Abstract
Chaperone-mediated autophagy (CMA) is a selective mechanism for the degradation of soluble cytosolic proteins in lysosomes. The limiting step of this type of autophagy is the binding of substrates to the lysosome-associated membrane protein type 2A (LAMP-2A). In this work, we identify a dynamic subcompartmentalization of LAMP-2A in the lysosomal membrane, which underlies the molecular basis for the regulation of LAMP-2A function in CMA. A percentage of LAMP-2A localizes in discrete lysosomal membrane regions during resting conditions, but it exits these regions during CMA activation. Disruption of these regions by cholesterol-depleting agents or expression of a mutant LAMP-2A excluded from these regions enhances CMA activity, whereas loading of lysosomes with cholesterol significantly reduces CMA. Organization of LAMP-2A into multimeric complexes, required for translocation of substrates into lysosomes via CMA, only occurs outside the lipid-enriched membrane microdomains, whereas the LAMP-2A located within these regions is susceptible to proteolytic cleavage and degradation. Our results support that changes in the dynamic distribution of LAMP-2A into and out of discrete microdomains of the lysosomal membrane contribute to regulate CMA.
Keywords: autophagy, lysosomes, membrane microdomains, proteases, protein degradation
Introduction
A subset of cytosolic proteins, which contain in their amino-acid sequence a pentapeptide motif biochemically related to KFERQ, can be selectively degraded by lysosomes via chaperone-mediated autophagy (CMA) (reviewed in Massey et al, 2006a). The CMA-targeting motif is first recognized by a cytosolic chaperone (the heat shock cognate protein of 70 kDa or hsc70) and its co-chaperones. This complex is then targeted to the lysosomal membrane, where the substrate binds to the lysosome-associated membrane protein type 2A or LAMP-2A (Cuervo and Dice, 1996). After undergoing unfolding, the substrate is translocated into the lysosomal lumen assisted by a luminal resident chaperone (lys-hsc70) (Agarraberes et al, 1997), and is rapidly degraded. Although the exact composition of the substrate translocation complex is still unknown, multimeric forms of LAMP-2A (Cuervo and Dice, 2000b), cytosolic chaperones (hsc70 and hsp90) (Agarraberes and Dice, 2001; Kiffin et al, 2004) and the luminal chaperone (Agarraberes et al, 1997; Cuervo et al, 1997) have all been shown to interact with the translocating substrate protein. CMA is maximally activated under stress conditions such as prolonged nutritional starvation, mild oxidative stress and exposure to toxic compounds (Massey et al, 2006a).
Binding of the substrate/chaperone complex to LAMP-2A is the limiting step of this lysosomal pathway (Cuervo and Dice, 2000b). In fact, changes in the levels of LAMP-2A at the lysosomal membrane modulate CMA activity (Cuervo and Dice, 2000a). Two different mechanisms contribute to regulate LAMP-2A levels at the lysosomal membrane, its turn over and its dynamic distribution between membrane and matrix. LAMP-2A is normally turned over in this compartment by a three-step process, requiring the cleavage of its cytosolic tail by a still unidentified membrane metalloprotease, followed by cleavage at the junction between the transmembrane and luminal regions of LAMP-2A. Cathepsin A is responsible for this second cleavage (Cuervo et al, 2003), which releases a truncated form of LAMP-2A, that is rapidly degraded by luminal proteases. Degradation of LAMP-2A at the lysosomal membrane decreases during CMA activation, resulting in higher levels of receptor available for substrate binding/translocation (Cuervo and Dice, 2000a). In addition, a percentage of intact LAMP-2A is present in the lysosomal lumen associated to lipid micelles. Under conditions requiring maximal activation of CMA, the luminal LAMP-2A is recruited to the lysosomal membrane, also contributing to increase the number of substrate binding/translocation units (Cuervo and Dice, 2000a). We have recently found that, during mild oxidative stress, de novo synthesized LAMP-2A is also delivered to lysosomes to increase rates of CMA (Kiffin et al, 2004).
Despite this myriad of regulatory mechanisms to control levels of LAMP-2A at the lysosomal membrane, the molecular basis of these processes remains, for the most part, unclear. It is not known whether the proteases randomly degrade molecules of LAMP-2A to downregulate CMA, or if a specific population of LAMP-2A is more susceptible to degradation. Monomers and multimers of LAMP-2A coexist at the lysosomal membrane (Cuervo and Dice, 2000b), but the relation between these different forms of LAMP-2A is unknown. As a percentage of LAMP-2A is always present at the lysosomal membrane, even during ‘resting' CMA conditions, it is likely that changes in the organization of this protein at the membrane are required for CMA activation. In this work, we describe the dynamic association of LAMP-2A with cholesterol/glycosphingolipid-rich microdomains in the lysosomal membrane. We have found that this bimodal distribution of LAMP-2A at the lysosomal membrane changes with changes in CMA activity, and that disruption of these lysosomal microdomains or mutations in LAMP-2A that exclude it from these membrane regions enhance CMA. In addition, the multimeric organization and proteolytic susceptibility of LAMP-2A changes in relation to its association with these particular membrane regions. We conclude that these discrete regions of the lysosomal membrane play a key role in CMA regulation.
Results
LAMP-2A is concentrated in microdomains at the lysosomal membrane
LAMP-2A maintains a very dynamic relation with the lysosomal membrane. Both, the regulated cleavage of this protein at the lysosomal membrane and its distribution between the membrane and lumen, change with changes in cellular conditions (Cuervo and Dice, 2000a, 2000b). To better elucidate the mechanisms that regulate these processes, we analyzed whether LAMP-2A localizes in areas of the lysosomal membrane of particular lipid composition. Treatment of membranes with different types of detergents at low temperature followed by sucrose gradient centrifugation has allowed identification of such areas in plasma membrane, Golgi, endosomes and even in secondary lysosomes (reviewed in Chamberlain, 2004). We used a similar approach in the population of rat liver lysosomes related to CMA (Cuervo et al, 1997). After incubating these lysosomes with different types of nonionic detergents, we found that, although for most of them it was possible to detect a translucent region in the sucrose gradient (particularly enriched in lipids) at 10–15% sucrose, immunoblot analysis revealed the presence of LAMP-2A only in membrane fractions resistant to extraction with Triton X-114 (1%) (Figure 1A). Flotillin-1, a protein commonly used as a maker for detergent-resistant microdomains (DRM) in cellular membranes, was present in the DRM enriched in LAMP-2A (about 50% of total lysosomal flotillin-1), as well as in other DRM that lacked LAMP-2A, obtained after extraction with different detergents (Figure 1A).
Figure 1.
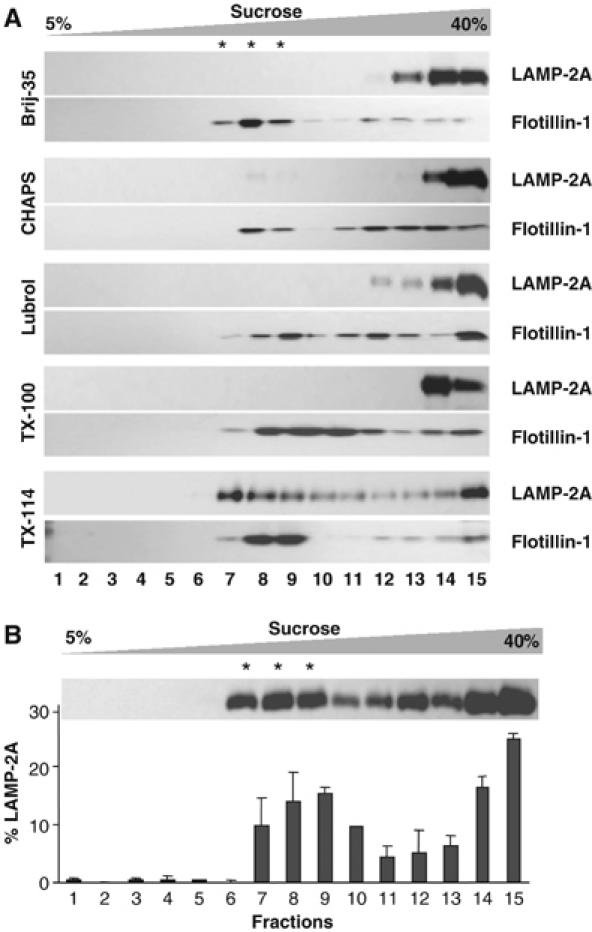
LAMP-2A localizes in detergent-resistant lipid microdomains of the lysosomal membrane. (A) Fed rat liver lysosomes were extracted with 1% of the indicated detergents and then subjected to sucrose gradient centrifugation. Aliquots collected from top to bottom were subjected to SDS–PAGE and immunoblotted for LAMP-2A and flotillin-1. Stars indicate the aliquots of the gradient of translucent appearance (highly enriched in lipids). (B) The distribution of LAMP-2A through the sucrose gradient after extraction with 1% Triton X-114 was calculated from the densitometric quantification of immunoblots as the one shown here. Values are expressed as percentage of the total lysosomal levels and are mean+s.e. of four experiments.
