Abstract
The periodic destruction of mitotic cyclins is triggered by the activation of the anaphase-promoting complex/cyclosome (APC/C) in mitosis. Although the ability of the APC/C to recognize destruction box (D-box) substrates oscillates throughout the cell cycle, the mechanism regulating APC/C binding to D-box substrates remains unclear. Here, we show that the APC/C inhibitor Emi1 tightly binds both the APC/C and its Cdh1 activator, binds to the D-box receptor site on the APC/CCdh1, and competes with APC/C substrates for D-box binding. Emi1 itself contains a conserved C-terminal D-box, which provides APC/C-binding affinity, and a conserved zinc-binding region (ZBR), which antagonizes APC/C E3 ligase activity independent of tight APC binding. Mutation of the ZBR converts Emi1 into a D-box-dependent APC/C substrate. The identification of a direct Emi1–APC/C complex further explains how Emi1 functions as a stabilizing factor for cyclin accumulation and the need to destroy Emi1 for APC/C activation in mitosis. The combination of a degron/E3 recognition site and an anti-ligase function in Emi1 suggests a general model for how E3 substrates evolve to become pseudosubstrate inhibitors.
Keywords: Mitosis, maturation-promoting factor, ubiquitin–protein ligases, Polo-like kinases, anaphase-promoting complex
Periodic accumulation and destruction of cyclins is essential for the oscillation of cyclin-dependent kinase activity that allows progression through the cell cycle. The anaphase-promoting complex or cyclosome (APC/C), an E3 ubiquitin ligase, is responsible for the timely protea-some-dependent destruction of cyclins, as well as other key substrates essential for cell cycle regulation (Harper et al. 2002). The APC/C is a multicomponent complex, composed of at least 10 subunits in mammals, including a Cullin homolog APC2 and a RING-H2 finger protein APC11 (Yu et al. 1998; Zachariae et al. 1998), which are at the core of the essential ubiquitin chain-forming activity.
Interestingly, the abundance of core APC/C subunits does not oscillate throughout the cell cycle, but APC/C substrate recognition activity is stimulated in mitosis (Harper et al. 2002; Yamano et al. 2004). The timing and regulation of APC/C activation in mitosis is not fully understood but certainly reflects a balance of positive and negative factors. APC/C activation requires the association of the APC/C with a WD40-repeat-containing activator protein. To date, two major activator proteins have been identified, Cdc20 and Cdh1 (Schwab et al. 1997; Visintin et al. 1997). The mechanism by which these proteins activate the APC/C is not fully understood. APC/C substrates are recognized through conserved degrons, either the destruction box (D-box) RxxL (Glotzer et al. 1991; King et al. 1996) or an alternative degron, the KEN box (Pfleger and Kirschner 2000). One site for recognition resides within the Cdc20 and Cdh1 activators (Pfleger et al. 2001). Specifically, APCCdc20 is thought to recognize substrates containing D-boxes, whereas APCCdh1 appears to have the additional capacity to bind substrates containing KEN boxes. Recent studies support that Cdc20 binds specifically to the D-box and Cdh1 to both KEN and D-boxes (Burton and Solomon 2000; Hilioti et al. 2001; Pfleger et al. 2001). A recent analysis, however, suggested that D-box peptides also bind directly to the core APC/C (Yamano et al. 2004), raising the possibility that the core APC/C contains a D-box receptor. While this finding suggested a primary interaction of D-box with the APC/C core, it does not exclude a multistep mechanism in which D-boxes interact with APC/C activators and with the APC/C core at distinct times during the ubiquitination process. The study by Burton et al. (2005) detected an association between the D-box/KEN box substrate Hsl1 and TAP-purified APC/C only in the presence of Cdh1. This study and another by Kraft et al. (2005) suggest that specific sequences on the WD40 domain of Cdh1 are required for direct D-box binding. Several models may reconcile these differences, including the possibility that there may be D-box-binding sites on both WD40 activators and the APC/C core itself. Indeed, the APC/C subunit APC10/Doc1 has been implicated as a D-box receptor, and evidence suggests it is critical for bridging the interaction between D-box and KEN-box substrates and APCCdh1(Passmore et al. 2003; Carroll et al. 2005).
To date, a number of inhibitors of the APC/C have been identified, including components of the spindle checkpoint (Hoyt et al. 1991; Li and Murray 1991; Castro et al. 2005; Nasmyth 2005), the APC/C inhibitor Emi1 (Reimann et al. 2001a, b), and the recently described RASSF1A (Jackson 2004; Song et al. 2004). The role of the spindle assembly checkpoint, notably the Cdc20-binding proteins Mad2 and BubR1, is important to restrain the activation of the APC/C and the destruction of the chromosome cohesion regulator securin at the metaphase–anaphase transition in vivo (Castro et al. 2005; Nasmyth 2005). Mad2 and the other components of the spindle checkpoint (Mad1, Bub1, BubR1, Mps1) do not appear important for the difference between inter-phase and mitotic levels of APC/C activity, but instead are implicated in the timing of events in mitosis (den Elzen and Pines 2001; Geley et al. 2001; Meraldi et al. 2004).
The primary inhibitor of the APC/C during interphase is the protein Emi1, which has been shown to be required for the accumulation of cyclins in HeLa cells (Hsu et al. 2002), for entry into mitosis in Xenopus embryos (Reimann et al. 2001a), and also to efficiently inhibit the APC/C in vitro (Reimann et al. 2001b). A homolog of Emi1, Emi2/Erp1, also directly inhibits the APC/C in meiosis (Schmidt et al. 2005; Tung et al. 2005). Previous studies showed that Emi1 could associate with the APC/C activators Cdc20 and Cdh1 (Reimann et al. 2001a, b; Hsu et al. 2002). Moreover, the mitotic destruction of Emi1 triggered by the SCFβTrCP ubiquitin ligase was shown to be required for the activation of cyclin A and cyclin B destruction (Guardavaccaro et al. 2003; Margottin-Goguet et al. 2003). Finally, the requirement of the polo kinase Plk1 for the mitotic destruction of Emi1 (Hansen et al. 2004; Moshe et al. 2004) and for full activation of the APC/C, suggests the model that polo kinases could affect APC/C activation by triggering the destruction of an interphase APC/C inhibitor.
Emi1 did not appear to be an APC/C substrate itself but instead required an extrinsic, SCF-dependent pathway for its destruction. Emi1 destruction is tightly linked to nuclear envelope breakdown, generally thought to the irreversible step in commitment to mitosis (Rieder and Maiato 2004) and a prerequisite for APC/C activation. Emi1 is thus also a strong candidate for the factor that provides a critical lag between activation of the mitosis-promoting activity (MPF) of cyclin B/Cdc2 and activation of the APC/C that causes cyclin B destruction. This lag is important for preventing premature destruction of accumulating cyclin, to allow full activation of MPF. Emi1’s role in controlling APC/C activity in early mitosis suggests it may participate in a recently described prometaphase timer (Meraldi et al. 2004).
To better understand how Emi1 inhibits cyclin destruction in interphase, we purified Emi1-associated proteins from interphase HeLa cells and found that Emi1 associates in a complex with the APC/C and Cdh1. Further, we find that Emi1 can bind to the APC/C via a conserved C-terminal destruction box and can compete for APC/C–substrate interaction. The ability of Emi1 to inhibit the APC/C derives from a combination of its conserved D-box, which provides strong APC/C-binding affinity and a block to substrate-APC/C binding, and a conserved zinc-binding region (ZBR), which provides an APC/C E3 ligase antagonizing activity. We find that the ZBR works by blocking substrate access to the APC/C, but through a possible steric mechanism independent of D-box binding. Inactivating this ZBR function turns Emi1 from an APC/C inhibitor into an APC/C substrate, supporting the idea that Emi1 functions as a pseudosubstrate inhibitor of the APC/C to allow cyclin accumulation.
