Abstract
The enzyme aromatase controls the androgen/oestrogen ratio by catalysing the irreversible conversion of testosterone into oestradiol (E2). Therefore, the regulation of E2 synthesis by aromatase is thought to be critical in sexual development and differentiation. Here, we demonstrate for the first time that experimental manipulation of E2 levels via the aromatase pathway induces adult sex change in each direction in a hermaphroditic fish that naturally exhibits bidirectional sex change. Our results demonstrate that a single enzymatic pathway can regulate both female and male sexual differentiation, and that aromatase may be the key enzyme that transduces environmental, including social, cues to functional sex differentiation in species with environmental sex determination.
Keywords: aromatase, oestradiol, sex change, Gobiodon, coral goby
1. Introduction
In vertebrates, the presence and activity of the enzyme aromatase controls the androgen/oestrogen ratio, by catalysing the irreversible conversion of testosterone (T) into oestradiol (E2). In a variety of vertebrates, including fishes, reptiles and birds, E2 supplements during early development can result in feminization, whereas the inhibition of E2 synthesis using aromatase inhibitors can result in masculinization (Yu et al. 1993; Lance 1997; Chardard & Dournon 1999; Pieau et al. 1999; Crews et al. 2001; Bruggeman et al. 2002; Devlin & Nagahama 2002). Therefore, it is commonly assumed that the regulation of E2 synthesis by aromatase plays a key role in the sexual development and differentiation of these vertebrates (Lange et al. 2002).
Unlike most vertebrates, many fishes are hermaphroditic and can change functional sex from female-to-male (protogyny) or from male-to-female (protandry; Devlin & Nagahama 2002). This phenomenon of adult sex change demonstrates that developmental pathways between testicular and ovarian differentiation can be influenced post-zygotically. One potential pathway comprises changes in E2 synthesis by specific (de)activation of the aromatase enzyme. Correlative support for a role of E2 in sex change has been revealed in numerous hermaphroditic fish species (Devlin & Nagahama 2002, and references therein; Kroon & Liley 2000; Lee et al. 2001; Godwin et al. 2003; Kroon et al. 2003). E2 administration has induced male-to-female sex change in some species (Devlin & Nagahama 2002, and references therein; Lee et al. 2000, 2001), while the administration of an aromatase inhibitor induced sex change in a protogynous goby (Kroon & Liley 2000) and grouper (Bhandari et al. 2004), but blocked natural sex change in a protandrous porgy (Lee et al. 2001). Moreover, elevated aromatase activity in the brain and gonads was associated with male-to-female sex change in this porgy (Chang & Lin 1998; Lee et al. 2000). Thus, it appears that the aromatase pathway is involved in mediating sex change in hermaphroditic fishes, possibly by modulating E2 concentrations that help control gonadal cell allocation.
Previous studies on the role of the aromatase pathway in sex change have focused on fish species characterized by unidirectional sex change. This has resulted in a comparison of endocrine processes mediating protandrous and protogynous sex change in different species, rather than directly testing for a single pathway controlling sexual differentiation in the same species. Recently, some species from the family Gobiidae (gobies) have been found to be able to change sex repeatedly in both directions, including several species of coral-dwelling gobies from the genera Gobiodon (Nakashima et al. 1996; Munday et al. 1998) and Paragobiodon (Kuwamura et al. 1994; Nakashima et al. 1996), several species of Lythrypnus (St. Mary 1993, 1996), and at least one species of Trimma (Sunobe & Nakazono 1993). This discovery of bidirectional sex change provides a unique opportunity to study the endocrine mechanisms underlying female and male sex differentiation, while avoiding issues associated with interspecific comparisons.
Here, we demonstrate that manipulating E2 levels via the aromatase pathway induces adult sex change in each direction in the coral goby, Gobiodon erythrospilus. Our experiments were successfully performed under natural social conditions, thereby removing confounding effects inherent to laboratory studies on species with socially controlled sex change. Specifically, we tested the hypotheses that an increase in E2 results in protandrous sex change, and a decrease in E2 results in protogynous sex change. We examined the effects of E2 on the gonadal cell allocation of mature females and males, through the administration of (i) E2, and (ii) an aromatase inhibitor (fadrozole), which blocks the conversion of T into E2.
2. Methods
(a) Study species
Previous studies on G. erythrospilus have confirmed that it can change sex in both directions (Munday 2002), and that E2 levels are correlated with sexual function (Kroon et al. 2003). One adult breeding pair generally occupies a coral colony (Munday et al. 1998; Hobbs et al. 2004), and natural sex change occurs as a result of changes in social conditions. Female-to-male sex change occurs in one individual when two females cohabit within a coral colony, while male-to-female sex change occurs when two males form a pair (Munday et al. 1998; Munday 2002). Furthermore, single, adult females always change sex to male, whereas single, adult males always remain male (J.-P. A. Hobbs 2002, unpublished thesis; Munday 2002; Hobbs et al. 2004). Here, we manipulated E2 levels in both natural, heterosexual pairs and single adults. Therefore, in addition to using pairs to determine if changing E2 levels can induce sex change in both males and females, we were also able to use single fish to determine if manipulating E2 levels could prevent naturally occurring sex change.
