |||||
| target genotype (TG) | pooled error | 126, 944 | 1.14 | 0.1499 | 63 | 78.63 | 74.53 |
| genetic distance | TG×genetic distance | 2, 62 | 7.25 | 0.0015 | 1 | 3.85 | 16.09*** |
| TG×genetic distance | pooled error | 126, 944 | 1.17 | 0.1142 | 63 | 74.63 | 72.79 |
| genetic distance2 | TG×genetic distance2 | 2, 62 | 12.26 | <0.0001 | 1 | 3.63 | 25.50*** |
| TG×genetic distance2 | pooled error | 126, 944 | 1.20 | 0.0788 | 63 | 74.24 | 72.60 |
| sex of TG | TG×sex of TG | 2, 63 | 1.11 | 0.3371 | 1 | 2.85 | 0.73 |
| TG×sex of TG | pooled error | 128, 944 | 1.27 | 0.0307 | 64 | 138.26* | 128.25*** |
| error | 473 | 692.62 | 522.70 | ||||
Significance of univariate tests is indicated: *p<0.05, ***p<0.001; n=746, R2=0.43 for sexual performance and R2=0.48 for clonal performance.
The model including cross-type instead of genetic similarity produced a similar outcome (table 2). Means (±s.d.) of sexual and clonal performance were 0.94±1.28 and 1.13±1.30 for within-population crosses (n=260), 1.02±1.34 and 1.15±1.19 for between-population crosses (n=352) and 1.06±1.13 and 0.36±0.41 for interspecific hybrid crosses (n=134). The main effect of cross-type arose mostly from reduced clonal growth in offspring of interspecific hybrid crosses in comparison with those of within-population and between-population crosses (table 2). No difference in performance was observed between offspring of within-population and between-population crosses of R. reptans, even though some offspring from within-population crosses suffered from inbreeding depression. Hybrid crosses were not exactly intermediate between the two parental species. The mean (±s.d.) sexual and clonal performance of R. flammula offspring was 0.58±0.85 and 0.12±0.10 (n=112). Hence, interspecific hybrid crosses showed—in comparison to the mid-value of intra-population R. reptans and R. flammula crosses—significantly increased sexual performance (d.f.=131, t=3.10 and p=0.0023) and significantly reduced clonal performance (d.f.=133, t=−7.58 and p<0.0001).
Table 2.
MANOVA examining effects of target genotype, sex of target genotype, cross-type and interaction terms on two estimates of relative fitness: sexual and clonal performance of offspring.
| source of variation | error term | d.f. | Wilks' F | p | type III sums of squares of univariate tests | ||
|---|---|---|---|---|---|---|---|
| d.f. | sexual performance | clonal performance | |||||
| target genotype (TG) | pooled error | 128, 942 | 1.18 | 0.0970 | 64 | 143.31** | 95.74* |
| cross-type | TG×cross-type | 4, 254 | 27.66 | <0.0001 | 2 | 1.41 | 63.87*** |
| TG×cross-type | pooled error | 256, 942 | 1.12 | 0.1285 | 128 | 178.24 | 122.48 |
| sex of TG | TG×sex of TG | 2, 63 | 0.86 | 0.4301 | 1 | 2.47 | 0.69 |
| TG×sex of TG | pooled error | 128, 942 | 1.28 | 0.0242 | 64 | 141.69* | 128.80*** |
| error | 472 | 695.36 | 527.48 | ||||
| t-values for contrasts between least-squared means of | within-population versus between-population crosses | −0.68 | −0.04 | ||||
| within-population versus hybrid crosses | −0.93 | 6.84*** | |||||
| between-population versus hybrid crosses | −0.39 | 6.99*** | |||||
Significance of univariate tests is indicated: *p<0.05, **p<0.01, ***p<0.001; n=746, R2=0.42 for sexual performance and R2=0.47 for clonal performance.