Results
Emi1 associates with the nuclear form of the APC
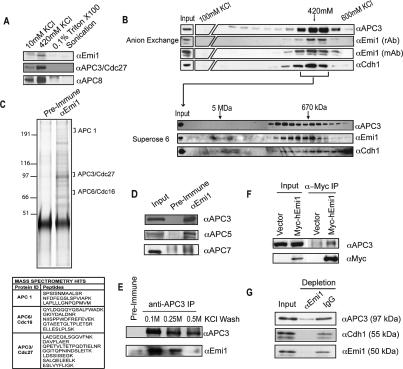
To better understand the mechanism of Emi1 control of the APC/C in interphase, we purified Emi1-associated proteins from interphase HeLa cells. Low salt and high salt extracts were made from asynchronous (mostly interphase) cells and examined by immunoblotting for Emi1 and the APC/C subunits APC3/Cdc27 and APC8 (Fig. 1A). The majority of these proteins were extracted from the cell pellets after 0.42M KCl extraction. Both Emi1 and APC/C are largely nuclear in interphase, which may require a nuclear or chromatin anchor.
Figure 1.
Emi1 associates with the nuclear form of the APC. (A) Emi1 and the APC/C are coextracted from the high-salt fraction of HeLa cells. HeLa cells were extracted by sequential treatments of nitrogen decompression, 420 mM KCl, 0.1% Triton X-100, and sonication to produce fractions as described in Materials and Methods. Fractions were examined by immunoblot analysis for Emi1 and APC/C subunits (APC3/Cdc27, APC8). (B) Emi1, Cdh1, and APC/C cofractionate during ion exchange and gel filtration chromatography. The nuclear fraction described in A was further fractionated by anion exchange (upper panel) chromatography and subsequently separated by gel filtration (lower panel). (C) Emi1 and APC/C coprecipitate from purified fractions. The fractions indicated in B were pooled and immunoprecipitated with anti-Emi1 antibodies and resolved by SDS-PAGE and silver stained. Excised bands were identified by mass spectrometry. APC/C subunits identified by mass spectromety are indicated by arrows. (D) Anti-Emi1 antibody immunoprecipitates were also examined by immunoblot analysis for Emi1 and APC/C subunits, APC3 (Cdc27), APC5, and APC7. (E) Anti-APC3/Cdc27 efficiently coprecipitates Emi1 from nuclear fractions. Immunoprecipitates were washed with 0.1, 0.25, or 0.5 M KCl; separated by SDS-PAGE; and examined by immunoblot analysis for Cdc27 and Emi1. (F) APC/C subunits associate with expressed epitope-tagged Emi1. HeLa cells were transfected with a construct expressing epitope-tagged Myc-hEmi1 or empty vector. Cell extracts were prepared and anti-Myc precipitates were examined by immunoblot analysis for the presence of Emi1 and APC/C subunits. (G) Immunodepletion of asynchronous nuclear extract by anti-Emi1 antibody results in the removal of the majority of APC/C and Cdh1. Nuclear extracts were incubated with anti-Emi1 or control antibody. Equal amounts of input and flow-through (nonbound) samples were examined by immunoblotting.
To examine whether Emi1 and the APC/C are directly associated, we separated the extracted protein complexes on a Qsepharose anion exchange (Q) column and observed coelution of Emi1, Cdc27, and the activator protein Cdh1 at ~420 mM KCl. Other APC/C subunits coeluted similarly (Supplementary Fig. 1A). This 0.42-M KCl fraction was then resolved by gel filtration and the APC/C subunit APC3/Cdc27 coeluted with Emi1, in a broad complex of ~1 MDa. We have not yet clearly determined any specific subcomplexes.
Sizing of the extracted Emi1 and APC/C directly on gel filtration chromatography showed cofractionation of these proteins in the 1 MDa complex (Supplementary Fig. 1B), although a more substantial fraction of the Emi1–APC/C complex appears in a high-molecular-mass complex of at least ~3 MDa. The physical behavior of this larger complex appears to reflect the association of Emi1–APC/C with additional factors (K. Ban, J.J. Miller, and P.K. Jackson, unpubl.). Separation of HeLa extract by sucrose gradient also revealed cosedimentation of Emi1 and APC/C in a large complex of ~1 MDa (Supplementary Fig. 1C).
There are two visible forms of the Emi1 protein (p51 and p49), consistently seen with either affinity-purified rabbit anti-Emi1 antibodies or with a specific Emi1 monoclonal antibody (Fig. 1B). The lower mobility form appears selectively associated with the APC in either the fractionated ~1-MDa complex (Fig. 1B) or in the high-molecular-mass complex (Supplementary Fig. 1B). Additional fractionation by anion exchange chromatography appears to separate Emi1 and the APC/C from a larger-molecular-weight complex, but we have not fully determined the composition of this larger complex. The consistent cofractionation of Emi1 and the APC/C following extraction, ion exchange, and gel filtration suggested that Emi1 and the APC/C remain specifically and tightly associated under a variety of conditions.
To characterize which proteins are associated with Emi1, we immunoprecipitated Emi1 from the 0.42-M KCl Q fraction and examined the identity of associated proteins by mass spectrometry (Fig. 1C) and by immunoblotting for candidate Emi1-associated proteins (Fig. 1D). Specific gel regions centered at 210, 100, and 75 kDa were excised, analyzed by mass spectrometry, and identified as APC/C subunits APC1, APC3/Cdc27, and APC6/Cdc16. We performed immunoblots of the Emi1 coimmunoprecipitated material for other APC/C subunits, and found that APC3/Cdc27, APC5, and APC7 (Fig. 1D), and APC1, APC4, APC6/Cdc16, APC8/Cdc23, and APC 11 (data not shown) were specifically associated with the complex. As further validation, Emi1 interacted with both Cdh1 and APC/C in a reconstitution of purified Emi1 with the APC/C (see Fig. 2).
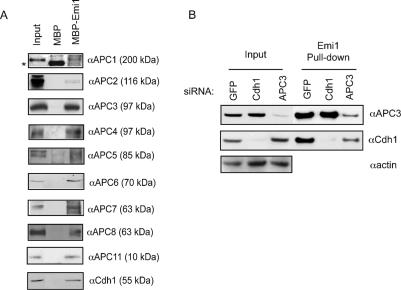
Figure 2.
Immobilized Emi1 can bind to the APC/CCdh1 in an “APC/C capture” assay. (A) Recombinant Emi1 protein immobilized on Sepharose beads (see Materials and Methods) was incubated with HeLa cell extracts, and precipitates were analyzed for APC/C binding by immunoblot analysis. The approximate molecular weight at which the indicated subunit migrates is indicated in parentheses. (B) Emi1 binds to the APC/C in the absence of Cdh1. Emi1 beads were incubated with extracts made from HeLa cells transfected with siRNA targeting Cdh1 or APC3 for 48 h.
We also observed Emi1–APC/C association when we immunoprecipitated APC3/Cdc27 from the high-salt-extracted HeLa fraction (Fig. 1E), demonstrating that the association can be seen by precipitation of either partner. Of interest, the association between Emi1 and the APC/C was stable in 0.25 M KCl but was partially disrupted in 0.5 M KCl, suggesting that the Emi1–APC/C interaction is more labile than interactions among the core subunits of the APC/C. A previous study noted that Emi1 can interact efficiently with the activator protein Cdc20 but did not detect an interaction between Emi1 and APC/C in Xenopus extracts (Reimann et al. 2001a). We suspect the Emi1–APC/C interaction was over-looked because of competition with the more prevalent Emi2/Erp1 protein, the meiosis-specific homolog of Emi1 (Schmidt et al. 2005; Tung et al. 2005).
