Abstract
Transgenic mice overexpressing PKCα in the epidermis (K5-PKCα mice) exhibit an inducible severe intraepidermal neutrophilic inflammation and systemic neutrophilia when PKCα is activated by topical 12-O-tetradecanoylphorbol-13-acetate (TPA). This inducible model of cutaneous inflammation was used to define mediators of skin inflammation that may have clinical relevance. Activation of cutaneous PKCα increased the production of the chemotactic factors cytokine-induced neutrophil chemoattractant (KC) and macrophage inflammatory protein 2 (MIP-2) in murine plasma. TPA treatment of cultured K5-PKCα keratinocytes also released KC and MIP-2 into culture supernatants through an NF-κB–dependent pathway. MIP-2 and KC mediated the infiltration of neutrophils into the epidermis, since this was prevented by ablating CXCR2 in K5-PKCα mice or administering neutralizing antibodies against KC or MIP-2. The neutrophilia resulted from PKCα-mediated upregulation of cutaneous G-CSF released into the plasma independent of CXCR2. These responses could be inhibited by topical treatment with a PKCα-selective inhibitor. Inhibiting PKCα also reduced the basal and TNF-α– or TPA-induced expression of CXCL8 in cultured psoriatic keratinocytes, suggesting that PKCα activity may contribute to psoriatic inflammation. Thus, skin can be the source of circulating factors that have both local and systemic consequences, and these factors, their receptors, and possibly PKCα could be therapeutic targets for inhibition of cutaneous inflammation.
Introduction
As the body’s largest and most exposed interface with the environment, the skin has a central role in host defense. The most numerous epidermal cells, keratinocytes, are major producers of local and systemic cytokines and can initiate an inflammatory response. However, cutaneous immune surveillance is achieved by a complex interplay among skin’s dendritic cells, infiltrating T cells, granulocytes, and keratinocytes (1). Neutrophils are considered to be the body’s first line of defense, as they are rapidly recruited at the inflammatory site. Chemotaxis of immune cells is mediated by a large group of structurally related proteins called chemokines. They are organized into 4 families (C, CC, CXC, CX3C) based on the position of 1 or 2 conserved cysteine residues in their N-terminal portion (2–4). Mouse neutrophils express a receptor homologous to human IL-8 (CXC chemokine ligand 8 [CXCL8]) receptor, or CXC chemokine receptor 2 (CXCR2). Mouse CXCR2 binds several CXCL8-like CXC chemokines, most notably cytokine-induced neutrophil chemoattractant (KC) and macrophage inflammatory protein 2 (MIP-2). KC and MIP-2 are related to the 3 human GRO chemokines (CXCL1/3) (5–7). Ablation of CXCR2 in mice prevents the migration of neutrophils in response to thioglycolate, revealing that it is critical for neutrophil chemotaxis (8).
Members of the PKC family of calcium and/or lipid activated serine-threonine kinases function downstream of nearly all membrane-associated signal transduction pathways. Six different PKC isozymes are expressed in mouse and human keratinocytes; they are broadly classified by their activation characteristics. The conventional PKC isozyme (PKCα) is calcium- and lipid-activated, whereas the novel isozymes (δ, ε, η) and atypical isozymes (ζ, μ) are calcium independent but activated by distinct lipids. We and others have previously demonstrated that transgenic mice that overexpress PKCα in basal keratinocytes exhibited an acute inflammatory response when mice were painted with the PKC activator 12-O-tetradecanoylphorbol-13-acetate (TPA) (9, 10). The conditional cutaneous inflammation was characterized by intraepidermal neutrophilic microabcesses that became confluent and trapped beneath the stratum corneum; such particular inflammation has not been described for transgenic mice with other PKC isoforms targeted to the epidermis (11–13). We have also reported that CCR1 or TNF receptor (TNFR) deficiency did not prevent the infiltration of neutrophils into the epidermis (9, 14). Because of its unique inducible character and the restriction to a single PKC isoform, this model provides a preclinical setting to study diseases such as psoriasis and acute generalized exanthematous pustulosis (AGEP) where intraepidermal neutrophilic infiltration and neutrophilia are important clinical manifestations. The current study was designed to define the factors responsible for the systemic and local recruitment of neutrophils in transgenic mice overexpressing PKCα in the epidermis (K5-PKCα mice).
Results
Activation of cutaneous PKCα causes systemic neutrophilia.
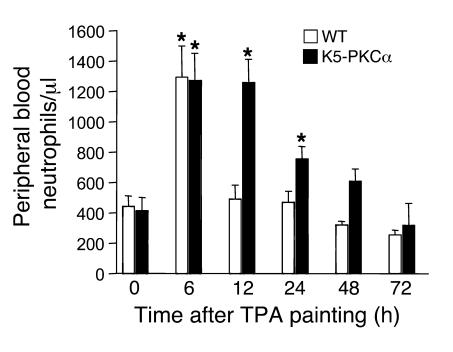
K5-PKCα transgenic mice are very sensitive to a single topical application of TPA to the skin, which causes severe neutrophilic infiltration into the epidermis (9). Previously we have shown this is independent of the TNF-α pathway and the chemokine receptor CCR1. We now find that concomitantly with the infiltration of neutrophils in the skin, TPA-treated K5-PKCα mice have elevated levels of circulating neutrophils in the peripheral blood (Figure 1). While there is a transient increase in TPA-treated WT animals, only K5-PKCα mice develop a sustained neutrophilia. In untreated K5-PKCα mice, the number of circulating neutrophils is identical to that in the WT mice.
Figure 1. K5-PKCα mice develop a sustained neutrophilia in response to TPA painting.
A single dose of TPA (1 μg) in acetone was applied to the shaved backs of K5-PKCα transgenic mice and WT littermates. Blood was drawn at various times, and differential wbc counts were performed. Bars represent the mean number of neutrophils per microliter of blood ± SEM for at least 7 mice per group for each time point. *P < 0.05 versus the respective control.
Activation of cutaneous PKCα increases MIP-2 and KC expression.
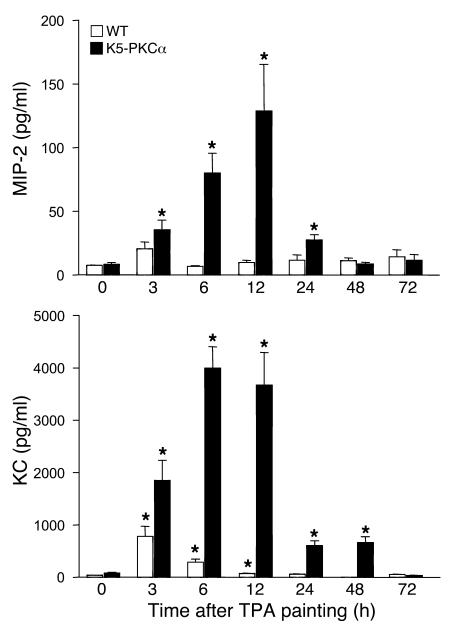
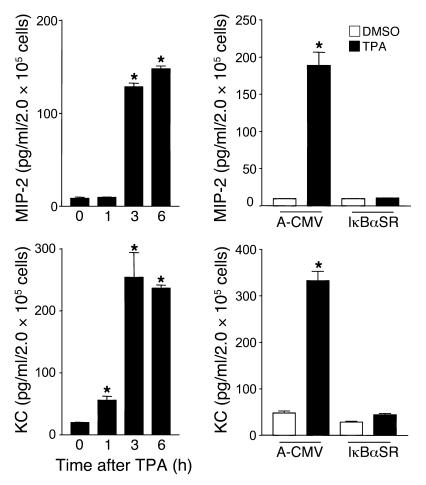
Chemokines are major regulators of neutrophil trafficking. Activation of cutaneous PKCα upregulates the transcription of KC and MIP-2 (10, 14). To determine whether the systemic recruitment of neutrophils and their infiltration in the skin was associated with an increased systemic level of these factors, MIP-2 and KC concentrations were measured in the serum of K5-PKCα mice and their WT littermates after a single topical TPA application. Levels of both chemokines were only modestly and transiently increased in the circulation of WT mice compared with the transgenic animals. Overall, KC peaked earlier and at a much higher concentration than MIP-2 (Figure 2). The kinetics of induction of these chemokines is consistent with the pattern of neutrophilia depicted in Figure 1. These results prompted further evaluation of the roles of both KC and MIP-2 in neutrophil recruitment to the skin. To determine whether PKCα activation could contribute to the increase in KC and MIP-2 levels in the serum, keratinocytes isolated from newborn K5-PKCα mice were treated with 5 ng/ml TPA for 1, 3, and 6 hours. KC and MIP-2 release was normalized to the number of cells at the collection time. Keratinocytes from K5-PKCα mice released KC and MIP-2 into the media in response to PKCα activation (Figure 3, left panels), and this release was sustained. Inhibition of NF-κB signaling in K5-PKCα keratinocytes transduced with an adenoviral IκBα mutant construct completely abrogated the secretion of MIP-2 and KC (Figure 3, right panels). This is consistent with the abrogation of MIP-2 and KC transcripts by NF-κB inhibition reported previously in this model (14).