(b) Fish capture and handling
Pairs of G. erythrospilus were collected from their host coral colonies by anaesthetization with clove-oil (Munday & Wilson 1997), at Lizard Island (14°40′ S, 145°28′ E) on the northern Great Barrier Reef. Fish were transported to the laboratory, reanaesthetized, sexed according to the shape of their genital papilla (Munday et al. 1998; Munday 2002), measured (standard length, 0.1 mm; weight, 0.1 g), and subsequently assigned to the various treatment groups. Previous studies have shown that there is a good relationship between functional sex, gonadal sex allocation, and the shape of the genital papilla. The gonads of adult males are dominated by spermatogenic tissue, including spermatozoa. Primary stage oocytes are often present, but never vitellogenic oocytes (Munday 2002; Kroon et al. 2003). The gonads of adult females are dominated by oocytes, including vitellogenic oocytes. Isolated crypts of spermatocytes may be present, but never spermatozoa (Munday 2002; Kroon et al. 2003). The genital papilla of adult males is long and tapered, while that of adult females is short and blunt, often with terminal processes (Munday et al. 1998; Hobbs et al. 2004). Only fish that were collected as (part of) a heterosexual pair and with a genital papilla characteristic of a mature male or female were used. Each fish was uniquely marked with visible fluorescent implant tags (North-West Technologies Inc.) injected into the dorsal musculature. Treatment fish were administered implants as described below. After treatment and overnight recovery, experimental fish were transported back to the field and placed on an empty coral colony. Each coral colony occupied by experimental fish was marked with a cable tie and a numbered tag to aid the subsequent recovery and identification of the experimental fish. To reduce any confounding effects of breeding on steroid concentrations, the study was conducted during the non-breeding season.
(c) Preparation and administration of implants
Elastomer implants containing E2 or fadrozole were prepared using established methods (Kroon & Liley 2000). The silicone elastomer mixed with fadrozole did not harden; therefore, the mixture was subsequently administered by injection rather than as solid implants. The administration of 17β-oestradiol (1,3,5[10]-oestratriene-3,17β-diol, Sigma Chemical Co., St Louis, MO, USA, catalogue number E-8875) in 1×1×6 mm3 implants (mean weight 5.0 mg±0.7 s.d., n=10) resulted in an average dose of 1.4 mg of E2 per fish. The aromatase inhibitor fadrozole, 4-(5,6,7,8-tetrahydroimidazole[1,5α] pyridin-5-yl)-benzonitrile monohydrochloride (Novartis, Switzerland) was administered in 2 units using a 3/10 ml syringe (mean weight 22.8 mg±1.5 s.d., n=10), resulting in an average dose of 3.3 mg fadrozole per fish. The efficacy of fadrozole as a potent and selective inhibitor of oestrogen biosynthesis has been demonstrated in fishes (Afonso et al. 1997, 1999). Implant-control fish received a 1×1×6 mm3 elastomer implant (mean weight 5.2 mg±0.7 s.d., n=10) containing neither E2 nor fadrozole.
To administer implants, a tiny incision was made on the ventro-lateral side of the fish, anterior to the genital papilla. Solid implants (E2 or control) were slipped gently inside the abdominal cavity using fine forceps, and moved frontally as far as possible. Fadrozole implants were administered by injection into the abdominal cavity, as far frontally as possible, through an 18.5 gauge (blunted) needle. The incision was sealed with Medical Adhesive (891 Type A Medical Adhesive, A 100, Factor II Inc., Lakeside, AZ, USA). After surgery, fish were placed into individual tanks with flow-through, natural seawater, at ambient sea temperature, for recovery.
(d) Experimental design
For the experiment using pairs, 60 heterosexual pairs were divided equally into four treatment groups, and re-established on their host coral. In the handling-control group, neither fish received an implant. In the implant-control group, both fish received a control implant (i.e. with no additive). In the experimental-control group, females were administered E2, and males fadrozole. In the experimental group, the females were administered fadrozole, and males E2. If E2 levels regulate sex differentiation of both female and male G. erythrospilus, we predicted that (i) reciprocal sex change would occur in pairs in the experimental group and (ii) no sex change would occur in the other groups (table 1).
Table 1.