Densitometric quantification of LAMP-2A blots revealed that approximately 34.6±2.6% of the LAMP-2A at the lysosomal membrane localized in the TX-114-resistant regions, whereas the rest distributed between the solubilized fractions (bottom of the gradient; 35.3±5.6%) and an intermediate region between the detergent-resistant and the solubilized fractions (30.1±4.3%) (Figure 1B). Although the meaning of this intermediate region is not clear, the ability of this fraction to float in the sucrose gradient suggests association of LAMP-2A to lipids. These results support that LAMP-2A associates to particular regions of the lysosomal membrane with defined lipid composition.
To explore any possible functional relevance of the association of LAMP-2A to these particular membrane regions, we compared the distribution of LAMP-2A in lysosomal membranes isolated from livers of fed rats (as in Figure 1) or after prolonged starvation, which activates CMA in this organ (Cuervo et al, 1995). Starvation considerably reduced the amount of LAMP-2A present in the DR region (<10% of total LAMP-2A in the membrane), which instead distributed between the soluble and intermediate regions (Figure 2A). To simplify the analysis, we collected together the three fractions corresponding to the DR regions of the lysosomal membrane (DR), and compared it to the remaining fractions pooled together (solubilized). As shown in Figure 2B, the distribution of LAMP-2A between these two fractions and the changes during starvation were comparable to the ones observed when all the individual fractions were collected (Figure 2A). For the rest of this study, we adopted this type of loading for the DR fractions, and divided the remaining fractions in a soluble fraction (Sol: fraction 15) and two intermediate fractions (Int 1: fractions 11–12; Int 2: fractions 13–14).
Figure 2.
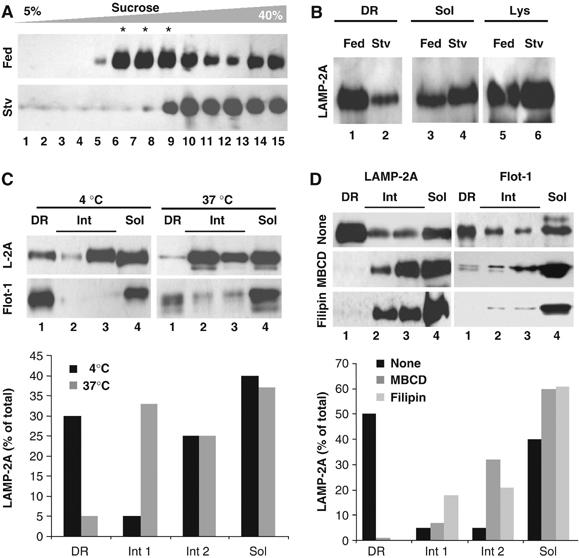
Properties of the LAMP-2A-containing lipid microdomains at the lysosomal membrane. (A) Lysosomes from livers of fed (Fed) or 24 h starved rats (Stv) were extracted with 1% Triton X-114 and processed as in Figure 1 or the aliquots in the translucent region were pooled together as the detergent-resistant (DR) fraction, and anything below this fraction was pooled, as the solubilized fraction (Sol) (B). Lanes 5–6 are one-tenth of the amount of lysosomes loaded in the gradient. (C) Distribution of LAMP-2A (L-2A) and flotillin-1 (Flot-1) in fed rats liver lysosomes extracted with 1% Triton X-114 at 4 or 37°C. DR fractions were pooled as in (B), and the aliquots below this region were divided in three fractions (a soluble (Sol; aliquot 15) and two intermediate fractions (light: aliquots 11–12; heavy: aliquots 13–14). (D) Fed rat liver lysosomes were incubated with MBCD or filipin, as described under Material and methods, and then processed as in (C). The mean value of the densitometric quantification of the LAMP-2A immunoblots from two experiments is shown at the bottom in (C) and (D).
As described for DRM in other membranes, incubation of the lysosomal membranes with the detergent at higher temperatures (37°C) disrupted these microdomains and reduced the content of both LAMP-2A and flotillin-1 in the lower density region of the sucrose gradient (Figure 2C). The LAMP-2A-enriched DRM of the lysosomal membrane were particularly rich in cholesterol, as LAMP-2A relocated from the DRM to the soluble and intermediate regions when lysosomes were incubated with the cholesterol-depleting agents methyl-betacyclodextrin (MBCD) or filipin (Figure 2D), suggesting that the association of LAMP-2A with lysosomal DRM is cholesterol dependent.
The location of LAMP-2A in DRM is not a result of partial solubilization of homogenous areas of the lysosomal membrane, because the two-dimensional electrophoretic protein pattern of the DRM was quite distinct from the one of the total lysosomal membranes (Figure 3A). In addition, immunoblot analysis for other lysosomal membrane proteins revealed their different distribution between DR and soluble fractions (Figure 3B). Of particular interest is the distribution of LAMP-2B, a splicing variant of the LAMP-2 gene, which shows some association to lipids (present in the intermediate region), but it could not be detected in the DRM. Cathepsin B, which associates in part with the lysosomal membrane, was also undetectable in the DRM (Figure 3B). Moreover, hsc70 and hsp90, the two major chaperones known to be part of the lysosomal CMA-binding/translocation complex (Agarraberes and Dice, 2001; Kiffin et al, 2004), were also absent in this region (Figure 3B).
Figure 3.
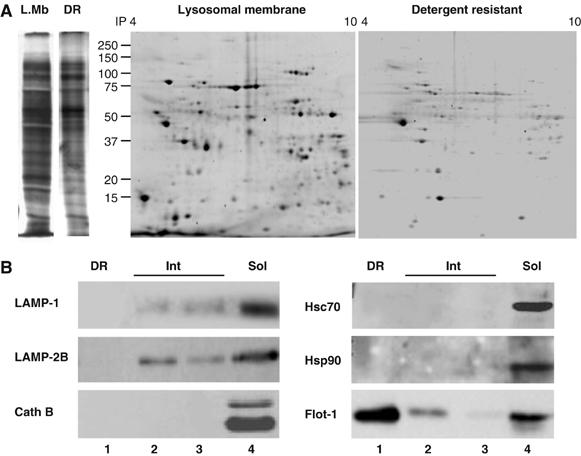
Protein composition of the LAMP-2A containing detergent-resistant lipid microdomains at the lysosomal membrane. (A) Lysosomal membranes from fed rat livers and their corresponding 1% Triton X-114-resistant regions, isolated as described under Material and methods, were subjected to one (left)- or two-dimensional (right) electrophoresis and stained with Sypro Ruby. (B) Immunoblot for the indicated proteins of the detergent resistant (DR), intermediate (Int) and solubilized (Sol) fractions of lysosomal membranes obtained as in Figure 2.
