Abstract
An immunofluorescence assay was developed to identify proteins specifically binding to oligonucleoside phosphorodithioate (ODN) aptamers from a bead-bound ODN library. Accordingly, NF-κB p50 protein was incubated with either bead-bound NF-κB consensus sequence or a bead-bound ODN combinatorial library and adsorption was then assessed using a specific primary antibody and a secondary antibody conjugated with Alexa 488 fluorescent dye. This assay avoids any problems related to fluorescently labeling target proteins. The method is straightforward and readily applicable to other transcription factors and proteins, and the feasibility of its application for high-throughput screening of large aptamer bead-based libraries by flow cytometry is demonstrated.
INTRODUCTION
Nucleic acid combinatorial chemistry (1) is an important technology in searching for nucleic acid pharmaceutical and diagnostic reagents. Over the last decade, an in vitro combinatorial selection procedure (2–4) has been used to identify high-affinity nucleic acid ligands (or aptamers) for a large number of different proteins (5–7) and other molecules (8–11). However, the identification of specific oligonucleoside phosphorodithioate (S2-ODN) aptamers (‘thioaptamers’) that bind proteins is not possible based upon the in vitro com binatorial selection method since the substrates, nucleoside dNTP(αS2), are not recognized by polymerases (12) which are required for re-amplification of selected combinatorial libraries by the polymerase chain reaction (PCR). Using a split synthesis method pioneered by Furka et al. (13) and Lam et al. (14), we have recently synthesized S2-ODN thioaptamer libraries in which only one oligonucleotide (ODN) species is attached to each bead (15). We have also demonstrated that the bead-based libraries can be selected by incubating the beads with a fluorescently labeled protein to select S2-ODN thioaptamers (15). Binding assays using fluorescently labeled substrates assume that the presence of labeled dye does not significantly alter their binding. Although being used in a number of important biological techniques (16), such labeling may affect the selection results since some of the binding groups on the proteins are potentially blocked by the dye molecule. In addition, appropriate labeling may be difficult or even impossible. Thus, the nucleic acid combinatorial approach requires not only the design and construction of libraries, but also the development of novel screening methodologies for library selection.
We recognized that immunofluorescence assays (17) have been used to detect the binding of proteins to specific DNA immobilized on a 96-well microplate (18). Here, we report the initial results to identify specific double-stranded (ds) S2-ODN thioaptamers from a previously studied S2-ODN combinatorial library (15) based on an immunofluorescence assay and demonstrate the application of a two-color immunofluorescence assay in a high-throughput flow cytometry selection. We compare this new screening method targeting transcription factor NF-κB p50 protein with that using the fluorescently labeled protein.
MATERIALS AND METHODS
Chemicals
The dA, dG, dC and dT phosphoramidites were purchased from Applied Biosystems (AB, Palo Alto, CA) or Glen Research (Sterling, VA). The Taq polymerase kits were from AB. The TA Cloning kit was from Invitrogen (Carlsbad, CA). The Klenow DNA polymerase I was from Promega (Madison, WI). Polystyrene beads (15–20 and 60–70 µm) with non-cleavable hexaethyleneglycol linkers with a loading of 70 µmol/g for 15–20 µm, 36 µmol/g for 60–70 µm were from ChemGenes Corp (Ashland, MA; we thank Dr Andrew D. Ellington, UT, Austin for help in the development of these specially constructed beads). Tris–HCl was purchased from Sigma. Sodium chloride, EDTA, ammonium hydroxide (28%) and other chemical reagents were obtained from Aldrich. 5′-Fluorescein phosphoramidite (catalog no. 10-5901-90) was purchased from Glen Research.
NF-κB p50 and p65 proteins
NF-κB p50 and p65 protein were expressed and purified as described previously (19–21).
Synthesis of bead-bound NF-κB consensus ODN
Bead-bound NF-κB IgκB consensus oligonucleotide (5′-AGTTGAGGGGACTTTCCCAGGC-3′) was synthesized on a 1 µmol scale on an Expedite 8909 DNA synthesizer (AB). The coupling yield was typically >99% as determined by the dimethoxytrityl cation assay. The fully protected ODN with the non-cleavable linker beads were treated with concentrated ammonia at 37°C for 21 h to remove the protecting groups while allowing the ODN to remain attached to the beads. A portion of the fully deprotected ODN with the non-cleavable linker beads (typically 1.0 mg) was converted to dsDNA by annealing at 100°C with its complementary sequence in annealing buffer (10 mM Tris–Cl, pH 7.4, 1 mM EDTA, 100 mM NaCl) for 2 min and gradually cooling to room temperature. The beads were washed twice with phosphate-buffered saline (PBS) buffer.
Construction of S2-ODN library
Standard phosphoramidite and thiophosphoramidite chemistry (22–26) was used for the S2-ODN library. The library was prepared on a 1 µmol scale of polystyrene beads. The downstream and upstream primers, 5′-GGATCCGGT GGTCTG-3′ and 5′-CCTACTCGCGAATTC-3′ were synthesized in parallel on a two-column DNA synthesizer (Expedite 8909, AB). The detailed synthesis protocol was described in Yang et al. (15). The fully deprotected S2-ODN with the non-cleavable linker bead-based single-stranded (ss)DNA library was washed twice with double distilled water. The ssDNA library (typically 1 mg of support beads) was converted to a dsDNA by a Klenow DNA polymerase I reaction in the presence of DNA polymerase buffer, dNTP mixture and reverse primer according to the manufacturer. The dsDNA library was washed twice with PBS buffer.