As an additional validation of Emi1–APC/C interaction, we expressed epitope-tagged Emi1 in human embryonic kidney 293T cells and found efficient coprecipitation of the APC3/Cdc27 protein (Fig. 1F). We were able to deplete the majority (>80%) of Emi1 from the extract using an anti-Emi1 antibody and found that Cdh1 (>80%) and the APC/C were efficiently codepleted (Fig. 1G), suggesting that a majority of Cdh1 and the APC/C are associated with Emi1 during interphase.
The APC/C binds to immobilized Emi1 in a ‘capture’ assay
Emi1 efficiently inhibits the APCCdh1 in vitro (Reimann et al. 2001b) and in vivo (Hsu et al. 2002). Here, the identification of an Emi1–APC/C complex suggests that Emi1 may inhibit the core APC/C directly. To test the mechanism of the Emi1–APC/C interaction, we used an in vitro binding or “capture” assay to reconstitute and study this interaction. This assay is similar to that used by Yamano et al. (2004) to study cyclin B–APC/C association. Purified, recombinant Emi1 was immobilized on Sepharose beads, and these beads were incubated with HeLa cell lysates to allow binding of the APC/C. The bound complex was then assayed by immunoblotting. As shown in Figure 2A, a full panel of known APC/C subunits, including the Cdh1 activator, was found to be associated with Emi1.
Previous reports had noted an interaction between Emi1 and the APC/C activators Cdh1 and Cdc20 (Reimann et al. 2001a, b; Hsu et al. 2002). We wondered if the interaction between Emi1 and the APC/C was strictly through this previously described association between Emi1 and activators, or if Emi1 is able to bind directly to the APC/C core, independently of Cdc20 and Cdh1. We tested whether loss of Cdh1 by RNA interference disrupts the Emi1–APC/C interaction in asynchronous HeLa cells. We found that we could efficiently deplete levels of Cdh1 in HeLa cells using small interfering RNA (siRNA) and, further, we found that Cdc20 does not detectably associate with the APC/C in asynchronous extracts (Supplementary Fig. 1I). Here, we observed that Emi1 could capture APC/C with equal efficiency in both the presence and absence of Cdh1 (Fig. 2B).
Yamano et al. (2004) found that the ability of the cyclin B D-box to bind to the APC/C occurred in Xenopus egg extracts depleted of Cdc20, which are absent of appreciable Cdh1. We also found that Emi1 could efficiently capture the APC/C in Xenopus egg extracts depleted of Cdc20 (Supplementary Fig. 1G). Thus Emi1, much like cyclin B, can capture the APC/C independent of activators Cdc20 and Cdh1.
To further characterize the interaction between Emi1 and APC/C, we targeted a number of APC/C subunits with siRNA. Overall, Emi1 capture of the APC/C was acutely sensitive to perturbations in the abundance of certain subunits. Depletion of APC3/Cdc27 significantly reduced the amount of APC/C captured by Emi1, as detected by immunoblotting with antibodies recognizing a number of other subunits (Fig. 2B; Supplementary Fig. 1H). Intriguingly, the reduced amount of APC3 also somewhat reduced the amount of Cdh1 captured by Emi1, suggesting that the APC/C can influence the Emi1–Cdh1 interaction.
Emi1 binding to the D-box receptor on the APC/C is more dependent on the D-box than on the ZBR
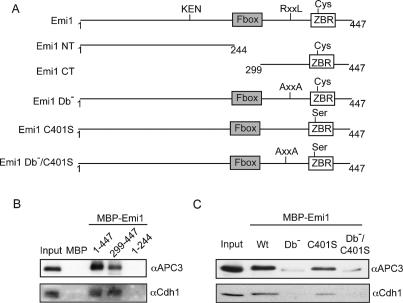
To define which structural elements in Emi1 are important for APC/C binding and inhibition, we examined the ability of immobilized Emi1 variants (Fig. 3A) to bind APC/C in the capture assay. We found that the Emi1 C terminus (amino acids 299–447) efficiently bound to both the APC/C and Cdh1, whereas the N-terminal portion (1–244) of Emi1 did not bind (Fig. 3B), consistent with earlier results showing the C terminus as the APC/C inhibitory domain (Reimann et al. 2001a).
Figure 3.
The APC/C binds to the C-terminal APC/C inhibitory domain of Emi1 via its conserved D-box. (A) Emi1 protein schematic, key features, and variant proteins. (B) The Emi1 C-terminal domain binds to the APC/C and Cdh1. Capture of the APC/C using recombinant MBP–Emi1 N terminus (1–244) or C terminus (299–447). (C) Efficient Emi1 binding to the APC/C and Cdh1 requires the C-terminal D-box. Capture of APC/C using MBP–Emi1 D-box mutant (Db−), MBP–Emi1 ZBR mutant (C401S), or double mutant (Db−/C401S).
The C terminus of Emi1 contains a ZBR (see Fig. 3A) conserved in homologs of Emi1 and Emi2/XErp1 (Schmidt et al. 2005; Tung et al. 2005) and also found in the TRIAD family of E3 ubiquitin ligases, including the E3 ubiquitin ligase parkin (van der Reijden et al. 1999). Examination of the Emi1 and Emi2/Erp1 primary sequences also showed the presence of conserved destruction boxes in the conserved C-terminal domain (Supplementary Fig. 2A), a region of Emi1 required for APC/C inhibition. Here, we found that the ability of Emi1 to capture the APC/C was strongly dependent on its D-box, as Emi1 protein containing a mutation of the D-box (R322xxL → AxxA) significantly reduced APC/C binding. Mutation of a conserved cysteine (C401) in the ZBR only reduced APC/C binding slightly. A double mutant with both the D-box and C401S mutations showed minimal binding to the APC/C, similar to the D-box mutant (Fig. 3C). The requirements for the interaction between Emi1 and Cdh1 were similarly strongly dependent on an intact D-box (Fig. 3C; Supplementary Fig. 2C).
We consistently observed two distinct forms of APC3 interacting differentially with the Emi1 wild-type and D-box mutant proteins. A faster migrating form of APC3 associates specifically with the Emi1 D-box mutant. While we do not fully understand the nature of this species, we suspect it may reflect a specific phosphorylation state of the APC/C that gates the Emi1–APC/C interaction.
Previous analyses of Emi1 showed that ZBR function was critical for APC/C inhibition (Reimann et al. 2001b). However, as we see here, the D-box contributes strongly to Emi1–APC/C binding and the ZBR has a modest or minimal contribution to binding, suggesting a distinct mechanism for the ZBR in APC/C inhibition. We were interested in further defining that distinct function.
Emi1 competes with D-box-dependent, APC/C substrate binding to the D-box receptor on the APC/C
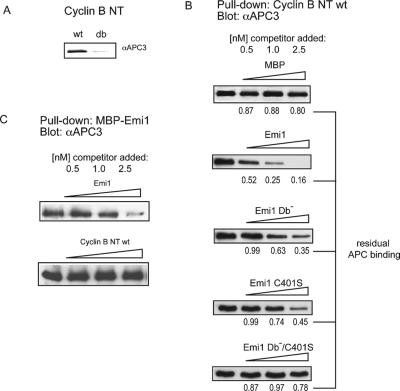
Substrate destruction boxes are important for both ubiquitination and binding to the APCCdh1 or APCCdc20 ligases (Burton et al. 2005; Kraft et al. 2005). To extend these analyses, we used cyclin B, a well-validated APC/C substrate, to demonstrate D-box-dependent binding to the APCCdh1. First, we validated that an immobilized N terminus of cyclin B efficiently captured the APC/C in HeLa extract (Fig. 4A). In this assay, similar to that in Xenopus extract published by Yamano et al. (2004), the binding of cyclin B to the APC/C is strongly dependent on the D-box.