The preferential location of LAMP-2A in cholesterol-enriched regions of the lysosomal membrane under normal nutritional conditions, defined so far by biochemical criteria (resistance to detergent solubilization), was also observed by fluorescence microscopy in whole cells. As shown in Figure 4, LAMP-2A colocalized in cultured mouse fibroblasts (NIH3T3 cells) with intracellular cholesterol (detected by filipin staining; Figure 4A) and with GM-1 ganglioside (detected with fluorescent-labeled cholera toxin B subunit; Figure 4B), both particularly enriched in DRM (Figure 4 insets show at high magnification LAMP-2A positive vesicles with ‘patched' membrane staining). In agreement with our biochemical data, colocalization of LAMP-2A with both lipids decreased considerably after serum removal (which activates CMA), or treatment with MBCD (which disrupts lipid-enriched microdomains) (Figure 4A and B). The reasons for the reduced size of the LAMP-2A-labeled structures observed often after MBCD treatment remains unknown. The changes in colocalization of LAMP-2A and lipids took place, at least in part, in CMA-related lysosomes, because using immunofluorescence with isolated lysosomes we also found that LAMP-2A colocalized in larger extent with cholesterol and GM1 in CMA-related lysosomes isolated from fed rats than in the ones from starved rats, and this colocalization decreased when lysosomes were treated with MBCD (Figure 1, Supplementary data).
Figure 4.
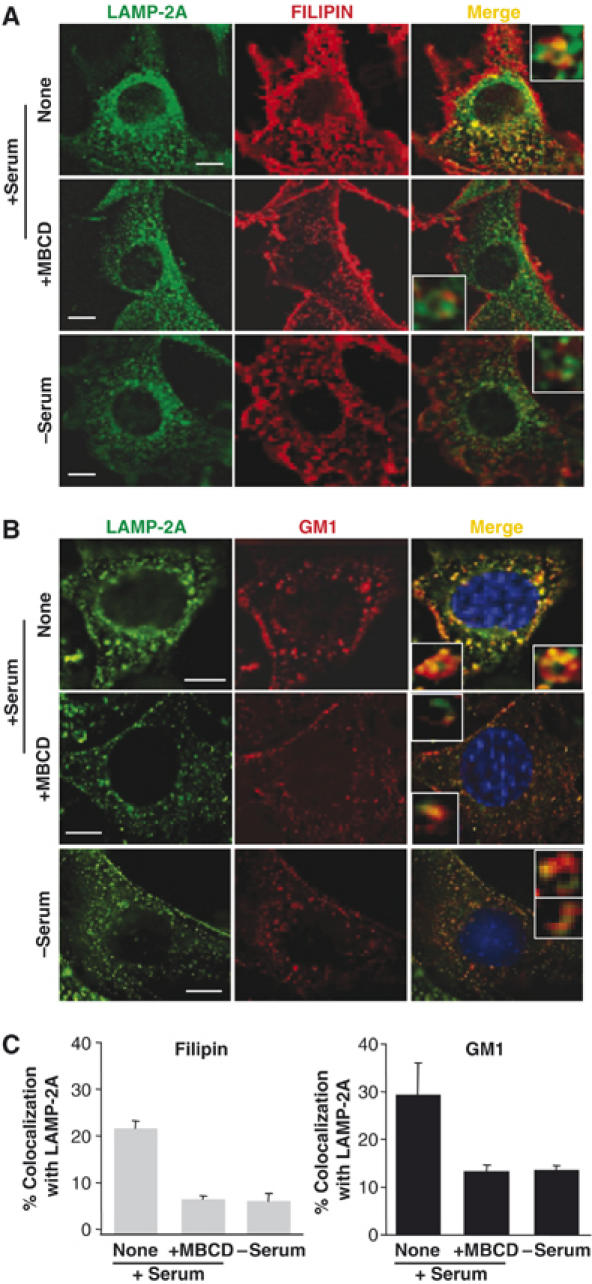
LAMP-2A localizes in cholesterol- and GM-1-enriched intracellular membrane domains. Mouse fibroblasts grown on coverslips in the presence (+) or absence (−) of serum were fixed and labeled with filipin (red) (A) or incubated with Texas-Red-cholera toxin B subunit to label GM-1-enriched membrane regions (red) and then fixed. (B) Both groups of cells were then subjected to indirect immunofluorescence for LAMP-2A (green). Where indicated, cells were treated with MBCD (25 mM) for 30 min before fixation. The merged images of both fluorophores are shown on the right. Insets show vesicles at higher magnification. Bar: 10 μm. The quantification of the colocalization of the two fluorophores is shown in (C). Values are expressed as the mean+s.e. of percentage of colocalization in 20–40 cells.
The ultrastructural analysis of the DRM of the lysosomal membrane revealed that, as described for DRM from other cellular membranes (Martin-Belmonte et al, 1999; Braccia et al, 2003), this fraction is mainly composed of a homogeneous population of spherical vesicle-like membranous structures in which the typical membrane bilayer was easily identifiable (Figure 5A). Electron microscopy analysis of unfixed samples (Figure 5B) revealed that membranes organize as ‘rods', which maintain spherical curvature (likely because of the asymmetric insertion of most lysosomal membrane proteins). These elongated structures probably result from the association of isolated microdomains, which often coalesce upon extraction with different detergents (Mayor and Maxfield, 1995). Immunogold staining for LAMP-2A of isolated rat liver lysosomes and their corresponding DRM supported the preferential localization of part of LAMP-2A in discrete DRM of the lysosomal membrane (LAMP-2A labeling per micron was four times higher in the profiles of the DR regions than in the profiles of complete lysosomal membranes (Figure 5C)). This ‘clustering' of LAMP-2A could even be detected in complete lysosomes, but it was not detected for LAMP-2B (Figure 5C, right panels), or if membranes were depleted of cholesterol by treatment with MBCD (data not shown). Double immunogold labeling confirmed that part of lysosomal LAMP-2A (45.5±6.5%) colocalizes with flotillin-1 (Figure 5D) in discrete membrane regions, which contain multiple labels for each protein (see table in Figure 2, Supplementary data).
Figure 5.
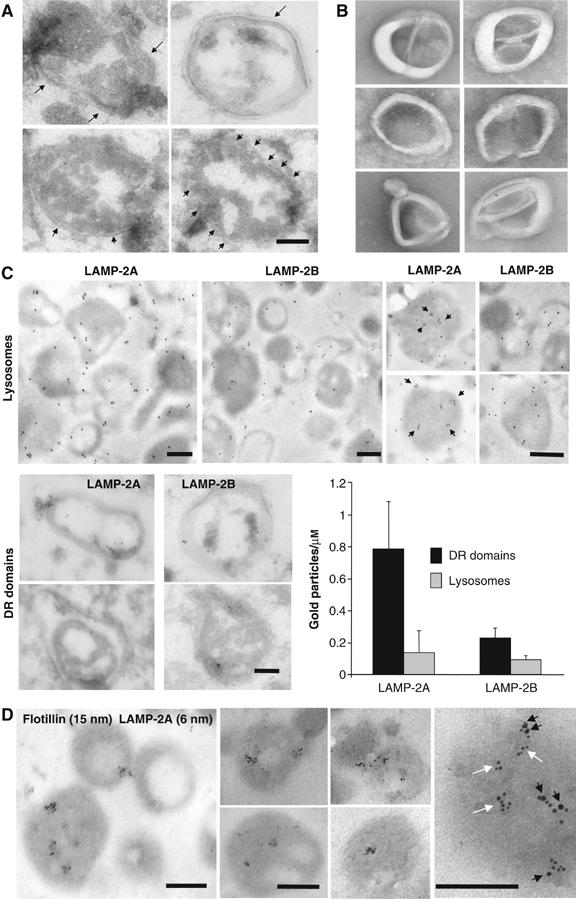
Distribution of LAMP-2A in discrete lysosomal membrane regions. (A, B) Regions of the lysosomal membrane resistant to 1% Triton X-114 recovered from the sucrose gradients were fixed and processed for electron microscopy (A) or visualized without fixation by electron microscopy (B). Arrows point to the membrane bilayer. (C) Intact rat liver lysosomes (top) and DR regions of the lysosomal membranes (bottom) were fixed and subjected to immunogold labeling for LAMP-2A and LAMP-2B, and a gold-conjugated secondary antibody as described under Material and methods. Top right panels show ‘clusters' of gold particles (black arrows) in individual lysosomes at higher magnification. Graph shows the labeling density (gold particles per μm) in the indicated fractions for both proteins. Values are mean+s.e. from the quantification of 15 different micrographs. (D) Intact rat liver lysosomes were fixed and subjected to double immunogold labeling for LAMP-2A and flotillin-1. Representative lysosomes are shown. Far right panel: zoomed-in image of the distribution of LAMP-2A in regions containing (black arrows) and not containing (white arrows) flotillin-1. Bars: 0.2 μm.
