Abstract
A requirement for integrin-mediated adhesion in cardiac physiology is revealed through targeted deletion of inte-grin-associated genes in the murine heart. Here we show that targeted ablation of the integrin-linked kinase (ILK) expression results in spontaneous cardiomyopathy and heart failure by 6 wk of age. Deletion of ILK results in disaggregation of cardiomyocytes, associated with disruption of adhesion signaling through the β1-inte-grin/FAK (focal adhesion kinase) complex. Importantly, the loss of ILK is accompanied by a reduction in cardiac Akt phosphorylation, which normally provides a protective response against stress. Together, these results suggest that ILK plays a central role in protecting the mammalian heart against cardiomyopathy and failure.
Keywords: Integrin-linked kinase, ILK, integrin, cardiomyopathy
Integrin-linked kinase (ILK) is a 59-kDa protein expressed in a wide variety of mammalian cell types and tissues, including colon, mammary gland, lung, liver, heart, pancreas, skeletal muscle, chondrocytes, lymphocytes, macrophages, and platelets (Hannigan et al. 1996; Li et al. 1997; Liu et al. 2005). The prototypical human isoform of ILK was originally characterized in terms of its oncogenic properties, which were attributed to serine/threonine kinase activity localized to the C terminus (Hannigan et al. 1996, 2005; Persad et al. 2001; White et al. 2001). Through the phosphorylation of downstream targets such as Akt and GSK-3β, ILK was shown to promote oncogenic transformation through the induction of anti-apoptotic pathways and cell cycle progression (Delcommenne et al. 1998; Novak et al. 1998; D'Amico et al. 2000; Persad et al. 2000; White et al. 2001).
In addition to a serine/threonine kinase domain, ILK contains structural motifs involved in protein–protein and protein–lipid interactions (Wu 2001). These domains include four tandem ankyrin repeats in the N terminus, which have been shown to bind the adaptor protein PINCH, a LIM-only protein that associates with the SH2/SH3-domain-containing Nck2 (Tu et al. 1999; Wu 2004, 2005). By binding molecules such as insulin receptor substrate-1 (IRS-1), DOCK180, and p21-activated kinase (PAK), the ILK/PINCH/Nck2 complex provides a bridge between receptor tyrosine kinases and the regulatory machinery of the cytoskeleton (Zhang et al. 2002). In addition, the C terminus of ILK has been shown to bind molecules such as paxillin, β1- and β3-integrins, and CH-ILKBP/actopaxin/affixin/parvin, an actin-binding focal adhesion protein (Hannigan et al. 1996; Tu et al. 2001; Yamaji et al. 2001; Wu 2004).
The nature of these interactions suggests that ILK plays a role as a molecular scaffold, necessary for maintaining the integrity of integrin-based cell adhesion complexes. Experiments involving the genetic ablation of ILK in Drosophila and Caenorhabditis elegans, as well as in the mouse epiblast and various cell types, have, indeed, confirmed that ILK plays a critical role in linking the actin-based cytoskeleton to the plasma membrane (Yamaji et al. 2001; Zervas et al. 2001; Mackinnon et al. 2002; Sakai et al. 2003). ILK-null flies, for example, show a phenotype of impaired muscle attachment at integrin-containing adhesion sites, due to a failure to recruit and assemble actin filaments on the inner side of the cell membrane (Zervas et al. 2001; Mackinnon et al. 2002).
The phenotype resulting from loss of ILK in these organisms suggests that ILK plays a central role as a bio-mechanical transducer of integrin-mediated adhesion in tissues requiring mechanical force or contractility (Zervas et al. 2001; Mackinnon et al. 2002). In this regard, contractile muscle cells of the mammalian heart represent ideal candidates for a critical physiological role for ILK. An important contribution of ILK in regulating the physiology of the heart is, indeed, suggested by several lines of evidence. First, ILK is expressed abundantly in both human and mouse cardiac tissue (Hannigan et al. 1996; Li et al. 1997). Second, the ILK-induced activation of Akt promotes cardiac survival and repair downstream from thymosin β4 following coronary artery ligation in mice (Bock-Marquette et al. 2004). Third, an ILK/ PINCH/parvin complex has been shown to regulate the integrin-mediated signaling pathways involved in hyper-trophic and apoptotic responses of cardiomyocytes in culture (Chen et al. 2005). In addition, ILK is indirectly implicated in cardiac physiology by reports that the targeted ablation of β1-integrin and associated molecules, including focal adhesion kinase (FAK) and melusin, results in cardiomyopathy and sudden death in response to aortic pressure overload (Shai et al. 2002; Brancaccio et al. 2003).
Despite this evidence, a physiological role for ILK in cardiac contractility has not been demonstrated. Consequently, we designed the present study to directly test the role of ILK in murine cardiac function. Using a gene-targeting approach, we show that loss of ILK in the mouse heart results in dilated cardiomyopathy and sudden death. In addition, this phenotype is associated with a decrease in FAK phosphorylation and Akt phosphorylation, both previously shown to be important regulators of the cardiac functional response to stress. Consistent with its role in β1-integrin-mediated adhesion complexes, the loss of ILK is accompanied by disaggregation of cardiomyocytes in vivo, and a concomitant down-regulation of β1-integrin protein. Together, these results suggest that ILK is essential for maintenance of normal cardiac function.