Bead-based immunofluorescence assay
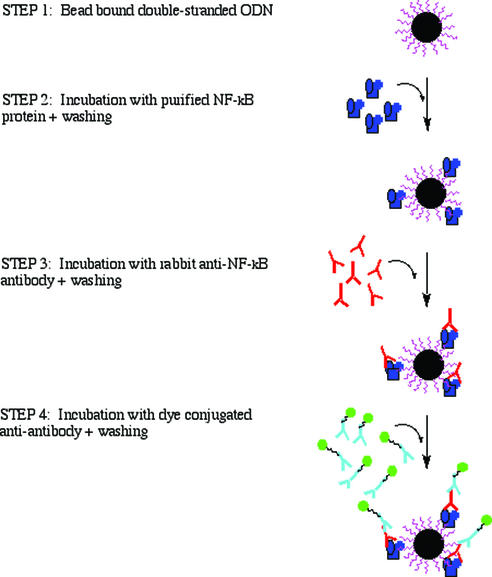
Figure 1 depicts a NF-κB–DNA binding assay schematic. The following parameters are the final conditions used for the bead-based assay after optimization. The optimization steps are briefly summarized in the text.
Figure 1.
The schematic procedure of the bead-bound NF-κB–thioaptamer binding assay.
Step 1: prepared bead-bound double-stranded ODN sequences. Beads (∼1500) were washed twice with 200 µl of PBS (pH 7.4) + 0.1% Tween-20 to remove impurities and block any non-specific binding and then once more with 200 µl PBS alone.
Step 2: binding of NF-κB p50 protein to the beads. The beads were then incubated with 200 µl of p50 protein (2 µg/ml) for 2 h at room temperature with occasional mild agitation (200 r.p.m. on a Vortex) to minimize sedimentation of the beads. The beads were washed three times with 200 µl of PBS (pH 7.4) + 0.1% Tween-20.
Step 3: binding of anti-p50 antibody to p50–DNA complex. Rabbit IgG antibody (Santa Cruz Biotechnology, Santa Cruz, CA; sc-114, each vial contains 200 µg of rabbit IgG in 1.0 ml of PBS containing 0.1% sodium azide and 0.2% gelatin), diluted 1000 times in PBS buffer, was incubated with the beads in the vial for 1 h at room temperature with occasional mild shaking. The beads were then washed three times with 200 µl of PBS (pH 7.4) + 0.1% Tween-20.
Step 4: binding of Alexa Fluor 488 labeled goat anti-rabbit IgG to anti-p50 antibody. Alexa Fluor 488 labeled goat anti-rabbit IgG [500 µl (2 mg/ml); Molecular Probes, Eugene, OR)], diluted 50 times in PBS buffer, was incubated with the beads in the vial for 1 h at room temperature with occasional mild shaking. The beads were then washed four times with 200 µl of PBS (pH 7.4) + 0.1% Tween-20.
Step 5: view or selection of positive beads. Approximately 50 µl of the bead solution was transferred to a slide. Beads were then viewed with an inverted Nikon Diaphot microscope equipped with a Mercury arc lamp. Optical filters from Omega Optical, Inc., specific for Alexa Fluor 488, were used to view all samples. Bright field and fluorescent photos were taken with a Nikon Coolpix 990 digital camera. Once a candidate bead was chosen, that bead was isolated from any contaminating beads nearby and lifted off the slide and placed in a PCR tube by micromanipulation. Borosilicate glass pipettes were pulled and manipulated with a pipet puller (Model PC-10) and a micromanipulator system (Model MMV-22D) from Narishige Co, Ltd. For the S2-ODN library, individual beads with the highest fluorescence intensity were washed with 8 M urea (pH 7.2) to remove the bound proteins.
One-bead one-PCR amplification and sequencing of PCR product
A selected single bead was mixed with the following PCR components: 8 µl of 25 mM MgCl2, 0.5 µl of Taq polymerase (5 U/µl), 1 µl of 8 mM dNTP, 10 µl of PCR buffer, 1 µl of 40 mM primers and water up to 100 µl. The PCR was run on a GeneAmp PCR system 2400 (Perkin Elmer, Gaithersburg, MD). The PCR mixtures were thermal cycled using the following scheme for amplification: 94°C for 5 min (1 cycle); 94°C for 2 min, 35°C for 2 min, 72°C for 2 min (35 cycles) and 72°C for 7 min (1 cycle). The PCR products were analyzed on a 15% native polyacrylamide gel. The PCR product was cloned using the TA Cloning procedure (Invitrogen) and sequenced on an ABI Prism 310 Genetic Analyzer (AB).