Predictions and results of implant experiments for (a) females, and (b) males, comparing gonadal sex allocation of treatment fish with handling—control fish, in heterosexual pairs and singles.
| (a) ♀ | gonadal cell allocation compared to handling controls | ||||
|---|---|---|---|---|---|
| implant | n | predicted final sex | pairs (n=8) | singles (n=3) | |
| pairs | E2 | 5 | ♀ | p=0.08 | n/a |
| AI | 5 | ♂ | p=0.004** | n/a | |
| singles | none | 3 | ♂ | p=0.03* | n/a |
| E2 | 8 | ♀ | n/a | p=0.009** | |
| AI | 5 | ♂ | n/a | p=0.16 | |
| (b) ♂ | gonadal cell allocation compared to handling controls | ||||
|---|---|---|---|---|---|
| implant | n | predicted final sex | pairs (n=12) | singles (n=3) | |
| pairs | E2 | 5 | ♀ | p=0.000 001*** | n/a |
| AI | 5 | ♂ | p=0.45 | n/a | |
| singles | none | 3 | ♂ | p=0.21 | n/a |
| E2 | 8 | ♀ | n/a | p=0.000 003*** | |
| AI | 10 | ♂ | n/a | p=0.16 | |
Fish in the handling-control group did not receive implants. Treatment fish received an implant containing either oestradiol (E2) or the aromatase inhibitor (AI) fadrozole. *p<0.05; **p<0.01; ***p<0.001.
For the experiment using single fish, 36 single females were established; four females in the handling-control group, 15 females in the experimental-control (i.e. fadrozole implant) group and 17 females in the experimental (i.e. E2 implant) group. In addition, 33 single males were established; four males in the handling-control group, 14 males in the experimental-control (i.e. fadrozole implant) group and 15 males in the experimental (i.e. E2 implant) group. If E2 levels regulate sex differentiation of both female and male G. erythrospilus, we predicted that (i) female-to-male sex change would occur in single females in the two control groups, but not in the E2 group and (ii) male-to-female sex change would occur in single males in the E2 group, but not in the other two groups (table 1).
(e) Classification of gonadal cells and data analysis
Tagged fish were recollected after six weeks, which is sufficient time for gonadal cell reallocation to occur in adult coral gobies (Munday et al. 1998; Munday 2002). Gonads from each fish were embedded in paraffin wax, longitudinally sectioned at 5 μm, mounted on glass microslides, stained with Mayer's haematoxylin and Young's eosin–erythrosin, and viewed with light microscopy. The proportion of each germ cell type was estimated, along a representative transect of the gonad, using established methods (Munday 2002). The individual responsible for quantifying gonadal cell allocation did not know the identity of the fish. Oocytes (eggs) were classified into three developmental stages: (i) previtellogenic (immature), (ii) cortical alveoli (developing), and (iii) mature oocytes, including vitellogenic oocytes. Previtellogenic oocytes comprised the chromatin nucleolar and perinucleolar stages, while the cortical alveoli stage was characterized by yolk vesicles in the cytoplasm (Munday 2002). Hydrated oocytes were not observed in any fish. Sperm cells were classified as (i) primary and secondary spermatocytes (developing), and (ii) mature spermatozoa.
To examine whether the implant procedure itself affected gonadal cell allocation in G. erythrospilus, we compared the gonadal cell allocation in the handling-control (no implant) and implant-control groups in females and males, respectively, using MANOVA (Statsoft 1999). Gonadal cell allocation did not differ significantly between the two groups (females: Rao's R4,10=0.27, p=0.89; males: Rao's R3,15=1.13, p=0.37), and was thus not affected by the implant procedure itself.
To test our predictions (table 1), we compared the gonadal cell allocation of experimental fish with those of handling-control fish (unless otherwise noted) using MANOVA (Statsoft 1999). Sex change was considered to have been induced if the experimental fish exhibited a significant shift in gonadal cell allocation towards the opposite sex. The proportions of oocytes and sperm cells were entered into the statistical analyses untransformed for both females and males, and α was set at 0.05. For heterosexual pairs, individual fish were included in the analyses only if, at the time of collection, (i) the original pair was present and (ii) both fish still had their implants (for the implant treatment groups only). In the handling-control group, failure to collect three of the females of the original pairs that were present at the time of collection, and the loss of another female during histological processing, resulted in unequal sample sizes in the female and male groups. For the single group, individual fish were included in the analyses only if the fish still had its implant at the time of collection (for implant treatment groups only).