Results and Discussion
Tissue expression patterns and experiments with cultured cardiomyocytes suggest that ILK may play a critical role in cardiac physiology (Hannigan et al. 1996; Li et al. 1997; Chen et al. 2005). To directly test this hypothesis, we have used a conditional gene-targeting approach to ablate ILK expression in the murine heart. To accomplish this, mice harboring a conditional loxP1-flanked allele of the ilk gene (ILKfl) (Terpstra et al. 2003; Troussard et al. 2003) were bred with a second line of mice expressing the bacteriophage CRE recombinase under transcriptional control of the muscle creatine kinase (mck) promoter (Wang et al. 1999). The mck promoter is expressed in skeletal and cardiac muscle, and has been used to generate CRE-mediated cardiac gene knockouts in previously published mouse models (Wang et al. 1999; Oudit et al. 2004). Through successive breeding of these two lines, we generated a large cohort of mice expressing the mckCRE transgene and harboring two copies of the loxP1-flanked ilk allele (mckCRE ILKfl/fl). In addition, littermate control animals representing various genetic combinations were simultaneously generated from these crosses. All mice were of the FVB/N genetic background.
CRE-mediated excision of the loxP1-flanked ilk alleles in tissues of mckCRE ILKfl/fl mice was confirmed using a PCR-based protocol designed to amplify the intact and recombined forms of the loxP1-flanked allele (Supplementary Fig. 1A). Consistent with known mck promoter specificity, CRE-mediated excision of the loxP1-flanked ilk allele was found to be highly efficient in skeletal and cardiac muscle from mckCRE ILKfl/fl mice (Supplementary Fig. 1A, lanes 3,4, respectively). In contrast, CRE-mediated recombination was absent in mammary gland tissue from these animals (Supplementary Fig. 1A, lane 2).
To confirm that excision of the conditional ilk allele in the hearts of mckCRE ILKfl/fl mice resulted in a corresponding loss of ILK protein, we performed immuno-blot analysis on cardiac tissue lysates from mckCRE ILKfl/fl and control animals (Supplementary Fig. 1B). Consistent with robust excision of the loxP1-flanked ilk alleles in hearts from mckCRE ILKfl/fl mice, ILK protein levels were greatly reduced in pooled cardiac tissue ly-sates from these animals, in contrast to those of control littermates (Supplementary Fig. 1B, top panel, cf. lanes 1 and 2). The dramatic difference in ILK levels was not due to protein loading, as Akt protein levels were comparable between the hearts of mckCRE ILKfl/fl and control mice (Supplementary Fig. 1B, bottom panel).
Given that the mckCRE transgene is expressed in both skeletal muscle and heart, we monitored a large cohort of mckCRE ILKfl/fl and control animals over a period of several months for signs of distress, including muscle weakness and behavioral abnormalities such as problems with breathing, gait, or eating habits. In addition, we established that the mckCRE ILKfl/fl combination had not resulted in embryonic lethality, since all genetic combinations were born according to expected Mendelian ratios (data not shown).
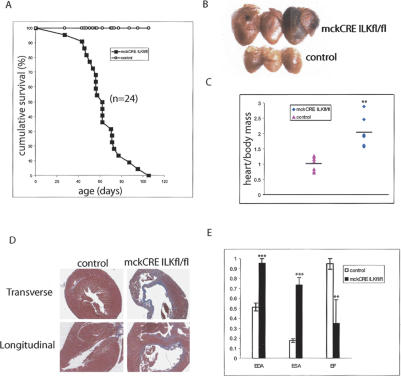
Despite efficient excision of the ilk gene in skeletal muscle, there was no evidence of skeletal muscle defects, both in terms of foreleg strength and muscle histology, in the mckCRE ILKfl/fl animals (data not shown). However, even though the overall behavior and size of mckCRE ILKfl/fl mice were indistinguishable from control animals (data not shown), we noticed that all mice of the mckCRE ILKfl/fl background died suddenly, starting at ~6 wk of age. As a result, a cohort of mckCRE ILKfl/fl animals (n = 24) and corresponding control littermates was monitored on a daily basis starting at the age of 4 wk. Strikingly, all mckCRE ILKfl/fl animals died between ~6 and 12 wk of age, with a median age of death being 2 mo (Fig. 1A). Control littermates, in contrast, lived up to 1 yr of age with no evidence of morbidity (Fig. 1A).
Figure 1.
