Abstract
The chromokinesin Kid is important in chromosome alignment at the metaphase plate. Here, we report that Kid function is regulated by phosphorylation. We identify Ser427 and Thr463 as M phase-specific phosphorylation sites and Cdc2–cyclin B as a Thr463 kinase. Kid with a Thr463 to alanine mutation fails to be localized on chromosomes and is only detected along spindles, although it retains the ability to bind DNA or chromosomes. Localization of rigor-type mutant Kid, which shows nucleotide-independent microtubule association, is also confined to the spindle, implying that strong association of Kid with the spindle can sequester it from chromosomes. T463A substitution in DNA-binding domain-truncated Kid consistently enhances its spindle localization. At physiological ionic strength, unphosphorylated Kid shows ATP-independent microtubule association, whereas Thr463-phosphorylated Kid shows ATP dependency. Moreover, the stalk region of unphosphorylated Kid interacts with microtubules and the interaction is weakened when Thr463 is phosphorylated. Our data suggest that phosphorylation on Thr463 of Kid downregulates its affinity for microtubules to ensure reversible association with spindles, allowing Kid to bind chromosomes and exhibit its function.
Keywords: Cdc2/Kid/kinesin-like motor/mitosis/phosphorylation
Introduction
Mitosis is the process in which a cell accurately segregates its genetic material into two daughter cells in a manner dependent on a dynamic structure known as the mitotic spindle. Errors in this process may result in cancer, aneuploidy or cell death. Both cytoplasmic dynein and members of the kinesin superfamily play critical roles in centrosome separation and assembly of bipolar spindles, spindle pole formation, maintaining spindle structure, positioning chromosomes at the spindle equator and chromosome segregation (reviewed in Heald, 2000; Mountain and Compton, 2000; Sharp et al., 2000). Apparently, coordinated actions of these motor proteins at the different parts of the spindle are important for proper M phase progression. Furthermore, extensive studies have shown that protein phosphorylation is an important regulatory mechanism for the initiation, progression and completion of mitosis. The Cdc2–cyclin B complex is the main regulator of G2/M progression, and it controls the activity or distribution of multiple proteins that are involved in spindle formation and chromosome segregation (Verde et al., 1990; Nigg, 1995; Nigg et al., 1996; Ohi and Gould, 1999). Cdc2–cyclin B phosphorylates microtubule (MT)-associated motors such as CENP-E or Eg5 family proteins (Liao et al., 1994; Blangy et al., 1995; Sawin and Mitchison, 1995) and MT-associated proteins, which are important for MT dynamics and stability (reviewed in Cassimeris, 1999; Andersen, 2000). In addition, several serine/threonine kinases, such as those of the Polo-like kinase family (reviewed in Glover et al., 1998; Nigg, 1998), Ipl1/aurora kinase family (reviewed in Bischoff and Plowman, 1999) and NIMA kinase family (Osmani et al., 1988; Lu and Hunter, 1995), have been identified through genetic analysis as mitosis-related kinases. Determination of the targets of these kinases and analysis of the mechanisms underlying their spatial and temporal coordination in mitotic cells would help us understand molecular events important for M phase progression.
We previously identified Kid as a member of the chromosome-associated kinesin family (Tokai et al., 1996). The N-terminal half of Kid contains a kinesin-like motor domain, and the C-terminal half contains a helix–hairpin–helix DNA-binding domain. Kid is co-localized with spindles and chromosomes during prophase through metaphase and is then enriched in the spindle pole-proximal side on the chromosomes at anaphase. The unique subcellular localization during mitosis and the structural features suggest that Kid plays an important role in chromosomal movement along MTs during mitosis (Tokai et al., 1996). Actually, recent studies have revealed that Xkid, a Xenopus homolog of Kid, is essential for chromosome alignment (Antonio et al., 2000; Funabiki and Murray, 2000) and that human Kid is necessary for chromosome orientation and oscillation of chromosome arms (Levesque and Compton, 2001). Although the data implicate Kid in chromosome movement along the spindle and alignment at metaphase plate, the mechanisms by which Kid is localized to various sites in the cell and by which Kid function is regulated during mitosis have remained to be addressed. In the present study, we have shown that phosphorylation of Kid modulates its mode of MT association and thereby contributes to its proper subcellular localization and function during mitosis.
Results
Ser427 and Thr463 on Kid are phosphorylated during M phase
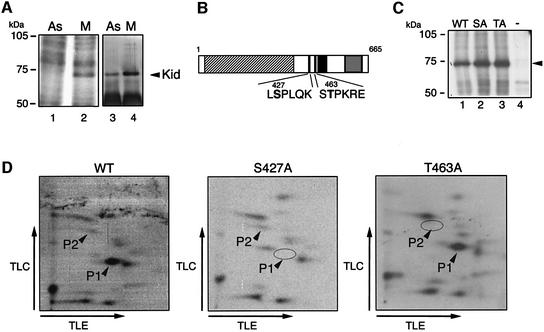
We previously reported that Kid shows dramatic changes in its localization during mitosis (Tokai et al., 1996). In addition, we have found that overexpression of Kid, which results in unusual subcellular localization, leads to mitotic abnormalities at multiple points during mitosis (Supplementary figure 1 available at The EMBO Journal Online). Thus, proper control of the amount and sub cellular localization of Kid during M phase is likely to be important for accurate chromosomal movement and, consequently, for accurate progression of mitosis. Because protein phosphorylation is implicated in control of spindle formation and chromosome segregation, we investigated whether Kid phosphorylation is related to its localization or function. First, to determine whether Kid is phosphorylated in vivo during M phase, nocodazole-treated or untreated 293T cells were metabolically labeled with [32P]orthophosphate, and anti-Kid immunoprecipitates of the cell lysates were analyzed by SDS–PAGE and autoradiography. The results suggested Kid to be prominently phosphorylated in M phase cells (Figure 1A). Inspection of the amino acid sequence of Kid revealed two possible target sites, Ser427 and Thr463, of M phase-specific kinases (Figure 1B). Ser427 was located within a consensus sequence (F/L/I-S/T-P-L/I/V/F/M-Q/K) found in epitopes for the MPM2 monoclonal antibody that recognizes M phase-specific phosphoproteins (Westendorf et al., 1994). Thr463 was located within the Cdc2 kinase consensus sequence (S/T-P-X-R/K) (Kennelly and Krebs, 1991).