Figure 2. K5-PKCα mice have increased circulating levels of MIP-2 and KC in response to TPA painting.
A single dose of TPA (1 μg) in acetone was applied to the shaved backs of K5-PKCα transgenic and WT littermates, blood was drawn at various times, and ELISA for KC and MIP-2 was performed on sera. Bars represent the mean ± SEM for 6 animals, and results are representative of at least 2 independent experiments. *P < 0.05 versus the respective control.
Figure 3. K5-PKCα primary keratinocytes produce MIP-2 and KC through PKCα-mediated NF-κB activation.
Left panels: Culture supernatants from K5-PKCα primary keratinocytes were collected at 1, 3, and 6 hours after TPA treatment. KC and MIP-2 concentrations were determined by ELISA. Bars represent the mean ± SEM of triplicate determinations. Results are representative of 3 independent experiments. *P < 0.05 versus time 0. Right panels: KC and MIP-2 concentrations in culture supernatant collected from K5-PKCα keratinocytes transduced with A-CMV (control) or degradation-resistant IκBα (IκBαSR) adenovirus after a 3-hour TPA or DMSO treatment. Bars represent the mean ± SEM of triplicate determinations. Results are representative of 3 independent experiments. *P < 0.05 versus the respective DMSO-treated/A-CMV control.
CXCR2 ligands mediate neutrophil recruitment to the epidermis of K5-PKCα mice but not neutrophilia.
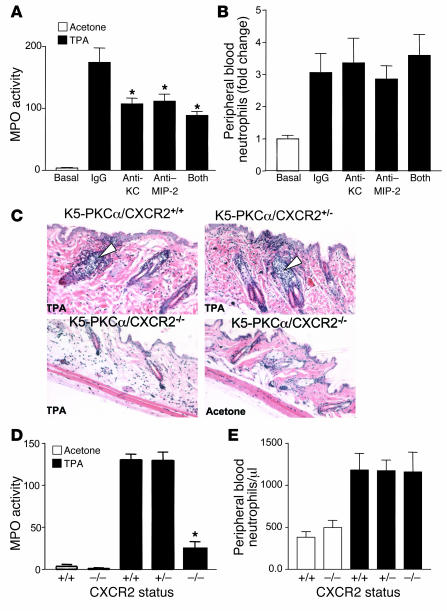
To determine whether MIP-2 and KC activities are required for leukocyte recruitment to the epidermis as well as neutrophilia, we analyzed neutrophil migration into the skin and peripheral blood neutrophil counts of mice that were pretreated systemically with neutralizing antibodies against MIP-2 or KC. These monoclonal antibodies are efficient at blocking MIP-2 and KC activities in various murine models of inflammation (15, 16). As shown in Figure 4A, antibodies given intravenously at 5 μg were effective at reducing neutrophil accumulation in the skin in response to PKCα activation as measured by myeloperoxidase (MPO) content. Combining the 2 neutralizing antibodies had no significant additive effect, suggesting a redundant function for KC and MIP-2. Blocking MIP-2 or KC activity did not prevent the development of neutrophilia in the blood of K5-PKCα mice (Figure 4B). These results suggest that while CXCR2 ligands contribute to the infiltration of neutrophils in the epidermis, they are not responsible for their recruitment to the bloodstream. To confirm the requirement for CXCR2 ligands to mediate PKCα-induced skin inflammation, we crossed K5-PKCα mice with mice homozygous for targeted disruption of CXCR2. The untreated skin of K5-PKCα/CXCR2-deficient mice appeared normal (Figure 4C, lower right panel). As shown in Figure 4C, the infiltration of neutrophils observed in the hair follicles of K5-PKCα/CXCR2+/+ or K5-PKCα/CXCR2+/– mice 6 hours after PKCα activation by topical TPA was absent in K5-PKCα/CXCR2–/– mice. The neutrophil content in skin was further evaluated by measurement of the MPO activity, which confirmed that CXCR2 deficiency prevented almost all of the infiltration of neutrophils in the epidermis of K5-PKCα mice (Figure 4D). The residual MPO activity detected at 6 hours after TPA treatment in K5-PKCα/CXCR2–/– mice most likely reflects neutrophils present in blood capillaries in the deep dermis collected as full-thickness biopsies were performed. Expansion of mature neutrophils has been observed in mice lacking CXCR2 (8), but circulating neutrophil levels are not elevated when mice are housed under germ-free condition (17, 18). Accordingly, circulating neutrophil counts of K5-PKCα/CXCR2–/– mice were indistinguishable from those of their K5-PKCα/CXCR2+/+ littermates (Figure 4E). As shown in Figure 4E, the neutrophilia elicited by activation of cutaneous PKCα was maintained in CXCR2-deficient mice. Collectively, our results demonstrated that PKCα mediates the recruitment of neutrophils to the epidermis through activation of CXCR2, while systemic neutrophilia is CXCR2 independent.
Figure 4. Blocking CXCR2 ligands or CXCR2 deficiency prevents neutrophil infiltration in the skin of K5-PKCα mice.
A single dose of TPA (1 μg) in acetone was applied to the shaved backs of K5-PKCα mice that had been injected intravenously with either control IgG, KC-neutralizing antibodies, MIP-2–neutralizing antibodies, or a combination of KC- and MIP-2–neutralizing antibodies. Skin was collected 6 hours later, and MPO activity was determined (A). Blood was collected at sacrifice, and the number of peripheral blood neutrophils was determined from differential wbc counts (B). For A and B, bars represent the mean ± SEM for 4 animals, and results are representative of 4 experiments. *P < 0.05 versus IgG control group. (C) K5-PKCα mice expressing CXCR2 (K5-PKCα/CXCR2+/+), K5-PKCα mice heterozygous for CXCR2 (K5-PKCα/CXCR2+/–), and K5-PKCα mice deficient for CXCR2 (K5-PKCα/CXCR2–/–) were TPA painted; skin was collected 6 hours later, and sections were stained with H&E. White arrowheads show early stage of neutrophil infiltration into the hair follicles. MPO was determined in skin extracts from the mouse groups depicted in C (D) as well as peripheral blood neutrophil counts (E). For D and E, bars represent the mean ± SEM for 7 animals, and results are representative of 2 experiments. *P < 0.05 versus TPA-treated K5-PKCα/CXCR2+/+ group.
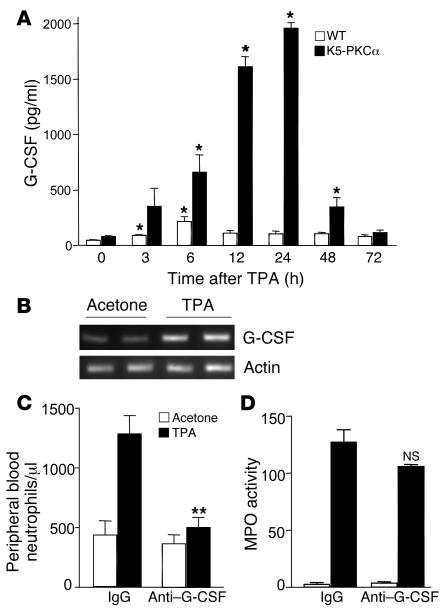
Systemic neutrophilia is dependent on PKCα-mediated upregulation of G-CSF.