Left ventricular dilation and impaired contraction in ILK-null hearts. (A) Mice of the mckCRE ILKfl/fl genetic combination (black squares) succumbed to sudden death between 6 and 12 wk of age (median = 8 wk). Control littermate animals are represented by open circles. (B) Hearts from mckCRE ILKfl/fl mice (top row) appear enlarged relative to control mice (bottom row). Hearts are representative of moribund mckCRE ILKfl/fl animals. (C) Hearts from mckCRE ILKfl/fl animals (blue diamonds) have an average mass twofold greater than those from control animals (pink triangles). Mass was determined for seven representative animals from mckCRE ILKfl/fl and control genotypes, and is presented as a ratio to overall body mass to correct for mouse size. (D) Sections of cardiac tissue were prepared from control (left panels) and mckCRE ILKfl/fl (right panels) animals, and stained with trichrome stain. Samples were prepared as cross (top panels) and longitudinal (bottom panels) sections. A dilated left ventricle is visible in each section prepared from mckCRE ILKfl/fl mice. (E) End diastolic (EDA) and end systolic (ESA) areas were increased in mckCRE ILKfl/fl animals (black bars), relative to controls (white bars). The ejection fraction (EF) was correspondingly reduced in mckCRE ILKfl/fl animals. These data are indicative of left ventricular dilation and impaired pumping capacity of hearts from mckCRE ILKfl/fl mice. Error bars represent SEM. (**) p < 0.01, (***) p < 0.001, Student's unpaired t-test.
During the 6-wk monitoring period, a small cohort of mckCRE ILKfl/fl animals (n = 7) was discovered in an acute phase of morbidity. This phenotype included labored breathing, lack of physical strength, disorientation, problems with balance, and a hunched, withdrawn behavior. Interestingly, these changes reflect classic human symptoms of dilated cardiomyopathy (DCM), which include shortness of breath, fatigue, light-headedness, fainting, lack of strength, and finally sudden death (Towbin and Bowles 2002). The moribund animals were sacrificed, and postmortem examinations were performed immediately. In all animals the hearts were grossly enlarged (Fig. 1B) and showed a twofold increase in the heart-to-body mass ratio (Fig. 1C). Consistent with the overall increase in size, trichrome-stained transverse and longitudinal sections of hearts from these mckCRE ILKfl/fl animals revealed dramatically dilated left ventricular chambers, with evidence of fibrosis (Fig. 1D).
The acute nature of the morbidity and mortality in mckCRE ILKfl/fl mice, combined with the overall dilated appearance of their hearts, suggests that the loss of ILK results in DCM. Since DCM is known to be associated with impaired cardiac function, we subjected four male mckCRE ILKfl/fl and four male control animals to echo-cardiographic analysis. The enlarged left ventricular chamber observed in the histological sections (Fig. 1D) was found to correspond to an increase in end diastolic (EDA) and end systolic (ESA) areas, as shown in Figure 1E. Importantly, the ejection fraction (EF) was greatly reduced in the mckCRE ILKfl/fl background, consistent with impaired pumping capacity of the hearts from these mice (Fig. 1E). Cardiac function, therefore, is, indeed, impaired in the mckCRE ILKfl/fl mice, consistent with a phenotype of DCM.
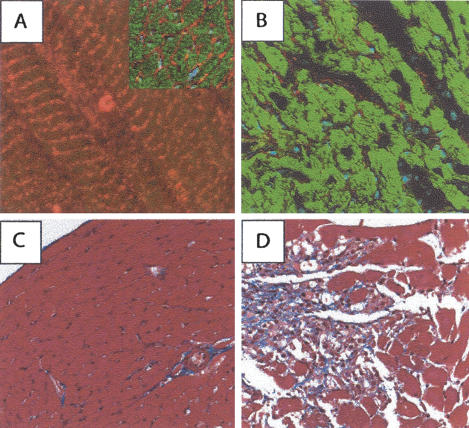
The phenotype resulting from cardiac-specific ablation of ILK closely resembles that reported following targeted deletion of β1-integrin, FAK, and melusin (Shai et al. 2002; Brancaccio et al. 2003; Peng et al. 2006). The similarity in the phenotype between these models is consistent with the colocalization of these proteins in β1-inte-grin-containing adhesion complexes. In cardiomyocytes in particular, these proteins, including ILK, have been shown to be localized to the costameres, regions of the sarcolemma coinciding with the Z-bands of striated cardiac muscle (Chen et al. 2005). Costameres are β1-inte-grin-dependent adhesion sites, and targeted deletion of β1-integrin results in failure of the adaptive cardiac response to pressure overload (Shai et al. 2002). In addition, the critical role of Z-band proteins in mediating the stress response of cardiomyocytes has been demonstrated for proteins such as muscle LIM protein (MLP) (Knoll et al. 2002). To confirm that ILK is expressed in the costameres of murine cardiac muscle, we performed immunofluorescence analysis on cryosections of hearts from wild-type animals. Using a polyclonal antibody directed against ILK, we, indeed, detected ILK protein in a pattern consistent with that of Z-bands and costameres of cardiomyocytes (Fig. 2A and inset).
Figure 2.