Fig. 1. Phosphorylation of Kid at multiple sites, including Ser427 and Thr463, in M phase. (A) Left panel: 293T cells were metabolically labeled with [32P]orthophosphate for the last 4 h of each condition followed by immunoprecipitation of Kid. The immunoprecipitates were analyzed by SDS–PAGE and autoradiography. Right panel: the same experiments were performed without [32P]orthophosphate to show the amounts of immunoprecipitated Kid. Lanes 1 and 3: asynchronized cells. Lanes 2 and 4: M phase-enriched cells. (B) A schematic representation of the Kid protein. The amino acids at putative phosphorylation sites are shown in bold. Hatched box, kinesin-like motor domain (amino acids 35–370); black box, coiled-coil domain (amino acids 466–506); shaded box, helix–hairpin–helix DNA-binding motifs (amino acids 594–647). (C) 293T cells were transfected with plasmids coding for Flag-Kid (lane 1), Flag-Kid-S427A (lane 2) and Flag-Kid-T463A (lane 3), or mock expression vector (lane 4), synchronized in M phase, and labeled with [32P]orthophosphate as in (A). Flag-Kid proteins were immunoprecipitated by anti-Flag antibody and analyzed by SDS–PAGE and autoradiography. (D) Phosphopeptide mapping. The Flag-Kid bands (shown in C) were cut out from the gel, eluted and digested with trypsin, followed by electrophoresis in the horizontal direction and chromatography in the vertical direction. Arrowheads denote the spots corresponding to phospho-Ser427 (P1) and phospho-Thr463 (P2).
To determine whether these sites are actually phosphorylated in vivo, we constructed expression vectors encoding Flag-tagged wild-type Kid and Kid mutants having Ser427 to alanine (Kid-S427A) or Thr463 to alanine (Kid-T463A) substitutions. After transfection of the constructs into 293T cells, cells were treated with nocodazole and metabolically labeled with [32P]ortho phosphate. Analysis of anti-Flag immunoprecipitates showed that both wild-type and mutant Kid proteins were phosphorylated (Figure 1C). Although only ∼50% of exogenous Flag-Kid proteins were solubilized, western blotting with anti-Flag, anti-PS427 and anti-PT463 antibodies (see below) revealed that solubilized and insolubilized Kid are phosphorylated to a similar extent (data not shown). Phosphorylated Kid proteins were digested with trypsin and subjected to two-dimensional peptide mapping. As shown in Figure 1D, wild-type Kid generated a number of spots, including P1 and P2, indicating that Kid was phosphorylated at multiple sites during M phase. Phosphopeptides P1 and P2 were no longer detected in the map of Kid-S427A and Kid-463A, respectively. In addition, phosphoamino acid analysis revealed that P1 and P2 contained phosphoserine and phosphothreonine, respectively (data not shown). On the basis of these results as well as charge differences in the peptides and relative positions in the chromatographic dimension, we concluded that P1 contains phospho-Ser427 and P2 contains phospho-Thr463.
Characterization of phosphorylated Kid with phosphoepitope-specific antibodies
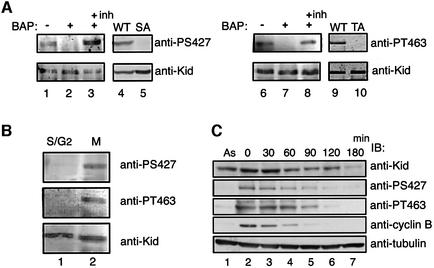
We developed and purified polyclonal antibodies that specifically recognized Kid protein phosphorylated on Ser427 (anti-PS427) or Thr463 (anti-PT463). Both antibodies recognized Kid immunoprecipitated from extracts of M phase-enriched cells, and antibody reactivity was diminished when immunoprecipitated Kid proteins were treated with phosphatase (Figure 2A, lanes 1–3 and 6–8). In addition, anti-PS427 and anti-PT463 did not react with Flag-tagged Kid-S427A and Kid-T463A purified from M phase-enriched cells, respectively (Figure 2A, lanes 4 and 5, and 9 and 10). Therefore, anti-PS427 and anti-PT463 were specifically reactive with the phosphorylated form of Kid. Using these antibodies, we measured Kid phosphorylation during the cell cycle. Western blots of extracts of HeLa cells, whose growth had been synchronized by thymidine block, with anti-PS427 and anti-PT463 showed that phosphorylated Kid was present exclusively at M phase (Figure 2B). When cells were released from nocodazole block, the amounts of Kid protein were reduced after M phase (Figure 2C). However, significant amounts of Kid were detected for 2–3 h after the release. The observation is in good agreement with the results of immunofluorescent staining of endogenous Kid (Tokai et al., 1996; our unpublished data). Downregulation of phosphorylated Kid occurred with a time frame similar to that of cyclin B degradation, with a short lag (Figure 2C). Based on these results, we concluded that phosphorylation of Kid at Ser427 and Thr463 occurs specifically at M phase.
Fig. 2. Identification of Ser427 and Thr463 as mitosis-induced phosphorylation sites. (A) Characterization of phospho-Ser427- and phospho-Thr463-specific antibodies. Endogenous Kid (lanes 1–3 and 6–8) or exogenously expressed Flag-tagged wild-type and mutant Kid (lanes 4 and 5, and 9 and 10) were immunoprecipitated from nocodazole-treated 293T cells with anti-Kid or anti-Flag antibodies, respectively. Immunoprecipitated endogenous Kid proteins were incubated with (lanes 2, 3, 7 and 8) or without (lanes 1 and 6) bacterial alkaline phosphatase (BAP) followed by immunoblotting with anti-PS427 (left panels) or anti-PT463 (right panels). Duplicate filters were blotted with anti-Kid antibodies. (B) S/early G2 phase- (lane 1) and M phase- (lane 2) enriched HeLa cell lysates were prepared in the presence of okadaic acid and used for immunoprecipitation with anti-Kid antibodies. Immunoprecipitates were analyzed by immunoblotting with antibodies as indicated. (C) HeLa cells were synchronized in M phase and then released to proceed to the next cell cycle. At the indicated time points, cells were harvested (lanes 2–7), directly lysed in SDS sample buffer, and analyzed by immunoblot analysis. Anti-cyclin B and anti-tubulin antibodies were used as controls. Lane 1 is a control of asynchronous cells.
