Abstract
Supported by a grant from the Agency for Healthcare Research and Quality, a University of Iowa Hospitals and Clinics interdisciplinary research team created an online data-capture-response tool utilizing wireless mobile devices and bar code technology to track and improve blood products administration process. The tool captures 1) sample collection, 2) sample arrival in the blood bank, 3) blood product dispense from blood bank, and 4) administration. At each step, the scanned patient wristband ID bar code is automatically compared to scanned identification barcode on requisition, sample, and/or product, and the system presents either a confirmation or an error message to the user. Following an eight-month, 5 unit, staged pilot, a ‘big bang,’ hospital-wide implementation occurred on February 7, 2005. Pilot period and preliminary house-wide data indicate improved error capture with the new barcode process over the old manual process.
INTRODUCTION
Patient safety associated with the blood transfusion process has received considerable attention in recent years due to the propensity of transfusion errors to cause catastrophic morbidity or mortality.1,2,3,4,5,6,7 Human error leading to potential or actual mistakes in blood administration can occur at any step in the process. Incorrect identification of patients is a major source of blood transfusion errors,1,5,6,7,8,10 as is inexact compliance with complex and time consuming processes.2,3,4,5,6
Error reductions are most likely to be obtained by a systems approach having the goals of reducing incomplete or erroneous identification of patients or blood products, simplifying processes, and obviating clerical or transcription errors.4,5,7,8,9,10 Systems that reduce reliance on human data entry and human double-checking through increased use of computer technology for these functions have the potential to substantially increase productivity and accuracy.5,7,8
Use of bar code technology for patient and product identification is not only a future requirement of the Joint Commission for Accreditation of Healthcare Organizations (JCAHO), but is also a major tool for error reduction.6,7,8,9 Wireless technology enables use of bar code equipment at the patient bedside, maximizing process efficiencies.8,10
The University of Iowa Hospitals and Clinics (UTHC) replaced its existing manual blood product process institution-wide with a point-of-care automated patient and blood product bar code scanning process, addressing the following specific areas of risk:
Patient identification,
Requisition, patient, and specimen sample matching,
Specimen sample encounter in the blood bank,
Blood product dispense from blood bank,
Blood product administration.
The goals of the project were to:
Increase patient safety through the use of bar code technology for patient identification,
Reduce errors in blood transfusion process using point-of-care wireless specimen and product tracking.
METHOD
The UIHC is a 772 bed tertiary care teaching facility that annually serves over 41,000 inpatient and 845,000 clinic and emergency room visits. The UTHC administers over 43,000 blood products annually. The hospital’s internally developed Electronic Health Record (EHR) sits on 2 IBM z/Series processors with a central data repository of almost 2 terabytes of storage, representing 2 million patient records. Information Network For On-line Retrieval & Medical Management (INFORMM) is the mainframe application upon which the INFORMM Patient Record (IPR), a PowerBuilder-based client-server application, is built. Collectively, the system supports over 6.1 million transactions daily performed by 7,100 health care providers and clerical staff in support of patient care processes, medical record documentation, order entry, and results reporting.
Under direction of a Hospital Advisory Committee task force, a research grant application was submitted to AHRQ requesting funding to support implementation of wireless bar code technology for blood products administration throughout the institution. Upon approval of this application, a project team was formed, lead by the research grant Co-Principle Investigators: the Senior Associate Director for Clinical Outcomes and Resource Management (CORM), and UIHC Chief Information Officer. Other team members were drawn from the Department of Nursing and Patient Care Services (DON), Department of Pathology, Health Care Information Systems (HCIS), and Epidemiology.
A project work group was formed that included nurses, physicians, and Pathology, Blood Center, and HCIS staff. This group developed detailed flow diagrams of current work-flow and the proposed bar-coded system work-flow, created and tested new programming, re-wrote policies and procedures, developed a roll-out plan and a down-time plan, and reported activity to the Grant Oversight Team. Members also reported progress to their respective departments. The work group identified a number of steps that needed to be accomplished prior to pilot of the new process, including institution of a new bar code ID band, installation of a wireless network, and selection of wireless devices.