G-CSFs and GM-CSFs stimulate proliferation of committed stem cells for granulocytes and macrophages. G-CSF receptor–deficient mice have decreased numbers of circulating neutrophils (19), with reduced chemokine responsiveness (20). Systemic levels of G-CSF and GM-CSF were measured by ELISA in the serum of K5-PKCα mice and their WT littermates after a single TPA application. GM-CSF levels were undetectable at all the time points (data not shown); however, a sustained increase in circulating G-CSF levels was detected in K5-PKCα mice (Figure 5A). PKCα activation led to increased expression of G-CSF transcripts in the skin of K5-PKCα mice (Figure 5B), and G-CSF mRNA induction and protein released upon PKC activation were reported previously (14, 21). To demonstrate the causal role of G-CSF on neutrophilia, we injected K5-PKCα mice with G-CSF–neutralizing antibodies prior to PKCα activation. As shown in Figure 5C, anti–G-CSF treatment decreased neutrophilia by 75% (P < 0.0001) in K5-PKCα mice compared with animals treated with IgG control antibodies. Pretreatment with neutralizing antibodies had no effect on the basal number of circulating neutrophils. As expected from the results obtained in CXCR2-deficient mice, blocking G-CSF had little effect on the infiltration of neutrophils in the skin (Figure 5D). The minor decrease observed could reflect the decrease in overall circulating neutrophils or their decreased responsiveness, as previously reported (20).
Figure 5. Blocking G-CSF prevents neutrophilia in K5-PKCα mice.
(A) A single dose of TPA (1 μg) in acetone was applied to the shaved backs of K5-PKCα transgenic mice and WT littermates. Blood was drawn at various times, and ELISA for G-CSF was performed on sera. Bars represent the mean ± SEM for 6 animals, and results are representative of at least 2 independent experiments. *P < 0.05 versus the respective control. (B) Agarose gel stained with ethidium bromide showing RT-PCR product for G-CSF and actin. RNA was extracted from K5-PKCα skin punch biopsy samples 3 hours after TPA or acetone treatment. Each lane represents an individual mouse. Peripheral blood neutrophil counts (C) and skin MPO activity (D) 6 hours after a single dose of TPA (1 μg) in acetone was applied to the shaved backs of K5-PKCα mice that had been injected intraperitoneally with either control IgG or G-CSF–neutralizing antibodies. Bars represent the mean ± SEM for 8 animals, and results are representative of 2 independent experiments. **P < 0.0001 versus TPA-treated IgG group. NS, not significant versus TPA-treated IgG group.
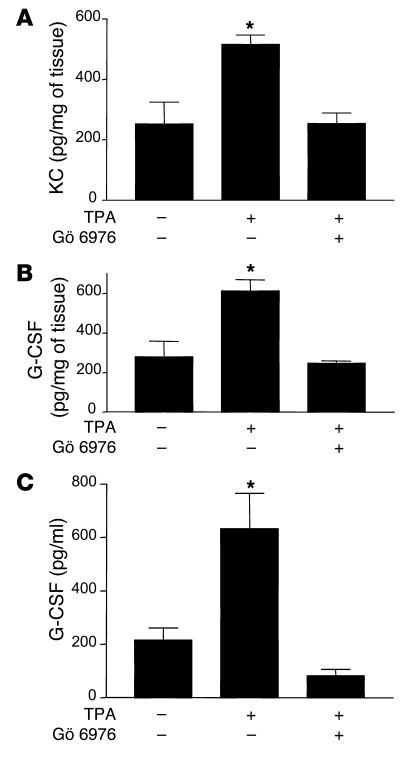
To further confirm that activation of PKCα in keratinocytes is the molecular event initiating the systemic neutrophilia as well as the local recruitment of neutrophils in the epidermis, we applied TPA in the presence or absence of the classical PKC inhibitor Gö 6976. KC and G-CSF production in the skin of K5-PKCα animals was prevented in Gö 6976–treated animals (Figure 6, A and B). Preventing G-CSF induction in the skin also prevented its accumulation in the bloodstream (Figure 6C). This result indicates that G-CSF measured in plasma at early time points after PKCα activation originates from the skin. Gö 6976 application to the back of WT mice prior to TPA painting reduced the 3-fold increase in MPO activity by 49% compared with the level in animals treated with TPA only (MPO activity: basal, 9.0 ± 1.9; TPA only, 28.3 ± 2.7; Gö 6976/TPA, 18.9 ± 4; P < 0.05).
Figure 6. Prevention of KC and G-CSF expression by the classical PKC inhibitor Gö 6976.
Gö 6976 was applied once topically just prior to TPA treatment. KC and G-CSF expression was quantified in skin extract (A and B, respectively), and levels circulating G-CSF (C) were determined in serum. The control group received the same volume of the solvent. Bars represent the mean ± SEM for 4 animals, and results are representative of 2 independent experiments. *P < 0.05 versus no TPA treatment.
PKCα regulates CXCL8 in cultured psoriatic keratinocytes.
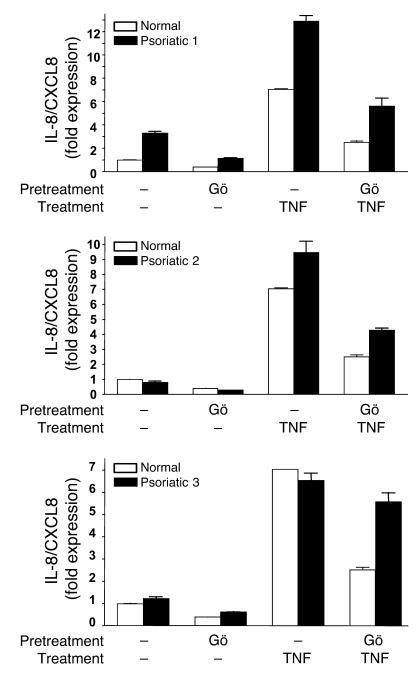
Intraepidermal inflammation and neutrophilia are manifested in several human dermatoses, with pustular psoriasis being a prominent example. The induction of CXCL8 in response to TNF-α is strongly associated with the syndrome. To test a possible function of PKCα in the regulation of CXCL8 in psoriatic keratinocytes, we took advantage of the earlier finding that keratinocytes cultured from lesional psoriatic skin display intrinsic abnormalities in their basal expression as well as their response to proinflammatory factors (22–24). Using real-time PCR, we observed elevated basal expression of transcripts for CXCL8 in keratinocytes from several patients with psoriasis and enhanced CXCL8 transcriptional response after stimulation with TNF-α (Figure 7 and Figure 8A). Blockade of PKCα activity with Gö 6976 reduced the level of CXCL8 mRNA by 80% in keratinocytes from all 3 psoriatic patients examined and substantially reduced the TNF-α–stimulated CXCL8 expression in 2 of the patients’ keratinocytes (Figure 7). Since similar results were detected in TNF-α–treated normal keratinocytes, PKCα may be a major pathway downstream from this proinflammatory cytokine in human epidermis.
Figure 7. PKCα inhibition attenuates abnormal levels of basal and induced CXCL8 in keratinocytes from psoriatic patients.
Real-time PCR analysis of CXCL8 mRNA expression in normal and psoriatic keratinocytes from lesional skin biopsy samples from psoriatic patients (Psoriatic 1–3) or normal skin of healthy controls treated as described in Methods. “Normal” is representative of cultured keratinocytes from 3 healthy controls. Bars represent the mean value of triplicate determinations from a single patient. Results are expressed as fold increase in expression compared with that in untreated normal keratinocytes.