Our results thus support the preferential localization of LAMP-2A in regions of the lysosomal membrane of defined lipid (enriched in cholesterol and GM1) and protein composition, and that changes in the abundance of LAMP-2A in these regions correlate with changes in the cellular nutritional status.
LAMP-2A exits the membrane microdomains when CMA is activated
The lower levels of LAMP-2A in the DRM of the lysosomal membrane during starvation did not correlate with changes in the total content of cholesterol in lysosomes (Figure 3, Supplementary data), but they could be owing to changes in the lysosomal system independent of changes in CMA. To analyze the consequences of CMA activation in the distribution of LAMP-2A in the lysosomal membrane, we incubated lysosomes from fed rats with different components known to activate CMA in isolated lysosomes (Figure 4, Supplementary data) (Cuervo et al, 1997, 1995). Incubation of lysosomes with two well-characterized CMA substrates, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Figure 6A) or ribonuclease A (data not shown), resulted in partial exclusion of LAMP-2A from the DRM toward the intermediate and soluble fractions (Figure 6A). This effect was considerably more pronounced when lysosomes were incubated with cytosol from starved animals, which contains potential CMA substrates and also, the cytosolic chaperone/cochaperone complexes, ATP and possibly other still unidentified components required for CMA activation (Figure 6A). In fact, as shown in Figure 6A, incubation with purified hsc70 or ATP also decreased the amount of LAMP-2A in DRM, although to a lesser extent, and combination of CMA substrates with these factors showed the closest effect to the one observed with the cytosol, supporting the additive effect of all of these components when added as part of the cytosolic fraction. The contribution of cytosolic ATP to CMA activation and/or LAMP-2A mobilization was confirmed when apyrase was added to the cytosol to deplete it of ATP. After this treatment, the effect observed with untreated cytosol was lost (Figure 6A, bottom right panel; note that apyrase alone does not change the distribution of LAMP-2A in the DRM). None of these treatments considerably changed the amount of flotillin-1 in the DRM (Figure 6A, left), suggesting that they did not result in disruption of these domains but rather, in the selective mobilization of LAMP-2A out of these discrete membrane regions.
Figure 6.
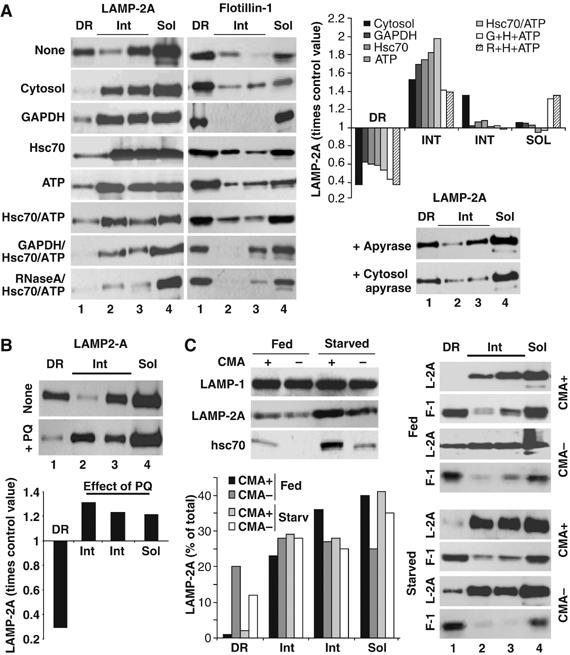
The distribution of LAMP-2A in discrete lysosomal membrane regions changes with changes in CMA. (A) Fed rat liver lysosomes were incubated in an isotonic buffer without additions, or in the presence of rat liver cytosol (25 μg), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (25 μg), GST-hsc70 (10 μg/ml), ATP (5 mM) or the indicated combinations for 15 min at 37°C. Lysosomes incubated directly with apyrase or with cytosol treated with apyrase are shown in the right bottom panel. At the end of the incubation, lysosomes were subjected to Triton X-114 extraction, sucrose density gradient centrifugation and immunoblot as in Figure 2C. Graph shows changes in LAMP-2A distribution expressed as times the value in untreated lysosomes and are the mean of the densitometric quantification of 3–6 experiments as the one shown here. Because all the incubations were performed in the presence of protease inhibitors, total levels of LAMP-2A in lysosomes remained constant throughout the incubation. (B) Lysosomes from livers of fed rats, untreated or treated with paraquat (+PQ), were processed as in (A). Graph shows changes in LAMP-2A distribution induced by the PQ treatment, expressed as times the value in untreated animals and are the mean of three experiments. (C) Lysosomes with high (CMA+) and low (CMA−) ability for CMA isolated from livers of fed or 48 h starved rats were processed as in (A). Top left: Levels of two CMA components (LAMP-2A and hsc70) and of an unrelated lysosomal membrane protein (LAMP-1) in each of the four groups of lysosomes. Bottom left: Percentage of total lysosomal LAMP-2A present in each fraction, determined by densitometric quantification of the immunoblots.
Mild-oxidative stress also activates CMA even under normal nutritional conditions (Kiffin et al, 2004). Consistent with the mobilization of LAMP-2A out of the DRM when CMA is activated in vitro, a larger percentage of LAMP-2A localized outside the DRM in lysosomes isolated from fed rats exposed to sublethal doses of paraquat, a pro-oxidant shown to induce activation of CMA (Kiffin et al, 2004) (Figure 6B). Likewise, when we subdivided the pool of CMA-related lysosomes used throughout this study into two subgroups, based on their CMA activity (Cuervo et al, 1997), we found that LAMP-2A was practically undetectable in DRM of the lysosomes with higher CMA activity (those enriched in the luminal hsc70 and LAMP-2A (as shown in Figure 6C, left)), whereas larger amounts of LAMP-2A could be detected in the DRM of lysosomes latent for CMA (Figure 6C, right). When maximal activation of CMA is needed, such as during prolonged starvation, this second group of lysosomes acquires the lumenal chaperone and become active (Figure 6C, left; Cuervo et al, 1997). Consistently, the amount of LAMP-2A in DRM decreased considerably in this reservoir pool of lysosomes after prolonged starvation (Figure 6C, right). In summary, activation of CMA both in vitro and in vivo associates with reduced levels of LAMP-2A in the DRM of the lysosomal membrane.
Changes in the lipid composition of the lysosomal membrane microdomains alter CMA
To determine how changes in the distribution of LAMP-2A between DR and soluble regions of the lysosomal membrane affect CMA activity, we disrupted these discrete cholesterol-enriched regions in cultured mouse fibroblasts. CMA is activated in these cells in response to removal of serum, increasing the degradation of long-lived cytosolic proteins, typically substrates for this pathway (Cuervo and Dice, 2000a; Cuervo et al, 2003; Massey et al, 2006b). Treatment with the cholesterol-depleting agent, MBCD, increased the proteolysis of long-lived proteins (Figure 7A). This increased degradation was mainly lysosomal, because it was significantly reduced in the presence of ammonium chloride and leupeptin, inhibitors of the lysosomal-dependent proteolysis (Figure 7A). Treatment with 3-methyladenine (3MA), which inhibits macroautophagy but not CMA (Massey et al, 2006b), partially reduced the observed MBCD-mediated increase in protein degradation (Figure 7B), suggesting that part of the increase in protein degradation (38%) was owing to activation of macroautophagy, whereas the other part (62%) was taking place via CMA. Thus, disruption of lipid microdomains resulted in activation of both CMA and macroautophagy. The effect on macroautophagy was, however, independent of changes in LAMP-2A redistribution, as MBCD treatment also increased protein degradation rates in cells stably RNA interfered for LAMP-2A, where the vast majority of the MBCD-induced increase took place via macroautophagy (89% sensitive to 3MA) (Figure 7B) (Massey et al, 2006b).
Figure 7.