Figure 4.
Emi1 can compete with substrate binding in D-boxand ZBR-dependent manner. (A) The normal N-terminal D-box region of cyclin B1 binds specifically to the APC/C. Recombinant N terminus of cyclin B1 (residues 1–100) or a variant containing a mutated D-box was immobilized on CNBr-activated Sepharose, incubated with cell extracts, and analyzed for APC/C binding with anti-Cdc27 antibody. (B) Emi1 competes with the ability of the cyclin B1 D-box region to bind the APC/ C. Recombinant wild-type or mutant MBP–Emi1 or MBP (0.5, 1, or 2.5 nmol) was added to 1 nmol bead-bound cyclin B1 and incubated with HeLa cell extracts as described in A. The amount of APC/C remaining bound to Cyclin B beads is indicated below, expressed as a percentage of control (no Emi1 addition) sample. Values represent mean of two experiments. (C) Cyclin B1 does not compete efficiently with Emi1 for APC/C binding. N terminus of cyclin B1 or MBP–Emi1 was added at doses of 0.5, 1.0, or 2.5 nmol to capture assay using 1 nmol of bead-bound MBP–Emi1.
Because Emi1 also binds to the D-box receptor, we tested whether Emi1 or a control protein (MBP) would compete with cyclin B binding to APCCdh1. We found that the Emi1 protein efficiently blocked D-box binding, but that the control protein did not (Fig. 4B). Emi1 was also able to compete for APCCdh1 binding with securin, another D-box APC/C substrate (Supplementary Fig. 2B). Emi1 was also able to compete with the interaction between cyclin B and Cdh1 (Supplementary Fig. 2D), similar to a previous report showing that Emi1 can compete with the ability of securin to interact with Cdc20 (Reimann et al. 2001b). Therefore, Emi1 protein can bind both Cdh1 and the APC/C and is capable of efficiently blocking both APC/C and Cdh1 capture by D-box substrates.
To examine whether the ability of Emi1 to compete D-box binding requires its D-box or ZBR, we tested the ability of single D-box (Db−) or ZBR (C401S) mutants and the double mutant (Db−/C401S) to compete cyclin B binding. We found that the D-box mutant and the ZBR mutant are both partially compromised in their ability to compete with cyclin B (Fig. 4B), but that the double mutant does not measurably compete. Although this demonstrates that the ZBR and D-box are each sufficient to inhibit substrate binding, they appear to inhibit by different mechanisms. The ability of the D-box mutant to compete with cyclin B binding to the APC/C suggests that the ZBR prevents substrate access to the APC/C in a D-box-independent manner. An interesting hypothesis is that the Emi1 ZBR is positioned within the APC/C holoenzyme to sterically exclude substrates from the APC/C D-box receptor and active site. Structural studies will be important to test this hypothesis.
We wanted to test if, as an interphase APC/C inhibitor, Emi1 was more effective in binding to the APC/C than its natural substrates, like cyclin B. Therefore, we tested the ability of cyclin B N terminus to compete with APC/C capture by immobilized Emi1 beads. We found that cyclin B competes for Emi1 binding to the APC/C poorly (Fig. 4C), suggesting that Emi1 has a significantly higher affinity than a natural substrate.
The results in Figure 4, B and C, also suggest that Emi1 uses the ZBR as an additional activity to prevent cyclin B binding. A fragment of Emi1 containing only the ZBR (amino acids 378–419) retains a modest ability to both capture the APC/C from HeLa extract and compete with cyclin B–APC/C binding (Supplementary Fig. 2E). Taken together, these results suggest the D-box enables Emi1 to bind efficiently to the APC/C whereas the ZBR can block access of APC/C substrates to the core enzyme. In addition to blocking substrate binding, this inhibition may work by inactivating other functions important for APC/C catalysis, although we have currently excluded E1–E2 charging and E2–APC/C binding (Supplementary Fig. 2F). Previous work has shown that Emi1 does not inhibit the activity of APC2/APC11 in a minimal reaction (Reimann et al. 2001b).
Emi1’s function as an inhibitor of APC/C E3 ligase activity requires contributions from its D-box and ZBR motifs
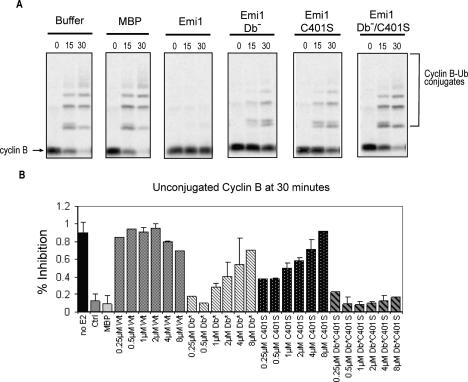
To further understand the contribution of the Emi1 D-box and ZBR to its APC/C inhibitory function, we tested the effect of mutations in these sequences on their ability to inhibit the APC/C using an in vitro ubiquitination assay. Here, we found Emi1 was able to inhibit the ability of the APC/C to ubiquitinate cyclin B to background levels at a dose of 0.5 μM (Fig. 5), consistent with previous reports (Reimann et al. 2001b). The ZBR mutant C401S required higher concentrations of ~4–8 μM to maximally inhibit APC/C activity. Likewise, mutation of the Emi1 D-box (RxxL → AxxA) also strongly reduced Emi1’s APC/C inhibitory activity, with concentrations of 8 μM now being required to maximally inhibit. A double mutant lacking both D-box and ZBR was unable to inhibit the APC/C, even at concentrations exceeding 8 μM. Thus, we find that both the D-box and ZBR are required for Emi1 to efficiently inhibit the APC/C.
Figure 5.
Emi1’s APC/C inhibitory function requires both the conserved D-box and ZBR functions. (A) APC/ CCdh1-dependent in vitro ubiquitination assays, using radiolabeled, in vitro translated cyclin B1 protein as a substrate, were incubated for the indicated times (0, 15, 30 min), and the formation of ubiquitinated cyclin B was visualized by autoradiography of SDS–polyacryl-amide gels. Wild-type, D-box mutant (Db−), ZBR mutant (C401S), and D-box, ZBR double-mutant Emi1 was added to the reaction mixture and assayed for the ability to inhibit cyclin B ubiquitination. (B) Quantitation of in vitro APC/C activity showing the amount of cyclin B remaining unconjugated at 30 min for a range of doses of Emi1 variants.
In these experiments, the Emi1 inhibitor was present throughout the ubiquitination experiment. As a more stringent test of Emi1’s APC/C inhibitory activity, we preincubated recombinant Emi1 wild-type or mutant versions with immunopurified APC/C for 30 min and then washed the beads to remove any unbound Emi1. Again, the Emi1 double mutant failed to inhibit APC/C activity, and the single D-box mutant and ZBR mutant both exhibited less activity. The D-box mutant appeared to be a less effective inhibitor in this assay, presumably because of a decreased ability to bind to the APC/C (Supplementary Fig. 3A,B). Thus, although both the ZBR and D-box can contribute to APC/C inhibitory activity in vitro at higher concentrations than are presumably seen in vivo, Emi1 requires both D-box-dependent APC/ C-binding function and ZBR-dependent ability to block substrate binding, presumably through an interaction outside the D-box receptor. We believe these interactions reflect the normal in vivo function of wild-type Emi1, which may remain associated with specific pools of the APC/C in interphase.