Cdc2 kinase phosphorylates Kid on Thr463
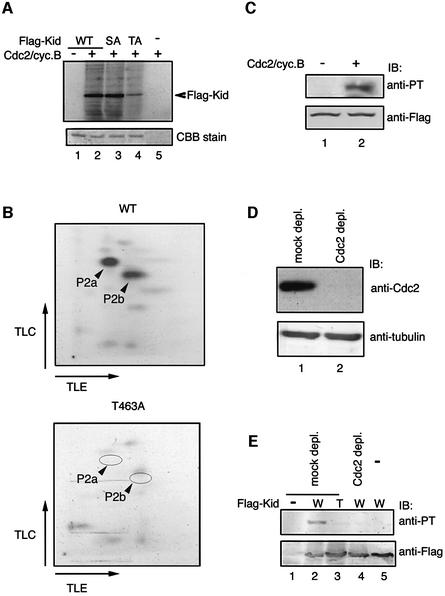
To determine whether Cdc2–cyclin B is responsible for Kid phosphorylation at Thr463, Flag-tagged Kid proteins were subjected to an in vitro phosphorylation reaction with purified Cdc2–cyclin B complex. Cdc2–cyclin B efficiently phosphorylated wild-type and Kid-S427A, but the level of Kid-T463A phosphorylation was relatively low (Figure 3A). Two major phosphopeptides, P2a and P2b, present in a two-dimensional tryptic phosphopeptide map of wild-type Kid were not present in the map of Kid-T463A (Figure 3B). P2a is in the same region as the spot P2 in Figure 1D. Both P2a and P2b contained Thr463 and resulted from incomplete or partial tryptic cleavage of phosphorylated Kid. Two basic residues, Lys465 and Arg466, in tandem are likely to cause partial digestion. Moreover, anti-PT463 antibodies reacted with Flag-Kid phosphorylated by purified Cdc2–cyclin B (Figure 3C), and Thr463 was not phosphorylated by other Cdk–cyclin complexes such as Cdk2–cyclin E, Cdk2–cyclin A or Cdk4–cyclin E (data not shown).
Fig. 3. Cdc2–cyclin B phosphorylates Kid at Thr463. (A) Dephosphorylated Flag-Kid(WT) (lanes 1 and 2), Flag-Kid(S427A) (lane 3) and Flag-Kid(T463A) (lane 4) were subjected to in vitro phosphorylation assay in the presence of [γ-32P]ATP with (lanes 2–5) or without (lane 1) purified Cdc2–cyclin B. Samples were then analyzed by SDS–PAGE and autoradiography. Duplicated precipitates were stained with Coomassie Blue, showing a similar amount of Flag-Kid (lower panel). (B) Phosphopeptide mapping. Flag-Kid (wild type and T463A) bands in (A) were cut from the gel and analyzed as in Figure 1C. (C) Flag-Kid(WT) was phosphorylated in vitro with (lane 2) or without (lane 1) Cdc2–cyclin B. Phosphorylation of Kid on Thr463 was analyzed by immunoblotting with anti-PT463 (upper panel). Duplicate filters were blotted with anti-Flag antibody (lower panel). (D) Mitotic cytosol was incubated with beads alone (lane 1) or agarose-coupled anti-Cdc2 antibody (lane 2). Unbound cytosolic proteins were immunoblotted with anti-Cdc2 antibody or anti-tubulin antibody as a loading control. (E) Wild-type (lanes 2, 4 and 5) or T463A (lane 3) Flag-tagged Kid was incubated in the presence of ATP with mock-depleted cytosol (lanes 2 and 3), Cdc2-depleted cytosol (lane 4) or without cytosol (lane 5). The reacted samples were analyzed by immunoblotting with anti-PT463 (upper panel). Duplicate filters were blotted with anti-Kid antibody (lower panel).
To show that Cdc2 kinase is essential for Kid phosphorylation at Thr463, we depleted Cdc2 from the cytosol of mitotic HeLa cells using anti-Cdc2 antibody (Figure 3D) and tested whether the depleted cytosol phosphorylated Kid. Whereas Flag-Kid incubated with the mock-depleted M phase cytosol was reactive with anti-PT463, Flag-Kid incubated with Cdc2-depleted cytosol was only a little reactive (Figure 3E). These results strongly suggest that Cdc2 is responsible for Kid phosphorylation at Thr463 in vivo.