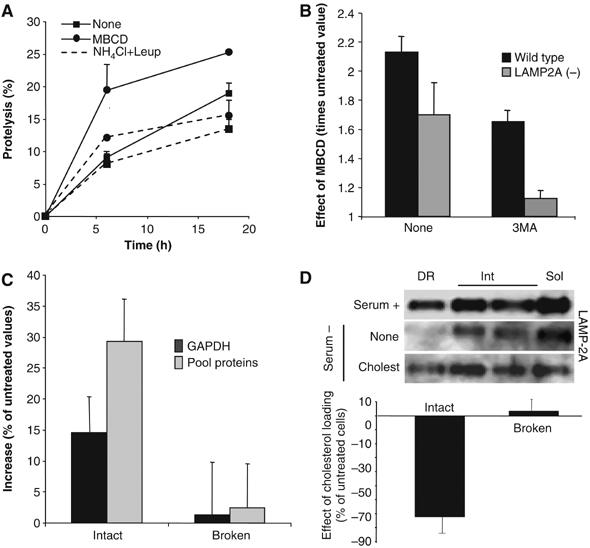
Consequences of changes in the lysosomal cholesterol content on CMA activity. (A) Degradation of long-lived proteins in mouse fibroblasts (NIH3T3 cells) treated or not with 25 mM MBCD. Where indicated, 15 mM NH4Cl/100 μM leupeptin (NH4Cl+Leup) were added into the medium. Values are the mean+s.e. of three experiments with triplicate samples. (B) Effect of MBCD on total rates of protein degradation, measured as in (A), in wild-type mouse fibroblasts and in fibroblasts stably RNA interfered for LAMP-2A (LAMP-2A(−)). The ratio of degradation in MBCD-treated and untreated cells is shown for cells maintained with or without 10 mM 3-methyladenine (3MA). (C) Proteolysis of [14C]GAPDH or a pool of [3H]labeled cytosolic proteins (pool proteins) by isolated rat liver lysosomes, treated or not with MBCD. Broken: lysosomes disrupted by a hypotonic shock before the incubation with the radiolabeled proteins. Values are expressed as percentage of the degradation by untreated lysosomes and are the mean+s.e. of three experiments with triplicate samples. (D) Lysosomal–mitochondrial fractions were isolated from mouse fibroblasts untreated or loaded with cholesterol (50 μM). Top: Distribution of LAMP-2A after Triton X-114 extraction and sucrose density gradient centrifugation. Bottom: Ability of intact or disrupted lysosomal–mitochondrial fractions to proteolize [14C]GAPDH. Values are expressed as percentage of the degradation in fractions from untreated cells and are the mean±s.e. of triplicate samples.
We further confirmed the effect of modifying the membrane distribution of LAMP-2A on CMA activity using isolated lysosomes. Lysosomes previously treated with MBCD, and consequently with higher percent of LAMP-2A in the soluble fraction of the membrane, showed higher rates of degradation of GAPDH and of a pool of radiolabeled cytosolic proteins than untreated lysosomes (Figure 7C). The increased degradation was not owing to leakage of lysosomal enzymes (we did not find changes in β-hexosaminidase latency (data not shown)) or to an increase in the activity of the lysosomal proteases (the MBCD-mediated increase in proteolysis was no longer evident when the lysosomal membrane was disrupted) (broken lysosomes; Figure 7C), suggesting that the enhanced degradation was, indeed, the result of higher rates of substrate binding and uptake inside lysosomes.
To determine whether sequestration of LAMP-2A in the DRM decreased CMA rates, we loaded cells with cholesterol by administering it directly to the culture medium. This treatment increases the concentration of cholesterol in lysosomes (Lusa et al, 2001). Because changes in the cholesterol content in lysosomes could modify their flotation in the discontinuous density gradients used for the isolation of the CMA-related lysosomes, we utilized instead differential centrifugation, which renders a more heterogeneous fraction of lysosomes with some light mitochondria. As in the fraction enriched in CMA-related lysosomes, we verified that (1) a percentage of LAMP-2A was located in the DRM in these lysosomes, (2) the amount of LAMP-2A associated to DRM was lower when CMA was activated by removal of serum and (3) treatment with cholesterol increased (4.5-folds) the amount of LAMP-2A in DRM (Figure 7D, top). CMA activity in this fraction is lower than in the highly purified lysosomes used in the rest of this study, but it can still be measured. As shown in Figure 7D (bottom), the ability to degrade GAPDH was significantly reduced (almost 70% less than control) in the lysosomes from cholesterol-loaded cells. This treatment did not affect the activity of the lysosomal proteases, as rates of GAPDH proteolysis were similar in lysosomes from treated and untreated cells, once the lysosomal membrane was disrupted (Figure 7D, bottom).
These results support that changes in the lipid composition of the lysosomal membrane alter LAMP-2A distribution in DRM and have an effect on CMA activity.
Exclusion of LAMP-2A from the membrane microdomains enhances lysosomal CMA activity
To directly analyze the effect on CMA of changes in the association of LAMP-2A with DRM, we carried out directed mutagenesis in LAMP-2A, in search of a mutation in the cytosolic or transmembrane region of LAMP-2A that altered its ability to associate to the DRM. We first verified that human LAMP-2A transfected into mouse fibroblasts behaved similarly to the endogenous protein: (1) 30% of the protein associated to lysosome membrane DRM and (2) serum removal reduced (0.6 times) the amount of LAMP-2A in DRMs (Figure 8A). As depicted in Figure 8B, we replaced with alanines (1) a proline residue (proline 378) close to the transmembrane/lumenal boundary (P/A), aiming to induce a pronounced conformational change; (2) two glycine residues in the transmembrane region (GG/AA), which resemble a previously described dimerization motif (GXXXG); (3) a tyrosine residue near the transmembrane/cytosolic tail boundary (Y/A), for possible phosphorylation events and (4) four positively charged residues in the cytosolic tail (4A), required for substrate binding to LAMP-2A (Cuervo and Dice, 2000b). The former three mutations did not alter the ability of LAMP-2A to bind CMA substrates (Figure 5, Supplementary data), or the targeting of LAMP-2A to lysosomes. Replacement of proline 378 by alanine markedly decreased association of LAMP-2A with DRM (Figure 8C and D). Furthermore, removal of serum from the culture medium to activate CMA, which reduced DRM levels of endogenous LAMP-2A and overexpressed wild-type, GG/AA and Y/A hLAMP-2A mutants, did not affect the membrane distribution of P/A hLAMP-2A (Figure 8E). Consistently with the lower sequestration of P/A hLAMP-2A into DRM, lysosomes from cells overexpressing this mutant showed higher CMA than lysosomes from cells overexpressing equivalent amounts of wild-type hLAMP-2A (Figure 8F shows higher degradation rates of GAPDH by intact but not by broken lysosomes), and lower increase in CMA activity after treatment of lysosomes with MBCD (Figure 8G) (the observed stimulatory effect is, for the most part, a consequence of the mobilization of the endogenous mLAMP-2A outside of the DRM). Although the 4A hLAMP-2A mutant unable to bind CMA substrates was detected in lysosomal DRM, levels of this protein in these regions remained unchanged upon serum-induced activation of CMA (Figure 8C–E), suggesting that binding of substrate proteins to the cytosolic tail of LAMP-2A could contribute to its mobilization from the DRM. As previously reported, the high levels of endogenous mLAMP-2A in lysosomes prevented any significant dominant-negative effect on CMA activity for this mutant.
Figure 8.