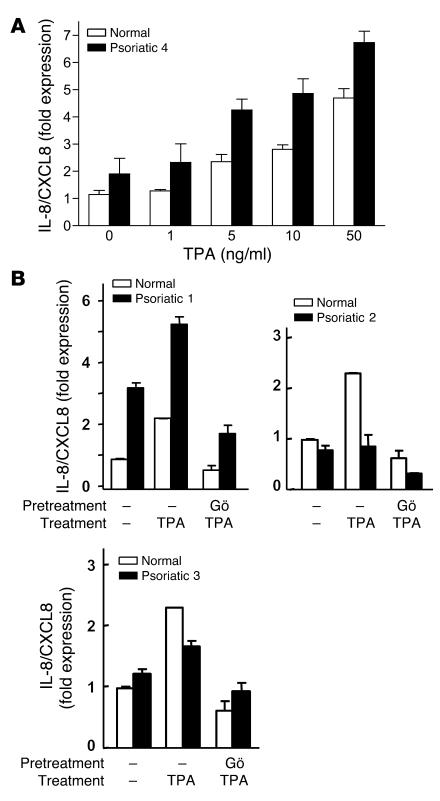
Figure 8. Keratinocytes from a subset of psoriatic patients express higher CXCL8 mRNA levels in response to TPA.
(A) Real-time PCR analysis of CXCL8 mRNA expression in keratinocytes cultured from lesional skin biopsy samples from a psoriatic patient or skin of a healthy control treated with increasing doses of TPA. (B) CXCL8 mRNA expression in patient and control keratinocyte cultures shown in Figure 7. Cultures were stimulated for 1 hour with TPA (5 ng/ml) with or without incubation with 5 μM Gö 6976 for the previous 30 minutes (B). “Normal” is representative of cultured keratinocytes from 3 healthy controls. Bars represent mean value of triplicate determinations from a single patient. Results are expressed as fold increase in expression compared with that in untreated normal keratinocytes.
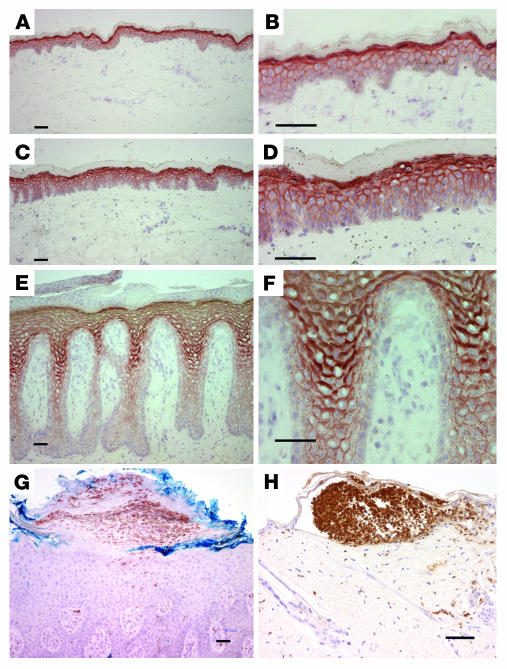
We also asked whether psoriatic keratinocytes were sensitive to TPA induction of CXCL8 transcripts. Figure 8A shows that TPA-induced CXCL8 message in both control and patient cultured keratinocytes is dose dependent, with generally higher levels in the patient cells. When the keratinocytes of patients and controls represented in Figure 7 were stimulated by TPA (Figure 8B), patient 1 was particularly sensitive to stimulation and inhibition by Gö 6976, while the other 2 patients were less sensitive to TPA, although Gö 6976 was inhibitory in both. These results suggest that PKCα participates in the induction of CXCL8 in both normal and patient keratinocytes, but there is variability in the level of contribution analogous to the variability of neutrophil infiltration in psoriatic skin among patients. Further support for a function of PKCα in the inflammatory infiltrate of specific human diseases is shown in Figure 9, A–F: a substantial increase in PKCα expression in multiple layers of psoriatic plaque was detected by immunohistochemistry. PKCα localizes mostly at cell-cell interfaces in the entire suprabasal compartment. Weak staining of lateral and apical surfaces of basal cells was observed, as previously reported (25). No staining was observed in the absence of the primary anti-PKCα antibody. In cases of pustular psoriasis (Figure 9G), neutrophils in macropustules were present beneath the stratum corneum. These pustules were nearly identical to the neutrophilic foci present in microabscesses in K5-PKCα mice upon PKCα activation (Figure 9H).
Figure 9. Prominent PKCα expression in the epidermis of psoriatic patients.
Frozen sections from normal (A and B), nonlesional (C and D), and psoriatic (E and F) epidermis were stained with monoclonal antibodies to PKCα and detected by immunoperoxidase. Results are typical of samples from 4 patients. Representative example of immunostaining for neutrophils in pustular psoriasis epidermis (G) and TPA-treated K5-PKCα mouse epidermis (H). PKCα staining in G (data not shown) was identical to E and F. Scale bars, 50 μm.
Discussion
Collectively these results identify PKCα activation in keratinocytes as the initiating event causing G-CSF release and CXCR2 ligand induction. Keratinocyte-derived G-CSF recruits neutrophils to the bloodstream, while CXCR2 ligands promote their infiltration into the epidermis. This model illustrates the capacity of keratinocyte to elaborate both a systemic and local inflammatory response and defines several pathways that may be therapeutically relevant to abrogating the intraepidermal form of cutaneous inflammation.
The cutaneous lesions of TPA-painted K5-PKCα mice resemble those of several human skin conditions, such as pustular psoriasis, AGEP (26), and cutaneous candidiasis (27). We now understand that in this mouse model, neutrophil infiltration and intraepidermal microabscesses result from a systemic increase in the level of circulating neutrophils induced by cytokines and chemokines of cutaneous origin. The critical mediator of cytokine/chemokine release is PKCα activation, which promotes intraepidermal chemotaxis of neutrophils via CXCR2 chemokines and systemic recruitment of neutrophils via G-CSF release. Although the regulatory function of CXCR2 on leukocyte influx in inflamed tissue is well appreciated (28), the present study suggests that systemic and local recruitment of neutrophils can be mediated by 2 independent mechanisms. Our data also suggest that a physiological function for PKCα in keratinocytes is to orchestrate a neutrophil response during inflammation. Interestingly, a secreted Salmonella effector protein activates a PKCα-dependent epithelial signaling pathway in enterocytes that promotes neutrophil transepithelial migration (29, 30).
NF-κB is a pivotal regulator of proinflammatory gene expression in K5-PKCα primary keratinocytes (14). As a consequence, NF-κB blockade abolishes the production of KC and MIP-2 in K5-PKCα keratinocytes. Our results also indicate that while the in vitro production of KC and MIP-2 are similar, circulating KC levels in vivo are far higher than MIP-2 levels. This observation is identical to the one described for thioglycollate- or glycogen-induced peritonitis (31). While TNF-α is a potent inducer of KC and MIP-2 expression in vivo (32), TNFR deficiency had no effect on PKCα-mediated induction of these 2 chemokines (14). PKCα has been described as being one of the downstream effectors of TNF-α (33–35). Direct activation of PKCα by TPA could explain TNF-α dispensability in our model (14). Our results indicate that PKCα also contributes to TNF-α–mediated induction of CXCL8 in human keratinocytes as a downstream effector. If in fact PKCα is an important downstream mediator of TNF-α proinflammatory activity, design of PKCα isoform–selective inhibitors may have potential clinical benefit in the context of antiinflammatory drug development.