Phosphorylation of Kid on Thr463 is required for chromosomal localization of Kid during prometaphase and metaphase
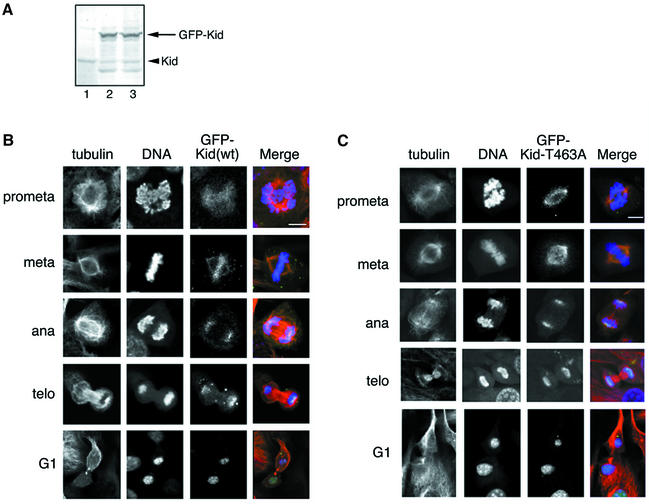
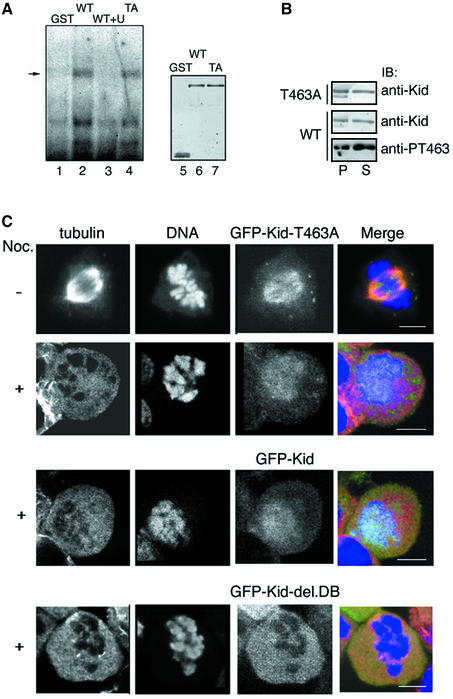
To examine the functional significance of Kid phosphorylation at Thr463, we analyzed the subcellular localization of Kid-T463A. NIH-3T3 cells were infected with retroviruses harboring the puromycin resistance gene and green fluoescent protein (GFP)–Kid (wild-type or T463A mutant) cDNA, and the cells expressing GFP–Kid were selected for puromycin resistance. By infecting the cells with the recombinant virus at appropriate multiplicity of infection (m.o.i.), a low but detectable level of GFP–Kid expression was achieved. Consequently, the subcellular distribution of GFP–Kid was more likely to mirror that of endogenous Kid and was less likely to disrupt progression of mitosis. It was important to keep the level of exogenous Kid as low as possible, because overexpressed Kid would disrupt proper spindle formation (Supplementary figure 1). Expression of GFP–Kid was confirmed by immunoblot analysis of whole-cell lysates, revealing that the concentration of exogenous GFP–Kid was 5- to 10-fold greater than that of endogenous Kid (Figure 4A, lane 2). GFP–Kid was localized to spindles and chromosomes during M phase progression from prophase to metaphase. In anaphase, a high concentration of GFP–Kid was observed at the boundary between the chromosomes and spindle MTs (Figure 4B). The localization pattern of GFP–Kid in infected cells was the same as that of endogenous Kid in uninfected cells (Tokai et al., 1996). In contrast, GFP–Kid-T463A was detected only along spindles and failed to be localized on chromosome arms during prometaphase and metaphase (Figure 4C). After the metaphase/anaphase transition, localization of GFP–Kid- T463A was virtually the same as that of wild-type Kid. With exogenous GFP–Kid-T463A expressed under the appropriate m.o.i. condition (Figure 4A, lane 3), mitosis was carried out by endogenous Kid. Therefore, phosphorylation of Kid at Thr463 appears to be necessary for association of Kid with chromosomes at prometaphase and metaphase.
Fig. 4. T463A mutation disrupts Kid localization on mitotic chromosome. (A) Extracts of NIH-3T3 cells infected with the retroviruses harboring GFP–Kid (lane 2), GFP–Kid-T463A (lane 3), or mock-infected cells (lane 1) were immunoblotted with anti-Kid antibodies. (B) Confocal fluorescence imaging of GFP–Kid. NIH-3T3 cells were infected with retrovirus harboring GFP–Kid, fixed at 40–48 h after infection, and stained for tubulin and DNA. Cells were observed for fluorescence of GFP–Kid (green), tubulin (red) and DNA (blue) by confocal microscopy. Prometa, prometaphase; meta, metaphase; ana, anaphase; telo, telophase; G1, G1 phase. (C) NIH-3T3 cells were infected with retrovirus harboring GFP–Kid-T463A, fixed, stained and observed as in (B). Scale bars = 10 µm.
Kid-T463A retains DNA- and chromosome-binding abilities
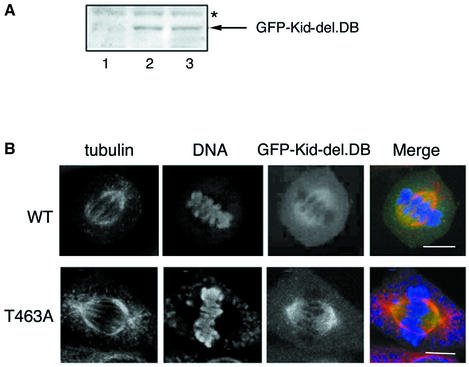
We first examined the effect of mutation or phosphorylation at Thr463 on the DNA-binding activity of Kid by electrophoretic mobility shift assay (EMSA). Kid proteins were purified from M phase-enriched 293T cells. More than 50% of the wild-type Kid proteins thus prepared were phosphorylated on Thr463. The DNA fragment of the c-erbB-2 promoter region, which contains the Kid-binding sequence (Tokai et al., 1996), was labeled with [γ-32P]ATP and used as a probe. A shifted band of a DNA–protein complex was observed in the presence of wild-type Kid and almost completely disappeared in the presence of an excess amount of unlabeled probe or anti-Kid antibody against the DNA-binding region of Kid (Figure 5A, lanes 2 and 3, and our unpublished data). There was little difference in the DNA-binding activity between wild-type Kid and Kid-T463A (Figure 5A, lanes 2 and 4). In addition, Kid with Thr463 changed to glutamate or aspartate, phospho-threonine-mimicking amino acids, also gave rise to the same level of shifted band (data not shown). We also performed a DNA–cellulose binding assay. Myc-His-tagged Kid proteins purified from M phase-enriched cells were incubated with double-stranded DNA–cellulose, and DNA–cellulose was then pelletted down by centrifugation. As shown in Figure 5B, T463A mutation did not decrease the association of Kid with DNA. Rather, it appeared that Thr463-phosphorylated Kid had slightly reduced affinity for DNA. Finally, we examined localization of Kid-T463A upon disruption of mitotic spindles by nocodazole. As shown in Figure 5C, Kid-T463A was co-localized with chromosomes in the absence of spindle, as was wild-type Kid, whereas localization of DNA-binding domain-truncated Kid (Kid-del.DB, amino acid residues 1–515) did not overlap with that of chromosomes. These data indicate that Kid-T463A retains the ability to associate with DNA or mitotic chromosomes both in vitro and in vivo, and that phosphorylation of Thr463 per se is not primarily related to Kid association with DNA and mitotic chromosomes.