Two-color flow-cytometry selection of dsDNA-conjugated bead
Flow-cytometic (FCM) analysis was performed on unbound and bound beads (p50 and p65 positive beads) to show that fluorescently labeled aptamer beads could be detected and differentiated from one another on our system, ultimately for high-throughput screening (HTS) purposes (27–31). The home-built High Resolution Cell Sorter (HiReCS) system was, in this case, set up for standard two-color analysis. A tunable argon-ion laser tuned to the 488 nm wavelength was used in all analyses with optical filters that were optimal for 488 Alexa dye and PE (phycoerythrin) excitation and emission. Samples were acquired on four parameters: Alexa 488, PE, FSC (forward scatter for size), and SSC (side scatter) and stored as listmode data in standard FCS 2.0 format for subsequent analysis. The flow-cytometer data analysis program WinList 5.0™ (Verity Software House, Inc.) was employed for color-gating analysis of bead populations of interest.
RESULTS AND DISCUSSION
Immunofluorescence assay on bead-bound NF-κB consensus ODN
The successive steps of the immunofluorescence assay screening for identifying specific S2-ODN thioaptamers binding to NF-κB p50 were first optimized by using bead-bound NF-κB consensus ODN duplex with purified p50 protein. Beads that are bound to a single ODN sequence were produced by incubating streptavidin-coated beads with a 5′-biotinylated ODN (32). Alternatively using the ChemGenes Corp. polystyrene beads with non-cleavable hexaethyleneglycol linker, synthesis of ss NF-κB consensus sequence on the beads is straightforward based on the established conditions. Importantly, the synthesized ODNs are still covalently attached to the beads with non-cleavable hexaethyleneglycol linker after full base and phosphate ester deprotection. To estimate how many picomoles ssDNA on a single bead, we counted the number of beads based on cell culture technique using a hemacytometer. We calculated that for the 15–20 µm beads there were 6 × 105 beads/mg. With a loading of 70 µmol of substrate/g (Chem Genes Corp.), we concluded that one bead contains ∼0.12 pmol of ssDNA (assuming 100% synthetic yield). Similarly, for the 60–70 µm beads, we obtained 4.7 × 104 beads/mg. Using a loading of 35 µmol of substrate/g, each bead contains 0.77 pmol ssDNA. Since the efficiency of nucleotide coupling was >99.5% monitored by the dimethoxytrityl cation assay, the final quantity of 22mer ssDNA on a single 15–20 µm bead was ∼0.1 pmol, while the quantity of 52mer ssDNA on a single 60–70 µm library bead was ∼0.7 pmol. To obtain beads bound to ds NF-κB consensus 22mer sequence, we annealed beads covalently attached to the ss ODN with an excess amount of complementary sequence. To verify production of the duplex bound on the beads, we have measured a decrease in the remaining amount of the complementary sequence. In addition, the duplex attached to the beads could be denatured by raising the temperature to 100°C for 5 min, then immediately quenching into an ice bath. The amount of the complementary strand freed in the denaturing process was comparable (±8%) with the amount originally bound.
Different parameters were then tested to optimize the binding of purified p50 protein to the ODN-bound bead (Fig. 1, step 2): incubation time, temperature, concentration of p50 protein and agitation (18). At a p50 protein concentration of 2 µg/ml, the signal reaches a plateau in ∼1 h at room temperature. The optimal incubation time was 2 h for maximal binding of the p50 protein.
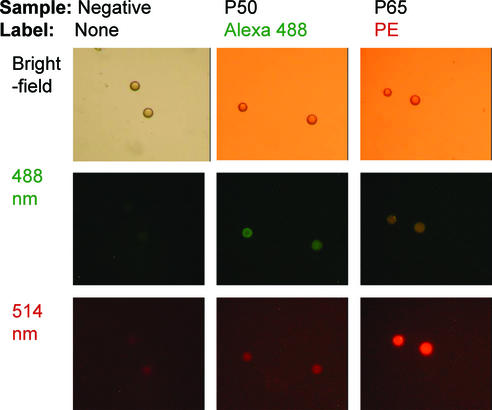
The binding at room temperature of the rabbit IgG antibody on the p50–DNA duplex (Fig. 1, step 3) was then optimized with varied antibody concentrations using the NF-κB DNA-binding assay in 96-well plates. The selected parameters for the incubation with the anti-p50 antibody were 1 h at room temperature with a 1000-fold dilution of anti-p50 antibody (0.2 ng/µl) in PBS buffer. Similarly, we optimized the parameters for step 4 (Fig. 1), the incubation with the anti-rabbit IgG conjugated with Alexa Fluor 488 dye. The selected parameters for the incubation with the anti-rabbit IgG were 1 h at room temperature with a 50-fold dilution of the anti-p50 antibody (40 ng/µl) in PBS buffer. The fluorescent image of Figure 2 shows that beads carrying NF-κB IgκB consensus sequence are lightly green, whereas blank beads appear unstained.
Figure 2.
The IgκB dsDNA consensus sequences were immobilized onto 15–20 µm polystyrene beads. DNA-bound beads were then incubated with purified p50 or p65 proteins. DNA transcription factor complexes were detected with primary antibody followed by an additional incubation with fluorescence-conjugated secondary antibody. The beads were viewed by fluorescent microscopy.