The previous hospital standard embossed ID band was replaced with the new bar-coded ID band. This change alone required multiple steps and decisions: testing and selection of new bands and labels, purchase and placement of nearly 200 label printers with requisite Ethernet lines, and in-servicing staff in creation and use of new labels and bands. The new band and label are latex free, impermeable to liquid, comfortable, multi-purpose, and inexpensive, in addition to supporting the patient-specific bar code.
To support the use of point-of-care wireless devices, the existing network infrastructure was augmented with an institution-wide wireless network. Need estimates were based upon 4 access points (AP’s) per 20K square feet.
The project team evaluated and tested an array of wireless devices. Primary features tested were reliability, ease of use, functionality, and total cost of ownership. A cart fair that was widely publicized internally was utilized to allow clinicians to gain experience with all devices under consideration and to give input through a formal evaluation process. Phlebotomy and Nursing departments ultimately selected different mobile carts, based on their different work processes. Personnel in some outpatient clinics selected hand held devices rather than carts based on space constraints or workflow needs. All devices were provided with tethered Symbol Bar Code Laser Readers after testing demonstrated that wireless, non-laser scanners had a much lower first time read rate than the tethered scanners.
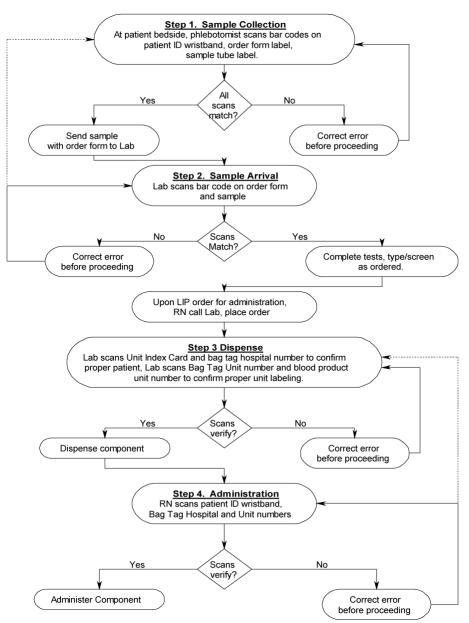
Once preliminary steps were completed, work group attention turned to the design of the new process. The newly designed online system captures and tracks activity at each step of the four step process:
Sample Collection. Bar code labels on the doctor’s order sheet and sample label are compared to that on the patient ID (PID) wristband, and must match, or a redraw is required.
Sample Arrival in Blood Bank. Bar code labels on the doctor’s order sheet and sample label are compared and must match, or sample is rejected.
Product Dispense. Order card bar code from patient care area and bag tag hospital number bar code are scanned and must match to confirm correct patient, and product bag unit number and blood product unit number are scanned and must match to confirm proper unit labeling. Any error must be corrected before dispensing.
Product Administration. PID wristband is scanned first, and PID bar code on the product bag is scanned and must match. The product bag unit number is also scanned, and must match what was dispensed from the blood bank for the patient, or the unit must be returned to the blood bank (Figure 1).
Figure 1.
Upon correct scan, the user is presented with the onscreen message “All scans match, verification is correct, please proceed.” Upon a mismatch, or incorrect scan, an error message appears on the screen, and the screen background turns bright red to alert the user. This red color change has been very effective in capturing the user’s attention for error correction.
The online history function automatically tracks all activity with fields for Events 1–4 (above), Error Flag, PID, Sample ID, Requisition ID, Order Card ID, datetime, Error Message, Operator, Collector (used for downtime), and Product Type. Misscans display in the history in red, and key-entered data are highlighted in yellow to make these entries easy to spot for follow-up. One team member from Nursing and one from Pathology were assigned responsibility for following up on errors in their respective departments, and addressing needs as dictated by the situation either on an individual or department-wide basis.