Fig. 5. Kid-T463A retains affinity for DNA and chromosome. (A) An EMSA of Kid. The radiolabeled DNA fragment containing the Kid-binding sequence was incubated with GST (lane 1), GST–Kid (lanes 2 and 3) or GST–Kid-T463A (lane 4) and analyzed by SDS–PAGE. A 100-fold molar excess of the unlabeled probe was added prior to addition of the labeled probe in lane 3. The amounts of proteins used for the assay were examined by immunoblotting with anti-GST antibody (right panel, lanes 5–7). (B) DNA–cellulose binding assay. Myc-His-tagged wild-type or T463A mutant Kid was incubated with DNA–cellulose and centrifuged. Supernatants (S; non-DNA bound) and pellets (P; DNA bound) were separated by SDS–PAGE and subjected to immunoblotting with anti-Kid or anti-PT463 antibodies. (C) GFP–Kid-T463A, GFP–Kid or GFP–Kid-del.DB was expressed in NIH-3T3 cells using the retrovirus system. Cells were fixed before (Noc. –) and after (Noc. +) 3 h of 100 ng/ml nocodazole treatment. Cells were then stained and observed as in Figure 4B. Scale bars = 10 µm.
Rigor-type mutant Kid shows mislocalization similar to the T463A mutant
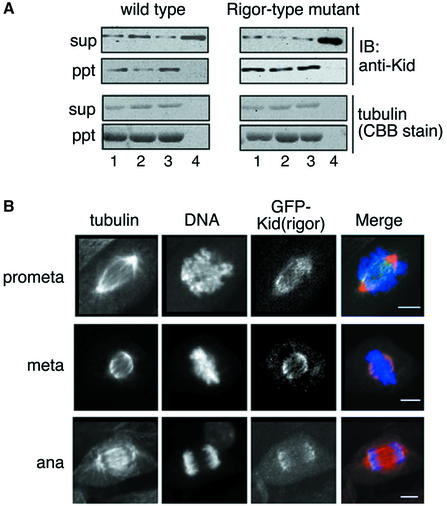
To examine the possibility that Kid-T463A has very high affinity for spindles, we constructed a rigor-type mutant of Kid. A threonine to asparagine mutation (rigor-type mutation) in the conserved ATP loop of the motor domain blocks ATP hydrolysis and, thereby, movement of kinesin-like motors along the MTs, but binding of the mutant with MTs is not abolished (Meluh and Rose, 1990; Rasooly et al., 1991; Nakata and Hirokawa, 1995; Blangy et al., 1998). The Thr134 to asparagine mutant Kid (Kid-T134N) was thus expected to interact with MTs constitutively, regardless of the presence of ATP or its analogs. By MT co-sedimentation assay, Kid-T134N versus wild-type Kid showed increased MT-binding activity (Figure 6A, lane 1). The MT-binding activity of Kid-T134N was not influenced by the presence of ATP or AMP-PNP, whereas the MT-binding activity of wild-type Kid was reduced in the presence of ATP (Figure 6A, lanes 2 and 3). In addition, the MT-dependent ATPase activity of Kid-T134N was impaired (Table I). These properties indicate that T134N mutation indeed conferred rigor-type MT-binding ability on Kid. As shown in Figure 6B, rigor-type mutant Kid showed almost the same mislocalization as Kid-T463A, implying that irreversibly strong MT-binding activity of Kid can result in the loss of chromosomal localization in prometaphase and metaphase cells. Therefore, we tentatively concluded that the ability of Kid to bind MTs is greatly enhanced by T463A mutation.
Fig. 6. Rigor-type and T463A mutant Kid show similar subcellular localization. (A) Equivalent amounts of GST–Kid-del.DB proteins with (right panel) or without (left panel) the T134N rigor-type mutation were subjected to MT co-sedimentation assay under the 200 mM K-Ac condition. MTs were added with no nucleotide (lane 1), 10 mM ATP (lane 2) or 3 mM AMP-PNP (lane 3). No MTs were added in lane 4. After centrifugation, supernatants (sup; non-MT bound) and pellets (ppt; MT bound) were separated by SDS–PAGE and analyzed by immunoblotting with anti-Kid antibodies or Coomassie Blue staining. (B) NIH-3T3 cells were infected with retrovirus carrying GFP–Kid-T134N, fixed, stained and observed as in Figure 4. Scale bars = 10 µm.
Table I. ATPase activity of wild-type and various Kid mutants.
| K-Ac(mM) | MTs |
||
|---|---|---|---|
| – | + | ||
| 100 | Wild typea | <0.05 | 38.0 ± 1.0 |
| T463Aa | <0.05 | 38.5 ± 0.9 | |
| T463Ea | <0.05 | 37.3 ± 1.2 | |
| T134Na | <0.05 | <0.05 | |
| Wild typeb | <0.05 | 53.0 ± 1.3 | |
| Wild typec | <0.05 | 50.3 ± 1.3 | |
| T463Ab | <0.05 | 51.2 ± 3.0 | |
| 200 | Wild typeb | <0.05 | 47.7 ± 1.2 |
| Wild typec | <0.05 | 48.8 ± 2.1 | |
| T463Ab | <0.05 | 48.0 ± 2.1 | |
Average values (per second) obtained from three or four independent experiments are shown with standard deviations.
aGST–Kid-del.DB proteins with or without T463A, T463E and T134N substitution were expressed in and purified from E.coli.
bHis-tagged, full-length Kid proteins with or without T463A substitution were purified from M phase-enriched HeLa cells.
cHis-tagged, full-length Kid proteins were purified from S phase-enriched HeLa cells.
GST–Kid-del.DBs prepared from E.coli and wild-type Kid prepared from S phase-enriched HeLa cells were not phosphorylated on Thr463.
Thr463 to alanine mutation of Kid results in its enhanced localization on spindle
To confirm further the idea described above, we examined the effect of T463A mutation on Kid-del.DB. When expressed at moderate and equal levels in NIH-3T3 cells (Figure 7A), GFP–Kid-del.DB with or without T463A mutation showed no chromosomal localization and was present on the spindle (Figure 7B). Notably, GFP–Kid-del.DB without T463A mutation was also distributed throughout the cytosol (upper panels). In contrast, GFP–Kid-del.DB with T463A substitution showed little cytoplasmic localization and localized prominently to the spindle (lower panels). These results strongly suggest that T463A mutation increases the affinity of Kid for spindle MTs in vivo.
Fig. 7. Enhanced MT binding of Kid in vivo by the T463A mutation. (A) GFP–Kid-del.DB with (lane 3) or without (lane 1) the T463A mutation was expressed in NIH-3T3 cells using the retrovirus system. Extracts of these cells and mock-infected cells (lane 1) were immunoblotted with anti-GFP antibody. Non-specific bands (*) were present as loading control. (B) NIH-3T3 cells expressing GFP–Kid-del.DB with (lower panels) or without (upper panels) the T463A mutation were fixed, stained and observed as in Figure 4. Scale bars = 10 µm.