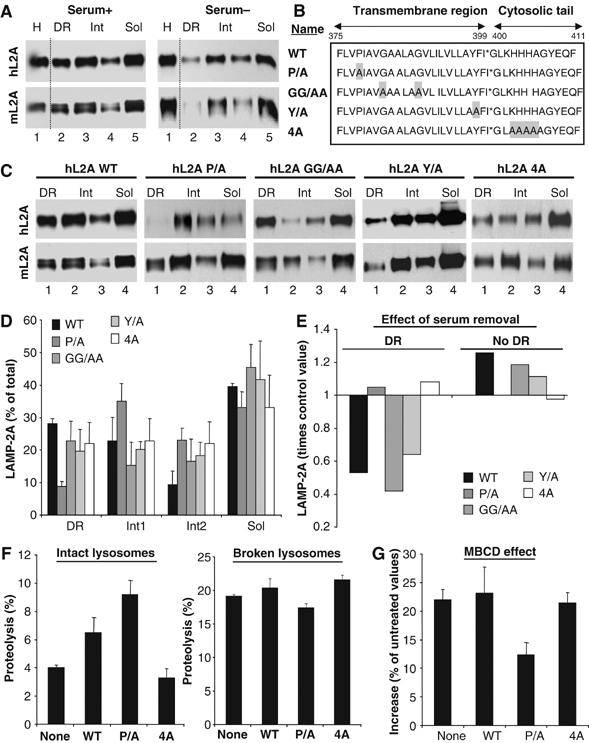
Lysosomes containing a mutant LAMP-2A unable to move into discrete lysosomal membrane regions show enhanced CMA activity. (A) Lysosomes from mouse fibroblasts transfected with wild-type hLAMP-2A and maintained in the presence (Serum+) or absence (Serum−) of serum were collected by centrifugation and subjected to Triton X-114 extraction, sucrose density gradient centrifugation and immunobloting for human (hL2A) or mouse (mL2A) LAMP-2A. Lane 1: homogenate. (B) Amino-acid sequence of the transmembrane and cytosolic region of hLAMP-2A and the mutations performed. Replaced amino acids are shadowed. (C) Lysosomes from mouse fibroblasts transfected with the indicated forms of hLAMP-2A and maintained in the presence of serum were processed as in (A). (D) The percentage of total lysosomal hLAMP-2A present in each fraction was determined by densitometric quantification of immunoblots as the ones shown here. Values, corrected for the amount of mLAMP-2A in each fraction, are mean+s.e. of 3–4 experiments. (E) Changes in hLAMP-2A distribution induced by serum removal. Values are expressed as times the values in cells maintained in the presence of serum and are the mean of two experiments. Changes in the three nonDR regions are shown together (no DR). (F) Proteolysis of a pool of 3[H]labeled cytosolic proteins by intact (left) or disrupted (right) lysosomes isolated from untransfected mouse fibroblasts (none) or fibroblasts transfected with wild-type (WT) or the indicated mutant forms of hLAMP-2A. Values are the mean+s.e. of two experiments with triplicate samples. (G) Part of the lysosomes from (F) was treated with MBCD before the incubation with the radiolabeled proteins. Values are expressed as percentage of degradation by untreated lysosomes and are the mean+s.e. of two experiments with triplicate samples.
Different organization of LAMP-2A in different regions of the lysosomal membrane
LAMP-2A organizes at the lysosomal membrane in high molecular weight protein complexes (Cuervo and Dice, 2000a), required for translocation of substrates into lysosomes via CMA (Meiseres N, Cuervo AM and Dice JF, unpublished results). We compared the multimeric state of LAMP-2A in the different regions of the lysosomal membrane by analyzing the ability of LAMP-2A to migrate through a continuous sucrose density gradient (10–80%), as reported before (Cuervo and Dice, 2000a). We detected a shift of part of LAMP-2A toward denser regions of the gradient in the membrane regions sensitive to detergent solubilization, whereas all the LAMP-2A in the DRM migrated in the gradient as a monomer (Figure 9A). This result suggests that sequestration of LAMP-2A in particular regions of the membrane decreases CMA activity, at least in part, by preventing LAMP-2A multimerization.
Figure 9.
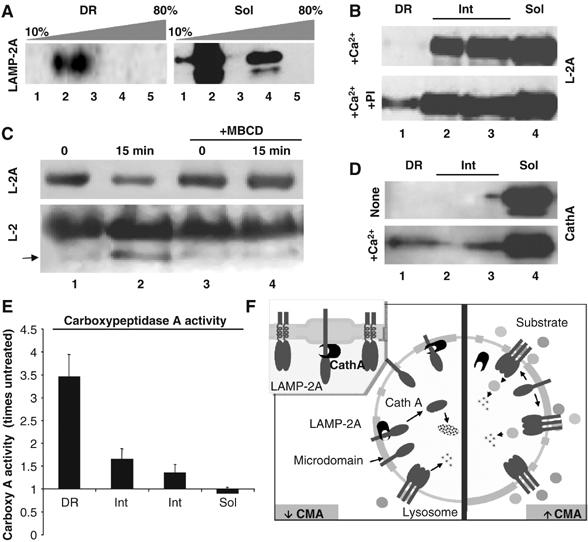
Organization of LAMP-2A in different regions of the lysosomal membrane. (A) The Triton X-114-resistant and soluble regions of the lysosomal membrane were subjected to centrifugation through a continuous (10–80%) sucrose density gradient, SDS–PAGE and immunoblot for LAMP-2A. The molecular weight range of each fraction is as follows: fraction 1 (<100 kDa); fraction 2 (100–200 kDa); fraction 3 (200–400 kDa); fraction 4 (400–600 kDa); fraction 5 (>600 kDa). (B) Fed rat liver lysosomes were incubated with 1 mM CaCl2 (+Ca2+) in an isotonic buffer in the absence or presence of a cocktail of protease inhibitors (PI). Samples were subjected to Triton X-114 extraction, sucrose density gradients centrifugation and LAMP-2A immunoblot. (C) Fed rat liver lysosomes treated or not with 25 mM MBCD were incubated in an isotonic buffer for 30 min and then subjected to SDS–PAGE and immunobloted with antibodies against the cytosolic (Top; L-2A) and luminal (Bottom; L-2) regions of LAMP-2A. Arrow: LAMP-2A proteolytic fragment (Cuervo and Dice, 2000a). (D, E) Rat liver lysosomes incubated or not with 1 mM CaCl2 (+Ca2+) were subjected to Triton X-114 extraction and sucrose density gradients centrifugation. Levels of cathepsin A (D) and carboxypeptidase A activity (E) were measured in each fraction by immunoblot or using a standardized colorimetric assay, respectively. Values in (E) are expressed as times the activity in untreated membranes and are mean+s.e. of two experiments. (F) Hypothetical model for the distribution of LAMP-2A in microdomains at the lysosomal membrane and its changes with CMA activity (see text for details).
LAMP-2A levels are regulated at the lysosomal membrane in part through a tightly controlled sequential cleavage by cathepsin A and a still unidentified membrane metalloprotease. Maximal degradation of LAMP-2A at the lysosomal membrane occurs when CMA is inactive (i.e. under normal nutritional conditions) (Cuervo and Dice, 2000a). Only monomers of LAMP-2A are susceptible to cleavage by the membrane proteases (Cuervo et al, 2003), and this degradation occurs in a Ca2+-dependent manner (required for the binding of cathepsin A to the lysosomal membrane) (Cuervo et al, 2003). The fact that LAMP-2A localizes preferentially in DRM during CMA inactivity, when LAMP-2A is typically degraded, and only in the form of monomers, the only ones susceptible to cleavage and degradation, led us to hypothesize that LAMP-2A degradation could take place preferentially in these particular regions of the lysosomal membrane. Indeed, when we incubated lysosomes with Ca2+ to promote the degradation of LAMP-2A by cathepsin A, we found a reduction in the amount of LAMP-2A in the DRM, which was prevented if a cocktail of protease inhibitors was added (Figure 9B). Under normal conditions, only full-size LAMP-2A is detected in the DRM, because truncated molecules of LAMP-2A, generated through cleavage by cathepsin A in the boundary between the transmembrane and lumenal regions, will fall off the membrane into the lysosomal lumen. Preincubation of intact lysosomes with MBCD, to promote exit of LAMP-2A from the DRM, resulted in almost complete blockage of the cleavage of LAMP-2A (measured as the decrease in the levels of the cytosolic tail of LAMP-2A (Figure 9C, top) and the presence of a truncated form of LAMP-2A of lower molecular weight, recognized only by an antibody against the luminal region of LAMP-2 (Figure 9C, bottom)) (note that total levels of LAMP-2A do not decrease when using this antibody as it also recognizes the LAMP-2B and LAMP-2C isoforms). Furthermore, addition of Ca2+ increased the levels (Figure 9D) and activity (Figure 9E) of cathepsin A in the DRM of the lysosomal membrane. These results suggest that degradation of LAMP-2A at the lysosomal membrane takes place preferentially in the DRM, where the protease responsible for LAMP-2A cleavage also localizes, and that the amount of LAMP-2A found normally associated to DRM corresponds to a steady-state picture of LAMP-2A molecules that have reached this compartment but have not been degraded yet. In support of this model is the fact that, the higher levels of LAMP-2A are found in the DRM of CMA lysosomes isolated from fed animals, the group of lysosomes with the lowest membrane levels of LAMP-2A and lowest CMA activity, and with the highest rates of LAMP-2A degradation (Cuervo and Dice, 2000b).