Mutation of the Emi1 ZBR converts Emi1 from an APC/C inhibitor to a D-box dependent APC/C substrate
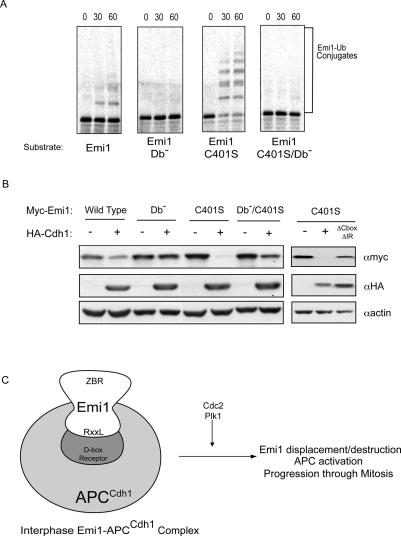
We found that the conserved D-box in Emi1 is able to bind to the APC/C D-box receptor, but that the ZBR provided an independent APC/C inhibitory function. We hypothesized that Emi1 might function as an APC/C substrate in the absence of the ZBR. Therefore, we tested the ability of wild type and the ZBR mutant of Emi1 to be ubiquitinated by the APC/C. We found that whereas wild-type Emi1 protein was a poor APC/C substrate, the ZBR mutant was efficiently ubiquitinated (Fig. 6A). Further, we found that the ZBR–D-box double mutant was not efficiently ubiquitinated, compared with the ZBR single mutant. Thus, lacking its essential ZBR-dependent APC/C inhibitory function, Emi1 becomes an efficient D-box-dependent APC/C substrate. Viewed in this context, by combining a D-box receptor-binding site and an additional ZBR-dependent inhibitory activity, Emi1 functions as a pseudo-substrate inhibitor of the APC/C.
Figure 6.
Loss of IBR/ZBR function in Emi1 converts the protein from APC/C inhibitor to APC/C substrate. (A) Standard APC/C-dependent ubiquitination assays, using radiolabled, in vitro translated C-terminal Emi1 wild-type or mutant protein as substrate. Reactions were incubated for 60 min, and the formation of ubiquitinated Emi1 was visualized by autoradiography of SDS–polyacrylamide gels. (Db−) D-box mutant. (B) Cdh1 over-expression reduces levels of cotransfected Emi1 C401S/ZBR. HA-Cdh1 and Myc-Emi1 variants were cotransfected into HEK293T cells. Lysates were harvested and Emi1 levels detected by anti-Myc immunoblot. A mutant version of Cdh1 lacking the C-terminal IR dipeptide and the C-box (ΔCboxΔIR) was used as a control. (C) A model for evolving APC/C substrates and inhibitors. Emi1 protein binds to the APC/C in a D-box-dependent manner to inhibit APC/C activity through G2 and early mitosis, permitting cyclin accumulation. The ZBR motif of Emi1 contributes to inhibition by blocking substrate access to the APC/C, as well as providing an additional inhibitory function that prevents the ubiquitination of substrates and Emi1 itself. The combination of D-box and ZBR allows Emi1 to compete with APC/C substrates for APC/C binding. Phosphorylation of Emi1 by Cdc2 and Plk1, potentially in conjunction with other events, results in the displacement and proteolytic destruction of Emi1, allowing for APC/C activation and progression through later stages of mitosis.
We also examined whether Emi1 lacking its ZBR function could serve as a substrate of the APC/C in vivo. Previous studies showed that overexpression of APC/C activators causes a decrease in levels of APCCdh1 substrates (Bashir et al. 2004). We cotransfected the HA-tagged APC/C activator Cdh1 in HEK293T cells along with Myc-Emi1 variants and found that levels of wild-type Myc-Emi1 protein were slightly reduced by Cdh1 overexpression (Fig. 6B, left panel). This suggests that Emi1 may have a limited ability to serve as an APCCdh1 substrate, or may simply represent a nonspecific effect of cotransfection. However, the levels of the Myc-Emi1 C401S mutant were strongly reduced by Cdh1 overexpression (Fig. 6B, right panel). In agreement with our in vitro results, the effect of Cdh1 overexpression on the ZBR mutant could be reversed by additionally mutating the D-box. Transfection of a form of Cdh1 containing mutations in APC/C-interacting motifs (ΔCbox, ΔIR), which have been shown to interfere with Cdh1 activator activity, failed to reduce Myc-Emi1 C401S levels (Fig. 6B) indicating that the observed changes in Myc-Emi1 levels upon Cdh1 overexpression are a result of APCCdh1-dependent destruction.
The Cdh1-induced decrease was not a result of the known Emi1–SCFβrCP destruction pathway (Margottin-Goguet et al. 2003; Hansen et al. 2004), as mutation of the conserved βTrCP degron DSGxxS in the Emi1 sequence (S145/9A) had no effect on the ability of Cdh1 to cause a decrease in Myc-Emi1 levels (Supplementary Fig. 3C).
We observed that the Emi1 ZBR mutant behaved similarly in Xenopus extracts in which the APC/C is activated by the addition of exogenous Cdh1 (Supplementary Fig. 3D). The bulk of in vitro translated Emi1 ZBR mutant was degraded by 30 min, while the ZBR–D-box double mutant remained stable even at 60 min.
Discussion
The function of Emi1 as an APC/C inhibitor is clearly important for the S-phase accumulation of cyclins and progression to mitosis, best demonstrated in HeLa cells (Hsu et al. 2002). Here, we find that Emi1 forms a natural, high-molecular-weight complex with the APCCdh1 complex. This complex may account for the majority of the APC/C in the nucleus of interphase cells. Within this context, our data support the idea that the normal function of Emi1 in interphase cells is to persistently associate with and inhibit the APC/C.
We studied the requirements for Emi1 binding to the APC/C using an immobilized Emi1-binding assay. Here, Emi1–APC/C association required a conserved D-box in the Emi1 C terminus to provide the majority of the APCCdh1-binding affinity, whereas the ZBR had a modest contribution to binding. Either the D-box or the ZBR is sufficient to partially antagonize substrate binding to the APC/C, and each contributes to the ability of Emi1 to block efficient ubiquitin chain formation. Because the Emi1 ZBR does not contribute strongly to Emi1–APC/C binding but nonetheless retains the ability to block substrate binding to the APC/C, it appears that the ZBR does not inhibit through the D-box receptor, but through a separate site on the APC/C. We suggest that the ZBR may be positioned to sterically exclude access to the D-box-binding site on the APCCdh1. Once properly oriented on the APC/C using its D-box, the Emi1 may use the ZBR to act as a plug to block substrates and/or key ubiquitination enzymes from reaching the active site of the APC/C.
Our previous work demonstrated a direct association of Emi1 with APC/C activators Cdc20 (Reimann et al. 2001a) and Cdh1 (Reimann et al. 2001b; Hsu et al. 2002), which provided one mechanism for APC/C inhibition. The ability of Emi1 to bind purified Cdh1 directly does require the Emi1 D-box. The ability of Emi1’s D-box to associate with the major APC/C complex, including Cdh1, suggests that Emi1 either binds the APC/C via a D-box receptor on Cdh1, through a separate D-box receptor on the APC/C, or a combination of the two. The possibility of a direct D-box receptor on the APC/C was suggested by Yamano et al. (2004) based on their finding that the cyclin B D-box associated with the APC/C in Xenopus extracts lacking Cdh1 and Cdc20. We similarly found that Emi1 can bind the APC/C in the absence of Cdc20 and Cdh1 in Xenopus egg extract. However, Burton et al. (2005) have shown that in yeast Cdh1 is required for a D-box substrate Hsl1 to bind to purified APC/C. It remains possible that an unidentified APC activator, possibly one specific to meiotic cells as found in other organisms, or additional factors in higher organisms may direct or enhance binding to D-box substrates directly with APC/C.