Phosphorylation on Thr463 regulates the association of a second MT-binding site of Kid to ensure reversible association between Kid and MTs
To understand the mechanisms underlying the enhanced MT association of Kid-T463A, we first analyzed the ATPase activity. Consistent with our previous report (Tokai et al., 1996), bacterially expressed, thus unphosphorylated, GST–Kid-del.DB showed MT-dependent ATPase activity. Thr463 to alanine mutation as well as Thr463 to glutamate mutation showed no obvious difference in ATPase activity, whereas rigor-type mutation impaired the ATPase activity (Table I). Moreover, there was no detectable difference in ATPase activity between full-length wild-type Kid and Kid-T463A purified from S phase or M phase cells (Table I). These results indicate that neither phosphorylation nor mutation at Thr463 affect the ATPase activity of Kid. Therefore, strong associations with MTs of Kid-T463A and rigor-type mutant of Kid are mediated by distinct mechanisms despite their similarity in subcellular localization.
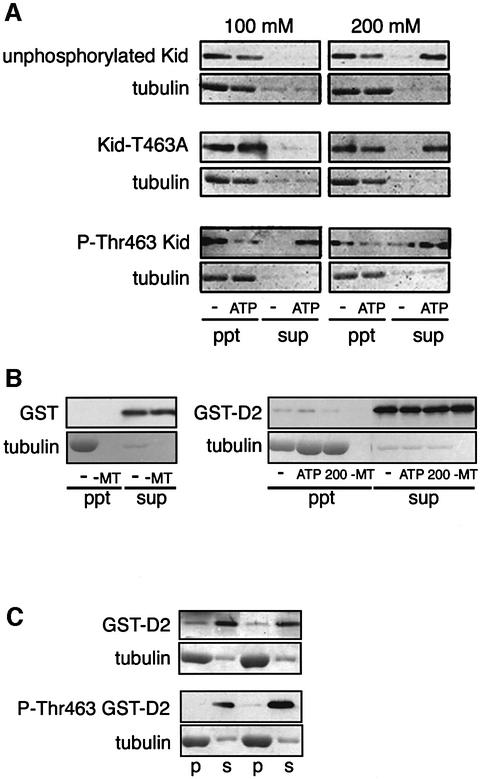
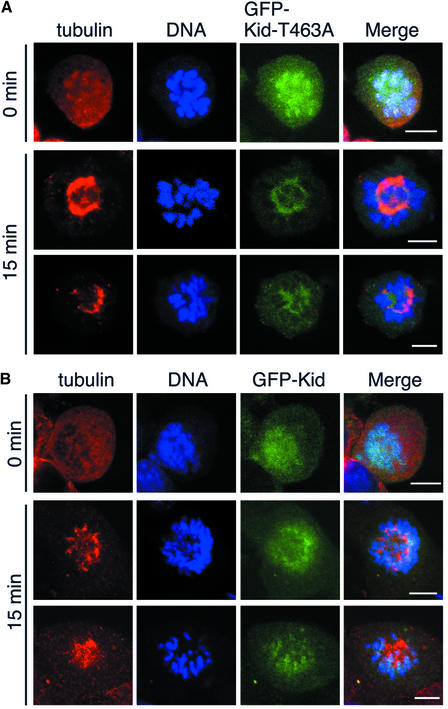
We next examined the nucleotide dependency of Kid binding to MTs in vitro by MT co-sedimentation assay. Under the low ionic strength conditions (100 mM K-Ac), unphosphorylated Kid prepared from S phase-enriched cells and Kid-T463A prepared from M phase-enriched cells showed strong interaction with MTs even in the presence of 10 mM Mg-ATP. Both proteins required high ionic strength (200 mM K-Ac) to be released from MTs in an ATP-dependent manner (Figure 8A, top and middle). In contrast, Thr463-phosphorylated Kid from M phase-enriched cells showed ATP-dependent release from MTs even under the low ionic strength conditions (Figure 8A, bottom). These results suggested that in addition to the interaction between MTs and the kinesin-like motor domain of Kid, there is an additional interaction site that prevents Kid from being detached from MTs in the presence of ATP under low ionic strength condition. To confirm the idea, we performed an MT co-sedimentation assay using GST–D2 that contained the stalk region of Kid (amino acids 407–548; Tokai et al., 1996). As shown in Figure 8B, GST–D2 showed weak but certain association with MTs, whereas GST was not bound to MTs. In addition, the association of GST–D2 and MTs was reduced under the high ionic strength conditions (Figure 8B, right panel). The association was also reduced when Thr463 was phosphorylated (Figure 8C). Moreover, a fraction of the GFP-fused stalk region of Kid became localized to the spindle when T463A substitution was introduced (Supplementary figure 2). It appeared that phosphorylation on Thr463 reduces interaction between the second binding site and MT so that MT binding of Kid can be reversible. To confirm this hypothesis, we performed a spindle reformation assay (Figure 9). NIH-3T3 cells expressing GFP–Kid or GFP–Kid-T463A were treated with nocodazole for 3 h, and then nocodazole was washed out to reform the spindle. Upon nocodazole treatment, both GFP–Kid and GFP–Kid-T463A were co-localized with chromosomes (Figures 5C and 9, top). At 15 min after the wash, most of the GFP–Kid-T463A was released from chromosomes and became confined to the reformed spindle (Figure 9A, middle and bottom), whereas the majority of GFP–Kid remained on the chromosomes (Figure 9B, middle and bottom).