3. Results
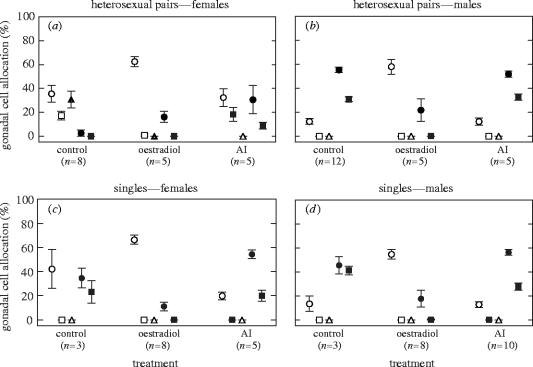
In heterosexual paired females, fadrozole administration induced female-to-male sex change (figures 1a and 2a,c). Gonadal cell allocation was significantly altered in the direction of a male gonad (Rao's R5,7=10.17, p=0.004; table 1), with the disappearance of vitellogenic oocytes, an increase in spermatocytes from 2.5 to 30.5%, and the appearance of spermatozoa. These gonads also contained a high proportion of previtellogenic oocytes, which is typical for individuals that have recently changed from female to male (Munday 2002). In contrast, E2 administration did not significantly affect overall gonadal cell allocation (figure 1a; Rao's R4,8=3.13, p=0.08; table 1). In these gonads, a negative E2 feedback exerted on gonadotrophin secretion may have resulted in the reduced allocation to cortical alveoli and vitellogenic oocytes (Peter & Yu 1997), and thus on oocyte maturation, and in an increased allocation to spermatocytes.
Figure 1.
Mean proportion of gonadal cells in heterosexual pairs and single Gobiodon erythrospilus in the three different treatment groups: (a) females established in heterosexual pairs; (b) males established in heterosexual pairs; (c) females established as singles; and (d) males established as singles. Note that single, adult females always change sex to male. Fish in the handling-control group did not receive implants (Control). Treatment fish received an implant containing either oestradiol (Oestradiol) or the aromatase inhibitor (AI) fadrozole. Open circle, previtellogenic oocytes; open square, cortical alveoli; triangle, mature oocytes; filled circle, spermatocytes; filled square, spermatozoa. Middle points are means, whiskers are standard errors. For results of statistical analyses, see table 1 and text.
Figure 2.
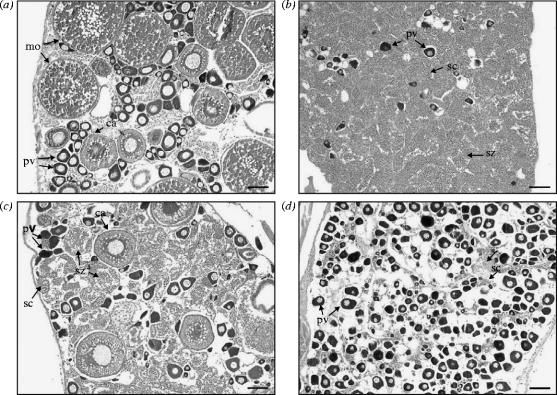
Gonad structure of Gobiodon erythrospilus established in heterosexual pairs: (a) control female; (b) control male; (c) female administered the aromatase inhibitor fadrozole; and (d) male administered oestradiol. pv, previtellogenic oocytes; ca, cortical alveoli; mo, mature oocytes; sc, spermatocytes; and sz, spermatozoa. Scale bar, 0.1 mm.
In heterosexual paired males, E2 administration induced male-to-female sex change (figures 1b and 2b,d). Gonadal cell allocation was significantly altered in the direction of a female gonad (Rao's R3,13=36.47, p=0.000 001; table 1), with the disappearance of spermatozoa, a decrease in spermatocytes from 55.2 to 21.7%, and an increase in previtellogenic oocytes from 12.1 to 57.9%. As in heterosexual paired females, a negative E2 feedback on gonadotrophin secretion may have regulated allocation to cortical alveoli, vitellogenic oocytes (Peter & Yu 1997) and spermatocytes. In contrast, fadrozole administration did not affect overall gonadal cell allocation (figure 1b; Rao's R3,13=0.94, p=0.45; table 1).
In single females, E2 administration prevented natural female-to-male sex change (figures 1c and 3a,b). Compared with the handling-control, paired females (figures 1a and 2a), gonadal cell allocation in single, control females had significantly altered in the direction of a male gonad (figures 1c and 3a; Rao's R5,5=6.57, p=0.03; table 1), with a high proportion of spermatocytes and spermatozoa. E2 administration resulted in single females retaining the characteristics of a female gonad (figures 1c and 3b; Rao's R3,7=8.70, p=0.009; table 1), including the complete absence of spermatozoa and only the occasional occurrence of spermatocytes. In contrast, fadrozole administration did not prevent natural sex change (figure 1c); the gonadal cell allocation of these fish did not differ from single, control females that naturally changed sex to male (Rao's R3,4=2.99, p=0.16; table 1).
Figure 3.
