Abstract
The idea that an aposematic prey combines crypsis at a distance with conspicuousness close up was tested in an experiment using human subjects. We estimated detectability of the aposematic larva of the swallowtail butterfly, Papilio machaon, in two habitats, by presenting, on a touch screen, photographs taken at four different distances and measuring the time elapsed to discovery. The detectability of larvae in these images was compared with images that were manipulated, using existing colours either to increase or decrease conspicuousness. Detection time increased with distance for all colourations. However, at the closest distance, detection time was longer for the larvae manipulated to be more cryptic than for the natural and more conspicuous forms. This indicates that the natural colouration is not maximally cryptic at a short distance. Further, smaller increments in distance were needed to increase detection time for the natural than for the conspicuous larva. This indicates that the natural colouration is not maximally conspicuous at longer distances. Taken together, we present the first empirical support for the idea that some colour patterns may combine warning colouration at a close range with crypsis at a longer range. The implications of this result for the evolution of aposematism are discussed.
Keywords: aposematism, crypsis, conspicuousness, predation, distance, background
1. Introduction
Protective prey colouration has usually been divided into two main categories, located at the opposite ends of the conspicuousness continuum. Accordingly, warning colouration (aposematic or mimetic) is generally assumed to be highly conspicuous to yield high signalling quality, whereas cryptic colouration is assumed to have evolved to minimize conspicuousness to decrease the risk of detection (e.g. Cott 1940; Edmunds 1974; Evans & Schmidt 1990; Endler 1991). Consequently, cryptic and warning colouration have often been considered as mutually exclusive and conflicting anti-predator strategies.
On the other hand, aposematic species seem to differ with respect to the strength of their warning signals, implying that not all aposematic prey have evolved to maximize their conspicuousness (e.g. Endler & Mappes 2004). One possible explanation for this variation, an explanation that we promote in this paper, is that some prey colouration has combined the apparently opposing functions of anti-predator signalling and camouflage (Edmunds 1974; Rothschild 1975; Papageorgis 1975; Endler 1978; Ruxton et al. 2004). Because the resolution of an eye is limited, colour patterns may appear different when viewed from a close distance than when viewed from further away. Thus, when viewed from afar, small pattern elements of a colour pattern may melt into the surrounding colour, or an area covered by a mixture of colours, such as dots or stripes, or two different colours may appear evenly coloured as a third colour (e.g. Endler 1978). Therefore, with an appropriate geometry and combination of colours, a pattern that appears conspicuous at a close range might turn to a cryptic one with increasing distance. In other words, the conspicuousness of any given prey colouration is affected by observation distance, but all patterns are not necessarily equally affected. Such variation among colour patterns may allow selection for distance-dependent combinations of signalling and camouflage.
Because the general conception seems to be that conspicuousness is the most characteristic feature of warning colouration, one discussion has revolved around how such costly traits could evolve, especially when a new mutation for the trait occurs in low frequency in the population (e.g. Harvey et al. 1982; Leimar et al. 1986; Mallet & Singer 1987; Guilford 1990; Yachi & Higashi 1998; Lindström et al. 1999; Servedio 2000; Speed 2001). Although we agree with Turner (1975) that the crucial feature in aposematism may not be conspicuousness per se, but to be recognized as unpalatable and discriminated from edible prey, it seems that some degree of conspicuousness may be necessary to attain that function (e.g. Gittleman & Harvey 1980; Guilford 1990). This does not imply, however, that conspicuousness is always maximized in aposematic animals; indeed previous researchers have suggested that some colourations could function to be conspicuous when seen close up and, at the same time, attain crypsis at a distance (Edmunds 1974; Papageorgis 1975; Rothschild 1975; Endler 1978). Such a combination of functions has been suggested, e.g. for the larvae of the cinnabar moth, Tyria jacobaea (Rothschild 1975), the queen, Danaus chrysippus (Edmunds 1974), Hyalophora cecropia (Deml & Dettner 2003, in Ruxton et al. 2004) and the swallowtail, Papilio machaon (Järvi et al. 1981). However, to our knowledge, the idea of such distance-dependent dual protective function has not been subjected to empirical study.
In this paper, we test the idea that the colouration of the larva of the swallowtail, P. machaon, may constitute a distance-dependent combination between conspicuousness related to aposematism on one hand and crypsis on the other hand. The larva is highly unpalatable to various birds which avoid it on sight after one or a few encounters (great tit, (Järvi et al. 1981; Wiklund & Järvi 1982); domestic hen, (Wiklund & Sillén-Tullberg 1985); quail, (Sillén-Tullberg 1990)). Moreover, the colouration includes the combination of orange dots and black bands, which has typically been considered a warning signal. Thus, the predator reactions, as well as some of the colours of the larva, suggest that it is aposematic. On the other hand, the green and black bands as well as the small size of the orange dots of the larva might suggest a cryptic function of the colour pattern, at least at a longer range. Thus, we wanted to study the function of the colouration of P. machaon and whether it changes with the distance to the viewer.