Fig. 8. MT co-sedimentation assay of full-length and the stalk region of Kid. (A) Unphosphorylated wild-type Kid protein (purified from S phase cells) and phosphorylated wild-type Kid and Kid-T463A mutant (purified from M phase cells) were subjected to MT co-sedimentation assays. MTs were added with no (–) or 10 mM ATP (ATP) under 100 or 200 mM K-Ac conditions. After centrifugation, supernatants (sup; non-MT bound) and pellets (ppt; MT bound) were separated by SDS–PAGE, and the amounts of Kid and tubulin were analyzed by immunoblotting with anti-Kid or anti-PT463 antibodies or Coomassie Blue staining. (B) Bacterially expressed GST (left panel) and GST–D2 (right panel) were subjected to MT co-sedimentation assays under 50 mM (–, ATP, –MT) or 200 mM (200) K-Ac conditions with no (–, 200, –MT) or 10 mM ATP (ATP) and analyzed as in (A). –MT represents negative control with no MTs. The amounts of proteins were analyzed by immunoblotting with anti-GST antibody or Coomassie Blue staining. (C) GST–D2 was incubated with (lower panels) or without (upper panels) Cdc2–cyclin B in the presence of ATP, subjected to MT co-sedimentation assay and analyzed as in (A). p, ppt; s, sup. Amounts of proteins were analyzed by immunoblotting with anti-GST or anti-PT463 antibodies or Coomassie Blue staining. Results of duplicate experiments are shown.
Fig. 9. Sequestration of Kid-T463A on the spindle. NIH-3T3 cells expressing GFP–Kid-T463A (A) or GFP–Kid (B) were treated with 100 ng/ml nocodazole for 3 h, as in Figure 5D, and then washed to remove the nocodazole. Cells were fixed for 0 (upper panels) or 15 min (middle and lower panels; two typical samples are shown for mutant and wild-type Kid, respectively) after the wash, stained and observed as in Figure 4. Scale bars = 10 µm.
Taking these findings together, we conclude that Kid possesses a second MT-binding site within the stalk region and that this binding site contributes to strong and irreversible association of unphosphorylated Kid with MTs. Cdc2 kinase phosphorylates Kid on Thr463 and regulates the mode of association between Kid and MTs.
Discussion
In the present study, we investigated the mechanisms by which localization of Kid is regulated during M phase. We showed that Kid is phosphorylated at multiple sites including Ser427 and Thr463 in an M phase- or G2/M phase-specific manner and that Cdc2 kinase catalyzes phosphorylation of Thr463 in vitro and in vivo. It is noteworthy that Thr463 is conserved among the species (Antonio et al., 2000; Funabiki and Murray, 2000; our unpublished data), suggesting the biological importance of Thr463 phosphorylation. Expression of T463A mutant Kid at moderate levels in NIH-3T3 cells revealed that the localization and possibly the function of Kid are regulated through its phosphorylation by Cdc2 kinase. During prometaphase and metaphase, localization of GFP–Kid- T463A is restricted to the spindle, whereas a large fraction of ectopically expressed wild-type GFP–Kid accumulates on chromosomes (Figure 4B and C). This unusual localization of Kid-T463A is due to the threonine residue being umphosphorylated rather than to the artificial change introduced by amino acid substitution, since Kid-T463A mutant and unphosphorylated wild-type Kid exhibit similar activities in the ATPase assay and the in vitro MT co-sedimentation assay (Table I; Figure 8). When cells are infected appropriately, it appears that ectopically expressed Kid mutants including S427A, T463A and rigor-type mutant Kid do not cause serious mitotic aberrations, since similar percentages of prophase and telophase cells are observed in infected and uninfected cells (data not shown). However, we cannot rule out the possibility of delay in the progression of mitosis. We observed that excess accumulation of GFP–Kid-T463A correlated with thickening of spindle MTs and breakage of the spindle in prometaphase (data not shown).
As mentioned above, phosphorylation on Thr463 is required for proper localization of Kid on chromosomes. However, mislocalization of Kid-T463A could not be ascribed to the loss or decrease of its chromosome-binding activity. Moreover, when DNA-binding activity is totally abolished by truncating the DNA-binding domain of Kid, the association with spindles is enhanced by the T463A mutation (Figure 7). Kid-T463A and unphosphorylated Kid, but not T463-phosphorylated Kid, showed nucleotide-independent MT association under condition of physiological ionic strength. These findings led us to speculate that Kid possesses a second MT-binding site that shows Thr463 phosphorylation dependency. Consistent with this hypothesis, the bacterially expressed GST- and GFP-fused stalk region of Kid was associated with MT in vitro and in vivo, respectively (Figure 8B; Supplementary figure 2). Direct interaction between α-tubulin and the C-terminal half (amino acids 404–665) of Kid has also been reported (Germani et al., 2000). In addition, the majority, but not all, of GST–D2 was dissociated from MT under the high ionic strength conditions or upon Thr463 phosphorylation. Con sistently, GST–Kid(1–515) purified from Escherichia coli and phosphorylated by Cdc2–cyclin B in vitro did not show clear ATP-dependent MT binding (data not shown). We assume that phosphorylation on Thr463 as well as other sites contributes to the reduced MT binding of Kid in vivo. Similar phosphoregulation has been reported between the interaction of mitogen-associated proteins (MAPs) with MTs (Andersen et al., 1994; Drewes et al., 1997) and that between the C-terminal domain of CENP-E and MTs (Liao et al., 1994). We also examined the effect of a Thr463 to glutamate or aspartate substitution on MT association. T463E and T463D mutants were localized on chromosomes during prophase to metaphase as efficiently as wild-type Kid (data not shown). The data suggest that mimicry of phosphorylation by a negatively charged amino acid side chain is effective to some extent. Besides Kid, Cdc2 kinase also phosphorylates several mitotic motor proteins and regulates their association with MTs. Phosphorylation of CENP-E by Cdc2 kinase inhibits its MT cross-linking activity before anaphase (Liao et al., 1994). Phosphorylation in the BimC box of human and Xenopus Eg5 by Cdc2 kinase is essential for localization of Eg5 to the centrosomes and spindle (Blangy et al., 1995; Sawin and Mitchison, 1995). In addition, Klp61F is phosphorylated at the Cdc2–cyclin B consensus sequence within the BimC box and localizes to spindles in a phosphorylation-dependent manner (Sharp et al., 1999). Thus, regulations of Kid and other motor proteins by Cdc2 kinase could occur coordinately, which would enable these motors to work cooperatively to achieve cellular processes underlying M phase progression.