ILK is required for cardiac muscle integrity. (A) Frozen section of heart from a wild-type FVB mouse that was incubated with an antibody specific for ILK, followed by a Cy3-conjugated secondary antibody. Note the expression of ILK (red) in the costa-meric regions of the murine cardiac myocytes. Inset shows the expression pattern in a cross-section of a wild-type heart, revealing ILK expression in the sarcolemma. Phalloidin stain appears green. (B) Frozen section of heart from an mckCRE ILKfl/fl mouse that was subjected to the same immunostaining protocol as in A. Note the absence of Cy3 signal in the heart tissue from this animal, indicating an absence of ILK expression. Cardiomyocytes from hearts of this genotype appear disaggregated following the loss of ILK. Hearts of this genetic combination were prepared from moribund animals exhibiting a dilated phenotype. (C,D) Hearts from control (C) and mckCRE ILKfl/fl (D) mice were sectioned and stained with trichrome stain. Note again the disaggregated tissue in the mckCRE ILKfl/fl genotype, in addition to large amounts of interstitial fibrosis (blue stain).
The localization of ILK to costameric regions of murine cardiac muscle suggests a role for ILK in cardiomyocyte adhesion complexes. To study the impact of ILK ablation on the architecture and structural integrity of the heart, we therefore performed immunofluorescence analysis on frozen heart sections, using tissue obtained from mckCRE ILKfl/fl mice (Fig. 2B). The absence of red staining along the sarcolemma of the myocytes confirmed that ILK had, indeed, been lost from this tissue. Strikingly, the loss of ILK resulted in disaggregation of adjacent cardiomyocytes within the heart tissue (Fig. 2B). In addition, cardiac tissue from control and mckCRE ILKfl/fl animals was subjected to trichrome staining to permit more accurate examination of the tissue architecture (Fig. 2C,D). Examination of the trichrome-stained sections confirmed dramatic disaggregation of cardiac tissue in mckCRE ILKfl/fl animals, compared with the compact arrangement of cardiomyocytes in control mice. In addition to a loss of structural integrity, trichrome staining revealed an accumulation of interstitial fibrotic tissue in hearts from mckCRE ILKfl/fl mice (Fig. 2D).
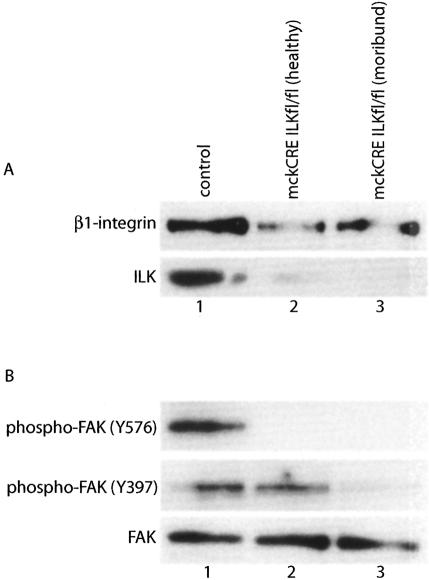
Since β1-integrin and FAK cardiac knockout models suffered pressure-induced cardiomyopathy similar to the DCM observed in the mckCRE ILKfl/fl animals, we decided to check the status of β1-integrin and FAK in the hearts of mckCRE ILKfl/fl mice by immunoblot analysis (Fig. 3). For this purpose, we prepared pooled cardiac protein lysates from mckCRE ILKfl/fl mice displaying a moribund phenotype and harboring dilated hearts, as well as from young (4- to 5-wk-old) healthy mckCRE ILKfl/fl animals not yet exhibiting a dilated cardiac phenotype. In addition, pooled lysates were prepared from littermate control animals. Probing of the lysates with an anti-β1-integrin monoclonal antibody revealed a reduction of total β1-integrin protein levels in phenotypically abnormal hearts from moribund mckCRE ILKfl/fl mice (Fig. 3A, top panel, cf. lane 3 and control sample in lane 1). Interestingly, total β1-integrin protein levels were also reduced in morphologically and histologically normal hearts from younger, healthy mckCRE ILKfl/fl mice (Fig. 3A, top panel, lane 2). A reprobing of the membrane was performed with an anti-ILK polyclonal antibody to confirm that ILK had been ablated in the hearts of these animals (Fig. 3A, bottom panel). The reduction in β1-integrin protein levels in pre-DCM hearts, as well as in diseased hearts, suggests that the down-regulation of β1-integrin in hearts of mckCRE ILKfl/fl mice may be due to ILK ablation directly, rather than indirectly to DCM-related consequences. Regardless of the mechanism, this result is consistent with an established role for β1-integrin in maintaining cardiac integrity.
Figure 3.