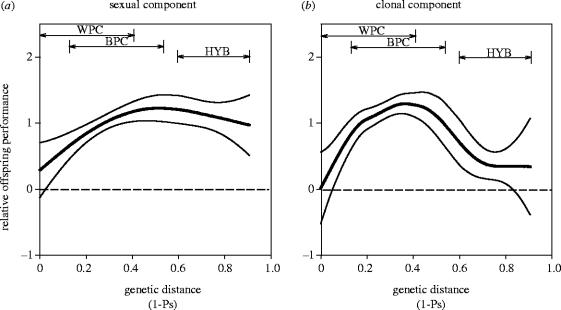
We used a spline-fitting approach to investigate the shape of the fitness curve around the optimum (figure 1). For clonal offspring performance, the spline was fairly flat over a wide range of outcrossing distances. Judging from the confidence intervals, the range extended from at least 0.15 to 0.6, which covered 86% of crosses within populations and 99% of crosses performed between populations of R. reptans. For sexual offspring performance, the range of optimal genetic similarity began at approximately 0.25 and fitness did not clearly decrease within the range of interspecific hybridization.
Figure 1.
Splines of relative sexual (a) and relative clonal (b) performance of offspring against the genetic distance between their parents. Thin lines represent upper and lower 95% confidence intervals. The double arrows indicate the range of genetic similarity of within-population crosses (WPC), between-population crosses (BPC) and interspecific hybrids (HYB). For sexual performance of offspring, summary statistics of the estimated curve were: n=732, log10 (n×λ)=−0.8085, d.f.=3.3343 and s.d.=1.2713. For clonal performance, summary statistics were: n=743, log10 (n×λ)=−2.0810, d.f.=6.0421 and s.d.=1.1209. Parental genetic similarity had very little influence on offspring performance over a broad range of intermediate genetic similarity values.
4. Discussion
This study found a broad range of genetic similarity among parents over which genetic compatibility occurs (figure 1). There was no single peak of optimal genetic outbreeding. Low genetic distances between parents caused inbreeding depression, leading to few offspring and low seedling performance. Inbreeding depression is expected to be high in obligatory outcrossing species such as R. reptans because recessive deleterious mutations usually occur in heterozygous state and are therefore rarely exposed to, and eliminated by, natural selection (Lande & Schemske 1985). Furthermore, because R. reptans is gametophytically self-incompatible and mutational loads are often increased at loci linked to the incompatibility locus (Glémin et al. 2001), it may be especially prone to accumulate deleterious mutations in the first place. Some crosses between close relatives probably had low fitness simply because the parents shared identical alleles at the incompatibility locus. However, inbreeding depression is not solely a result of incompatibility in R. reptans, a point illustrated by the fact that our results were essentially unchanged when we excluded crosses without seed set (also see Willi et al. 2005).
Outbreeding over large genetic distances, in this case hybridization between two closely-related species, led to a decline in the clonal component of fitness. The sexual component showed no significant decline at large genetic distances. Outbreeding depression probably arose from a combination of expression of intermediate phenotypes in hybrids and a breakdown of co-adapted gene complexes affecting clonal growth. Evidence for co-adaptation comes from less-than-intermediate clonal performance of interspecific hybrids. Recombination of selfish genetic elements that evolved in allopatry can also cause outbreeding depression but there was no indication of this mechanism in our results. Cytoplasmic sex ratio distorters in plants are located on mitochondria and can produce male sterility in hybrids (Budar et al. 2003). Meiotic drive genes are thought to occur mainly on sex chromosomes and cause hybrid sterility in the heterogametic sex (Hurst & Pomiankowski 1991). We observed no patterns in flower and seed production or pollen fertility to suggest systematic F1 hybrid breakdown in sexual performance and sterility.