Does Emi1 bind to the APC only through Cdh1? Interestingly, we find that Emi1 depletion removes almost 100% of Cdh1 from HeLa extracts and at least 80% of the APC/C. However, the levels of Cdh1 appear to be a small, substoichiometric fraction of the levels of the APC holoenzyme. Further, depletion of Cdh1 from HeLa extract did not affect Emi1 capture of the APC/C. Thus, it is unlikely that Cdh1 serves as a persistent physical bridge between Emi1 and the APC/C. We favor the model that Emi1 interacts first with Cdh1 to be loaded onto the APC/C and that there is an independent D-box receptor, such as APC10/Doc1, on the APC that will allow binding of D-box proteins like Emi1 and cyclin B. The D-box receptor within the core APC/C revealed by this study and that of Yamano et al. (2004), which both used a high concentration of D-box protein immobilized on beads, is of significantly lower affinity than that of the D-box receptor on the activators. The apparently higher affinity of Emi1 to Cdh1 than the APC/C may be important given the lower abundance of Cdh1, or may suggest an ordered interaction with Cdh1 first and the APC/C second.
The stable high-affinity association of Emi1 and the APC/C in interphase raises the critical questions of when and how Emi1 is displaced from the APC/C to relieve inhibition. The destruction of Emi1 by a SCF ubiquitin ligase is triggered by the Polo-like kinase Plk1 and accelerated by cyclin B/Cdc2 kinase (Hansen et al. 2004; Moshe et al. 2004), leading to the activation of the APC/C in early mitosis (Fig. 6C). Because Emi1 tightly binds the APC/C core in interphase, the displacement of Emi1 from the APC/C may be required before it can be targeted for ubiquitin-dependent destruction. This displacement step could be regulated by the same mitotic kinases required for Emi1 destruction.
Recent studies have shown that a highly conserved homolog of Emi1, called Erp1/Emi2, plays a critical role in inhibiting the APC/C in Meiosis II to prevent activation of cyclin destruction in unfertilized eggs (Schmidt et al. 2005; Tung et al. 2005). Like Emi1, Erp1/Emi2 also contains a D-box and ZBR domain within the C-terminal inhibitory domain (Fig. 3A; Supplementary Fig. 2A). We find that Emi2 does associate with the APC/C (J.J. Miller and P.K. Jackson, unpubl.), supporting the idea that Emi2 would also form a complex with the APC/C as part of its APC/C inhibitory function in meiosis II.
The ability of a conserved degradation sequence, or degron, paired with a catalysis-inhibitory function suggests a general model for evolving substrates of ubiquitin ligases into regulatory inhibitors. Similar models for pseudosubstrate inhibition of other regulatory enzymes, notably protein kinases (Russo et al. 1996), suggest that coupling a high-affinity binding site or catalysis inhibitory domain to a typical peptide substrate site provides an efficient enzyme inhibitor. A recent study suggested that the SCFβTrCP ubiquitin ligase may have a pseudosubstrate regulator as the chromatin-associated hnRNP-U protein may regulate substrate binding (Davis et al. 2002), although a specific βTrCP degron within this protein was not identified. The possibility that other pseudosubstrate inhibitors of E3 ubiquitin ligases may couple conserved degrons with specific E3 inhibitory domains suggests a means for how these inhibitors may be identified.
The ordered destruction of APC/C substrates, notably cyclin A, securin, and cyclin B, ensures that specific steps in mitosis occur in the correct sequence. One way to achieve this ordered timing is by tuning the affinity or Km of each substrate to allow the “best” substrate to be destroyed first and lower-affinity substrates would be destroyed later. Here, the queue of destruction of substrates would be linked to the rank order of Km values for each substrate. Maximum discrimination of timing is further enhanced when the APC/C is limiting and the rate of substrate destruction is saturated. Here, the “best” substrates serve as inhibitors to push lower-affinity substrates further back in the queue. Additional regulatory events and localization mechanisms may contribute to and expand the relative timing. However, given that the time cells spend in interphase may vary greatly, there must be a mechanism to release the queue at the appropriate time in mitosis. Further, if the “best” substrate is destroyed first in the queue, what prevents this substrate from binding to the APC/C and being destroyed prematurely? By evolving a high-affinity D-box protein like Emi1 into an inhibitor, the cell can use the single event of destroying the inhibitor—cued by mitotic entry and nuclear envelope breakdown—to initiate the timed and ordered destruction of cyclin A, securin, and cyclin B after the highly variable time spent in inter-phase.
Materials and methods
Antibodies
The following antibodies were used for immunoblotting: rabbit anti-hEmi1 (Hsu et al. 2002), mouse monoclonal anti-hEmi1 (raised against full-length MBP–Emi1 fusion protein), mouse anti-Cdc27 (610454; BD Transduction Laboratories), rabbit anti-APC1, APC2, APC5, APC8 (Hongtao Yu, University of Texas Southwestern Medical Center), rabbit anti-APC4, APC6, APC7, APC11 (Jan-Michael Peters, Research Institute of Molecular Pathology), mouse monoclonal anti-Myc 9E10 antibody (Teresa Wang, Stanford University), rabbit anti-Cdc20 (Edgar Kramer, Max-Planck Institute), rabbit anti-Cdc20 (Zymed), and mouse anti-Cdh1 (NeoMarkers). Rabbit anti-Emi1, rabbit anti-Cdc27 raised against GST-hCdc27, and rabbit anti-myc (sc-789; Santa Cruz Biotechnology) were used for immunoprecipitation.
Plasmids and purified proteins
Constructs for wild-type and D-box mutant cyclin B N terminus were obtained from Guowei Fang (Stanford University) and purified by standard methods. pCS2 + Myc-hEmi1 was previously described (Hansen et al. 2004) and site-directed mutagenesis was performed using QuikChange protocol. pMal-Emi1 site-directed mutants were created by standard methods and verified by sequencing. Recombinant MBP–Emi1 fusion proteins were purified as described previously (Hsu et al. 2002). Cdh1 was expressed in Sf9 cells and purified by standard methods.
HeLa cell extraction
HeLa whole-cell pellet was resuspended in Buffer A (10 mM Tris at pH 7.4, 10 mM KCl, 1.5 mM MgCl2, 1 mM DTT, LPC cocktail [Leupeptin, Pepstatin, and Chymotrypsin, each at 10 μg/ mL]) and subjected to nitrogen decompression using a Parr Instruments Cell Disruption Bomb. After centrifugation at 12,000g, the soluble fraction (Cytoplasmic) was removed and the remaining pellet resuspended in Buffer B (10 mM Tris at pH 7.4, 420 mM KCl, 1.5 mM MgCl2, 25% Glycerol, 1 mM DTT, LPC). Samples were spun again (12,000g for 10 min), the supernatant was removed (Nuclear fraction), and the pellet was re-suspended in Buffer B plus 0.1% Triton X-100. Samples were spun again, the supernatant was removed, and the pellet was resuspended in Buffer B + 0.1% Triton X-100 and sonicated (20% duty cycle, 40 sec). After a final spin, the supernatant from each step was dialyzed into 20 mM Tris (pH 7.4), 100 mM KCl, 10% Glycerol, 0.5 mM DTT, and 0.1 mM PMSF.
Chromatography
HeLa cell nuclear fractions were fractionated on a Q-Sepharose column and eluted with a 0.1–0.6 M KCl gradient. Pooled fractions containing Emi1 were separated on a Superose 6 gel filtration column (Amersham Biosciences) or a Superdex 200 gel filtration column (Amersham Biosciences). In addition, nuclear extracts were fractionated directly by Superose 6 gel filtration.
Immunoprecipitation
Affinity-purified anti-Emi1 antibody, mouse monoclonal IgG (Jackson Immunoresearch), anti-Emi1 rabbit polyclonal sera, anti-Cdc27 rabbit polyclonal sera, or preimmune sera were covalently coupled to protein A Affiprep beads (Bio-Rad). Beads were washed with IP Buffer (20 mM Tris at pH 7.4, 100 mM KCl, 0.1% NP-40, 10% Glycerol, 1 mM MgCl2, LPC) and incubated with HeLa cell nuclear fractions or pooled fractions from anion exchange (4°C for 45 min). Immunoprecipitates were washed three times with IP Buffer, eluted with 2 M MgCl2 and separated by SDS-PAGE.