Similarly, the beads were incubated with NF-κB p65 protein (2 µg/ml), followed by anti-p65 antibody (Mouse IgG antibody, BD Biosciences, San Jose, CA, 610868). Binding to the anti-p65 antibody was complete with anti-mouse IgG1 antibody conjugated with R-phycoerythrin (PE) fluorochrome. Lightly red beads can also be viewed in Figure 2.
Immunofluorescence assay on bead-based S2-ODN library
To synthesize one-bead one-ODN/S2-ODN library, we utilize a ‘mix and separate’ split synthesis method (15). A two-column DNA synthesizer was used for constructing the library. The normal phosphate backbone linkages were generated using standard phosphoramidite monomers via oxidation in column 1, while the phosphorodithioate linkages were synthesized using thiophosphoramidite monomers via sulfurization in column 2, respectively. Two sequences of the same length are programmed for each column and are designed such that the bases are different at every equal position not only for diversifying base compositions but also for coding a phosphate or phosphorodithioate linkage. We have also created S2-ODN libraries that differ in the position of phosphate or dithioate but not in their base sequence. In such cases, the approach to identify the location of dithioate linkage(s) is based on the reactivity of dithioate towards iodoethanol in the base condition. By the split/pool method with two columns we can create 2N different members of the library for N split/pool steps. Utilizing more columns (M) would allow us to synthesize MN different beads with one unique aptamer sequence on each bead. For verification of multiple column approach, a large library containing 412 (or ∼107) ODN aptamers has been synthesized by using four columns on the Expedite 8909 Synthesizer.
Recently, we have also demonstrated the principal of creating a library of libraries in which each bead contains a defined library of aptamers to achieve library sizes comparable with those created by in vitro combinatorial selection methods by utilizing mixtures of phosphoramidites/thiophosphoramidites at selected positions in a given synthesis step. We could thus easily create ∼108 beads with 106 combinatorial library members on each bead; the total diversity in principle is thus 1014, the same as in in vitro combinatorial selection libraries. As an example, a library of libraries was prepared on a 1 µmol scale of polystyrene beads (60–70 µm). The downstream and upstream primers, 5′-d(GGATCCGGTGGTCTG)-3′ and 5′-d(CCTACTCGCGAATTC)-3′ were synthesized in parallel on a two-column DNA synthesizer (Expedite 8909, Applied Biosystems). Following the 5′-primer, the sequences programmed on the synthesizer for the combinatorial library were 5′-AT*GN*GA*AT*TT*NC*CA-3′ on column 1 and 5′-GG*AG*NG*CN*CA*GG*AC-3′ on column 2. The 3′-primer sequence completed the 44mer programmed on the synthesizer. A ‘split and pool’ occurred at each position indicated by an asterisk in order to synthesize the combinatorial region for the library of libraries. The letter N indicates an equimolar mixture of four bases (A, C, G and T). Five of the beads were randomly selected from the library and ‘one bead one PCR’ was run, cloned and sequenced. The desired sequences showing random bases at the N positions were obtained. The results indicated the successful construction of the library of libraries.
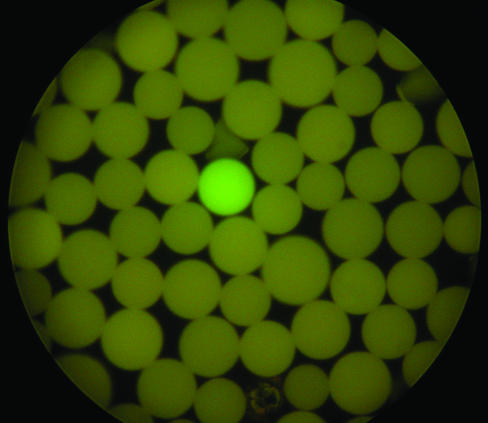
The bead S2-ODN library has been screened previously (15) against a NF-κB p50 protein labeled with a fluorescent dye, and several S2-ODN sequences binding NF-κB p50 protein have been identified. The binding of NF-κB p50 to the bead library has been demonstrated by screening directly against the NF-κB p50 protein transcription factor. Thus, the binding of the NF-κB p50 to a specific sequence was detected using a primary anti-NF-κB antibody followed by a secondary antibody conjugated with Alexa Fluor 488. As shown in Figure 3, only one positive bead was intensely stained when viewed by fluorescent microscopy, while the majority of the beads remained unstained. Individual beads from the library having fluorescence intensities comparable with the positive control beads bound to the NF-κB consensus sequence described above were picked up with the aid of a micropipette coupled to a micromanipulator.
Figure 3.
An aliquot of S2-ODN bead library was incubated with purified p50 protein. DNA transcription factor complexes were detected with primary antibody (rabbit IgG) specific for the p50 protein followed by an additional incubation with Alexa 488-conjugated secondary antibody for rabbit IgG. The beads were viewed by fluorescent microscopy.
The bead-bound 52mer S2-ODN library contains a 22mer variable region, potentially large enough to bind two dimers of NF-κB (21). Since the two sequences of the same length of the S2-ODN library were programmed for each column and were designed so that the bases were different at every equal position, sequencing the phosphorodithioate linkage(s) was straightforward. Shown in Table 1 are the variable region sequences obtained from the screening with the fluorescently labeled NF-κB p50 (15) and immunofluorescence assay, respectively. When comparing the base sequences listed at rows 5, 10, 15 and 21, we found that the 5′-terminal 15-base sequences are identical. The remaining variable 7-base sequences (indicated by underlining) are different between the two selection methods. The difference may be caused by the two screening assays. Perhaps one or more amino groups on the p50 protein have been modified by the fluorescent label, which precludes the second p50 dimer from binding to the full 22mer site? Studies on the quantitative affinities of the selected dithioaptamers to the protein along with selection from a large library (106∼108) to NF-κB are in progress to understand these differences.