There is an obvious problem involved in the measurement of the detectability of aposematic prey to its natural predators: the predators may avoid the prey, which would obviously flaw the measurement. Furthermore, in the present study, in which we are interested in the distance dependence of the function of prey colouration, an additional problem would arise, because the measurements of detectability should be taken only at given distances between prey and the predator. To overcome these problems, we chose to use photographs of the prey taken at given distances and presented on a touch-sensitive computer screen. Because computer screens and digital cameras are optimized for human colour vision, we used humans as ‘predators’. An additional benefit of the use of photographs and humans was that we could include image manipulation in our methods.
Because conspicuousness is a relative concept, we set out to study the colouration of the swallowtail larva by comparing its detectability with that of alternative colourations. These alternative colourations were produced by the manipulation of colour pattern elements of the larvae in the digital photographs taken in two natural habitats of the swallowtail. Detectabilities at the given distances were measured using human subjects that were individually tested for how fast they could discover a larva in an image exposed on a screen. We ask how the detectability of the swallowtail larva varies with distance in comparison to the alternative colourations. Specifically, we ask whether the colouration is maximally cryptic or could be made more cryptic at a close distance, and whether it is maximally conspicuous or could be made more conspicuous at a longer distance. Our results strongly suggest that it represents a distance-dependent combination of protective functions.
2. Material and methods
(a) Papilio machaon and its habitat
The European swallowtail has a Holarctic distribution, with host plants belonging to the families Apiaceae and Rutaceae. In Sweden it uses two different major breeding habitats (cf. Wiklund 1974). One habitat type is coastal localities in the immediate vicinity of open water such as rocky shores. Here, the broad-leafed triennial apiaceous plant Angelica archangelica is the dominant host plant, and larvae are commonly found where the stalks of A. archangelica grow in crevices among rocks or between boulders, with no other plants growing nearby. The other habitat type is humid inland localities such as fens, bogs and wet meadows where the perennial apiaceous plant Peucedanum palustre is the major host plant. In Sweden, the life cycle of the swallowtail is generally univoltine, with hibernation in the pupal stage and mating and oviposition taking place during May and June. The first three larval instars are coloured black with a white saddle and are regarded as mimics of bird droppings. The fourth and fifth instars are coloured green with broad black transversal stripes that are punctuated with orange spots (figure 1a,d).
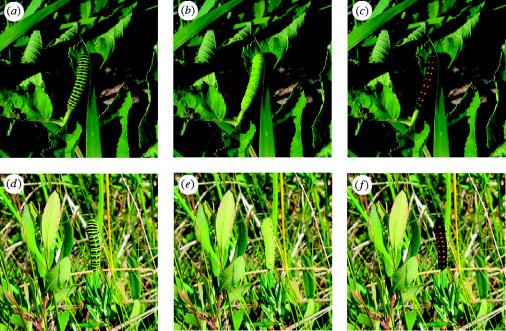
Figure 1.
A fifth instar larva of the swallowtail butterfly, Papilio machaon with the colour patterns used in the experiment. Images (a)–(c) on Angelica archangelica in the shore habitat and images (d)–(f) on Peusedanum palustre in the fen habitat. The three larval colourations used were the normal, unmanipulated colour pattern (black and green bands and orange dots; (a) and (d)), the manipulated, uniformly green colour pattern (b) and (e) and the manipulated, black colouration with enlarged orange spots (c) and (f).
In the experiments, we used images of natural as well as manipulated larval colourations. It is important to note that the image manipulations carried out only used colours that the larva can produce and that they represent colourations that are similar to those that occur in the wild and hence lie within the natural range of variation in larval colouration of P. machaon. In the final (fifth) instar, the natural variation of larval colour includes all of the three larval colour elements, the hue of the green basic colour, the tone of the orange spots of the black stripes, and the width of the black stripes themselves. The green basic colour varies from dark green when ambient temperatures are low, to a creamy light green when ambient temperatures are high (Christer Wiklund, personal observation), and the orange spots that derive their colour from carotenoids sequestered from larval feeding have a deeper tone in low compared with high ambient temperatures. Occasionally, larvae can be found that have lost the ability to synthesize the black pigment completely, and these larvae are uniformly green with small orange spots (cf. figure 2a). The breadth of the black stripes is variable (cf. the American swallowtail, Papilio polyxenes; Hazel 2002), and occasionally these bands are so broad that the larvae are virtually black (cf. figure 2b).