Identification of SDS-PAGE bands by mass spectrometry
Protein samples eluted from anti-Emi1 antibody beads were separated by SDS-PAGE and stained with Colloidal Blue Staining Kit (Invitrogen). Individual bands were excised from gel, reduced with DTT, and alkylated with acrylamide, resulting in propionamide-modified cysteines. Promega-modified trypsin was used for the proteolysis. Liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) was run with a peptide trap for online desalting, followed by a 0.150 × 100-mm C18 column, and elution directly into the ESI-ion trap mass spectrometer. The online Mascot MS/MS Ions Search from Matrix Science (http://www.matrixscience.com) was used for the database search.
Plasmid and siRNA transfections
HEK293T cells were transfected with pCS2 + myc or pCS2 + HA constructs and harvested as described previously (Hansen et al. 2004). siRNA oligoduplexes (Genentech) were transfected at 100 nM final concentration using oligofectamine (Invitrogen). The target sequence of the Cdh1 oligoduplex is GCAACGATGTGTCTCCCTATT. The target sequence of the APC3 oligoduplex is GGAAATAGCCGAGAGGTAATT.
Capture assay
Immobilization of protein Recombinant proteins were coupled to CNBr-activated Sepharose 4B (Amersham Biosciences) in Coupling Buffer (0.1 M NaHCO3 at pH 8.3, 0.5 M NaCl) at a protein concentration of 0.1 μmol/mL medium (16 h at 4°C) and washed three times with Coupling Buffer, and remaining active groups on medium were blocked with 0.1 M Tris (pH 8.0) and 0.5 M NaCl (16 h at 4°C).
Binding assay CNBr-Sepharose containing 1 μmol immobilized protein was incubated with 100 μL of HeLa cell extract (10 μg/μL) in 10 mM Hepes (pH 7.4), 100 mM KCl (2 h at 4°C). The samples were washed three times with 10 mM Hepes (pH 7.4) and 250 mM KCl and bound proteins were resolved by SDS-PAGE and immunoblotting. Reactions involving immobilized cyclin B were incubated at 20°C.
Quantitation Levels of APC binding were measured by densiometry using ImageQuant program (GE Healthcare).
Cdc20 depletion
Stage VI oocytes were obtained and injected with 0.4 ng each of two antisense oligonucleotides against Cdc20 (Taieb et al. 2001) in a maximum volume of 50 nL. Oocytes were matured with 10 μg/mL progesterone in OR2, and lysates were prepared as described (Hochegger et al. 2001).
In vitro ubiquitination assay
The assay was performed as described previously (Reimann et al. 2001b) using anti-Cdc27 immunoprecipitates from interphase Xenopus extract and hCdh1 as activator. Assays contained E1, E2, ubiquitin, ATP, and the 35S-labeled in vitro translated substrate (cyclin B for Fig. 5 and Supplementary Fig. 3A, Emi1 for Fig. 6.) Recombinant MBP fusion proteins were incubated for 30 min with activated anti-Cdc27 immunoprecipitates before addition of substrate. MBP fusion proteins were present throughout incubation with substrate for reactions presented in Figure 5. MBP fusions were removed before incubation with substrate for reactions presented in Supplementary Figure 3A.
Acknowledgments
We thank Jan-Michael Peters for anti-APC antibodies and Cdh1 ΔCbox and ΔIR constructs; Hongtao Yu for anti-APC antibodies; Jeff Tung for assistance with the Cdc20 depletion experiment; Allis Chen, Andrew Guzzetta, and Evelyn Wang for performing mass spectrometry analyses; and Guowei Fang for critical reading of the manuscript. This work was supported by NIGMS grants RO1 GM60439 and RO1 GM54811 (to P.K.J.), NIGMS Medical Scientist Training Grant GM07365 (to J.J.M.), and the Tumor Biology Training Grant CA09151 (to M.K.S.).
Footnotes
Supplemental material is available at http://www.genesdev.org.
Article published online ahead of print. Article and publication date are online at http://www.genesdev.org/cgi/doi/10.1101/gad.1454006.
References
- Bashir T., Dorrello N.V., Amador V., Guardavaccaro D., Pagano M. Control of the SCF(Skp2–Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature. 2004;428:190–193. doi: 10.1038/nature02330. [DOI] [PubMed] [Google Scholar]
- Burton J.L., Solomon M.J. Hsl1p, a Swe1p inhibitor, is degraded via the anaphase-promoting complex. Mol. Cell. Biol. 2000;20:4614–4625. doi: 10.1128/mcb.20.13.4614-4625.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton J.L., Tsakraklides V., Solomon M.J. Assembly of an APC–Cdh1–substrate complex is stimulated by engagement of a destruction box. Mol. Cell. 2005;18:533–542. doi: 10.1016/j.molcel.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Carroll C.W., Enquist-Newman M., Morgan D.O. The APC subunit Doc1 promotes recognition of the substrate destruction box. Curr. Biol. 2005;15:11–18. doi: 10.1016/j.cub.2004.12.066. [DOI] [PubMed] [Google Scholar]
- Castro A., Bernis C., Vigneron S., Labbe J.C., Lorca T. The anaphase-promoting complex: A key factor in the regulation of cell cycle. Oncogene. 2005;24:314–325. doi: 10.1038/sj.onc.1207973. [DOI] [PubMed] [Google Scholar]
- Davis M., Hatzubai A., Andersen J.S., Ben-Shushan E., Fisher G.Z., Yaron A., Bauskin A., Mercurio F., Mann M., Ben-Neriah Y. Pseudosubstrate regulation of the SCF(β-TrCP) ubiquitin ligase by hnRNP-U. Genes & Dev. 2002;16:439–451. doi: 10.1101/gad.218702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Elzen N., Pines J. Cyclin A is destroyed in prometaphase and can delay chromosome alignment and anaphase. J. Cell Biol. 2001;153:121–136. doi: 10.1083/jcb.153.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geley S., Kramer E., Gieffers C., Gannon J., Peters J.M., Hunt T. Anaphase-promoting complex/cyclosome-dependent proteolysis of human cyclin A starts at the beginning of mitosis and is not subject to the spindle assembly checkpoint. J. Cell Biol. 2001;153:137–148. doi: 10.1083/jcb.153.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M., Murray A.W., Kirschner M.W. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Guardavaccaro D., Kudo Y., Boulaire J., Barchi M., Busino L., Donzelli M., Margottin-Goguet F., Jackson P.K., Yamasaki L., Pagano M. Control of meiotic and mitotic progression by the F box protein β-Trcp1 in vivo. Dev. Cell. 2003;4:799–812. doi: 10.1016/s1534-5807(03)00154-0. [DOI] [PubMed] [Google Scholar]
- Hansen D.V., Loktev A.V., Ban K.H., Jackson P.K. Plk1 regulates activation of the anaphase promoting complex by phosphorylating and triggering SCFβTrCP-dependent destruction of the APC Inhibitor Emi1. Mol. Biol. Cell. 2004;15:5623–5634. doi: 10.1091/mbc.E04-07-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J.W., Burton J.L., Solomon M.J. The anaphase-promoting complex: It's not just for mitosis any more. Genes& Dev. 2002;16:2179–2206. doi: 10.1101/gad.1013102. [DOI] [PubMed] [Google Scholar]
- Hilioti Z., Chung Y.S., Mochizuki Y., Hardy C.F., Cohen-Fix O. The anaphase inhibitor Pds1 binds to the APC/ C-associated protein Cdc20 in a destruction box-dependent manner. Curr. Biol. 2001;11:1347–1352. doi: 10.1016/s0960-9822(01)00399-2. [DOI] [PubMed] [Google Scholar]