The two mechanisms of inbreeding depression and hybrid breakdown apparently have no overlapping area of influence on the genetic similarity axis. On the contrary, there is a large range of intermediate genetic similarity where outcrossing is more or less equally optimal. This result supports Crow & Kimura's (1979) model of truncation selection, suggesting that an increase in a character value, here parental genetic distance and presumably offspring heterozygosity, increases offspring performance only to a threshold value; thereafter, no increase in fitness is expected from a further increase in genetic distance and heterozygosity (Mitton 1997). Alternatively, both inbreeding depression and hybrid breakdown may affect fitness within this middle region but they compensate for one another, as found in Arabian oryx (Marshall & Spalton 2000). As the environment can modulate the strength of inbreeding and outbreeding depression (e.g. Norman et al. 1995), the wide optimum we observed could be a consequence of low inbreeding and outbreeding depression in the common garden.
Even considerably outbred crosses barely experienced a decrease in fitness, as long as they occurred within the same species (figure 1). According to Lynch (1991), fitness reductions due to outbreeding should become more obvious in the F2 generation, since F2 individuals benefit only half as much as F1 individuals from between-population dominance effects. Two recent studies tested this idea by performing interpopulation crosses over geographical distances from a few metres to a few thousand kilometres. Fenster & Galloway (2000) found heterosis in outbred F1 plants and hybrid breakdown in the F3 generation independent of geographical distance. Edmands (1999) also found no relationship between fitness and geographical or genetic distance in the F1 of a marine copepod, but decreased fitness measures with increasing geographical and genetic distance in the F2. These studies show that the pattern of optimal outbreeding may change between F1 and later generations. On the other hand, two sequential generations of outbreeding between populations or species is unrealistic because the F2 and later generations probably consist of backcrosses with individuals of a parental population. In this case, hybrid breakdown is less likely (Edmands 1999; Schweitzer et al. 2002).
Our data support the hypothesis that, in comparison to frequently selfing species, obligate outbreeders are less likely to suffer from outbreeding depression when crossed with fairly unrelated individuals. Outbreeding depression may result from a reproductive system which allows selfing or biparental inbreeding, even within local populations (reviewed in Waser 1993). The conclusion here is that the mating system can influence the shape of the relationship between fitness and genetic similarity. In turn, the shape of this same relationship can influence how selection acts on the mating system. Our finding of genetic compatibility over a wide range of genomic divergence should favour the evolution of a mating system that only avoids inbreeding and interspecific hybridization. Inbreeding is more likely than hybridization in natural populations and a crude incompatibility system, such as that found in R. reptans, is sufficient to accomplish inbreeding avoidance. Inbreeding avoidance may also be the main selective force for female multiple mating strategies in animal species (Tregenza & Wedell 2002).
However, this raises the issue of why outcrossing plants and animals can show remarkably subtle discrimination of gametes (Bernasconi et al. 2004). Waser & Price (1994) found an optimal geographical outcrossing distance in the range of 10 m and pollen originating from this distance was favoured in female mate choice (Waser et al. 1987; Waser & Price 1991). Likewise, if carrying different alleles at a resistance locus is advantageous for defence against parasites and pathogens, we might expect mechanisms similar to those in vertebrates favouring partners with different MHC genotypes (Tregenza & Wedell 2000). This implies that the existence and location of an optimal genetic similarity for outbreeding may depend on exposure of offspring to parasites or other sources of environmental stress. We hypothesize that the shape of the curve between genetic similarity and offspring performance strongly depends on the environment.
Taken at face value, our results imply that conservation biologists designing breeding programmes for outcrossing species should expect that nearly any non-inbred conspecific cross will yield offspring of equivalent F1 performance. If true, this conclusion would simplify the process of arranging matings by increasing the proportion of feasible matings. However, the conclusion may not be justified if the shape of the outcrossing curve varies among species and it will certainly be wrong if populations are locally adapted. Clearly, experiments similar to ours must be repeated for other species and should include variation in test environments. In the meantime, we believe that the main implications of our work lie in more theoretical realms, such as the evolution of incompatibility systems, mate choice and dispersal.