Table 1. S2-ODN sequences selected from screening with NF-κB p50 proteina.
| Automated sequences from screening with fluorescently labeled NF-κB p50 protein | ||||||||||||||||||||||
| 1b | C | G | C | C | A | G | C | C | A | A | A | G | G | T | G | C | T | G | T | C | A | G |
| 2b | C | G | C | C | C | A | G | T | G | G | C | T | A | G | T | G | A | A | C | C | C | C |
| 3b | A | T | G | T | A | G | C | C | G | A | A | G | G | T | G | G | A | A | C | C | C | C |
| 4b | C | G | C | C | A | G | C | C | G | A | A | G | G | T | G | G | A | A | C | C | C | C |
| Ac | 1 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 1 | 3 | 3 | 0 | 1 | 0 | 0 | 0 | 3 | 3 | 0 | 0 | 1 | 0 |
| C | 3 | 0 | 3 | 3 | 1 | 0 | 3 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 4 | 3 | 3 |
| G | 0 | 3 | 1 | 0 | 0 | 3 | 1 | 0 | 3 | 1 | 0 | 3 | 3 | 1 | 3 | 3 | 0 | 1 | 0 | 0 | 0 | 1 |
| T | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 3 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| 5d | C | G | C | C | A | G | C | C | G | A | A | G | G | T | G | G | A | A | C | C | C | C |
| Deduced sequence from screening with fluorescently labeled NF-κB p50 protein | ||||||||||||||||||||||
| 6b | C | G | C | C | A | G | C | C | A | a | A | G | G | T | G | C | T | G | T | C | A | G |
| 7b | C | G | C | C | c | A | G | T | g | G | C | T | A | G | T | g | a | A | C | C | C | C |
| 8b | A | T | G | T | A | G | C | C | g | a | A | G | G | T | G | g | a | A | C | C | C | C |
| 9b | C | G | C | C | A | G | C | C | g | a | A | G | G | T | G | g | a | A | C | C | C | C |
| 10e | C | G | C | C | A | G | C | C | g | a | A | G | G | T | G | g | a | A | C | C | C | C |
| Automated sequences from immunofluorescence assay | ||||||||||||||||||||||
| 11 | A | T | G | T | A | G | C | C | A | A | A | G | G | T | G | G | A | A | C | C | C | C |
| 12 | C | G | C | C | A | G | C | C | G | A | A | G | G | T | G | C | T | G | T | C | A | G |
| 13 | C | G | C | C | C | A | G | T | G | A | A | G | G | T | G | C | T | G | T | C | A | G |
| 14 | C | G | C | C | C | A | G | T | A | G | C | T | A | G | T | C | T | G | T | C | A | G |
| Ac | 1 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 2 | 3 | 3 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 3 | 0 |
| C | 3 | 0 | 3 | 3 | 2 | 0 | 2 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 4 | 1 | 1 |
| G | 0 | 3 | 1 | 0 | 0 | 2 | 2 | 0 | 2 | 1 | 0 | 3 | 3 | 1 | 3 | 1 | 0 | 3 | 0 | 0 | 0 | 3 |
| T | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 3 | 1 | 0 | 3 | 0 | 3 | 0 | 0 | 0 |
| 15d,f | C | G | C | C | A | G | C | C | G | A | A | G | G | T | G | C | T | G | T | C | A | G |
| 16f | C | A | G | T | A | |||||||||||||||||
| Deduced S2-ODN sequence from immunofluorescence assay | ||||||||||||||||||||||
| 17 | A | T | G | T | A | G | C | C | A | a | A | G | G | T | G | g | a | A | C | C | C | C |
| 18 | C | G | C | C | A | G | C | C | g | a | A | G | G | T | G | C | T | G | T | C | A | G |
| 19 | C | G | C | C | c | A | G | T | g | a | A | G | G | T | G | C | T | G | T | C | A | G |
| 20 | C | G | C | C | c | A | G | T | A | G | C | T | A | G | T | C | T | G | T | C | A | G |
| 21e | C | G | C | C | A | G | C | C | g | a | A | G | G | T | G | C | T | G | T | C | A | G |
aThe upper case letter indicates a 3′-phosphate linkage and the lower case bold letter indicates a 3′-dithioate linkage.
bThe sequences are cited from Yang et al. (15).
cNumber of times a given residue appears.
dConsensus sequence based on the residue which appears the majority of the time in the position.
eSequences in rows 10 and 21 are deduced from rows 5 and 15, respectively.
fThe italic letters in rows 15 and 16 have equal probability from selection.