Our results support that a dynamic subcompartmentalization of LAMP-2A in different microdomains at the lysosomal membrane contributes to modulate CMA activity. Sequestration of LAMP-2A in cholesterol-enriched regions favors the cleavage of LAMP-2A by the membrane-associated proteases and its consequent degradation (Figure 9F, left), whereas exclusion of LAMP-2A from these regions allows the multimerization of LAMP-2A, required for the uptake of CMA substrate by lysosomes (Figure 9F, right).
Discussion
We have identified the preferential association, under particular conditions, of LAMP-2A with discrete microdomains of the lysosomal membrane of different properties than the surrounding membrane. The combination of both, a biochemical approach (Figures 1 and 2) and imaging methods (Figures 4 and 5), has allowed us to define the LAMP-2A-containing microdomains as regions of the lysosomal membrane of particular lipid (enriched in cholesterol and glycosphingolipids) (Figures 4 and 5) and protein (Figure 3) composition. This bimodal distribution of LAMP-2A at the lysosomal membrane changes with changes in CMA activity (Figure 6), and likewise, perturbation of the lipid organization of the lysosomal membrane (Figure 7) or of the ability of LAMP-2A to associate to discrete membrane microdomains (Figure 8) affects CMA. By providing an ordered membrane microenvironment, lysosomal membrane microdomains may facilitate the interaction of LAMP-2A with the protease(s) responsible for its cleavage, resulting in decreased levels of LAMP-2A at the lysosomal membrane and downregulation of CMA (Figure 9B–E). On the other hand, the exclusion of multimeric forms of LAMP-2A from the membrane microdomains (Figure 9A) strongly supports that exit of LAMP-2A from these regions is required for CMA activation.
Membrane microdomains have been identified in the plasma membrane and also in intracellular compartments such as Golgi, late endosomes and phagosomes (reviewed in Lusa et al, 2001; Schroeder et al, 2001), where they play diverse roles in cell signaling, protein sorting and antigen presentation, among others. In this report, we identify a novel role for the microdomains at the lysosomal membrane in the regulation of a selective form of autophagy.
The presence of DRM in lysosomes has been previously reported, although a more general pool of lysosomes, rather than this selective group of CMA-related lysosomes, was used (Taute et al, 2002). As in other membranes (Pike, 2004), DRM with very different properties are likely to exist in lysosomes. In fact, as shown in Figure 1, we were able to isolate lysosomal membrane regions resistant to different detergents, supporting slightly different physicochemical characteristics, but LAMP-2A localized only in those resistant to Triton X-114. Flotillin-1 acts as a resident protein as it remains in the lysosomal DRM even under conditions in which LAMP-2A is mobilized out of these regions.
Growing evidence supports the dynamic nature of membrane microdomains (reviewed in Helms and Zurzolo, 2004), implicating the recruitment of small microdomains (approximately 50 nm) into more stable pre-existing membrane domains. It is possible that the molecules of LAMP-2A located in the intermediate region, between the Triton X-114-resistant and the solubilized regions, are associated with some type of small microdomains. In fact, we have previously described the presence of LAMP-2A complexes of different sizes at the lysosomal membrane (Cuervo and Dice, 2000b). Differences in activity and composition among the LAMP-2A complexes at the lysosomal membrane could explain the apparent discrepancy in the effect of DRM stabilizers and destabilizers on CMA. As shown in Figure 7, disruption of the DRMs released almost all LAMP-2A in these regions (about 30% of total) but only had a modest effect (15–20%) on CMA activity, whereas sequestration of only 20% of the LAMP-2A in DRM after cholesterol treatment resulted in almost 70% inhibition of CMA (Figure 7D). Because mutimerization of LAMP-2A (in 6–8 molecules/complex) is required for substrate uptake, larger amounts of LAMP-2A need to be released to attain activation of this pathway, whereas dissociation of 1–2 molecules from the multimeric LAMP-2A complex is likely enough to inactivate uptake (Bandyopadhyay U and Cuervo AM, unpublished observations). Further studies are needed to elucidate the dynamics of assembly and disassembly of the LAMP-2A complexes and their relationship with different lysosomal lipid-microdomains. In this respect, although the inability of the LAMP-2A P/A mutant to associate to DRM likely results from the pronounced conformational change introduced by this mutation (replacement by alanine, a strong α-helix promoter, may result in the elimination of the kink introduced by proline, and thus extension of the transmembrane helix), this mutant form could be a valuable tool in the future study of the mechanisms that regulate the dynamic association of LAMP-2A to membrane microdomains.
Of particular interest is the preferential cleavage of LAMP-2A in the lysosomal membrane microdomains. There are other examples of proteins that get recruited into lipid-enriched domains to undergo cleavage (i.e. transfer of amyloid and prion proteins to plasma membrane microdomains favors their cleavage by secretases or zinc metalloproteases, respectively) (reviewed in Hooper, 2005). How the recruitment of LAMP-2A into cholesterol-enriched regions favors its cleavage and prevents its multimerization remains to be elucidated. It is possible that, as described for other proteins (O'Shea, 2005), the sequestration of LAMP-2A in these regions facilitates a conformational change which prevents its association into multimeric complexes, and at the same time, exposes particular regions susceptible to cleavage by the proteases (Figure 9F, inset). The cholesterol-enriched regions could also favor the recruitment of the proteases into these areas, and the exclusion of components required for LAMP-2A multimerization. In fact, several proteases have been described to associate specifically to membrane lipid microdomains (Wong and Stevens, 2005). LAMP-2A-enriched lipid microdomains at the lysosomal membrane are also enriched in cathepsin A, required for LAMP-2A cleavage, and exclude other components known to participate in CMA (chaperones).
In conclusion, we have identified for the first time a role for specific regions at the lysosomal membrane in the regulation of CMA. Alterations in the organization of LAMP-2A into discrete lysosomal lipid microdomains could be behind the malfunctioning of CMA in aging and in some familiar forms of Parkinson's disease (reviewed in Massey et al, 2006a). This novel finding opens new possibilities to modulate CMA activity by inducing changes in the lipid organization of the lysosomal membrane.
Materials and methods
Animals and cells
Male Wistar rats (200–250 g) were used. Paraquat (40 mg/kg body weight) was administered in two consecutive doses separated by 24 h. Mouse fibroblasts (NIH3T3) were from the American Type Culture Collection (Manassas), and the LAMP-2A RNAi stable clone was generated as described before (Massey et al, 2006b).
Chemicals
Sources of chemicals were as described previously (Cuervo and Dice, 2000a; Cuervo et al, 2003; Kiffin et al, 2004). The antibodies against the cytosolic tail of rat and mouse LAMP-2A and -2B were prepared in our laboratory (Cuervo and Dice, 1996; Massey et al, 2006b). The antibodies against human LAMP-2 and mouse LAMP-1 were from the Developmental Studies Hybridoma Bank (Iowa University), against cathepsin D was from Santa Cruz Biotechnology, against hsc70 and hsp90 from Stressgen and against flotillin-1 from BD Transduction Laboratories. The polyclonal antibody against cathepsin A was a gift from Dr A D'Azzo (SJCRH, Memphis). MBCD, filipin, cholesterol and apyrase were from Sigma. Texas-Red-conjugated cholera toxin, Amplex Red Cholesterol Assay kit and SyproRuby were from Molecular Probes (Invitrogen).