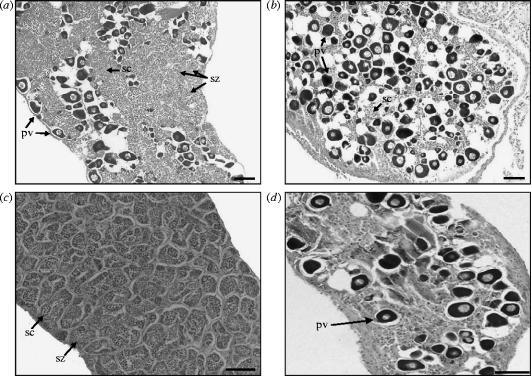
Gonad structure of Gobiodon erythrospilus established as single females or males: (a) female control; (b) female administered oestradiol; (c) male control; and (d) male administered oestradiol. Note that single, adult females always change sex to male. pv, previtellogenic oocytes; sc, spermatocytes; and sz, spermatozoa. Scale bar, 0.1 mm.
In single males, E2 administration induced male-to-female sex change (figures 1d and 3c,d). Gonadal cell allocation in single control males remained similar to handling-control, paired males (figure 1b,d; Rao's R3,11=1.77, p=0.21; table 1). E2 administration resulted in a significant shift in gonadal cell allocation in the direction of a female gonad (figures 1d and 3c,d; Rao's R3,7=107.31, p=0.000 003; table 1), with the disappearance of spermatozoa, a decrease in spermatocytes from 45.5 to 17.6%, and an increase in previtellogenic oocytes from 13.3 to 54.7%. In contrast, fadrozole administration did not affect overall gonadal cell allocation (figure 1d; Rao's R3,9=2.18, p=0.16; table 1).
4. Discussion
The results from paired fish support our predictions, that (i) reciprocal sex change occurs in pairs where the females were administered fadrozole and males E2 and (ii) no sex change occurs in the reverse or control treatments. Moreover, the results from single fish demonstrate that E2 administration (i) prevents natural sex change in single females and (ii) induces sex change in single males. Thus, our results support the hypothesis that manipulating E2 levels induces sex change in either direction in G. erythrospilus, and demonstrate that a single enzymatic pathway can regulate both female and male sexual differentiation.
We propose that, at least in G. erythrospilus, exposure to dominant behaviour helps mediate sex change by the regulation of E2 synthesis through the aromatase pathway. In male–male pairs of G. erythrospilus, the larger individual remains male and the smaller changes sex to female, whereas, in female–female pairs, the larger individual changes sex to male and the smaller remains female (Munday 2002). Single females change sex to male and single males remain male (J.-P. A. Hobbs 2002, unpublished thesis; Munday 2002; Hobbs et al. 2004). These patterns can be explained if dominant behaviour activates E2 synthesis and the absence of dominant behaviour deactivates E2 synthesis. The presence of a dominant male should thus suppress sex change in subordinate females and induce sex change in subordinate males by up-regulating E2 synthesis. The absence of dominant behaviour should induce sex change in dominant and single females by downregulating E2 synthesis. In fishes, exposure to dominant behaviour can result in a rise of cortisol levels in subordinate individuals (Overli et al. 1999; Elofsson et al. 2000; Sloman et al. 2001; Doyon et al. 2003). Moreover, the glucocorticoid response element is a regulatory element of the ovarian isoform of the aromatase gene in Gobiodon histrio (Gardner et al. 2003), and may thus directly regulate aromatase activity. Socially controlled sex change may thus be mediated by changing cortisol levels (Perry & Grober 2003) through the aromatase pathway, a proposition that deserves further study.
Importantly, G. erythrospilus in heterosexual pairs do not change sex, regardless of the relative sizes of the male and female (Munday 2002). Therefore, it seems that size-based dominance alone does not control sex change in G. erythrospilus, and that the presence of a male is sufficient to prevent sex change in normal social groups. This suggests that male behaviour, or perhaps other sensory cues from males, could also affect the regulation of E2 synthesis.
Recently, two aromatase genes that encode two distinct cytochrome P450 aromatase isoforms, the ovarian isoform and the brain isoform, have been documented in the bidirectional sex changing gobies G. histrio (Gardner et al. 2003) and Trimma okinawae (Kobayashi et al. 2004). In T. okinawae, the ovarian isoform mRNA was present in ovary and testis, and the ovarian isoform transcripts correlated with ovarian development, suggesting that the ovarian isoform, but not the brain isoform, is involved in the regulation of ovarian vitellogenesis in this species (Kobayashi et al. 2004). Hence, if two aromatase isoforms are also present in G. erythrospilus, the results of our study suggest that fadrozole blocks the ovarian isoform in this species. In contrast, the oestrogen response element is a regulatory element of the brain isoform, but not the ovarian isoform, in G. histrio (Gardner et al. 2003), and E2 administration can result in upregulation of this isoform expression in the goldfish brain (Gelinas et al. 1998). This suggests that, in E2-treated G. erythrospilus, at least part of the changes in gonadal cell allocation may have resulted from E2 regulation of the brain isoform in the brain region.