Preliminary studies on multi-color flow-cytometry sorting of aptamer-conjugated beads
Manual screening under a microscope and picking the most intensely fluorescently stained thioaptamer beads within a large library of potentially billions of beads is time consuming and limited to one-color selection, and therefore clearly limits the scope of bead-based library applications. We recognize that multicolor flow-cytometry and cell-sorting instrumentation potentially provides a rapid method for cell/bead sorting (33) and identification of ligands from large combinatorial peptide libraries as well (34,35). Therefore, a HTS method for identification of high-affinity aptamers to target proteins by flow cytometry and cell sorting was essential. Using the split-synthesis protocol, we have prepared a library (∼1.6 × 107) attached on 15–20 µm beads. The smaller polystyrene beads have proved to be ideal for this purpose.
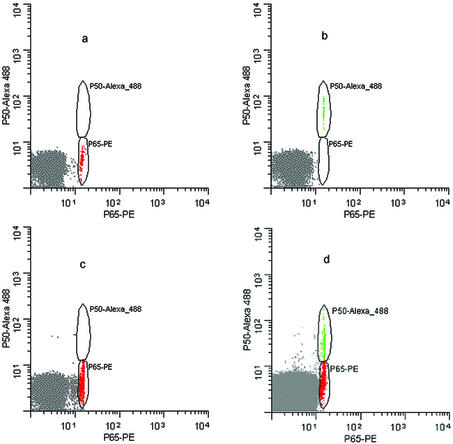
For flow-cytometric analysis separate sets of identical beads, each containing the same 22mer IgκB dsDNA consensus sequence were incubated with either purified p50 and p65 proteins, respectively. The transcription factor–DNA complexes were detected with primary antibodies specific for the p50 and p65 proteins followed by an additional incubation with Alexa 488-conjugated secondary antibody for p50 and PE-conjugated secondary antibody for p65. Samples containing p50-PE labeled beads alone or p65-Alexa 488 beads alone were run separately, followed by a mix of the two bead populations. Color gating on each separate bead population using WinList™ clearly exhibited the fact that the ‘positive’ beads were easily separated from ‘negative’ beads based on scatter and fluorescent properties. As shown in Figure 4a–d, we demonstrate the feasibility of dual-color flow-cytometric detection for the one-bead, one-ODN aptamer selection.
Figure 4.
Flow cytometric analysis of NF-κB p50 and p65 proteins to ∼15–20 µm beads bearing IgκB dsDNA consensus sequences. Alexa-488 fluorescence intensity is shown on the vertical axis, while the PE fluorescence intensity is shown on the horizontal axis. (a) Negative control beads; (b) 0.35% of p50 Alexa 488 positive beads; (c) 17% of p65 PE positive beads; (d) 0.04% of p50 Alexa 488 positive beads and 0.09% of p65 PE positive beads.
In Figure 4a, a control data file shows the autofluorescent microspheres as the negative control sample where the beads were unbound. Figure 4b shows the p50 positive beads only (green), while Figure 4c shows the p65 positive beads only (red). Finally, Figure 4d shows a mixed bead population. The larger autofluorescent bead population is the result of 0.8 µm non-fluorescent ‘carrier beads’ that were added to all samples in order to bring up the volume of the samples for a more optimal sample flow rate since the aptamer beads were present in numbers insufficient for conventional flow-cytometric analysis. Table 2 shows the population statistics for the bead analysis.
Table 2. Statistics for flow cytometry aptamer bead selection.
| Sample | Total | Region | % Gate |
|---|---|---|---|
| Figure 4a. CONTROL.FCS | |||
| R1: Autofluorescent beads | 10 000 | 9530 | 95.3 |
| Figure 4b. FCS | |||
| R2: p50 Alexa 488 positive beads | 10 000 | 35 | 0.35 |
| Figure 4c. FCS | |||
| R3: p65 PE positive beads | 2000 | 3488 | 17.44 |
| Figure 4d. FCS | |||
| R1: Autofluorescent beads and carrier beads | 1 000 000 | 963 321 | 96.33 |
| R2: p50 Alexa 488 positive beads | 1 000 000 | 354 | 0.04 |
| R3: p65 PE positive beads | 1 000 000 | 935 | 0.09 |
Thus, individual beads from a library having high fluorescence intensities can be easily detected by flow cytometry for the ultimate sorting of aptamer beads into microcentrifuge tubes for rapid PCR based sequencing (15). The home-built HiReCS flow cytometer instrumentation constitutes a HTS device that can analyze beads at rates in excess of 106 beads/s (27–31), allowing the analysis of a very small fraction of fluorescence aptamer beads to be detected and sorted from large libraries (e.g. >108 beads). The multi-color HTS of aptamer libraries will play a critical role for identification of different combinations of transcription factors such as NF-κB p50/p65 homo- and heterodimers.
CONCLUSIONS
The present results demonstrate that the immunofluorescence-based assay provides a fast and efficient approach for identifying lead thioaptamers (or unmodified aptamers) for new agents targeting proteins such as NF-κB. Our results demonstrate that dye labels can potentially modulate part of the binding properties of the attached substrates. The slightly different binding sequences from two independent screening methods may result from the dye molecules altering the p50 protein molecular binding capacity by partially blocking some of the thioaptamer–protein binding groups such as lysine primary amines. The immunofluorescence based assay method described here is thus favored over selection of beads from directly fluorescently labeled substrates. However, the identity of the 15-nt sequence between the two assay methods demonstrates that direct labeling of the selected protein is feasible when antibodies are not conveniently available.