Once preliminary work was completed, a pilot was initiated in one outpatient clinic. The initial pilot utilized a dual process, requiring staff to first perform the new bar code process for all steps, and then to perform the old manual process. Based on feedback from this pilot unit, both programming and process were refined, and the pilot was extended, one unit at a time, to an adult inpatient unit, a pediatric inpatient unit, an intensive care unit, and an adult transplant unit. Data from all units were aggregated and analyzed by the research team. Results were discussed with UIHC leadership staff.
During the pilot patients moved throughout the hospital (e.g., from emergency to operating room to intensive care) from new bar code process units to old manual process units. This dictated either a dual process until universal roll out was accomplished, or a house wide synchronized conversion to new process. Hospital leadership determined that a ‘big bang’ rollout model would be utilized. This decision necessitated that staff education and security access preparation, wireless network readiness, mobile device configuration and testing, and planning for 24/7 user support upon go-live all be accomplished institution-wide prior to go-live. DON, HCIS, and Pathology staff worked together to provide either user or super user training to 2000 nurses, phlebotomists, anesthesiologist, perfusionists, and blood bank and critical care laboratory personnel. All trained staff were given access to the online tool based on requirements of their particular job. The Clinical Applications Support Team (CAST) configured, tested, and dispensed 97 mobile and hand held devices with scanners. Testing included ensuring network connectivity in each individual patient care room. HCIS hired and trained additional Help Desk staff to support wireless network and hardware issues around the clock.
RESULTS
System activity was used as a rate denominator for Relative Risk comparison in an historic cohort study design. From the pilot period, analysis of Relative Risk for the difference of two proportions was performed using PROC FREQ in SASv.9 with asymptotic confidence intervals. The 4/21/04 -12/27/04 pilot period included 8,824 instances of system activity. The relative risk (RR) of detecting a misidentification prior to administration (95% CI):
2004 (Study Year) vs. 2003: 9.98 (1.28, 78.0)
2004 (Study Year) vs. 2002: 3.33 (0.92, 12.1)
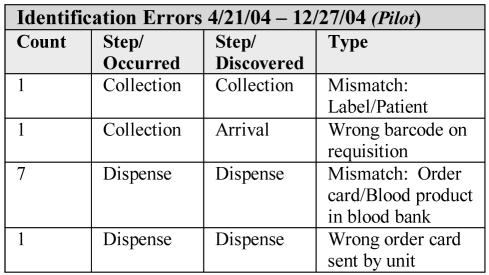
Thus, from the pilot data, we estimated that we were between 3 and 10 times more likely to catch an identification error using the barcode system than with the manual system. Although these numbers are small, they are indicative of improvement. See Figure 2 for actual errors caught during pilot. Note that all but one were discovered at the moment of occurrence, a major improvement over the manual process.
Figure 2.
Post house-wide go-live, sample rejection decreased from an average of 1.82% in the manual system to 0.17% in the automated system. Comparing scanner-detected prevented errors to voluntarily-reported incident reports from the same time period in previous years (2/7 to 4/21 in 2003 and 2004), the relative risk of finding a misidentification event: (95% confidence interval)
AT SAMPLE PROCESSING increased 10 fold (RR= 9.98 (2.9 – 34.5)). Total activity = 6,953
-
IN ANY STEP OF THE PROCESS increased 30 fold
(RR= 30.6 (9.5 – 98.4)). Total activity = 22,569
Thus, we estimate that we are 30 times more likely to catch an identification error using the barcode system than with the manual system.
DISCUSSION
In the manual system, identification errors were generally discovered in the blood bank, often resulting in a delay of care, an additional sample collection, or a wastage of blood product. A major benefit of the computer system is immediate notification to the clinician of a mismatch that can then be corrected immediately.