CXCR2 ligands have been implicated in the etiology of a number of pathological conditions in humans as well as in various mouse models of inflammation (for review, see refs. 36, 37). Increased IL-8 expression and neutrophil recruitment have been reported in bronchoalveolar fluid of patients with bacterial pneumonia or bronchiolitis obliterans syndrome (38). Similarly, human CXCR2 ligands (CXCL1, CXCL5, and CXCL8) are strongly increased in the synovial fluid of patients suffering from rheumatoid arthritis. In psoriasis, the skin lesions contain activated T cells and neutrophils (39), and it is thought that the latter could be recruited by elevated expression of CXCL8 and CXCL1 (40). We now show by immunohistochemistry that PKCα demonstrates a strong expression in upper keratinocytes of psoriatic epidermal lesions, with a staining pattern consistent with membrane localization and gradation in the suprabasal compartments and the superior aspect of basal cells. Phospholipase C and diacylglycerol levels are elevated in psoriatic lesions (41–43), which could explain the membrane localization of PKCα. It is worth noting that GROα and CXCL8 mRNA are expressed in suprapapillary layers of psoriatic lesions (44, 45), which further substantiates a possible regulatory link between PKCα and these 2 chemokines. However, enhanced sensitivity for CXCL8 mRNA induction by TNF-α or TPA was not displayed by all lines of cultured psoriatic keratinocytes. The intraepidermal inflammatory component of psoriasis is also variable, with the pustular form being the extreme. The similarity in epidermal pustule formation between humans with this form of psoriasis and mice with cutaneous activation of PKCα suggests that PKCα activation may contribute to the more inflammatory subset of psoriatic intraepidermal lesions.
Consistent with our mouse model, expression of CXCR2 mRNA and protein are increased in lesional psoriatic keratinocytes (46, 47). Although the role of neutrophils in the psoriasis phenotype is not fully understood, their depletion in the flaky skin mouse model dramatically improves the skin lesions (48). Neutrophils infiltrate the skin of K14-KC transgenic mice, but the inflammatory reaction is moderate due to downregulation of CXCR2 (49). Inducible expression of KC in transgenic grafts causes a greater inflammatory response by circumventing CXCR2 desensitization (50). Thus, maintaining the level of CXCR2 is critical for propagating the inflammatory response in skin. Since CXCR2 is expressed by keratinocytes as well as neutrophils, the high level expression of this receptor in psoriasis could imply an autocrine or paracrine effect (51). The high level of expression of IL-8 and CXCR2 in psoriasis prompted clinical trials with anti–IL-8 antibodies for this disease. However, the treatment was ineffective, and the approach was abandoned. In our study, we also found that systemic antibody treatment to block KC or MIP-2 was only partially effective and combined antibodies could not improve efficacy. The lack of complete effectiveness in the human and mouse studies could have a number of explanations, including the upregulation of GRO in human psoriasis (40) or the stoichiometry of antibody-ligand interactions in our mouse model. Our studies suggest that targeting the receptor CXCR2 may have better therapeutic potential. This K5-PKCα model may be useful to screen small molecules as CXCR2 antagonists. The recent generation of human CXCR2 knock-in mice (52) and targeting CXCR2 using cell-penetrating lipopeptides (53) (pepducins) are additional approaches that show promise.
The continuous supply of granulocytes to the circulating blood depends on their rate of production and their subsequent release into the marrow sinuses. Although neutrophils play a critical role in innate immunity, inappropriate/excessive recruitment could contribute to tissue damage during inflammatory situations. Various factors with distinct cellular targets and activity can induce the release of neutrophils from the bone marrow (cytokines, chemokines, fMLP, C5a), but the mechanisms controlling neutrophil release from the bone marrow are still incompletely understood. Several investigators have shown that the intravenous administration of CXCL8 causes a release of neutrophils from the bone marrow (54, 55). More recently it was suggested that KC could mediate recruitment of neutrophils from the bone marrow by stimulating their chemotaxis or by blocking the bone marrow–retaining effect of stromal cell–derived factor 1 (SDF-1)/CXCR4 interaction (56, 57). Our data indicate that CXCR2 signaling from cutaneous sources is not responsible for the systemic recruitment of neutrophils, as CXCR2 deficiency or CXCR2 ligand blockade do not prevent neutrophilia. Clearly, CXCR2 signaling is not universally required for neutrophil release from the bone marrow. We then tested the hypothesis that systemically elevated levels of G-CSF could be responsible for the neutrophilia. G-CSF is widely used to treat or prevent neutropenia in a variety of clinical situations. Generation of G-CSF– and G-CSF receptor–deficient mice by gene targeting has demonstrated unequivocally the importance of G-CSF in the regulation of baseline granulopoiesis (19, 58). G-CSF–deficient also mice have reduced neutrophilic responses to listeria infection (58), suggesting a role for G-CSF in “emergency” situations. Consistent with this observation, we show in the present study that the inflammatory response initiated by cutaneous PKCα leads to the systemic release of G-CSF from the skin and recruitment of neutrophils from the bone marrow. While the exact mechanism of action of G-CSF is beyond the scope of this article, recent data suggest that G-CSF action on neutrophils is likely indirect and could involve trans-acting signals on SDF-1/CXCR4 (59).
In summary, these data provide evidence that PKCα activation in keratinocytes is a crucial event orchestrating cutaneous neutrophil responses. Both KC and MIP-2 are PKCα regulated through NF-κB activation, and they direct neutrophil migration through the stromal compartment into the epidermis. Collectively our data provide what we believe to be the first proof of concept for CXCR2 targeting in skin lesions that present an extensive intraepidermal neutrophil infiltration. Concomitantly, PKCα activation causes the release into the bloodstream of G-CSF from keratinocytes that recruit neutrophils from the bone marrow. It appears that targeting the concerting action of PKCα on neutrophil trafficking could have therapeutic benefit in some cutaneous inflammatory situations.
Methods
Subjects.
Epidermal keratinocytes were collected from skin biopsy samples from 4 adult patients with moderate-to-severe chronic plaque psoriasis (3 women and 1 man; age range, 33–73 years) and 3 healthy age-matched control subjects. Skin sections were obtained from skin biopsies of lesional and nonlesional skin of 4 psoriatic patients (1 woman and 3 men; age range, 48–69) with plaque psoriasis and of the skin of 3 healthy age-matched subjects. Informed consent was obtained from all subjects, and the study was approved by the Istituto Dermopatico dell’Immacolata (Rome, Italy) and University of Düsseldorf (Düsseldorf, Germany). Skin sections of anonymous patients with pustular psoriasis were obtained from the National Cancer Institute Laboratory of Pathology.
Human keratinocyte cultures.
Epidermal cell suspensions were prepared from lesional skin biopsy samples from psoriatic patients or normal skin of healthy controls. After trypsinization of the epidermis, isolated epidermal cells were seeded on a feeder layer of mitomycin C–treated (M0503; Sigma-Aldrich) NIH 3T3 fibroblasts and cocultured using an optimized Rheinwald and Green technique (60). Human primary keratinocytes were used at the fourth passage after 24 hours in the absence of hydrocortisone with serum replaced with 0.1% BSA. Total RNA was harvested after 1 hour stimulation with TNF-α (50 ng/ml) or TPA (5 ng/ml) with or without incubation with 5 μM Gö 6976 for the previous 30 minutes (Calbiochem; EMD Biosciences).
Immunohistochemistry.
Five-micrometer cryostatic sections were fixed with acetone for 20 minutes at –20°C, treated with 0.3% hydrogen peroxide for 15 minutes, and blocked with PBS solution containing 3% BSA. Sections were then incubated for 1 hour at room temperature with mouse anti-human PKCα (05-154; Upstate USA Inc.) diluted 1:500 in 1% BSA; CD15 (clone C3D-1; Dako) diluted 1:25 in 1% BSA, rinsed, and incubated with 1:200 biotinylated anti-mouse antibody (Vector Laboratories); or 1:250 rabbit anti-mouse IgM biotinylated (E4065; Dako). Ly6G staining was previously described (9). Immunoreactivity was revealed using an ABC avidin-biotin-peroxidase system and AEC substrate (Vector Laboratories). Sections were counterstained with Mayer hematoxylin. For negative control experiments, primary antibody was omitted.
Mice and treatments.
Mouse studies were performed under a protocol approved by the National Cancer Institute and the NIH Animal Care and Use Committee. The construction and characterization of K5-PKCα mice were previously described (9). These mice express a full-length murine PKCα cDNA under the control of the bovine keratin 5 promoter (9) that targets the transgene to the basal layer of the epidermis and the outer root sheath of the hair follicle. For the studies reported here, an FVB/N strain expressing a 10-fold excess of PKCα over the endogenous level in the target sites was used (9).