Acknowledgments
We thank S. Hoebee, R. Holderegger, B. Liebst and S. Röthlisberger for an introduction to allozyme electrophoresis. V. Summa, G. Vergnerie and A. Weidt helped transplant seedlings. D. Lang, G. Vergnerie, A. Willi, C. Willi and E. Willi helped measure offspring performance. Many thanks to G. Bernasconi, M. Fischer, B. R. Grant and D. Hosken for constructive comments on the manuscript. We were supported by the Swiss Nationalfonds (31-56 809.99, 31-67876.02 to M. Fischer) and the Institute of Environmental Sciences, University of Zurich.
References
- Bateson P. Sexual imprinting and optimal outbreeding. Nature. 1978;273:659–660. doi: 10.1038/273659a0. [DOI] [PubMed] [Google Scholar]
- Bernasconi G, et al. Evolutionary ecology of the prezygotic stage. Science. 2004;303:971–975. doi: 10.1126/science.1092180. [DOI] [PubMed] [Google Scholar]
- Bowcock A.M, Ruiz-Linares A, Tomfohrde J, Minch E, Kidd J.R, Cavalli-Sforza L.L. High resolution of human evolutionary trees with polymorphic microsatellites. Nature. 1994;368:455–457. doi: 10.1038/368455a0. [DOI] [PubMed] [Google Scholar]
- Budar F, Touzet P, De Paepe R. The nucleo-mitochondrial conflict in cytoplasmic male sterilities revisited. Genetica. 2003;117:3–16. doi: 10.1023/a:1022381016145. [DOI] [PubMed] [Google Scholar]
- Charlesworth D, Charlesworth B. Inbreeding depression and its evolutionary consequences. Annu. Rev. Ecol. Syst. 1987;18:237–268. [Google Scholar]
- Crow J.F, Kimura M. Efficiency of truncation selection. Proc. Natl Acad. Sci. USA. 1979;76:396–399. doi: 10.1073/pnas.76.1.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmands S. Heterosis and outbreeding depression in interpopulation crosses spanning a wide range of divergence. Evolution. 1999;53:1757–1768. doi: 10.1111/j.1558-5646.1999.tb04560.x. [DOI] [PubMed] [Google Scholar]
- Fenster C.B, Galloway L.F. Population differentiation in an annual legume: genetic architecture. Evolution. 2000;54:1157–1172. doi: 10.1111/j.0014-3820.2000.tb00551.x. [DOI] [PubMed] [Google Scholar]
- Glémin S, Bataillon T, Ronfort J, Mignot A, Olivieri I. Inbreeding depression in small populations of self-incompatible plants. Genetics. 2001;159:1217–1229. doi: 10.1093/genetics/159.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosberg R.K. Limited dispersal and proximity-dependent mating success in the colonial ascidian Botryllus schlosseri. Evolution. 1987;41:372–384. doi: 10.1111/j.1558-5646.1987.tb05804.x. [DOI] [PubMed] [Google Scholar]
- Hamrick J.L, Godt M.J.W. Effects of life history traits on genetic diversity in plant species. Phil. Trans. R. Soc. B. 1996;351:1291–1298. [Google Scholar]
- Hebert P.D.N, Beaton M.J. Helena Laboratories; Beaumont, Texas: 1993. Methodologies for allozyme analysis using cellulose acetate electrophoresis: a practical handbook. [Google Scholar]
- Hess H.E, Landolt E, Hirzel R. Birkhäuser; Basel: 1980. Flora der Schweiz und angrenzender Gebiete, Band 2. [Google Scholar]
- Heywood J.S. Spatial analysis of genetic variation in plant populations. Annu. Rev. Ecol. Syst. 1991;22:335–355. [Google Scholar]
- Hurst L.D, Pomiankowski A. Causes of sex ratio bias may account for unisexual sterility in hybrids: a new explanation of Haldane's rule and related phenomena. Genetics. 1991;128:841–858. doi: 10.1093/genetics/128.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S.K. The evolution of inbreeding plants. Annu. Rev. Ecol. Syst. 1976;7:469–494. [Google Scholar]