E2 regulation by the aromatase enzyme appears to be a key process in the sex differentiation of vertebrate species with environmental sex determination (Baroiller et al. 1999; Devlin & Nagahama 2002; Crews 2003; Godwin et al. 2003), including temperature-dependent sex determination in fishes (Kitano et al. 1999; Kwon et al. 1999; Baroiller & D'Cotta 2001; D'Cotta et al. 2001) and reptiles (Pieau et al. 1999; Crews et al. 2001; Gabriel et al. 2001; Crews 2003; but see Murdock & Wibbels 2003), and has now been shown to mediate sexual differentiation in a species with socially controlled sex change. Given the pivotal role of aromatase in E2 synthesis, this enzyme may play a key role in transducing environmental information into sex differentiation responses in species with environmental sex determination.
Acknowledgments
We are grateful to the Lizard Island Research Station for providing excellent facilities. We thank Novartis Pharma A. G., Switzerland, for providing the aromatase inhibitor, fadrozole. Comments by Bob Delvin, John Godwin, Peter Gehrke and two anonymous referees improved the manuscript. Thanks to Heinz Buettikofer for finalizing the figures. This research was supported by the Australian Research Council, James Cook University and the Lucie Burgers Foundation for Comparative Behaviour Research, Arnhem, The Netherlands. This work was conducted in F.J.K. and D.A.W.'s own time, and is not part of their CSIRO responsibilities.
References
- Afonso L.O.B, Campbell P.M, Iwama G.K, Devlin R.H, Donaldson E.M. The effect of the aromatase inhibitor fadrozole and two polynuclear aromatic hydrocarbons on sex steroid secretion by ovarian follicles of coho salmon. Gen. Comp. Endocrinol. 1997;106:169–174. doi: 10.1006/gcen.1996.6855. [DOI] [PubMed] [Google Scholar]
- Afonso L.O.B, Iwama G.K, Smith J, Donaldson E.M. Effects of the aromatase inhibitor fadrozole on plasma sex steroid secretion and ovulation rate in female coho salmon, Oncorhynchus kisutch, close to final maturation. Gen. Comp. Endocrinol. 1999;113:221–229. doi: 10.1006/gcen.1998.7198. [DOI] [PubMed] [Google Scholar]
- Baroiller J, D'Cotta H. Environment and sex determination in farmed fish. Comp. Biochem. Physiol. C. 2001;130:399–409. doi: 10.1016/s1532-0456(01)00267-8. [DOI] [PubMed] [Google Scholar]
- Baroiller J.F, Guigen Y, Fostier A. Endocrine and environmental aspects of sex differentiation in fish. Cell. Mol. Life Sci. 1999;55:910–931. [Google Scholar]
- Bhandari R, Higa M, Nakamura S, Nakamura M. Aromatase inhibitor induces complete sex change in the protogynous honeycomb grouper (Epinephelus merra) Mol. Reprod. Dev. 2004;67:303–307. doi: 10.1002/mrd.20027. [DOI] [PubMed] [Google Scholar]
- Bruggeman V, Van As P, Decuypere E. Developmental endocrinology of the reproductive axis in the chicken embryo. Comp. Biochem. Physiol. A. 2002;131:839–846. doi: 10.1016/s1095-6433(02)00022-3. [DOI] [PubMed] [Google Scholar]
- Chang C.F, Lin B.Y. Estradiol-17β stimulates aromatase activity and reversible sex change in protandrous black porgy, Acanthopagrus schlegeli. J. Exp. Zool. 1998;280:165–173. [Google Scholar]
- Chardard D, Dournon C. Sex reversal by aromatase inhibitor treatment in the newt Pleurodeles waltl. J. Exp. Zool. 1999;283:43–50. doi: 10.1002/(sici)1097-010x(19990101)283:1<43::aid-jez6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Crews D. Sex determination: where environment and genetics meet. Evol. Dev. 2003;5:50–55. doi: 10.1046/j.1525-142x.2003.03008.x. [DOI] [PubMed] [Google Scholar]
- Crews D, Fleming A, Willingham E, Baldwin R, Skipper J. Role of steroidogenic factor I and aromatase in temperature-dependent sex determination in the red-eared slider turtle. J. Exp. Zool. 2001;290:597–606. doi: 10.1002/jez.1110. [DOI] [PubMed] [Google Scholar]