K5-PKCα mice were crossed to homozygous mice deficient in CXCR2 (8). F1 K5-PKCα/CXCR2+/– mice were backcrossed to CXCR2–/– mice to generate K5-PKCα mice deficient for CXCR2. At weaning, tail samples were collected and screened by separate PCR reactions for each of the alleles. The K5-PKCα allele was identified by PCR using primers TGCATATAAATTCTGGCTGGCG and GCATGAACATGGTTAGCAGAGGG that span a 166-nucleotide sequence of the β-globin intron. CXCR2 alleles were detected using the following primers: CXCR2 WT allele, GGTCGTACTGCGTATCCTGCCTCAG and TAGCCATGATCTTGAGAAGTCCATG; CXCR2-KO allele, GAACCTCATTCTGCCCTCTT and CGAGATCAGCAGCCTCTGTTC.
TPA (Axxora) was dissolved in acetone, and 1 μg was applied in 200 μl. Gö 6976 (Calbiochem; EMD Biosciences) was dissolved in acetone, and 13 nmol was applied 5 minutes prior to TPA. Ten micrograms of G-CSF–neutralizing antibodies (Novus Biologicals) were injected intraperitoneally 3 hours prior to TPA painting. Five micrograms of anti-mouse KC (R&D Systems) and/or 5 μg anti-mouse MIP-2 antibodies (R&D Systems) were administered intravenously 5 minutes prior to TPA painting.
Peripheral blood analysis.
The mice were anesthetized with Nembutal (Abbott). Blood for differential white blood cell counts was collected by intracardiac puncture using heparinized syringes to prevent coagulation. Cell analysis was performed at the Department of Laboratory Medicine at the NIH.
Cell culture.
Primary mouse keratinocytes and hair follicle buds were isolated from newborn transgenic and WT littermate epidermis as described previously (61). Primary keratinocytes were seeded at a density of 5 × 106 cells per 60-mm dish (or equivalent concentrations) in Ca2+- and Mg2+-free MEM (Invitrogen) supplemented with 8% Chelex-treated (Bio-Rad) fetal bovine serum (Gemini Bio-Products) and 0.2 mM Ca2+. After 24 hours, cultures were switched to the same medium with 0.05 mM Ca2+ to select for basal cells. TPA was reconstituted in DMSO, and primary keratinocytes were treated with TPA (5 ng/ml) for various times as indicated in the figures.
Skin histology and MPO assay.
Shaven transgenic and WT animals were treated topically with a single dose (1 μg) of TPA in 200 μl of acetone. Skin was excised at various times after treatment and fixed in 10% formalin solution (Sigma-Aldrich), paraffin embedded, sectioned, and stained with H&E. Back whole skin samples were used for MPO assays as previously described (14, 62). In brief, samples were homogenized in potassium phosphate buffer (pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide (HTAB), sonicated, and freeze thawed 3 times, after which sonication was repeated. The suspension was centrifuged at 40,000 g for 15 minutes, and 10 μl of supernatant was added to 290 μl of potassium phosphate buffer (pH 6.0) containing 0.167 mg/ml o-dianisidine dihydrochloride (Sigma-Aldrich) and 0.0005% hydrogen peroxide. Changes in OD were monitored at 460 nm at 25°C, over a 4-minute period. Isolated neutrophils obtained from mice with thioglycollate-induced peritonitis were processed in the same manner and used to generate a calibration curve for neutrophils, which were enumerated by light microscopy.
RT-PCR analysis.
RNA was isolated from cultured keratinocytes or skin-punch biopsies with Tri zol following manufacturer’s protocol (Invitrogen). For cDNA synthesis, 2 μg of total RNA was reverse transcribed using SuperScript II Reverse Transcriptase (Invitrogen). PCR amplifications were performed in a volume of 20 μl using Platinum PCR SuperMix (Invitrogen). The primers used for this analysis were as follows: actin, CAGATCATGTTTGAGACCTTC and ACTTCATGATGGAATTGAATG; G-CSF, TCTGCCCCGAAGCTTCCTGCTTAAG and CAGCAACACCAGCTCCTCGGGGTGA. To avoid saturation or plateau effect of amplification, PCR was limited to a total of 20 cycles for actin and 32 cycles for G-CSF. Results are representative of at least 3 independent experiments.
For real-time PCR analysis, the expression level of IL-8/CXCL8 cDNA was determined using a Bio-Rad iCycler iQ and Gene Expression Macro (version 1.1) from Bio-Rad. cDNA (diluted 1:200 in the final volume reaction) was measured from triplicate samples using iQ SYBR Green Supermix (Bio-Rad). The primer sequences were as follows: IL-8/CXCL8, GCTCTAGAATGACTTCCAAGCTGGCCG and CGGGATCCTTATGAATTCTCAGCCCTCT; GAPDH primers were from Gene Link (catalog 40-1005-10). Relative standard curves were generated from log input (serial dilutions of pooled cDNA) versus the cycle threshold (Ct). The linear correlation coefficients (r2) were 0.997 and 0.987 for CXCL8 and GAPDH, respectively. The slope of the standard curve was used to determine the efficiency of target amplification (E) using the equation E = (10–1/slope–1) × 100. Similar high efficiency was obtained for CXCL8 and GAPDH, allowing for the comparative Ct method to be used. Relative quantitation was used to calculate the 2–(ΔDCt) formula, where ΔDCt represents the cycle difference corrected for GAPDH, used as an internal control (63). The data are presented as fold change in gene expression normalized to GAPDH and relative to untreated control. Electrophoresis of the amplified product from real-time PCR showed a single band.
Adenovirus transduction.
The IκBαSR (64) adenovirus was introduced into primary keratinocytes using an adenoviral construct driven by a CMV promoter and empty adenovirus was used as control (A-CMV). The cells were infected for 30 minutes in serum-free medium with an MOI of 5 viral particles/cell and 2.5 μg/ml of Polybrene (Sigma-Aldrich) to enhance uptake. Serum-containing medium was added to the cells for the next 48 hours after the infection. The IκBαSR adenovirus was a generous gift from D.C. Guttridge of the University of North Carolina, Chapel Hill, North Carolina, USA.
ELISA.
Cytokine/chemokine levels in the serum, skin extracts and culture supernatants were quantified by ELISA. For collection of serum, blood was drawn by intracardiac puncture, incubated at room temperature for 30 minutes, and centrifuged at 5,000 g for 10 minutes. Full-thickness skin punch biopsies were homogenized in protein extraction buffer M-PER (Pierce Biotechnology) supplemented with a cocktail of protease inhibitors (Complete Mini; Roche Diagnostics). The samples were spun for 5 minutes at maximum speed, and supernatants were collected for analysis. Quantikine ELISA kits for MIP-2, KC, and G-CSF were used according to the manufacturer’s protocol (R&D Systems).
Statistics.
Results are expressed as mean ± SEM. Data were analyzed by prism software and significance values assigned using 2-tailed Student’s t test. P < 0.05 was considered to be statistically significant.