- Knowlton N, Jackson J.B.C. Inbreeding and outbreeding in marine invertebrates. In: Thornhill N.W, editor. The natural history of inbreeding and outbreeding. University of Chicago Press; Chicago: 1993. pp. 200–249. [Google Scholar]
- Lande R, Schemske D.W. The evolution of self-fertilization and inbreeding depression in plants. I. Genetic models. Evolution. 1985;39:24–40. doi: 10.1111/j.1558-5646.1985.tb04077.x. [DOI] [PubMed] [Google Scholar]
- Lynch M. The genetic interpretation of inbreeding depression and outbreeding depression. Evolution. 1991;45:622–629. doi: 10.1111/j.1558-5646.1991.tb04333.x. [DOI] [PubMed] [Google Scholar]
- Marshall T.C, Spalton J.A. Simultaneous inbreeding and outbreeding depression in reintroduced Arabian oryx. Anim. Conserv. 2000;3:241–248. [Google Scholar]
- Mitton J.B. Oxford University Press; Oxford: 1997. Selection in natural populations. [Google Scholar]
- Neff B.D. Stabilizing selection on genomic divergence in a wild fish population. Proc. Natl Acad. Sci. USA. 2004;101:2381–2385. doi: 10.1073/pnas.0307522100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman J.K, Sakai A.K, Weller S.G, Dawson T.E. Inbreeding depression in morphological and physiological traits of Schiedea lydgatei (Caryophyllaceae) in two environments. Evolution. 1995;49:297–306. doi: 10.1111/j.1558-5646.1995.tb02242.x. [DOI] [PubMed] [Google Scholar]
- Prati D, Peintinger M. Biological flora of Central Europe: Ranunculus reptans L. Flora. 2000;195:135–145. [Google Scholar]
- SAS Institute Inc. Cary, NC; SAS Institute Inc.: 1999. SAS OnlineDoc®, Version 8. [Google Scholar]
- Schweitzer J.A, Martinsen G.D, Whitham T.G. Cottonwood hybrids gain fitness traits of both parents: a mechanism for their long-term persistence? Am. J. Bot. 2002;89:981–990. doi: 10.3732/ajb.89.6.981. [DOI] [PubMed] [Google Scholar]
- Tregenza T, Wedell N. Genetic compatibility, mate choice and patterns of parentage: invited review. Mol. Ecol. 2000;9:1013–1027. doi: 10.1046/j.1365-294x.2000.00964.x. [DOI] [PubMed] [Google Scholar]
- Tregenza T, Wedell N. Polyandrous females avoid costs of inbreeding. Nature. 2002;415:71–73. doi: 10.1038/415071a. [DOI] [PubMed] [Google Scholar]
- Waddington K.D. Pollen flow and optimal outbreeding distance. Am. Nat. 1983;122:147–151. [Google Scholar]
- Waser N.M. Population structure, optimal outbreeding, and assortatice mating in Angiosperms. In: Thornhill N.W, editor. The natural history of inbreeding and outbreeding. University of Chicago Press; Chicago: 1993. pp. 173–199. [Google Scholar]
- Waser N.M, Price M.V. Outcrossing distance effects in Delphinium nelsonii: pollen loads, pollen tubes, and seed set. Ecology. 1991;72:171–179. [Google Scholar]
- Waser N.M, Price M.V. Crossing-distance effects in Delphinium nelsonii: outbreeding and inbreeding depression in progeny fitness. Evolution. 1994;48:842–852. doi: 10.1111/j.1558-5646.1994.tb01366.x. [DOI] [PubMed] [Google Scholar]
- Waser N.M, Price M.V, Montalvo A.M, Gray R.N. Female mate choice in a perennial herbaceous wildflower, Delphinium nelsonii. Evol. Trend. Plant. 1987;1:29–33. [Google Scholar]
- Willi Y, Van Buskirk J, Fischer M. A threefold genetic Allee effect: population size affects cross-compatibility, inbreeding depression and drift load in the self-incompatible Ranunculus reptans. Genetics. 2005;169:2255–2265. doi: 10.1534/genetics.104.034553. [DOI] [PMC free article] [PubMed] [Google Scholar]