Project costs included purchase and installation of barcode label printers on every in-patient and outpatient area, purchase and installation of the house-wide wireless network, purchase and configuration of mobile computing devices and carts, and house-wide, multi-departmental staff education. These costs, only partially offset by AHRQ grant monies, can pose a significant barrier to implementation.6'7'8 UIHC intends to leverage these devices by extending their use to bar-code medication administration and CPOE. Additional benefits include process simplification for nursing staff, who have embraced the process change.
Success of any new computer-based process depends at least in part on the project team’s ability to anticipate and obviate potential workflow issues, and to respond rapidly to unanticipated user needs to forestall process breakdown and workaround development. The work group anticipated that it would be possible for staff to scan a bar code label that was not on the patient wrist as a workaround. The critical importance of identifying the patient correctly and scanning only the bar code on the patient wrist was stressed during training. In addition, each trainee signed a mandatory attestation form. This form stated that the clinician signer agreed to scan as the patient identifier only the PID on the patient wrist.
Unanticipated issues and solutions included:
Inaccessible Wristbands in the OR. Often patients’ arms are tucked under sterile drapes and completely inaccessible to anesthesiologists. To support this reality, special programming for the anesthesiologists allows this group to scan first the PID wristband, and then a label on the anesthesiology record. After a match is recorded by the system, the bar code on the anesthesiology record can be used as a PID proxy for the duration of the case.
OR Rapid Infusion (‘blood bath’) Situations. Functionality was created to support those instances when it is necessary to give a number of blood products in rapid succession. The programming accommodation of this anesthesiology requirement was a major factor in process acceptance by that group.
Separate Equipment Requirement for Perfusionists. Even though all clinicians saw and selected equipment and received training on the new application prior to go-live, they were not always able to kinesthetically visualize the process as it affected them in their work areas. Perfusionists in particular did not realize until after go-live that they would be unable to physically access the scanning equipment in the OR. Additional equipment was purchased and placed for this group.
Dual Process until Rollout Complete. A phased pilot is difficult if patients transfer through the pilot and non-pilot care areas, and the system edits against mid-process introductions. A dual process was required of the pilot units until the ‘big bang’ implementation.
Idiosyncratic Workflow in Same Day Surgery. AABB regulations require that the date on the sample tube equal the date of sample collection. Workflow in the Day of Surgery area (same day surgery) necessitates previous day paperwork, including label printing. Programming to accommodate this need was created and implemented.
Surprise Bar Code Symbology. The current UIHC pathology vendor system utilizes American Blood Commission (ABC) Codabar bar code labels. Several weeks after go-live, the blood band received a product with an International Society of Blood Transfusion (ISBT 128) label. This format was longer than what had been specified as the maximum expected unit label character length. Program adjustments had to be made to the software to accommodate the ISBT 128 label format.
Dueling Scanners. The new programming included functionality that can differentiate a blood unit label number from the blood bag unit number. In order to leverage that advantage, and to preserve the required functionality of the blood bank legacy system, it was necessary to attach two scanners to five blood bank workstations. Additionally, on nursing units, existing legacy barcode scanners for medical record and radiology film tracking require very different hard-coding, making it impossible to use the same scanner for both processes. Programmers modified the legacy application software to enable scanners to read all clinical bar codes.
Broad Scope of Project. The project piloted not only a new application and process but also numerous types of new equipment, new equipment configurations, and a new wireless network. It would have been less intensive to pilot the hardware and network with an existing application and then implement the new application and process after all the new wireless network and hardware issues were identified and addressed.
SUMMARY
There are a number of system, workflow, hardware and software issues that must be addressed to ensure the successful implementation of a wireless, barcode blood products administration process. Specific risk areas in the blood products administration process include the points of patient identification; requisition, patient, and specimen sample matching; specimen sample encounter; blood product dispense; and blood product administration. The goal of this project was to reduce risks in those areas. Using wireless, bar code technology for point-of-care patient identification and specimen and product tracking, the system is successful in increasing patient safety and capture of errors over the manual process. The new system captures errors at the point of occurrence, improving the safety and efficiency of clinical care. It is up to 30 times more effective in capturing errors than the manual process. Because the process is effective and efficient, it has received broad acceptance from clinicians.