Acknowledgments
The authors thank Philip M. Murphy for critical reading of the manuscript, Gail McMullen for excellent technical assistance, and Marta Custer for care of the mouse colonies. We would like also to acknowledge Ronald Wolf for contributing human psoriatic skin samples. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Nonstandard abbreviations used: CXCL8, CXC chemokine ligand 8; CXCR2, CXC chemokine receptor 2; KC, cytokine-induced neutrophil chemoattractant; MIP-2, macrophage inflammatory protein 2; MPO, myeloperoxidase; TPA, 12-O-tetradecanoylphorbol-13-acetate.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 116:2757–2766 (2006). doi:10.1172/JCI27514
References
- 1.Kupper T.S., Fuhlbrigge R.C. Immune surveillance in the skin: mechanisms and clinical consequences. Nat. Rev. Immunol. 2004;4:211–222. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy P.M., et al. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol. Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- 3.Murphy P.M. International Union of Pharmacology. XXX. Update on chemokine receptor nomenclature. Pharmacol. Rev. 2002;54:227–229. doi: 10.1124/pr.54.2.227. [DOI] [PubMed] [Google Scholar]
- 4.Zlotnik A., Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 5.Bozic C.R., et al. Expression and biologic characterization of the murine chemokine KC. . J. Immunol. 1995;154:6048–6057. [PubMed] [Google Scholar]
- 6.Bozic C.R., et al. The murine interleukin 8 type B receptor homologue and its ligands. Expression and biological characterization. J. Biol. Chem. 1994;269:29355–29358. [PubMed] [Google Scholar]
- 7.Lee J., et al. Chemokine binding and activities mediated by the mouse IL-8 receptor. J. Immunol. 1995;155:2158–2164. [PubMed] [Google Scholar]
- 8.Cacalano G., et al. Neutrophil and B cell expansion in mice that lack the murine IL- 8 receptor homolog. Science. 1994;265:682–684. doi: 10.1126/science.8036519. [DOI] [PubMed] [Google Scholar]
- 9.Cataisson C., et al. Activation of cutaneous protein kinase C alpha induces keratinocyte apoptosis and intraepidermal inflammation by independent signaling pathways. J. Immunol. 2003;171:2703–2713. doi: 10.4049/jimmunol.171.5.2703. [DOI] [PubMed] [Google Scholar]
- 10.Wang H.Q., Smart R.C. Overexpression of protein kinase C-alpha in the epidermis of transgenic mice results in striking alterations in phorbol ester-induced inflammation and COX-2, MIP-2 and TNF-α expression but not tumor promotion. J. Cell Sci. 1999;112:3497–3506. doi: 10.1242/jcs.112.20.3497. [DOI] [PubMed] [Google Scholar]
- 11.Reddig P.J., et al. Transgenic mice overexpressing protein kinase C delta in the epidermis are resistant to skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1999;59:5710–5718. [PubMed] [Google Scholar]
- 12.Reddig P.J., et al. Transgenic mice overexpressing protein kinase C epsilon in their epidermis exhibit reduced papilloma burden but enhanced carcinoma formation after tumor promotion. Cancer Res. 2000;60:595–602. [PubMed] [Google Scholar]
- 13.Jansen A.P., et al. Relation of the induction of epidermal ornithine decarboxylase and hyperplasia to the different skin tumor-promotion susceptibilities of protein kinase C alpha, -delta and -epsilon transgenic mice. Int. J. Cancer. 2001;93:635–643. doi: 10.1002/ijc.1395. [DOI] [PubMed] [Google Scholar]
- 14.Cataisson C., Pearson A.J., Torgerson S., Nedospasov S.A., Yuspa S.H. Protein kinase C alpha-mediated chemotaxis of neutrophils requires NF-kappa B activity but is independent of TNF alpha signaling in mouse skin in vivo. J. Immunol. 2005;174:1686–1692. doi: 10.4049/jimmunol.174.3.1686. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X.W., Wang Y., Liu Q., Thorlacius H. Redundant function of macrophage inflammatory protein-2 and KC in tumor necrosis factor-alpha-induced extravasation of neutrophils in vivo. Eur. J. Pharmacol. 2001;427:277–283. doi: 10.1016/s0014-2999(01)01235-3. [DOI] [PubMed] [Google Scholar]
- 16.Xue M.L., et al. Role and regulation of CXC-chemokines in acute experimental keratitis. Exp. Eye Res. 2003;76:221–231. doi: 10.1016/s0014-4835(02)00270-1. [DOI] [PubMed] [Google Scholar]
- 17.Shuster D.E., Kehrli M.E., Ackermann M.R. Neutrophilia in mice that lack the murine IL-8 receptor homolog. Science. 1995;269:1590–1591. doi: 10.1126/science.7667641. [DOI] [PubMed] [Google Scholar]
- 18.Broxmeyer H.E., et al. Involvement of interleukin (IL) 8 receptor in negative regulation of myeloid progenitor cells in vivo: evidence from mice lacking the murine IL-8 receptor homologue. J. Exp. Med. 1996;184:1825–1832. doi: 10.1084/jem.184.5.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu F., Wu H.Y., Wesselschmidt R., Kornaga T., Link D.C. Impaired production and increased apoptosis of neutrophils in granulocyte colony-stimulating factor receptor-deficient mice. Immunity. 1996;5:491–501. doi: 10.1016/s1074-7613(00)80504-x. [DOI] [PubMed] [Google Scholar]
- 20.Betsuyaku T., et al. A functional granulocyte colony-stimulating factor receptor is required for normal chemoattractant-induced neutrophil activation. J. Clin. Invest. 1999;103:825–832. doi: 10.1172/JCI5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wheeler D.L., et al. Overexpression of protein kinase C-epsilon in the mouse epidermis leads to a spontaneous myeloproliferative-like disease. Am. J. Pathol. 2005;166:117–126. doi: 10.1016/s0002-9440(10)62237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nickoloff B.J., Mitra R.S., Varani J., Dixit V.M., Polverini P.J. Aberrant production of interleukin-8 and thrombospondin-1 by psoriatic keratinocytes mediates angiogenesis. Am. J. Pathol. 1994;144:820–828. [PMC free article] [PubMed] [Google Scholar]
- 23.Giustizieri M.L., et al. Keratinocytes from patients with atopic dermatitis and psoriasis show a distinct chemokine production profile in response to T cell-derived cytokines. J. Allergy Clin. Immunol. 2001;107:871–877. doi: 10.1067/mai.2001.114707. [DOI] [PubMed] [Google Scholar]
- 24.Giustizieri M.L., Albanesi C., Scarponi C., De Pita O., Girolomoni G. Nitric oxide donors suppress chemokine production by keratinocytes in vitro and in vivo. Am. J. Pathol. 2002;161:1409–1418. doi: 10.1016/S0002-9440(10)64416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tibudan S.S., Wang Y., Denning M.F. Activation of protein kinase C triggers irreversible cell cycle withdrawal in human keratinocytes. . J. Invest. Dermatol. 2002;119:1282–1289. doi: 10.1046/j.1523-1747.2002.19625.x. [DOI] [PubMed] [Google Scholar]
- 26.Britschgi M., Pichler W.J. Acute generalized exanthematous pustulosis, a clue to neutrophil-mediated inflammatory processes orchestrated by T cells. Curr. Opin. Allergy Clin. Immunol. 2002;2:325–331. doi: 10.1097/00130832-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Wilson B.D., Sohnle P.G. Participation of neutrophils and delayed hypersensitivity in the clearance of experimental cutaneous candidiasis in mice. Am. J. Pathol. 1986;123:241–249. [PMC free article] [PubMed] [Google Scholar]
- 28.Esche C., Stellato C., Beck L.A. Chemokines: key players in innate and adaptive immunity. J. Invest. Dermatol. 2005;125:615–628. doi: 10.1111/j.0022-202X.2005.23841.x. [DOI] [PubMed] [Google Scholar]
- 29.Lee C.A., et al. A secreted Salmonella protein induces a proinflammatory response in epithelial cells, which promotes neutrophil migration. Proc. Natl. Acad. Sci. U. S. A. 2000;97:12283–12288. doi: 10.1073/pnas.97.22.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva M., Song C., Nadeau W.J., Matthews J.B., McCormick B.A. Salmonella typhimurium SipA-induced neutrophil transepithelial migration: involvement of a PKC-alpha-dependent signal transduction pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;286:G1024–G1031. doi: 10.1152/ajpgi.00299.2003. [DOI] [PubMed] [Google Scholar]