Finally, our preliminary results from dual-color flow cytometry bead identification and analysis suggests that this high-throughput selection technique may be used to replace manual visual fluorescence microscope identification of beads containing bound target protein and the need to isolate the individual ‘positive’ beads with the micromanipulator described previously. As we have demonstrated previously single bead sequencing, the flow-sorted ‘positive’ beads could then be subjected to, e.g. one-bead PCR analysis techniques to identify the thioaptamer that binds the target protein, thus automating and vastly expanding the speed of selection of aptamers compared to in vitro combinatorial selection methods. A multi-color flow-cytometry assay could be easily expanded to multiple proteins and even multiple proteins in cell proteome extracts. Such an assay could be especially useful in drug discovery and diagnostics.
Acknowledgments
ACKNOWLEDGEMENTS
We are grateful to Matthew Tacker and Danyel Tacker for contributions to this work. We also thank C. J. Orlea for her help in manuscript preparation and Dr Andrew Ellington (U. Texas, Austin) and ChemGenes for providing the non-cleavable linker beads. This research was supported by DARPA (9624-107 FP), NIH (AI27744 and N01-HV-28184), NIEHS (ES06676), NIBIB (EB00245), the Welch Foundation (H-1296) and DTRA (DAAD13-02-C-0079 and DAAD17-01-D-0001). S.E.B. was supported by a postdoctoral fellowship from an NIEHS grant T32-07254.
REFERENCES
- 1.Osborne S.E. and Ellington,A.D. (1997) Nucleic acid selection and the challenge of combinatorial chemistry. Chem. Rev., 97, 349–370. [DOI] [PubMed] [Google Scholar]
- 2.Ellington A.D. and Szostak,J.W. (1990) In vitro selection of RNA molecules that bind specific ligands. Nature, 346, 818–822. [DOI] [PubMed] [Google Scholar]
- 3.Tuerk C. and Gold,L. (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophase T4 DNA polymerase. Science, 249, 505–510. [DOI] [PubMed] [Google Scholar]
- 4.Bock L.C., Griffin,L.C., Latham,J.A., Vermaas,E.H. and Toole,J.J. (1992) Selection of single-stranded DNA molecules that bind and inhibit human thrombin. Nature, 355, 564–566. [DOI] [PubMed] [Google Scholar]
- 5.Jellinek D., Green,L.S., Bell,C., Lynott,C.K., Gill,N., Vargeese,C., Kirschenheuter,G., McGee,D.P.C., Abesinghe,P., Pieken,W.A., Shapiro,R., Rifkin,D.B., Moscatelli,D. and Janjic,N. (1995) Potent 2′-amino-2′-deoxypyrimidine RNA inhibitors of basic fibroblast growth factor. Biochemistry, 34, 11363–11372. [DOI] [PubMed] [Google Scholar]
- 6.Lee S.W. and Sullenger,B.A. (1997) Isolation of a nuclease-resistant decoy RNA that can protect human acetylcholine receptors from myasthenic antibodies. Nat. Biotechnol., 15, 41–45. [DOI] [PubMed] [Google Scholar]
- 7.Lebruska L.L. and Maher,L.L.,III (1999) Selection and characterization of an RNA decoy for transcription factor NF-κB. Biochemistry, 38, 3168–3174. [DOI] [PubMed] [Google Scholar]
- 8.Koizumi M. and Breaker,R.R. (2000) Molecular recognition of cAMP by an RNA aptamer. Biochemistry, 39, 8983–8992. [DOI] [PubMed] [Google Scholar]
- 9.Gold L., Singer,B., He,Y. and Brody,E. (1997) SELEX and the evolution of genomes. Curr. Opin. Genet. Dev., 7, 848–851. [DOI] [PubMed] [Google Scholar]
- 10.Nolte A., Klußmann,S., Bald,R., Erdmann,V.A. and Furste,J.P. (1996) Mirror-design of L-oligonucleotide ligands binding to l-arginine. Nat. Biotechnol., 14, 1116–1119. [DOI] [PubMed] [Google Scholar]
- 11.Ye X., Gorin,A., Ellington,A.D. and Patel,D. (1996) Deep penetration of an α-helix into the widened RNA major groove in the HIV-1 Rev peptide-RNA aptamer complex. Nature Struct. Biol., 3, 1026–1033. [DOI] [PubMed] [Google Scholar]
- 12.Ludwig L. and Eckstein,F. (1991) Synthesis of nucleoside 5′-O-(1,3-dithiotriphosphates) and 5′-O-(1,1-dithiotriphosphates). J. Org. Chem., 56, 1777–1783. [Google Scholar]
- 13.Furka A., Sebestyen,F., Asgedom,M. and Dibo,G. (1988) In Highlights of Modern Biochemistry. Proceedings of the 14th International Congress of Biochemistry. VSP, Utrecht, The Netherlands, Vol. 5, p. 47.