Figure 2.
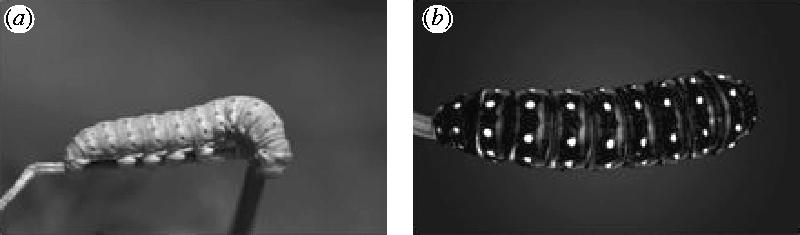
Two rare, natural colouration variants of the fifth instar P. machaon larvae. These individuals are offspring of two different females that were wild-caught in southern Sweden. (a) A uniformly green larva (but with orange spots present) that has lost the ability to synthesize the black pigment, (b) a black larva in which the black, orange-studded stripes are so broad that the normally green area is completely covered by black.
In contrast to the trichromatic vision of humans, many passerine birds have a fourth class of photoreceptors that are sensitive to light in the UV part of the spectrum (e.g. Cuthill et al. 2000; Ödéen & Håstad 2003). Therefore, if humans do not detect a difference between two colour samples this may be either because there is no difference or because there is a difference that humans cannot detect. In other words, if a larva seen against a given background appears cryptic to humans, it is not necessarily cryptic for passerine birds. Because such birds are likely potential predators on insect larvae, we wanted to find out how the exclusion of UV in our experiment, due to use of humans as predators, affects our conclusions. For this purpose, we compared the reflectance spectrum of the fifth instar P. machaon larva and leaves from one of the hosts, using an S1000-2LOS25U spectrometer (Ocean Optics, Dunedin, FL, USA) and a DH-2000 deuterium–halogen light source (215–1700 nm). The Reflectance probe (FCR-7UV200-1.5×100-2, Avantes, Eerbeek, The Netherlands) was held at an angle of 45° from the measured surface to minimize mirror reflectance. The maximum sensitivity in UV-sensitive birds ranges from 355 to 376 nm (Cuthill et al. 2000; Hart 2001). Within the range invisible to humans but visible to some birds, from 355 to 380 nm, the reflectance of the green areas of the larva and the leaves was similar and relatively low. The orange spots reflected somewhat more light within this range and the black stripes had the lowest reflectance. Generally, these reflectances were low compared with the reflectances within the range visible to humans, except for the black stripes, which had a very low reflectance even within that range. In sum, there appears not to be any dramatic differences in the UV range that could make the seemingly cryptic elements of the pattern conspicuous to passerine birds.
In late June to early July, we photographed the fifth instar larvae in two localities representing the two different habitats. The first locality, Nothamn, is located on Väddö, a large island in the northeastern part of the landscape Uppland, and is characterized by cliffs and rocky shores with A. archangelica. The other locality, Hillebolamossen, is a large fen in a nature reserve, Florarna, in northern Uppland. Here P. palustre grows among Sphagnum spp. on the dryer parts. The swallowtail has been observed for several years in both localities.
(b) Photography
We used a 3.2 megapixel digital camera (Canon PowerShot S30) and took several series of photographs in both localities. Photographs were taken around midday in full sunlight. We used 7.1 mm focal length, which yields an angle of view approximately equivalent to a 35 mm lens in the traditional 35 mm film format, an angle wider than that of the human eye. One series of pictures was taken of each larva at different distances, starting from a distance of 20 cm (measured from the lens of the camera to the middle of the larva using a standard), continuing with 30 cm and so on, increasing the distance in 10 cm steps up to 120 cm. Photographs were finally taken at a distance of 150 cm. Four pictures, each with the larva positioned in a different quadrant of the photo, were taken at each distance.
Because the aim of the study was to compare the detectability of P. machaon at different relative distances, we wanted to find a suite of distances ranging from easy to difficult for our human subjects. Therefore, we excluded distances at which the detection task appeared to be too easy or too difficult. Accordingly, we used 30 cm as the shortest and 90 cm as the furthest distance and we also used two distances in between these, 50 and 70 cm, respectively. However, it should be noted that a wide-angle lens makes any real distance appear longer to us in the photographs. Therefore, we refer to these distances, from the shortest one, as distance 1, 2, 3 and 4, respectively.