- 31.Call D.R., et al. Differential local and systemic regulation of the murine chemokines KC and MIP2. Shock. 2001;15:278–284. doi: 10.1097/00024382-200115040-00005. [DOI] [PubMed] [Google Scholar]
- 32.Tessier P.A., et al. Chemokine networks in vivo: involvement of C-X-C and C-C chemokines in neutrophil extravasation in vivo in response to TNF-alpha. J. Immunol. 1997;159:3595–3602. [PubMed] [Google Scholar]
- 33.Arnott C.H., et al. Tumour necrosis factor-alpha mediates tumour promotion via a PKC alpha- and AP-1-dependent pathway. Oncogene. 2002;21:4728–4738. doi: 10.1038/sj.onc.1205588. [DOI] [PubMed] [Google Scholar]
- 34.Huang W.C., Chen J.J., Chen C.C. c-Src-dependent tyrosine phosphorylation of IKKbeta is involved in tumor necrosis factor-alpha-induced intercellular adhesion molecule-1 expression. . J. Biol. Chem. 2003;278:9944–9952. doi: 10.1074/jbc.m208521200. [DOI] [PubMed] [Google Scholar]
- 35.Huang W.C., Chen J.J., Inoue H., Chen C.C. Tyrosine phosphorylation of I-kappa B kinase alpha/beta by protein kinase C-dependent c-Src activation is involved in TNF-alpha-induced cyclooxygenase-2 expression. J. Immunol. 2003;170:4767–4775. doi: 10.4049/jimmunol.170.9.4767. [DOI] [PubMed] [Google Scholar]
- 36.Luster A.D. Chemokines — chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 37.Murdoch C., Finn A. Chemokine receptors and their role in inflammation and infectious diseases. Blood. 2000;95:3032–3043. [PubMed] [Google Scholar]
- 38.Belperio J.A., et al. Role of CXCR2/CXCR2 ligands in vascular remodeling during bronchiolitis obliterans syndrome. J. Clin. Invest. 2005;115:1150–1162. doi: 10.1172/JCI200524233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nickoloff B.J., Nestle F.O. Recent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunities. J. Clin. Invest. 2004;113:1664–1675. doi: 10.1172/JCI200422147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gillitzer R., Ritter U., Spandau U., Goebeler M., Brocker E.B. Differential expression of GRO-alpha and IL-8 mRNA in psoriasis: a model for neutrophil migration and accumulation in vivo. J. Invest. Dermatol. 1996;107:778–782. doi: 10.1111/1523-1747.ep12371803. [DOI] [PubMed] [Google Scholar]
- 41.Nanney L.B., Gates R.E., Todderud G., King L.E., Carpenter G. Altered distribution of phospholipase C-gamma 1 in benign hyperproliferative epidermal diseases. Cell Growth Differ. 1992;3:233–239. [PubMed] [Google Scholar]
- 42.Bergers M., Van de Kerkhof P.C., Happle R., Mier P.D. Membrane-bound phospholipase C activity in normal and psoriatic epidermis. Acta Derm. Venereol. 1990;70:57–59. [PubMed] [Google Scholar]
- 43.Fisher G.J., Talwar H.S., Baldassare J.J., Henderson P.A., Voorhees J.J. Increased phospholipase C-catalyzed hydrolysis of phosphatidylinositol-4,5-bisphosphate and 1,2-sn-diacylglycerol content in psoriatic involved compared to uninvolved and normal epidermis. J. Invest. Dermatol. 1990;95:428–435. doi: 10.1111/1523-1747.ep12555582. [DOI] [PubMed] [Google Scholar]
- 44.Gillitzer R., et al. Upper keratinocytes of psoriatic skin lesions express high levels of NAP-1/IL-8 mRNA in situ. J. Invest. Dermatol. 1991;97:73–79. doi: 10.1111/1523-1747.ep12478128. [DOI] [PubMed] [Google Scholar]
- 45.Kulke R., et al. Co-localized overexpression of GRO-alpha and IL-8 mRNA is restricted to the suprapapillary layers of psoriatic lesions. J. Invest. Dermatol. 1996;106:526–530. doi: 10.1111/1523-1747.ep12343916. [DOI] [PubMed] [Google Scholar]
- 46.Schulz B.S., et al. Increased expression of epidermal IL-8 receptor in psoriasis. Down-regulation by FK-506 in vitro. J. Immunol. 1993;151:4399–4406. [PubMed] [Google Scholar]
- 47.Kulke R., et al. The CXC receptor 2 is overexpressed in psoriatic epidermis. J. Invest. Dermatol. 1998;110:90–94. doi: 10.1046/j.1523-1747.1998.00074.x. [DOI] [PubMed] [Google Scholar]
- 48.Schon M., Denzer D., Kubitza R.C., Ruzicka T., Schon M.P. Critical role of neutrophils for the generation of psoriasiform skin lesions in flaky skin mice. J. Invest. Dermatol. 2000;114:976–983. doi: 10.1046/j.1523-1747.2000.00953.x. [DOI] [PubMed] [Google Scholar]
- 49.Lira S.A., et al. Expression of the chemokine N51/KC in the thymus and epidermis of transgenic mice results in marked infiltration of a single class of inflammatory cells. J. Exp. Med. 1994;180:2039–2048. doi: 10.1084/jem.180.6.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wiekowski M.T., et al. Disruption of neutrophil migration in a conditional transgenic model: evidence for CXCR2 desensitization in vivo. . J. Immunol. 2001;167:7102–7110. doi: 10.4049/jimmunol.167.12.7102. [DOI] [PubMed] [Google Scholar]
- 51.Tuschil A., Lam C., Haslberger A., Lindley I. Interleukin-8 stimulates calcium transients and promotes epidermal cell proliferation. J. Invest. Dermatol. 1992;99:294–298. doi: 10.1111/1523-1747.ep12616634. [DOI] [PubMed] [Google Scholar]
- 52.Mihara K., et al. Human CXCR2 (hCXCR2) takes over functionalities of its murine homolog in hCXCR2 knockin mice. Eur. J. Immunol. 2005;35:2573–2582. doi: 10.1002/eji.200526021. [DOI] [PubMed] [Google Scholar]
- 53.Kaneider N.C., Agarwal A., Leger A.J., Kuliopulos A. Reversing systemic inflammatory response syndrome with chemokine receptor pepducins. Nat. Med. 2005;11:661–665. doi: 10.1038/nm1245. [DOI] [PubMed] [Google Scholar]
- 54.Jagels M.A., Hugli T.E. Neutrophil chemotactic factors promote leukocytosis. A common mechanism for cellular recruitment from bone marrow. J. Immunol. 1992;148:1119–1128. [PubMed] [Google Scholar]
- 55.Terashima T., English D., Hogg J.C., van Eeden S.F. Release of polymorphonuclear leukocytes from the bone marrow by interleukin-8. Blood. 1998;92:1062–1069. [PubMed] [Google Scholar]
- 56.Martin C., et al. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19:583–593. doi: 10.1016/s1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]
- 57.Suratt B.T., et al. Role of the CXCR4/SDF-1 chemokine axis in circulating neutrophil homeostasis. Blood. 2004;104:565–571. doi: 10.1182/blood-2003-10-3638. [DOI] [PubMed] [Google Scholar]
- 58.Lieschke G.J., et al. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84:1737–1746. [PubMed] [Google Scholar]
- 59.Semerad C.L., Liu F., Gregory A.D., Stumpf K., Link D.C. G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity. 2002;17:413–423. doi: 10.1016/s1074-7613(02)00424-7. [DOI] [PubMed] [Google Scholar]
- 60.Pastore S., et al. Granulocyte macrophage colony-stimulating factor is overproduced by keratinocytes in atopic dermatitis. Implications for sustained dendritic cell activation in the skin. J. Clin. Invest. 1997;99:3009–3017. doi: 10.1172/JCI119496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dlugosz, A.A., Glick, A.B., Tennenbaum, T., Weinberg, W.C., and Yuspa, S.H. 1995. Isolation and utilization of epidermal keratinocytyes for oncogene research. InMethods in enzymology . P.K. Vogt and I.M. Verma, editors. Academic Press. New York, New York, USA. 3–20. [DOI] [PubMed] [Google Scholar]
- 62.Bradley P.P., Priebat D.A., Christensen R.D., Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 63.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 64.Guttridge D.C., Albanese C., Reuther J.Y., Pestell R.G., Baldwin A.S. NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol. Cell. Biol. 1999;19:5785–5799.. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]