- D'Cotta H, Fostier A, Guiguen Y, Govoroun M, Baroiller J. Aromatase plays a key role during normal and temperature-induced sex differentiation of Tilapia Oreochromis niloticus. Mol. Reprod. Dev. 2001;59:265–576. doi: 10.1002/mrd.1031. [DOI] [PubMed] [Google Scholar]
- Devlin R.H, Nagahama Y. Sex determination and sex differentiation in fish. Aquaculture. 2002;208:191–364. [Google Scholar]
- Doyon C, Gilmour K.M, Trudeau V.L, Moon T.W. Corticotropin-releasing factor and neuropeptide Y mRNA levels are elevated in the preoptic area of socially subordinate rainbow trout. Gen. Comp. Endocrinol. 2003;133:260–271. doi: 10.1016/s0016-6480(03)00195-3. [DOI] [PubMed] [Google Scholar]
- Elofsson U.O, Mayer I, Damsgard B, Winberg S. Intermale competition in sexually mature arctic charr: effects on brain monoamines, endocrine stress responses, sex hormone levels, and behavior. Gen. Comp. Endocrinol. 2000;118:450–460. doi: 10.1006/gcen.2000.7487. [DOI] [PubMed] [Google Scholar]
- Gabriel W, Blumberg B, Sutton S, Place A, Lance V. Alligator aromatase cDNA sequence and its expression in embryos at male and female incubation temperatures. J. Exp. Zool. 2001;290:439–448. doi: 10.1002/jez.1087. [DOI] [PubMed] [Google Scholar]
- Gardner L, Anderson T, Place A.R, Elizur A. Sex change strategy and the aromatase genes. Fish Physiol. Biochem. 2003;28:147–148. doi: 10.1016/j.jsbmb.2004.12.045. [DOI] [PubMed] [Google Scholar]
- Gelinas D, Pitoc G.A, Callard G.V. Isolation of a goldfish brain cytochrome P450 aromatase cDNA: mRNA expression during the seasonal cycle and after steroid treatment. Mol. Cell. Endocrinol. 1998;138:81–93. doi: 10.1016/s0303-7207(98)00015-x. [DOI] [PubMed] [Google Scholar]
- Godwin J, Luckenbach J.A, Borski R.J. Ecology meets endocrinology: environmental sex determination in fishes. Evol. Dev. 2003;5:40–49. doi: 10.1046/j.1525-142x.2003.03007.x. [DOI] [PubMed] [Google Scholar]
- Hobbs J.-P.A, Munday P.L, Jones G.P. Social induction of maturation and sex determination in a coral reef fish. Proc. R. Soc. B. 2004;271:2109–2114. doi: 10.1098/rspb.2004.2845. 10.1098/rspb.2004.2845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano T, Takamune K, Kobayashi T, Nagahama Y, Abe S.I. Suppression of P450 aromatase gene expression in sex-reversed males produced by rearing genetically female larvae at a high water temperature during a period of sex differentiation in the Japanese flounder (Paralichthys olivaceus) J. Mol. Endocrinol. 1999;23:167–176. doi: 10.1677/jme.0.0230167. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Kobayashi T, Nakamura M, Sunobe T, Morrey C.E, Suzuki N, Nagahama Y. Characterization of two types of cytochrome P450 aromatase in the serial-sex changing gobiid fish, Trimma okinawae. Zool. Sci. 2004;21:417–425. doi: 10.2108/zsj.21.417. [DOI] [PubMed] [Google Scholar]
- Kroon F.J, Liley N.R. The role of steroid hormones in protogynous sex change in the blackeye goby, Coryphopterus nicholsii. Gen. Comp. Endocrinol. 2000;118:273–283. doi: 10.1006/gcen.2000.7459. [DOI] [PubMed] [Google Scholar]
- Kroon F.J, Munday P.L, Pankhurst N.W. Steroid hormone levels and bi-directional sex change in the coral-dwelling goby Gobiodon histrio (Teleostei: Gobiidae) J. Fish Biol. 2003;62:153–167. [Google Scholar]
- Kuwamura T, Nakashima Y, Yogo Y. Sex change in either direction by growth rate advantage in a monogamous coral goby Paragobiodon echinocephalus. Behav. Ecol. 1994;5:434–438. [Google Scholar]
- Kwon J.Y, McAndrew B.J, Penman D.J. Inhibition of aromatase activity suppresses high-temperature feminisation of genetic male nile tilapia, Oreochromis niloticus. In: Norberg B, Kjesbu O.S, Taranger G.L, Andersson E, Stefansson S.O, editors. Proceedings of the 6th International Symposium on the Reproductive Physiology of Fish 6. 1999. [Google Scholar]
- Lance V. Sex determination in reptiles: an update. Am. Zool. 1997;37:504–513. [Google Scholar]