- 14.Lam K.S., Salmon,S.E., Hersh,E.M., Hruby,V.J., Kazmierskl,W.M. and Knapp,R. (1991) A new type of synthetic peptide library for identifying ligand-binding activity. Nature, 354, 82–84. [DOI] [PubMed] [Google Scholar]
- 15.Yang X., Bassett,S.E., Li,X., Luxon,B.A., Herzog,N.K., Shope,R.E., Aronson,J., Prow,T.W., Leary,J.F., Kirby,R., Ellington,A.D. and Gorenstein,D.G. (2002) Construction and selection of bead bound combinatorial oligonucleoside phosphorothioate and phosphorodithioate aptamer libraries designed for rapid PCR-based sequencing. Nucleic Acids Res., 30, e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swartz D.R. (1996) Covalent labeling of proteins with fluorescent compounds for imaging applications. Scanning Microscopy Suppl., 10, 273–284. [PubMed] [Google Scholar]
- 17.Gosling J.P. (2000) Immunoassays: A Practical Approach. Oxford University Press, Oxford, UK.
- 18.Renard P., Ernest,I., Houbio,A., Art,M., Calvez,H.L., Raes,M. and Remacle,J. (2001) Development of a sensitive multi-well colorimetric assay for NFκB. Nucleic Acids Res., 29, e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh G., Duyne,G.V., Ghosh,S. and Sigler,P.B. (1995) Structure of NF-κB p50 homodimer bound to a κB site. Nature, 373, 303–310. [DOI] [PubMed] [Google Scholar]
- 20.Muller C.W., Rey,F.A., Sodeoka,M., Verdine,G.L. and Harrison,S.C. (1995) Structure of the NF-κB p50 homodimer bound to DNA. Nature, 373, 311–317. [DOI] [PubMed] [Google Scholar]
- 21.King D., Bassett,S.E., Li,X., Fennewald,S.A., Herzog,N.K., Luxon,B.A., Shope,R. and Gorenstein,D.G. (2002) Combinatorial selection and binding of phosphorothioate aptamers targeting human NF-κB RelA (p65) and p50. Biochemistry, 41, 9696–9706. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen J., Brill,W.K.D. and Caruthers,M.H. (1988) Synthesis and characterization of dinucleoside phosphorodithioates. Tetrahedron Lett., 29, 2911–2914. [Google Scholar]
- 23.Farschtschi N. and Gorenstein,D.G. (1988) Preparation of a deoxynucleoside thiophosphoramidite intermediate in the synthesis of nucleoside phosphorodithioates. Tetrahedron Lett., 29, 6843–6846. [Google Scholar]
- 24.Wiesler W.T. and Caruthers,M.H. (1996) Synthesis of phosphorodithioate DNA via sulfur-linked base labile protecting groups. J. Org. Chem., 61, 4272–4281. [DOI] [PubMed] [Google Scholar]
- 25.Yang X., Fennewald,S., Luxon,B.A., Aronson,J., Herzog,N.K. and Gorenstein,D.G. (1999) Aptamers containing thymidine 3′-O-phosphorodithioates: synthesis and binding to nuclear factor-κB. Bioorg. Med. Chem. Lett., 9, 3357–3362. [DOI] [PubMed] [Google Scholar]
- 26.Yang X., Hodge,H.P., Luxon,B.A., Shope,R. and Gorenstein,D.G. (2002) Separation of synthetic oligonucleotide dithioates from monothiophosphate impurities by anion-exchange chromatography on a Mono Q column. Anal. Biochem., 306, 92–99. [DOI] [PubMed] [Google Scholar]
- 27.Leary J.F., Reece,L.N., Szaniszlo,P., Prow,T. and Wang,N. (2001) High-throughput cell analysis and sorting technologies for clinical diagnostics and therapeutics. Proc. SPIE, 4255, 16–27. [Google Scholar]
- 28.Leary J.F., Corio,M.A. and McLaughlin,S.R (1993) US Patent 5,204,884.
- 29.Leary J.F., Corio, M.A. and McLaughlin, S.R (1998) US Patent 5,804,143.
- 30.Corio M.A. and Leary,J.F. (1993) US Patent 5,199,576.
- 31.Corio M.A. and Leary,J.F. (1996) US Patent 5,550,058.
- 32.Drewett V., Molina,H., Millar,A., Muller,S., von Hesler,F. and Shaw,P.E. (2001) DNA-bound transcription factor complexes analysed by mass-spectrometry: binding of novel proteins to the human c-fos SRE and related sequences. Nucleic Acids Res., 29, 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Givan A.L. (2001) Cells from without: lymphocytes and the strategy of gating. Flow Cytometry: First Principles. Wiley-Liss Press, New York, NY.
- 34.Needels M.C., Jones,D.G., Tate,E.H., Heinkei,G.L., Kochersperger,L.M., Dower,W.J., Barrett,R.W. and Gallop,M.A. (1993) Generation and screening of an oligonucleotide-encoded synthetic peptide library. Proc. Natl Acad. Sci. USA, 90, 10700–10704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller K., Gombert,F.O., Manning,U., Grossmuller,F., Graff,P., Zaegel,H., Zuber,J.F., Freuler,F., Tschopp,C. and Baumann,G. (1996) Rapid identification of phosphopeptide ligands for SH2 domins. Proc. Natl Acad. Sci. USA, 271, 16500–16505. [PubMed] [Google Scholar]