(c) Image manipulations
We chose two suites of photographs, one from each locality. Each suite consisted of one individual larva photographed at different distances. The criteria of choice were high photographic quality and about equal size of the larva in the two suites. In the experiment, we used both unmanipulated and manipulated photographs from the two suites. The aim of the manipulations was to change larval detectability in both directions. The manipulations were done using the software Adobe Photoshop Elements (1.0.1), which enabled the copying of the colours from pixels in one part of the image, and layering these over other parts. Both larvae in the suites were illuminated from the side, and it was thus important to copy pixels from areas with the same brightness as those overlaid. In the aim to decrease detectability, we copied pixels from nearby green areas and pasted these to cover black and orange areas completely. Thus, in these photographs, the larva was uniformly green (figure 1b,e). To increase detectability, we manipulated two traits (figure 1c,f). First we copied pixels from black areas and completely covered the green areas. Second, we enlarged the orange spots by approximately 50%. Because of the smallness of the orange spots, the orange in the more distant pictures was diluted by colours from nearby fields. Therefore, we copied pixels from the orange spots in the closest photograph and pasted them on to the more distant photographs.
(d) Image presentation
There were two main reasons for using human subjects in our experiment. First, training of subjects to the experimental protocol, presentation of the images at controlled distances and measurement of time to detection (instead of having to measure time to attack) are obviously much less problematic and less time consuming with humans than with other organisms. Second, digital cameras and computer screens are optimized for human colour vision. Because we have studied the effect of the apparent size on the detectability of the larval colour pattern and the depth cues were equal for all three colourations, we have no reason to expect that the two-dimensionality of the images would have biased our results.
The subjects in our experiments were 150 undergraduate students of psychology at Stockholm University. We preferred non-biology students because of their supposedly greater naivety with regard to insects. We chose psychology students because they are rewarded with university points for partaking in experiments with a psychological bearing.
In order to avoid effects of learning (e.g. search image formation), each subject was presented only one photograph on a touch screen (15 in.). This photograph was taken in one of the two habitats at one of the four distances, and represented the natural larva or one of the two manipulations. The subject's task was to detect and point at the larva on the screen. A computer program presented the images as well as measuring and recording the detection times (i.e. touch on larva). All the combinations of habitat, larval colouration and distance were presented approximately the same number of times.
Before the experiment, the subjects were informed about the aim of the experiment, namely measurement of detectability of different colourations at different distances and in different habitats, and that their task was to find and touch a caterpillar/larva in the image presented as quickly as possible. We also illustrated the general appearance of lepidopteran larvae by allowing the subjects to have a brief look at drawn illustrations of randomly chosen larvae in a handbook covering over 500 species of lepidopteran caterpillars (Carter & Hargreaves 1986). Thus, we wanted to convey the form but give no prenotion of the colouration of the object to be discovered. The subjects were also informed about the variation in degree of difficulty among photographs.
Each subject was tested separately in a room with electric lights turned off and with closed venetian blinds to prevent reflectance on the screen. The person was asked to sit down at a comfortable distance in front of the screen. He/she was instructed about how to use the touch screen and practised it a couple of times before confirming that he/she was ready for the presentation. Then, the person was free to start the task by touching a start button that appeared on the screen, whereupon the image was shown. The subject was allowed to touch the screen wherever he/she believed to have discovered a larva. However, the presentation program only responded to the touch on the larva, not on other areas of the screen. As soon as the correct area was touched, the elapsed presentation time was recorded and the image on the screen was turned off. A maximum of 120 s search time was allowed for a subject, and if he/she did not detect the larva before the time was up, the program turned off the image and recorded 120 s as search time. After the experiment, the test person was free to discuss his/her experience of the test with us.
(e) Statistical analyses
To test the effect of colouration, distance and habitat on detection time we used an analysis of variance. To test our specific hypotheses we used planned comparisons. Because these comparisons were not orthogonal, we used the Dunn–Sidák method to adjust the significance levels in order to correct for type I error (Sokal & Rohlf 1995). We applied logarithmic transformation on the data to meet the conditions of the statistical analyses.
3. Results
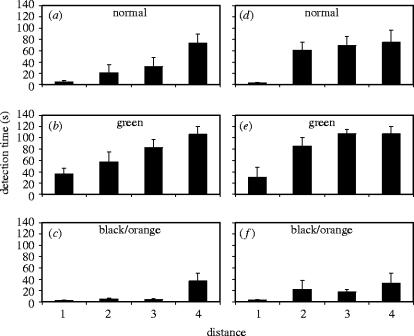
Our manipulation of colouration did indeed affect conspicuousness, and so did distance, as was shown by the analysis of variance of detection time (table 1; figure 3). Moreover, detection time differed between the two habitats (table 1; figure 3); the larvae were in general easier to detect in the shore habitat as was indicated by the shorter detection time (mean=38.75, s.e.=4.96, n=79) as compared with the fen habitat (mean=50.15, s.e.=5.68, n=71). In addition, distance interacted significantly with colouration and with habitat (table 1; figure 3).