- Hochegger H., Klotzbucher A., Kirk J., Howell M., le Guellec K., Fletcher K., Duncan T., Sohail M., Hunt T. New B-type cyclin synthesis is required between meiosis I and II during Xenopus oocyte maturation. Development. 2001;128:3795–3807. doi: 10.1242/dev.128.19.3795. [DOI] [PubMed] [Google Scholar]
- Hoyt M.A., Totis L., Roberts B.T. S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell. 1991;66:507–517. doi: 10.1016/0092-8674(81)90014-3. [DOI] [PubMed] [Google Scholar]
- Hsu J.Y., Reimann J.D., Sorensen C.S., Lukas J., Jackson P.K. E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APC(Cdh1) Nat. Cell Biol. 2002;4:358–366. doi: 10.1038/ncb785. [DOI] [PubMed] [Google Scholar]
- Jackson P.K. Linking tumor suppression, DNA damage and the anaphase-promoting complex. Trends Cell Biol. 2004;14:331–334. doi: 10.1016/j.tcb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- King R.W., Glotzer M., Kirschner M.W. Mutagenic analysis of the destruction signal of mitotic cyclins and structural characterization of ubiquitinated intermediates. Mol. Biol. Cell. 1996;7:1343–1357. doi: 10.1091/mbc.7.9.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft C., Vodermaier H.C., Maurer-Stroh S., Eisenhaber F., Peters J.M. The WD40 propeller domain of Cdh1 functions as a destruction box receptor for APC/C substrates. Mol. Cell. 2005;18:543–553. doi: 10.1016/j.molcel.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Li R., Murray A.W. Feedback control of mitosis in budding yeast. Cell. 1991;66:519–531. doi: 10.1016/0092-8674(81)90015-5. [DOI] [PubMed] [Google Scholar]
- Margottin-Goguet F., Hsu J.Y., Loktev A., Hsieh H.M., Reimann J.D., Jackson P.K. Prophase destruction of Emi1 by the SCF(βTrCP/Slimb) ubiquitin ligase activates the anaphase promoting complex to allow progression beyond prometaphase. Dev. Cell. 2003;4:813–826. doi: 10.1016/s1534-5807(03)00153-9. [DOI] [PubMed] [Google Scholar]
- Meraldi P., Draviam V.M., Sorger P.K. Timing and checkpoints in the regulation of mitotic progression. Dev. Cell. 2004;7:45–60. doi: 10.1016/j.devcel.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Moshe Y., Boulaire J., Pagano M., Hershko A. Role of Polo-like kinase in the degradation of early mitotic inhibitor 1, a regulator of the anaphase promoting complex/cyclosome. Proc. Natl. Acad. Sci. 2004;101:7937–7942. doi: 10.1073/pnas.0402442101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K. How do so few control so many? Cell. 2005;120:739–746. doi: 10.1016/j.cell.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Passmore L.A., McCormack E.A., Au S.W., Paul A., Willison K.R., Harper J.W., Barford D. Doc1 mediates the activity of the anaphase-promoting complex by contributing to substrate recognition. EMBO J. 2003;22:786–796. doi: 10.1093/emboj/cdg084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfleger C.M., Kirschner M.W. The KEN box: An APC recognition signal distinct from the D box targeted by Cdh1. Genes& Dev. 2000;14:655–665. [PMC free article] [PubMed] [Google Scholar]
- Pfleger C.M., Lee E., Kirschner M.W. Substrate recognition by the Cdc20 and Cdh1 components of the anaphase-promoting complex. Genes & Dev. 2001;15:2396–2407. doi: 10.1101/gad.918201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann J.D., Freed E., Hsu J.Y., Kramer E.R., Peters J.M., Jackson P.K. Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell. 2001a;105:645–655. doi: 10.1016/s0092-8674(01)00361-0. [DOI] [PubMed] [Google Scholar]
- Reimann J.D., Gardner B.E., Margottin-Goguet F., Jackson P.K. Emi1 regulates the anaphase-promoting complex by a different mechanism than Mad2 proteins. Genes & Dev. 2001b;15:3278–3285. doi: 10.1101/gad.945701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder C.L., Maiato H. Stuck in division or passing through: What happens when cells cannot satisfy the spindle assembly checkpoint. Dev. Cell. 2004;7:637–651. doi: 10.1016/j.devcel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Russo A.A., Jeffrey P.D., Patten A.K., Massague J., Pavletich N.P. Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A–Cdk2 complex. Nature. 1996;382:325–331. doi: 10.1038/382325a0. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Duncan P.I., Rauh N.R., Sauer G., Fry A.M., Nigg E.A., Mayer T.U. Xenopus polo-like kinase Plx1 regulates XErp1, a novel inhibitor of APC/C activity. Genes & Dev. 2005;19:502–513. doi: 10.1101/gad.320705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M., Lutum A.S., Seufert W. Yeast Hct1 is a regulator of Clb2 cyclin proteolysis. Cell. 1997;90:683–693. doi: 10.1016/s0092-8674(00)80529-2. [DOI] [PubMed] [Google Scholar]
- Song M.S., Song S.J., Ayad N.G., Chang J.S., Lee J.H., Hong H.K., Lee H., Choi N., Kim J., Kim H., et al. The tumour suppressor RASSF1A regulates mitosis by inhibiting the APC–Cdc20 complex. Nat. Cell Biol. 2004;6:129–137. doi: 10.1038/ncb1091. [DOI] [PubMed] [Google Scholar]
- Taieb F.E., Gross S.D., Lewellyn A.L., Maller J.L. Activation of the anaphase-promoting complex and degradation of cyclin B is not required for progression from Meiosis I to II in Xenopus oocytes. Curr. Biol. 2001;11:508–513. doi: 10.1016/s0960-9822(01)00145-2. [DOI] [PubMed] [Google Scholar]
- Tung J.J., Hansen D.V., Ban K.H., Loktev A.V., Summers M.K., Adler J.R., III, Jackson P.K. A role for the anaphase-promoting complex inhibitor Emi2/XErp1, a homolog of early mitotic inhibitor 1, in cytostatic factor arrest of Xenopus eggs. Proc. Natl. Acad. Sci. 2005;102:4318–4323. doi: 10.1073/pnas.0501108102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Reijden B.A., Erpelinck-Verschueren C.A., Lowenberg B., Jansen J.H. TRIADs: A new class of proteins with a novel cysteine-rich signature. Protein Sci. 1999;8:1557–1561. doi: 10.1110/ps.8.7.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visintin R., Prinz S., Amon A. CDC20 and CDH1: A family of substrate-specific activators of APC-dependent proteolysis. Science. 1997;278:460–463. doi: 10.1126/science.278.5337.460. [DOI] [PubMed] [Google Scholar]
- Yamano H., Gannon J., Mahbubani H., Hunt T. Cell cycle-regulated recognition of the destruction box of cyclin B by the APC/C in Xenopus egg extracts. Mol. Cell. 2004;13:137–147. doi: 10.1016/s1097-2765(03)00480-5. [DOI] [PubMed] [Google Scholar]
- Yu H., Peters J.M., King R.W., Page A.M., Hieter P., Kirschner M.W. Identification of a cullin homology region in a subunit of the anaphase-promoting complex. Science. 1998;279:1219–1222. doi: 10.1126/science.279.5354.1219. [DOI] [PubMed] [Google Scholar]
- Zachariae W., Schwab M., Nasmyth K., Seufert W. Control of cyclin ubiquitination by CDK-regulated binding of Hct1 to the anaphase promoting complex. Science. 1998;282:1721–1724. doi: 10.1126/science.282.5394.1721. [DOI] [PubMed] [Google Scholar]