Loss of ILK impacts β1-integrin/FAK signaling in mckCRE ILKfl/fl mice. (A) Pooled protein lysates from control (lane 1) and mckCRE ILKfl/fl (lanes 2,3) mice were subjected to immunoblot analysis, by probing with antibodies to β1-integrin (top panel) and ILK (bottom panel). Mice of the mckCRE ILKfl/fl genotype were selected at two time points: young, healthy animals not yet exhibiting evidence of cardiomyopathy (lane 2) and older mice showing evidence of morbidity due to cardiac failure (lane 3). Note that loss of ILK protein is accompanied by a corresponding reduction in β1-integrin protein levels. (B) Pooled lysates from A were subjected to immunoblot analysis for levels of FAK phospho-Tyr 576 (top panel) and phospho-Tyr 379 (bottom panel).
Since FAK kinase activity also plays an important role downstream of β1-integrin and is required for protection against pressure-induced DCM (Peng et al. 2006), we probed the same set of protein lysates with antibodies directed against two major phosphorylation sites on the FAK molecule (Fig. 3B). Using an antibody specific for phospho-Tyr 576 in the kinase domain of FAK, we found that phosphorylation of this residue was not induced in the ILK-null hearts of mckCRE ILKfl/fl mice (Fig. 3B, top panel, cf. lanes 2,3 and the control sample in lane 1). Phosphorylation of this residue normally occurs after FAK is recruited to β1-integrin-containing adhesion complexes, and is required for activation of FAK kinase activity. These results are therefore consistent with disruption of β1-integrin-dependent adhesion signaling in hearts of mckCRE ILKfl/fl animals, and may explain in part the DCM phenotype in these mice.
When the lysates were probed with an antibody directed against phosphotyrosine residue 397 in the amino portion of FAK, we found that a decrease in phosphorylation occurred only in hearts exhibiting DCM, and not in pre-DCM hearts from mckCRE ILKfl/fl animals (Fig. 3B, middle panel, cf. lanes 2 and 3). This phosphotyro-sine residue represents the binding site for major effectors of cell adhesion, such as the c-Src kinase and PI3′ kinase. These results therefore suggest residual maintenance of the FAK-containing adhesion complexes in pre-DCM hearts, even though FAK kinase activity has been dramatically reduced. As expected from the dramatic disaggregation of cardiac tissue in moribund mckCRE ILKfl/fl animals, the onset of DCM coincides with inhibition of FAK phosphorylation on this second residue. The loss of ILK therefore results in a progressive disruption of the β1-integrin/FAK adhesion signaling complex, which may play an important role in the onset of DCM in mckCRE ILKfl/fl mice.
As expected, hearts from healthy mckCRE ILKfl/fl mice appeared normal in terms of size and histology (data not shown). However, since all mice of this geno-type failed to survive beyond 12 wk of age, we hypothesized that the dilated cardiac phenotype and subsequent death represented a failed response to normal physiological stresses. The spontaneous death of mckCRE ILKfl/fl mice, starting at ~6 wk of age, is in contrast to other models involving cardiac-specific ablation of β1-inte-grin-related proteins, such as melusin and FAK (Brancaccio et al. 2003; Peng et al. 2006). In the case of these models, a progressive change in cardiac physiology, from hypertrophy to DCM occurs in response to artificially applied aortic pressure overload, with little or no evidence of spontaneous heart failure (Brancaccio et al. 2003; Peng et al. 2006). In the model involving cardiac-specific FAK inactivation, evidence of spontaneous DCM did not appear until 9 mo of age. The mckCRE ILKfl/fl mice, however, die at a relatively early age (median age = 2 mo), and in the absence of externally applied stresses.
The relatively rapid decline in cardiac function in mckCRE ILKfl/fl mice would therefore suggest that ILK plays a critical role in protecting the heart from normal physiological stress. Indeed, the sensitivity of ILK-null hearts was apparent by the fact that the mckCRE ILKfl/fl mice often died during mating and during attempted surgical procedures such as implantation of telemetry devices (data not shown). We were able to detect evidence of cardiac stress at the molecular level by performing immunoblot analysis on the pooled lysates from control and mckCRE ILKfl/fl animals, using antibodies directed against phospho-ERK1/2 (Fig. 4A). The status of ERK1/2 phosphorylation was chosen as an indicator of cardiac stress in the mckCRE ILKfl/fl model due to previously published reports that ERK1/2 phosphorylation provides a protective response in the mammalian heart (Wang and Proud 2002; Proud 2004). By probing cardiac protein ly-sates with an antibody recognizing the phosphorylated form of ERK1/2, we found that ERK1/2 phosphorylation was elevated in hearts of moribund mckCRE ILKfl/fl animals showing DCM (Fig. 4A, lane 3), as well as in hearts from young, healthy mckCRE ILKfl/fl mice not yet showing phenotypic evidence of cardiomyopathy (Fig. 4A, lane 2). This result demonstrates that hearts from mckCRE ILKfl/fl mice show evidence of stress at the molecular level prior to phenotypic changes at the histological and physiological levels.
Figure 4.