ACKNOWLEDGEMENTS
This work is the result of a large team, with major contributions by the following individuals: Dr. Loreen Herwaldt, Professor, Dept. of Internal Medicine, Co-Principle Investigator; Lee Carmen, Chief Information Officer, Co-Principle Investigator; Linda Chase, Associate Director, Administration, DON; Lynn Comreid, Advance Practice Nurse, DON; Susan Dane, Pathology Informatics - Clinical Laboratory Manager Department of Pathology; Deborah Greene, Manager, Quality Assurance Department of Pathology; Dr. Charles Helms, Professor, Chief of Staff; Dr. John Kemp, Professor of Pathology; Judith Levitt, Section Manager, DeGowin Blood Center Department of Pathology; Steve McGrane, Application Technical Lead on Bar Code Projects; Sheri Swartzendruber, Risk Project Manager; Dr. Marita Titler, Director, Nursing Research, Quality and Outcomes Management; Jeffrey Vande Burg, Program Associate, Statistician, Program of Hospital Epidemiology;
This research was funded through a major grant from the Agency for Healthcare Research and Quality, HS-03-005, Blood Product Transfusions & Safe Practice Implementation.
REFERENCES
- 1.Linden J, Paul B, Dressier K. A report of 104 transfusion errors in New York State. Transfusion. 1992;32:601–606. doi: 10.1046/j.1537-2995.1992.32792391030.x. [DOI] [PubMed] [Google Scholar]
- 2.Sazama K. Reports of 355 transfusion-associated deaths: 1976 through 1985. Transfusion. 1990;30:583–590. doi: 10.1046/j.1537-2995.1990.30790385515.x. [DOI] [PubMed] [Google Scholar]
- 3.Murphy WG, McClelland BDL. Deceptively low morbidity from failure to practice safe blood transfusion: An analysis of serious blood transfusion errors. Vox Sang. 1998;57:59–62. doi: 10.1111/j.1423-0410.1989.tb04985.x. [DOI] [PubMed] [Google Scholar]
- 4.Linden JV, Kaplan HS. Transfusion Errors: Causes and Effects. Transfusion Medicine Reviews. 1994 Jul;8( 3):169–183. doi: 10.1016/s0887-7963(94)70109-7. [DOI] [PubMed] [Google Scholar]
- 5.Turner CL, Casbard AC, Murphy MF. Barcode technology: its role in increasing the safety of blood transfusion. Transfusion. 2003 Sep;43:1200–1209. doi: 10.1046/j.1537-2995.2003.00428.x. [DOI] [PubMed] [Google Scholar]
- 6.Murphy MF, Kay JDS. Barcode identification for transfusion safety. Current opinion in hematology. 2004 Sep;11(5):334–8. doi: 10.1097/01.moh.0000142801.38087.e5. [DOI] [PubMed] [Google Scholar]
- 7.Dohnalek LJ, Cusaac L, Westcott J, Langeberg A, Sandler SG. The Code to Safer Transfusions. Nursing Management. 2004:33–36. doi: 10.1097/00006247-200406000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Making Health Care Safer: A Critical Analysis of Patient Safety Practices (AHRQ Publication No. 01-E058) Agency for Healthcare Research and Quality. Available at: http://www.ahrq.gov/clinic/ptsafety/spotlight.htm Accessed June 25, 2003.
- 9.JCHAO Sentinel Event Alert. Blood transfusion errors: Preventing future occurrences. . Sentinel Event Alert. 1999:10. [PubMed] [Google Scholar]
- 10.Butch SH. Computerization in the Transfusion Service. Vox Sanguinis. 2002;83(Suppl 1):105–110. doi: 10.1111/j.1423-0410.2002.tb05279.x. [DOI] [PubMed] [Google Scholar]