Table 1.
Analysis of variance of detection time of P. machaon larvae.
| effect | d.f. | F | p |
|---|---|---|---|
| distance | 3, 126 | 44.39 | 0.000 |
| colouration | 2, 126 | 73.78 | 0.000 |
| habitat | 1, 126 | 12.47 | 0.001 |
| distance×colouration | 6, 126 | 2.24 | 0.043 |
| distance×habitat | 3, 126 | 6.14 | 0.001 |
| colouration×habitat | 2, 126 | 1.24 | 0.293 |
| distance×colouration×habitat | 6, 126 | 0.84 | 0.541 |
Figure 3.
Time to detection by human subjects of the larva of P. machaon from photographs taken at four distances in a shore habitat (a)–(c) and in a fen habitat (d)–(f). Detection time increased with increasing distance for the normal larva (a) and (d), as well as for manipulated images of the larva (green: (b) and (e); black/orange: (c) and (f)).
To analyse the function of P. machaon larval colouration closer, we first wanted to test whether it is maximally cryptic at a short distance. Because our manipulated green colouration provided a longer detection time than the normal larval colouration at the shortest distance in both habitats, we conclude that the normal colouration has not been maximized for short distance crypsis (shore: F1,126=16.18, p<0.001; fen: F1,126=12.00, p<0.01; figure 3). Detection time at this distance did not differ significantly between the normal colouration and the manipulated black/orange colouration in either of the habitats (shore: F1,126=0.57, p=n.s.; fen: F1,126=0.006; p=n.s.; figure 3).
Next, we wanted to find out whether the natural larval colouration is maximally conspicuous at a longer range, or if detectability could be increased by manipulating larval colouration. Therefore, we tested whether the increase in detection time with increasing distance took place at a higher rate for the normal colouration than for the manipulated black–orange colouration. In the shore habitat there was no significant difference in detection time of the natural colouration between distances 1 and 2 (F1,126=2.60, p=n.s.; figure 3), but at distance 3 its detection time had increased significantly from distance 1 (F1,126=11.98, p<0.01; figure 3). In contrast, the detection time of the manipulated black–orange colouration did not increase significantly between distance 1 and distance 2 (F1,126=0.85, p=n.s.; figure 3), nor between distance 1 and distance 3 (F1,126=0.64, p=n.s.; figure 3), and so we conclude that the long range conspicuousness of the typical P. machaon larval colouration has not been maximized in the shore habitat. The result is qualitatively similar for the fen habitat; the detection time of the natural colouration increased significantly already between distance 1 and 2 (F1,126=35.53, p<0.001; figure 3). In contrast, the detection time for the manipulated black–orange colouration did not differ significantly between distances 1 and 2 (F1,126=5.58, p=n.s.; figure 3).
4. Discussion
Previous studies have pointed out that animal colouration used for anti-predator purposes may serve other functions too. However, the dual functions in these cases are usually ascribed to different receivers being targeted for each function, such as conspecifics at close range and predators at a longer range (Endler 1978; Marshall 2000). Here, instead, we have studied colouration that may function in two different ways for a single purpose, namely as an anti-predator device, and moreover, against the same potential predator species or even individuals. In this study, we have shown that, by using naturally occurring colours of P. machaon larva, it is possible to increase the short distance crypsis of the larval colouration. This shows that the larva is more conspicuous than it potentially could be at a short distance, suggesting that it has not evolved to maximize its crypsis. We have also shown that at the shortest distance the detectability of the natural colouration and the black–orange manipulation did not differ, and further, the detectability of the black–orange manipulation decreased with increasing distance at a lower rate than it did for the natural colouration. This suggests that conspicuousness of the larval colouration is not maximized at longer distances. From these results we can draw some conclusions about the evolution of anti-predator colouration.
First and foremost, on a general level our study lends empirical support for the plausibility of the idea about distance-dependent dual function of anti-predator colouration. We have shown that there are differences among colour patterns in how their conspicuousness varies with distance. As a result of this variation, colour patterns are not only suitable for pure cryptic or pure warning function, but there are colour patterns that can be used for combining the two functions in a distance-dependent manner. The idea that protective colouration may comprise a combination between being conspicuous at close range and being cryptic at a longer distance has been forwarded by several researchers (Edmunds 1974; Papageorgis 1975; Rothschild 1975; Endler 1978; Deml & Dettner 2003, in Ruxton et al. 2004), but to our knowledge the present study is the first to lend experimental support for the existence of such colour patterns.