Loss of ILK in murine hearts results in impaired molecular stress response. (A, top panel) Pooled cardiac protein lysates from control (lane 1) and mckCRE ILKfl/fl animals (lanes 2,3) were probed for phospho-ERK1/2 levels. mckCRE ILKfl/fl mice were chosen at a young, healthy stage (lane 2) and an older moribund stage (lane 3). Bottom panel shows levels of total ERK1/2 protein. Elevated levels of phosphor-ERK1/2 reflect a molecular response to cardiac stress. (B, top panel) Lysates from A were subjected to immunoblot analysis for levels of Akt phospho-Tyr 473. Bottom panel shows total Akt protein levels. Note the reduction in Akt phosphorylation, which normally provides a protective role by promoting hypertrophy during cardiac stress.
In addition to ERK1/2, Akt represents another important regulator of cardiac stress (Proud 2004). The role of Akt in promoting cardiac survival is related to the induction of protein synthesis and cardiac hypertrophy, which facilitates a compensatory increase in pumping capacity. Since Akt phosphorylation at Ser 473 has been shown to be regulated downstream from ILK kinase activity (Delcommenne et al. 1998), we examined the status of Akt phosphorylation in hearts of mckCRE ILKfl/fl mice (Fig. 4B). In contrast to elevated levels of ERK1/2 phosphorylation in the hearts of mckCRE ILKfl/fl mice, levels of phospho-Ser 473 of Akt are dramatically reduced in response to ILK ablation (Fig. 4B, top panel). This result is consistent with the published role for ILK in the induction of Akt phosphorylation (Delcommenne et al. 1998). More importantly, the inhibition of Akt phosphorylation in hearts of mckCRE ILKfl/fl animals indirectly suggests that there may be an impaired stress response in the ILK-null hearts. Since Akt is important for protein synthesis and cardiac hypertrophy in response to increased load on the heart (Proud 2004), the inhibition of Akt phosphorylation in ILK-null hearts may explain the rapid induction of DCM and sudden death in mckCRE ILKfl/fl mice. In conclusion, the phenotype of mckCRE ILKfl/fl mice provides a direct demonstration that ILK plays a critical role in the maintenance of mammalian cardiac physiology. Given the unique cardiac phenotype exhibited by these transgenic mice, these mice may prove to be a useful model to test the efficacy of cardiac stem cell therapies. In addition, the mckCRE ILKfl/fl mice represent an excellent model to confirm the role that ILK plays downstream from thymosin β4 in promoting cardiac survival and repair following coronary artery ligation (Bock-Marquette et al. 2004).
Materials and methods
Generation of mckCRE ILKfl/fl mice
The generation of mice harboring a conditional loxP1-flanked allele of ILK has been described previously (Terpstra et al. 2003; Troussard et al. 2003). These mice were crossed with animals expressing the mckCRE transgene (Wang et al. 1999) to generate mckCRE ILKfl/fl mice. Both strains were backcrossed into the FVB/N background prior to breeding. Mice were housed in the Animal Resource Center of the Royal Victoria Hospital. Animals undergoing echocardiographic analysis were housed at the Montreal Heart Institute, Université de Montréal. All mice were housed and treated according to CCAC guidelines.
PCR analysis of CRE-mediated excision
Total genomic DNA was prepared from cardiac and skeletal muscle and mammary gland tissue using a standard protocol. PCR primer sequences and amplification parameters were described elsewhere (Terpstra et al. 2003; Troussard et al. 2003).
Echocardiographic analysis
Area and M mode echocardiographic measurements were performed in anesthetized (isofluorane) mice using a Hewlett-Packard Sonos 5500 and S-12 transducer. The ejection fraction was estimated as EF = (EDA − ESA)/ EDA × 100%, where EDA and ESA are the end diastolic and systolic areas, respectively.
Immunofluorescence and trichrome staining
For immunofluorescence analysis, 8-μm cryosections were prepared from flash-frozen cardiac tissue. Sections were incubated for 1 h with anti-ILK polyclonal antibody (Upstate Biotechnology 06-592) at a dilution of 1:100, followed by 1 h with anti-rabbit Cy3-conjugated secondary antibody (1:1000). Sections were counterstained with DAPI (blue) and Phalloidin (green). For trichrome staining of cardiac tissue, hearts were fixed in buffered formalin and embedded in paraffin. Sections (8 μm) were then stained with trichrome according to the manufacturer's instructions.
Statistical analysis
Data were presented as mean ± SEM. Statistical significance was calculated using the Student's unpaired t-test.
Acknowledgments
We thank Cynthia Lavoie and Vasilios Papavasiliou for help with animal care and transport. W.J.M. is a recipient of a CRC Chair in Molecular Oncology. This work was funded by the Canadian Institutes of Health Research.
Footnotes
Supplemental material is available at http://www.genesdev.org.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1458906.