- Lange I, Hartel A, Meyer H. Evolution of oestrogen functions in vertebrates. J. Steroid Biochem. Mol. Biol. 2002;83:219–226. doi: 10.1016/s0960-0760(02)00225-x. [DOI] [PubMed] [Google Scholar]
- Lee Y.H, Lee F.Y, Yueh W.S, Tacon P, Du J.L, Chang C.N, Shan J.R, Tanaka H, Chang C.F. Profiles of gonadal development, sex steroids, aromatase activity, and gonadotropin II in the controlled sex change of protandrous black porgy, Acanthopagrus schlegeli Bleeker. Gen. Comp. Endocrinol. 2000;119:111–120. doi: 10.1006/gcen.2000.7499. [DOI] [PubMed] [Google Scholar]
- Lee Y.H, et al. Sex change in the protandrous black porgy, Acanthopagrus schlegeli: a review in gonadal development, estradiol, estrogen receptor, aromatase activity and gonadotropin. J. Exp. Zool. 2001;290:715–726. doi: 10.1002/jez.1122. [DOI] [PubMed] [Google Scholar]
- Munday P.L. Bi-directional sex change: testing the growth-advantage model. Behav. Ecol. Sociobiol. 2002;52:247–254. [Google Scholar]
- Munday P.L, Wilson S.K. Comparative efficacy of clove oil and other chemicals in anaesthetization of Pomacentrus amboinensis, a coral reef fish. J. Fish Biol. 1997;51:931–938. [Google Scholar]
- Munday P.L, Caley M.J, Jones G.P. Bi-directional sex change in a coral-dwelling goby. Behav. Ecol. Sociobiol. 1998;43:371–377. [Google Scholar]
- Murdock C, Wibbels T. Cloning and expression of aromatase in a turtle with temperature-dependent sex determination. Gen. Comp. Endocrinol. 2003;130:109–119. doi: 10.1016/s0016-6480(02)00573-7. [DOI] [PubMed] [Google Scholar]
- Nakashima Y, Kuwamura T, Yogo Y. Both-way sex change in monogamous coral gobies, Gobiodon spp. Environ. Biol. Fish. 1996;46:281–288. [Google Scholar]
- Overli O, Harris C.A, Winberg S. Short-term effects of fights for social dominance and the establishment of dominant-subordinate relationships on brain monoamines and cortisol in rainbow trout. Brain Behav. Evol. 1999;54:263–275. doi: 10.1159/000006627. [DOI] [PubMed] [Google Scholar]
- Perry A.N, Grober M.G. A model for social control of sex change: interactions of behavior, neuropeptides, glucocorticoids, and sex steroids. Horm. Behav. 2003;43:31–38. doi: 10.1016/s0018-506x(02)00036-3. [DOI] [PubMed] [Google Scholar]
- Peter R.E, Yu K.L. Neuroendocrine regulation of ovulation in fishes: basic and applied aspects. Rev. Fish Biol. Fish. 1997;7:173–197. [Google Scholar]
- Pieau C, Dorizzi M, Richard-Mercier N. Temperature-dependent sex determination and gonadal differentiation in reptiles. Cell. Mol. Life Sci. 1999;55:887–900. doi: 10.1007/s000180050342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloman K.A, Metcalfe N.B, Taylor A.C, Gilmour K.M. Plasma cortisol concentrations before and after social stress in rainbow trout and brown trout. Physiol. Biochem. Zool. 2001;74:383–389. doi: 10.1086/320426. [DOI] [PubMed] [Google Scholar]
- St Mary C.M. Novel sexual patterns in two simultaneously hermaphroditic gobies, Lythrypnus dalli and Lythrypnus zebra. Copeia. 1993;4:1062–1072. [Google Scholar]
- St Mary C.M. Sex allocation in a simultaneous hermaphrodite, the zebra goby Lythrypnus zebra: insights gained through a comparison with its sympatric congener Lythrypnus dalli. Environ. Biol. Fish. 1996;45:177–190. [Google Scholar]
- Statsoft. Statsoft Inc; Tulsa, OK: 1999. Statistica for Windows version 5.5. [Google Scholar]
- Sunobe T, Nakazono A. Sex change in both directions by alteration of social dominance in Trimma okinawae (Pices: Gobiidae) Ethology. 1993;94:339–345. [Google Scholar]
- Yu N.W, Hsu C.Y, Ku H.H, Chang L.T, Liu H.W. Gonadal differentiation and secretions of estradiol and testosterone of the ovaries of Rana catesbeiana tadpoles treated with 4-hydroxyandrostene-dione. J. Exp. Zool. 1993;265:252–257. doi: 10.1002/jez.1402650307. [DOI] [PubMed] [Google Scholar]