Second, previous studies have shown that P. machaon larvae gain protection from aposematism (Järvi et al. 1981; Wiklund & Järvi 1982; Wiklund & Sillén-Tullberg 1985; Sillén-Tullberg 1990), and the present study suggests that its conspicuousness, which is relatively high at close range, steeply decreases with increasing distance. To be able to carry out our experiment we had to use humans as predators in our experiment. However, we did not find any indication that P. machaon would be using a signalling channel that is invisible to humans but not to its natural predators, and therefore appear conspicuous to its predators when it appears cryptic to us. Therefore, we think that the fourth and fifth instar larvae of P. machaon most probably represent an example of prey that uses a distance-dependent combination of anti-predator signalling and camouflage.
The results in the two habitats were generally in agreement. However, detection times were consistently longer in the fen habitat. A reason for this difference may be that the fen habitat appears visually more complex than the shore habitat, and visual background complexity may decrease the risk of detection for a cryptic prey (Merilaita 2003). Also, the patch size of the larval colouration might better match the patch size in the fen habitat than the patch size in the shore habitat.
The adaptive explanation for the use of prey colouration with dual, distance-dependent function is that in some prey, such dual function may yield better protection than pure cryptic or pure aposematic colouration. Although it is possible that a trade-off between the two functions is involved (i.e. crypsis at long distance may reduce the efficacy of the close-distance aposematic function and vice versa), in some cases, a distance-dependent combination of the functions may yield the best overall protection. To put it more generally, it may be that the optimal detection range varies among different aposematic prey. Thus, for an aposematic colouration with a strong warning signal (i.e. ‘pure’ aposematic colouration) the optimal detection range would be long, whereas for an aposematic colouration that gains some benefit from crypsis at longer distance, the optimal detection range would be shorter.
Also, it is possible that a distance-dependent combination of crypsis and warning colouration substantially reduces the initial cost of aposematism, a cost that may arise when an aposematic phenotype first appears and is still rare. At this stage, the new phenotype may suffer from its relatively high detectability, that is, its warning colouration attracts the attention of predators, but due to its rarity, predators have not effectively learnt to avoid it (e.g. Fisher 1958; Harvey et al. 1982; Mallet & Singer 1987; Lindström et al. 2001). Thus, instead of decreasing predation risk, aposematic colouration may initially increase it and counteract the spread of the phenotype (but see Merilaita & Tullberg 2005). However, for colouration with distance-dependent dual function, such initial cost may be substantially reduced, because of its low detectability compared with ‘pure’ aposematic colouration.
We think that there is strong empirical support for the importance of conspicuousness, both in enhancing avoidance learning and strengthening initial avoidance of prey (e.g. Gittleman & Harvey 1980; Roper & Wistow 1986; Roper & Redston 1987; see also review in Guilford 1990; but see Sherratt & Beatty 2003). However, although it is often assumed that aposematic colouration is also easy to detect at longer distances, there is only one hypothesis that has been formulated to address the question as to why this should be, namely the ‘detection distance hypothesis’ (Guilford 1986). The hypothesis is based on the idea that the sooner an unprofitable prey is discovered, the more time is given for making the correct decision about the status of the prey, whereas less time increases the probability of committing errors in this decision, e.g. recognition errors. This hypothesis seems highly reasonable and it is partly supported in experiments showing that chicks commit fewer errors when given more time to view the unprofitable prey (Guilford 1986; Gamberale-Stille 2000). However, little support is given for the idea that an increased distance per se could have such an effect (Guilford 1989). Although it should be advantageous for aposematic prey to be discovered sufficiently early to give the predator enough time to make the correct decision not to attack, it is not necessarily advantageous to be detected at a long distance. This is so because signal strength, e.g. the area of aposematic signal exposed, gives rise to a more pronounced inhibition to attack prey (Gamberale & Tullberg 1996; Gamberale-Stille & Tullberg 1999) and a longer detection distance is expected to decrease the strength of a given aposematic signal. In our opinion, the effect of distance on the warning function of prey colouration needs more empirical investigation.
To conclude, our study lends empirical support to the idea that anti-predator colouration may serve a distance-dependent dual function. It is conceivable that a combination of warning and cryptic colouration may be quite common among distasteful prey—it certainly has been suggested to apply to quite a few species by previous workers, but whether it is the rule rather than the exception will have to await further empirical investigation.