References
- Bock-Marquette I., Saxena A., White M.D., Dimaio J.M., Srivastava D. Thymosin β4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432:466–472. doi: 10.1038/nature03000. [DOI] [PubMed] [Google Scholar]
- Brancaccio M., Fratta L., Notte A., Hirsch E., Poulet R., Guazzone S., De Acetis M., Vecchione C., Marino G., Altruda F., et al. Melusin, a muscle-specific integrin β1-interacting protein, is required to prevent cardiac failure in response to chronic pressure overload. Nat. Med. 2003;9:68–75. doi: 10.1038/nm805. [DOI] [PubMed] [Google Scholar]
- Chen H., Huang X.N., Yan W., Chen K., Guo L., Tummalapali L., Dedhar S., St-Arnaud R., Wu C., Sepulveda J.L. Role of the integrin-linked kinase/PINCH1/α-parvin complex in cardiac myocyte hypertrophy. Lab. Invest. 2005;85:1342–1356. doi: 10.1038/labinvest.3700345. [DOI] [PubMed] [Google Scholar]
- D'Amico M., Hulit J., Amanatullah D.F., Zafonte B.T., Albanese C., Bouzahzah B., Fu M., Augenlicht L.H., Donehower L.A., Take-maru K., et al. The integrin-linked kinase regulates the cyclin D1 gene through glycogen synthase kinase 3β and cAMP-responsive element-binding protein-dependent pathways. J. Biol. Chem. 2000;275:32649–32657. doi: 10.1074/jbc.M000643200. [DOI] [PubMed] [Google Scholar]
- Delcommenne M., Tan C., Gray V., Rue L., Woodgett J., Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc. Natl. Acad. Sci. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan G.E., Leung-Hagesteijn C., Fitz-Gibbon L., Coppolino M.G., Radeva G., Filmus J., Bell J.C., Dedhar S. Regulation of cell adhesion and anchorage-dependent growth by a new β 1-integrin-linked protein kinase. Nature. 1996;379:91–96. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- Hannigan G., Troussard A.A., Dedhar S. Integrin-linked kinase: A cancer therapeutic target unique among its ILK. Nat. Rev. Cancer. 2005;5:51–63. doi: 10.1038/nrc1524. [DOI] [PubMed] [Google Scholar]
- Knoll R., Hoshijima M., Hoffman H.M., Person V., Lorenzen-Schmidt I., Bang M.L., Hayashi T., Shiga N., Yasukawa H., Schaper W., et al. The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell. 2002;111:943–955. doi: 10.1016/s0092-8674(02)01226-6. [DOI] [PubMed] [Google Scholar]
- Li F., Liu J., Mayne R., Wu C. Identification and characterization of a mouse protein kinase that is highly homologous to human integrin-linked kinase. Biochim. Biophys. Acta. 1997;1358:215–220. doi: 10.1016/s0167-4889(97)00089-x. [DOI] [PubMed] [Google Scholar]
- Liu E., Sinha S., Williams C., Cyrille M., Heller E., Snapper S.B., Georgopoulos K., St-Arnaud R., Force T., Dedhar S., et al. Targeted deletion of integrin-linked kinase reveals a role in T-cell chemotaxis and survival. Mol. Cell. Biol. 2005;25:11145–11155. doi: 10.1128/MCB.25.24.11145-11155.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackinnon A.C., Qadota H., Norman K.R., Moerman D.G., Williams B.D. C. elegans PAT-4/ILK functions as an adaptor protein within integrin adhesion complexes. Curr. Biol. 2002;12:787–797. doi: 10.1016/s0960-9822(02)00810-2. [DOI] [PubMed] [Google Scholar]
- Novak A., Hsu S.C., Leung-Hagesteijn C., Radeva G., Papkoff J., Montesano R., Roskelley C., Grosschedl R., Dedhar S. Cell adhesion and the integrin-linked kinase regulate the LEF-1 and β-catenin signaling pathways. Proc. Natl. Acad. Sci. 1998;95:4374–4379. doi: 10.1073/pnas.95.8.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudit G.Y., Sun H., Kerfant B.G., Crackower M.A., Penninger J.M., Backx P.H. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J. Mol. Cell. Cardiol. 2004;37:449–471. doi: 10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Peng X., Kraus M.S., Wei H., Shen T.L., Pariaut R., Alcaraz A., Ji G., Cheng L., Yang Q., Kotlikoff M.I., et al. Inactivation of focal adhesion kinase in cardiomyocytes promotes eccentric cardiac hypertrophy and fibrosis in mice. J. Clin. Invest. 2006;116:217–227. doi: 10.1172/JCI24497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persad S., Attwell S., Gray V., Delcommenne M., Troussard A., Sang-hera J., Dedhar S. Inhibition of integrin-linked kinase (ILK) suppresses activation of protein kinase B/Akt and induces cell cycle arrest and apoptosis of PTEN-mutant prostate cancer cells. Proc. Natl. Acad. Sci. 2000;97:3207–3212. doi: 10.1073/pnas.060579697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persad S., Attwell S., Gray V., Mawji N., Deng J.T., Leung D., Yan J., Sanghera J., Walsh M.P., Dedhar S. Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase: Critical roles for kinase activity and amino acids arginine 211 and serine 343. J. Biol. Chem. 2001;276:27462–27469. doi: 10.1074/jbc.M102940200. [DOI] [PubMed] [Google Scholar]