Isolation of subcellular fractions
Rat liver lysosomes were isolated from a light mitochondrial–lysosomal fraction in a discontinuous metrizamide density gradient, and lysosomal fractions with different activities for CMA were further separated by differential centrifugation as described (Cuervo et al, 1997). Lysosomes from cultured cells were isolated as described (Storrie and Madden, 1990). A crude fraction containing lysosomes and mitochondria was prepared after cell lysis by differential centrifugation (2500 g 15 min; 17 000 g 10 min). Lysosomal matrices and membranes were isolated after hypotonic shock (Cuervo et al, 1995).
Isolation of detergent resistant lysosomal membrane microdomains
Lysosomal membranes (150 μg protein) from rat liver or mouse cultured fibroblasts were incubated with 1% of nonionic detergents in 150 mM NaCl, 50 mM Tris–HCl and 5 mM EDTA pH 7.4 (incubation buffer) on ice for 30 min and then subjected to centrifugation in a step-wise discontinuous sucrose gradient (5–35%). Gradient fractions, collected from top to bottom, were concentrated by precipitation in acid or by high-speed centrifugation and then subjected to SDS–PAGE and immunoblot (for details see Supplementary material and methods). The LAMP-2A complexes in the DR and soluble fractions were resolved by centrifugation on a continuous density (10–80%) sucrose gradient as described before (Cuervo and Dice, 2000b).
General methods
Uptake and degradation of substrate proteins by isolated lysosomes, and of intracellular protein turnover, were measured as previously described (Cuervo and Dice, 1996; Cuervo et al, 1997) (for details see Supplementary Materials and methods). Electron microscopy and immunogold, fluorescence and immunocytochemical staining, and immunoblot of samples were carried out using standard procedures (for details see Supplementary Materials and methods). Quantification of intracellular cholesterol was carried out using the Amplex Red Cholesterol Assay kit (Molecular Probes) following manufacturer's instructions. Point mutations were performed with the QuikChangeTM Site-Directed Mutagenesis Kit (Stratagene) and were verified by DNA sequence. Cells were transfected with the cDNAs for native and mutated human LAMP-2A subcloned in the pCR3 mammalian expression vector (Invitrogen) using Lipofectamine (Invitrogen). Degradation of LAMP-2A at the lysosomal membrane was analyzed, as described before, after incubation for 15 min at 37°C using two different antibodies against the cytosolic and lumenal regions of LAMP-2A. Degradation of LAMP-2A is linear with time for the first 20 min of incubation (Cuervo and Dice, 2000a).
Supplementary Material
Supplementary material and methods
Supplementary Data
Acknowledgments
We are grateful to Frank Macaluso and Leslie Gunther (Analytical Imaging Facility) for their valuable assistance with cryoelectron microscopy and immunogold procedures, to Ms Urmi Bandyopadhyay for her generous help isolating lysosomes, and to Dr Nancy Carrasco for her assistance in the interpretation of the mutation-induced conformational changes in LAMP-2A. This work was supported by grants NIH/NIA AG021904 and AG25355 and EMF AG-NS-0163.
References
- Agarraberes F, Terlecky S, Dice J (1997) An intralysosomal hsp70 is required for a selective pathway of lysosomal protein degradation. J Cell Biol 137: 825–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarraberes FA, Dice JF (2001) A molecular chaperone complex at the lysosomal membrane is required for protein translocation. J Cell Sci 114: 2491–2499 [DOI] [PubMed] [Google Scholar]
- Braccia A, Villani M, Immerdal L, Niels-Christiansen L-L, Nystrøm B, Hansen G, Danielsen E (2003) Microvillar membrane microdomains exist at physiological temperature. J Biol Chem 278: 15679–15684 [DOI] [PubMed] [Google Scholar]
- Chamberlain L (2004) Detergents as tools for the purification and classification of lipid rafts. FEBS Lett 559: 1–5 [DOI] [PubMed] [Google Scholar]
- Cuervo A, Dice J (1996) A receptor for the selective uptake and degradation of proteins by lysosomes. Science 273: 501–503 [DOI] [PubMed] [Google Scholar]
- Cuervo A, Dice J (2000a) Regulation of lamp2a levels in the lysosomal membrane. Traffic 1: 570–583 [DOI] [PubMed] [Google Scholar]
- Cuervo A, Dice J (2000b) Unique properties of lamp2a compared to other lamp2 isoforms. J Cell Sci 113: 4441–4450 [DOI] [PubMed] [Google Scholar]
- Cuervo A, Dice J, Knecht E (1997) A lysosomal population responsible for the hsc73-mediated degradation of cytosolic proteins in lysosomes. J Biol Chem 272: 5606–5615 [DOI] [PubMed] [Google Scholar]
- Cuervo A, Knecht E, Terlecky S, Dice J (1995) Activation of a selective pathway of lysosomal proteolysis in rat liver by prolonged starvation. Am J Physiol 269: C1200–C1208 [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Mann L, Bonten E, d'Azzo A, Dice J (2003) Cathepsin A regulates chaperone-mediated autophagy through cleavage of the lysosomal receptor. EMBO J 22: 12–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J, Zurzolo C (2004) Lipids as targeting signals: lipid rafts and intracellular trafficking. Traffic 5: 247–254 [DOI] [PubMed] [Google Scholar]
- Hooper N (2005) Roles of proteolysis and lipid rafts in the processing of the amyloid precursor protein and prion protein. Biochem Soc Trans 33: 335–338 [DOI] [PubMed] [Google Scholar]
- Kiffin R, Christian C, Knecht E, Cuervo A (2004) Activation of chaperone-mediated autophagy during oxidative stress. Mol Biol Cell 15: 4829–4840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusa S, Blom T, Eskelinen E, Kuismanen E, Mansson J, Simons K, Ikonen E (2001) Depletion of rafts in late endocytic membranes is controlled by NPC1-dependent recycling of cholesterol to the plasma membrane. J Cell Sci 114: 1893–1900 [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F, Lopez-Guerrero J, Carrasco L, Alonso M (1999) The amino-terminal nine amino acid sequence of poliovirus capsid VP4 prtoein is sufficient to confer N-Myristoylation and targeting to detergent-insoluble membranes. Biochemistry 39: 1083–1090 [DOI] [PubMed] [Google Scholar]
- Massey A, Zhang C, Cuervo A (2006a) Chaperone-mediated autophagy in aging and disease. Curr Top Dev Biol 73: 205–235 [DOI] [PubMed] [Google Scholar]
- Massey AC, Kaushik S, Sovak G, Kiffin R, Cuervo AM (2006b) Consequences of the selective blockage of chaperone-mediated autophagy. Proc Nat Acad Sci USA 103: 5905–5910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S, Maxfield FR (1995) Insolubility and redistribution of GPI-anchored proteins at the cell surface after detergent treatment. Mol Biol Cell 6: 929–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea P (2005) Physical landscapes in biological membranes: physico-chemical terrains for spatio-temporal control of biomolecular interactions and behaviour. Philos Transact A Math Phys Eng Sci 363: 575–588 [DOI] [PubMed] [Google Scholar]
- Pike L (2004) Lipid rafts: heterogeneity on the high seas. Biochem J 378: 281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder F, Gallegos A, Atshaves B, Storey S, McIntosh A, Petrescu A, Huang H, Starodub O, Chao H, Yang H, Frolov A, Kier A (2001) Recent advances in membrane microdomains: rafts, caveolae, and intracellular cholesterol trafficking. Exp Biol Med 226: 873–890 [DOI] [PubMed] [Google Scholar]
- Storrie B, Madden E (1990) Isolation of subcellular organelles. Meth Enzymol 182: 203–225 [DOI] [PubMed] [Google Scholar]
- Taute A, Watzig K, Simons B, Lohaus C, Meyer H, Hasilik A (2002) Presence of detergent-resistant microdomains in lysosomal membranes. Biochem Biophys Res Commun 298: 5–9 [DOI] [PubMed] [Google Scholar]
- Wong GW, Stevens RL (2005) Identification of a subgroup of glycosylphosphatidylinositol-anchored tryptases. Biochem Biophys Res Comm 336: 579–584 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material and methods
Supplementary Data