Acknowledgments
We thank Fredrik Dalerum, Georg Nygren, Jesper Nyström and Fredrik Stjernholm for valuable help with the experiment. Our great thanks to the students from the Department of Psychology at Stockholm University who struggled to detect the caterpillars. John Endler and Gabriella Gamberale-Stille provided valuable comments on the manuscript. This study was financially supported by the Swedish Research Council (B.T., S.M. and C.W.).
References
- Carter D.J, Hargreaves B. Collins; London: 1986. Caterpillars of butterflies and moths in Britain and Europe. [Google Scholar]
- Cott H.C. Methuen; London: 1940. Adaptive colouration in animals. [Google Scholar]
- Cuthill I.C, Partridge J.C, Bennett A.T.D, Church S.C, Hart N.S, Hunt S. Ultraviolet vision in birds. Adv. Study Behav. 2000;29:159–214. [Google Scholar]
- Deml R, Dettner K. Comparative morphology and secretion chemistry of the scoli in caterpillars of Hyalophora cecropia. Naturwissenschaften. 2003;90:460–463. doi: 10.1007/s00114-003-0458-8. [DOI] [PubMed] [Google Scholar]
- Edmunds M.E. Longman; Harlow, Essex: 1974. Defence in animals: a survey of anti-predator defences. [Google Scholar]
- Endler J.A. A predator's view of animal coloration. Evol. Biol. 1978;11:319–364. [Google Scholar]
- Endler J.A. Interactions between predators and prey. In: Krebs J.R, Davies N.B, editors. Behavioural ecology. 3rd edn. Blackwell Scientific Publications; Oxford: 1991. pp. 169–196. [Google Scholar]
- Endler J.A, Mappes J. Predator mixes and the conspicuousness of aposematic signals. Am. Nat. 2004;163:532–547. doi: 10.1086/382662. [DOI] [PubMed] [Google Scholar]
- Evans D.L, Schmidt J.O, editors. Insect defenses: adaptive mechanisms and strategies of prey and predators. State University of New York Press; New York: 1990. [Google Scholar]
- Fisher R.A. 2nd edn. Dover; New York: 1958. The genetical theory of natural selection. [Google Scholar]
- Gamberale-Stille G. Decision time and prey gregariousness influence attack probability in naive and experienced predators. Anim. Behav. 2000;60:95–99. doi: 10.1006/anbe.2000.1435. [DOI] [PubMed] [Google Scholar]
- Gamberale G, Tullberg B.S. Evidence for a peak-shift in predator generalization among aposematic prey. Proc. R. Soc. B. 1996;263:1329–1334. doi: 10.1098/rspb.1996.0195. [DOI] [PubMed] [Google Scholar]
- Gamberale-Stille G, Tullberg B.S. Experienced chicks show biased avoidance of stronger signals: an experiment with natural colour variation in live aposematic prey. Evol. Ecol. 1999;13:579–589. [Google Scholar]
- Gittleman J.L, Harvey P.H. Why are distasteful prey not cryptic? Nature. 1980;286:149–150. [Google Scholar]
- Guilford T. How do warning colours work—conspicuousness may reduce recognition errors in experienced predators. Anim. Behav. 1986;34:286–288. [Google Scholar]
- Guilford T. Studying warning signals in the laboratory. In: Blanchard R.J, Brain P.F, Blanchard D.C, Parmigiani S, editors. Ethoexperimental approaches to the study of behaviour. Kluwer Academic; Dordrecht: 1989. pp. 87–103. [Google Scholar]
- Guilford T. The evolution of aposematism. In: Evans D.L, Schmidt J.O, editors. Insect defenses: adaptive mechanisms and strategies of prey and predators. State University of New York Press; New York: 1990. pp. 23–61. [Google Scholar]
- Hart N.S. The visual ecology of avian photoreceptors. Prog. Retin. Eye Res. 2001;20:675–703. doi: 10.1016/s1350-9462(01)00009-x. [DOI] [PubMed] [Google Scholar]
- Harvey P.H, Bull J.J, Pemberton M, Paxton R.J. The evolution of aposematic coloration in distasteful prey—a family model. Am. Nat. 1982;119:710–719. [Google Scholar]
- Hazel W.N. The environmental and genetic control of seasonal polyphenism in larval colour and its adaptive significance in a swallowtail butterfly. Evolution. 2002;56:342–348. doi: 10.1111/j.0014-3820.2002.tb01344.x. [DOI] [PubMed] [Google Scholar]
- Järvi T, Sillén-Tullberg B, Wiklund C. The cost of being aposematic. An experimental study of predation on larvae of Papilio machaon by the great tit Parus major. Oikos. 1981;36:267–272. [Google Scholar]