- Proud C.G. Ras, PI3-kinase and mTOR signaling in cardiac hypertrophy. Cardiovasc. Res. 2004;63:403–413. doi: 10.1016/j.cardiores.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Sakai T., Li S., Docheva D., Grashoff C., Sakai K., Kostka G., Braun A., Pfeifer A., Yurchenco P.D., Fassler R. Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes & Dev. 2003;17:926–940. doi: 10.1101/gad.255603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shai S.Y., Harpf A.E., Babbitt C.J., Jordan M.C., Fishbein M.C., Chen J., Omura M., Leil T.A., Becker K.D., Jiang M., et al. Cardiac myocyte-specific excision of the β1 integrin gene results in myocar-dial fibrosis and cardiac failure. Circ. Res. 2002;90:458–464. doi: 10.1161/hh0402.105790. [DOI] [PubMed] [Google Scholar]
- Terpstra L., Prud'homme J., Arabian A., Takeda S., Karsenty G., Dedhar S., St-Arnaud R. Reduced chondrocyte proliferation and chondrodysplasia in mice lacking the integrin-linked kinase in chondrocytes. J. Cell Biol. 2003;162:139–148. doi: 10.1083/jcb.200302066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin J.A., Bowles N.E. The failing heart. Nature. 2002;415:227–233. doi: 10.1038/415227a. [DOI] [PubMed] [Google Scholar]
- Troussard A.A., Mawji N.M., Ong C., Mui A., St-Arnaud R., Dedhar S. Conditional knock-out of integrin-linked kinase demonstrates an essential role in protein kinase B/Akt activation. J. Biol. Chem. 2003;278:22374–22378. doi: 10.1074/jbc.M303083200. [DOI] [PubMed] [Google Scholar]
- Tu Y., Li F., Goicoechea S., Wu C. The LIM-only protein PINCH directly interacts with integrin-linked kinase and is recruited to integrin-rich sites in spreading cells. Mol. Cell. Biol. 1999;19:2425–2434. doi: 10.1128/mcb.19.3.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y., Huang Y., Zhang Y., Hua Y., Wu C. A new focal adhesion protein that interacts with integrin-linked kinase and regulates cell adhesion and spreading. J. Cell Biol. 2001;153:585–598. doi: 10.1083/jcb.153.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Proud C.G. Ras/Erk signaling is essential for activation of protein synthesis by Gq protein-coupled receptor agonists in adult cardiomyocytes. Circ. Res. 2002;91:821–829. doi: 10.1161/01.res.0000041029.97988.e9. [DOI] [PubMed] [Google Scholar]
- Wang J., Wilhelmsson H., Graff C., Li H., Oldfors A., Rustin P., Bruning J.C., Kahn C.R., Clayton D.A., Barsh G.S., et al. Dilated cardiomyopathy and atrioventricular conduction blocks induced by heart-specific inactivation of mitochondrial DNA gene expression. Nat. Genet. 1999;21:133–137. doi: 10.1038/5089. [DOI] [PubMed] [Google Scholar]
- White D.E., Cardiff R.D., Dedhar S., Muller W.J. Mammary epithelial-specific expression of the integrin-linked kinase (ILK) results in the induction of mammary gland hyperplasias and tumors in transgenic mice. Oncogene. 2001;20:7064–7072. doi: 10.1038/sj.onc.1204910. [DOI] [PubMed] [Google Scholar]
- Wu C. ILK interactions. J. Cell Sci. 2001;114:2549–2550. doi: 10.1242/jcs.114.14.2549. [DOI] [PubMed] [Google Scholar]
- Wu C. The PINCH–ILK–parvin complexes: Assembly, functions and regulation. Biochim. Biophys. Acta. 2004;1692:55–62. doi: 10.1016/j.bbamcr.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Wu C. PINCH, N(i)ck and the ILK: Network wiring at cell-matrix adhesions. Trends Cell Biol. 2005;15:460–466. doi: 10.1016/j.tcb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Yamaji S., Suzuki A., Sugiyama Y., Koide Y., Yoshida M., Kanamori H., Mohri H., Ohno S., Ishigatsubo Y. A novel integrin-linked kinase-binding protein, affixin, is involved in the early stage of cell–substrate interaction. J. Cell Biol. 2001;153:1251–1264. doi: 10.1083/jcb.153.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervas C.G., Gregory S.L., Brown N.H. Drosophila integrin-linked kinase is required at sites of integrin adhesion to link the cytoskeleton to the plasma membrane. J. Cell Biol. 2001;152:1007–1018. doi: 10.1083/jcb.152.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Guo L., Chen K., Wu C. A critical role of the PINCH–integrin-linked kinase interaction in the regulation of cell shape change and migration. J. Biol. Chem. 2002;277:318–326. doi: 10.1074/jbc.M108257200. [DOI] [PubMed] [Google Scholar]