- Leimar O, Enquist M, Sillén-Tullberg B. Evolutionary stability of aposematic coloration and prey unprofitability—a theoretical analysis. Am. Nat. 1986;128:469–490. [Google Scholar]
- Lindström L, Alatalo R.V, Mappes J, Riipi M, Vertainen L. Can aposematic signals evolve by gradual change? Nature. 1999;397:249–251. [Google Scholar]
- Lindström L, Alatalo R.V, Lyytinen A, Mappes J. Strong antiapostatic selection against novel rare aposematic prey. Proc. Natl. Acad. Sci. USA. 2001;98:9181–9184. doi: 10.1073/pnas.161071598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet J, Singer M.C. Individual selection, kin selection, and the shifting balance in the evolution of warning colours—the evidence from butterflies. Biol. J. Linn. Soc. 1987;32:337–350. [Google Scholar]
- Marshall N.J. Communication and camouflage with the same ‘bright’ colours in reef fishes. Phil. Trans. R. Soc. B. 2000;355:1243–1248. doi: 10.1098/rstb.2000.0676. 10.1098/rstb.2000.0676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merilaita S. Visual background complexity facilitates the evolution of camouflage. Evolution. 2003;57:1248–1254. doi: 10.1111/j.0014-3820.2003.tb00333.x. [DOI] [PubMed] [Google Scholar]
- Merilaita, S. & Tullberg, B. S. 2005 Constrained camouflage facilitates the evolution of conspicuous warning coloration. Evolution.59, 38–45. [PubMed]
- Ödéen A, Håstad O. Complex distribution of avian colour vision systems revealed by sequencing the SWS1 opsin from total DNA. Mol. Biol. Evol. 2003;20:855–861. doi: 10.1093/molbev/msg108. [DOI] [PubMed] [Google Scholar]
- Papageorgis C. Mimicry in neotropical butterflies. Am. Sci. 1975;63:522–532. [Google Scholar]
- Roper T.J, Redston S. Conspicuousness of distasteful prey affects the strength and durability of one-trial avoidance learning. Anim. Behav. 1987;35:739–747. [Google Scholar]
- Roper T.J, Wistow R. Aposematic coloration and avoidance learning in chicks. Q. J. Exp. Psychol. 1986;38:141–149. [Google Scholar]
- Rothschild M. Remarks on carotenoids in the evolution of signals. In: Gilbert L.E, Raven P.H, editors. Coevolution in animals and plants. University of Texas Press; Austin, Texas: 1975. pp. 20–52. [Google Scholar]
- Ruxton G.D, Sherratt T.N, Speed M.P. Oxford University Press; Oxford: 2004. Avoiding attack: the evolutionary ecology of crypsis, warning signals and mimicry. [Google Scholar]
- Servedio M.R. The effects of predator learning, forgetting, and recognition errors on the evolution of warning coloration. Evolution. 2000;54:751–763. doi: 10.1111/j.0014-3820.2000.tb00077.x. [DOI] [PubMed] [Google Scholar]
- Sherratt T.N, Beatty C.D. The evolution of warning signals as reliable indicators of prey defense. Am. Nat. 2003;162:377–389. doi: 10.1086/378047. [DOI] [PubMed] [Google Scholar]
- Sillén-Tullberg B. Do predators avoid groups of aposematic prey? An experimental test. Anim. Behav. 1990;40:856–860. [Google Scholar]
- Sokal R.R, Rohlf F.J. Freeman; New York: 1995. Biometry. [Google Scholar]
- Speed M.P. Can receiver psychology explain the evolution of aposematism? Anim. Behav. 2001;61:205–216. doi: 10.1006/anbe.2000.1558. [DOI] [PubMed] [Google Scholar]
- Turner J.R.G. A tale of two butterflies. Nat. Hist. 1975;84:28–37. [Google Scholar]
- Wiklund C. The concept of oligophagy and the natural habitats and host plants of Papilio machaon L. in Fennoscandia. Entomol. Scand. 1974;5:151–160. [Google Scholar]
- Wiklund C, Järvi T. Survival of distasteful insects after being attacked by naive birds: a reappraisal of the theory of aposematic coloration evolving through individual selection. Evolution. 1982;36:998–1002. doi: 10.1111/j.1558-5646.1982.tb05468.x. [DOI] [PubMed] [Google Scholar]
- Wiklund C, Sillén-Tullberg B. Why distasteful butterflies have aposematic larvae and adults, but cryptic pupae: evidence from predation experiments on the monarch and European swallowtail. Evolution. 1985;39:1155–1158. doi: 10.1111/j.1558-5646.1985.tb00456.x. [DOI] [PubMed] [Google Scholar]
- Yachi S, Higashi M. The evolution of warning signals. Nature. 1998;394:882–884. [Google Scholar]