The second MT-binding site of Kid appeared to contribute to its high-affinity MT association during ATP hydrolysis. In agreement with this idea, we recently found the KmMT of ATPase activity of bacterially expressed Kid(1–515) to be 5–6 times lower than that of Kid(1–388) (K.Shiroguchi, M.Ohsugi, M.Edamatsu, T.Yamamoto and Y.Y.Toyoshima, submitted), suggesting that the second MT-binding site contributes to the high-affinity MT binding of unphosphorylated Kid. Further more, spindle-restricted localization of rigor-type mutant Kid and the result of spindle reformation assays strongly support the idea that reversible association of Kid with MTs is required to maintain its proper chromosomal localization. Strong association of Thr463-unphosphorylated Kid with MT might be important to catch spindle MTs on mitotic chromosome arms where the majority of Kid exists at the beginning of mitosis. Subsequent phosphoregulation might be required for a reversible association of Kid with MTs, and thus for proper localization on both spindle and chromosomes and generating the polar ejection force during prometaphase and metaphase (Antonio et al., 2000; Funabiki and Murray, 2000; Levesque and Compton, 2001). After the metaphase/anaphase transition, localization of GFP–Kid- T463A as well as rigor-type mutant Kid is indistinguishable from that of wild-type Kid (Figures 4C and 6B). These results indicate that neither the phosphorylation on Thr463 nor the reversible association with MTs is required for proper localization of Kid to the boundary of chromosomes and MTs during anaphase, when Cdc2–cyclin B kinase activity is lost. Phosphorylation on Thr463 is decreased consistently at the end of M phase (Figure 2C). In Xenopus egg extracts, degradation of Xkid is required for the migration of chromosomes to the poles during anaphase (Funabiki and Murray, 2000). In contrast, in somatic cells, Kid as well as Xkid on chromosome arms disappears after the metaphase/anaphase transition, but significant amounts of Kid and Xkid are maintained and concentrated at the boundary between the chromosomes and spindle MTs (Figure 4B; Tokai et al., 1996; Antonio et al., 2000). Consistently, the amount of Kid protein was not so dramatically decreased after metaphase/anaphase transition (Figure 2C). These data suggest that Kid also has a role after metaphase/anaphase transition in somatic cells. During anaphase, Thr463-unphosphorylated Kid may be important in anchoring chromosomes to the shortening plus ends of MTs with its strong affinity for MTs. These data also suggest that the localization and functions of Kid are controlled throughout M phase by multiple means, such as degradation, phosphorylation and possibly dephosphorylation.
Unlike Nod, which is also a chromosome-associated kinesin-like protein and recently has been shown to lack motility (Matthies et al., 2001), Kid does have plus end-directed motor activity (Yajima et al., 2003). As several studies have shown that the neck linker and stalk regions are critical mechanical elements for motor force generation (Romberg et al., 1998; Endow and Higuchi, 2000), the phosphorylation state of Thr463 might also affect the motility of Kid.
S427A mutation did not notably affect Kid localization (data not shown). Kid did not become reactive with anti-PS427 antibody after incubation with Cdc2–cyclin B (data not shown). In addition, besides Ser427 and Thr463, Kid was phosphorylated on other sites during M phase (Figure 1D), suggesting involvement of other mitotic kinases in the regulation of Kid function. Several other MT-associated proteins, such as MAPs (reviewed in Andersen, 2000) and Eg5 (Blangy et al., 1995; Giet et al., 1999), are also phosphorylated at multiple sites by multiple mitotic kinases. Thus, progression of mitosis is regulated by a sequence of concerted protein phosphorylation events. Identification of kinases that catalyze Kid phosphorylation and the biological role of Kid phosphorylation at Ser427 as well as other sites will provide important insight into the functions of Kid in mitosis.
Materials and methods
Cell culture and synchronization
Cells were cultured in Dulbecco’s modified Eagle’s medium, then synchronized in S phase by 2.5 mM thymidine block for 20–24 h or in M phase by 100 ng/ml nocodazole treatment for 14–18 h. S/early G2 phase HeLa cells were harvested 2 h after the release from thymidine block. Mitotic HeLa cells were collected by shake-off 9–11 h after the release from thymidine block. For metabolic labeling, 293T cells were cultured with [32P]orthophosphate (0.5 mCi/ml) in phosphate-free medium for the last 4 h of culture. Synchronization of the cells was checked by immunoblotting of the cell lysates with anti-cyclin A, B or D3.
Immunocytochemistry
Cells were fixed and immunostained as previously described (Tokai et al., 1996) except that DNAs were stained with TOTO3 (Molecular Probes), and observed with a confocal microscope (MRC-1024 and Radiance 2000; Bio-Rad).
Plasmid and retrovirus constructions
To prepare plasmids for Kid expression in mammalian cells or in E.coli, full-length Kid cDNA or a part of the coding region corresponding to amino acids 1–515 (for Kid-del.DB) flanked by EcoRI and BamHI sites at the 5′ and 3′ ends, respectively, was amplified by PCR. The resulting cDNA was cloned into the mammalian expression vector pME18S-FLAG, pME18S-GST (Tokai et al., 1996), GFP fusion protein expression vector pCMX-SAP/Y145F (a gift from Dr K.Umezono), pcDNA3.1/Myc-His (Invitrogen) or pGex vector (Amersham Pharmacia). Site-directed mutagenesis was performed by a PCR-mediated method. DNA fragments encoding GFP–Kid fusion proteins were recloned into pMX-puro vector (Onishi et al., 1996; Morita et al., 2000) for retrovirus production. Construction, expression and purification of GST–KidKD and GST–D2 were as previously described (Tokai et al., 1996). All constructs were confirmed by sequencing.
Immunobiochemistry and antibodies
Cells were lysed in lysis buffer (50 mM Tris–HCl pH 8.0, 150 mM NaCl, 1% NP-40, 1 mM EDTA, 50 U/ml aprotinin and 30 µg/ml DNase I) containing 0.1 µM okadaic acid when noted. For immunoprecipitation, lysates were incubated with 1–3 µg of anti-Kid antibody at 4°C for 1 h. Immune complexes were collected on protein G– or protein A–Sepharose (Amersham Pharmacia) and washed with lysis buffer. Immunoblots were performed as previously described (Tokai et al., 1996). For immunodepletion, mitotic HeLa cells were lysed in buffer A [20 mM Tris–HCl pH 7.5, 10 mM MgCl2, 1 mM EGTA, 1 mM EDTA, 0.5 mM Na3VO4, and 5 mM dithiothreitol (DTT)] containing 0.1% NP-40 and 30 µg/ml DNase I, and insoluble materials were removed by centrifugation. A 200 µl cytosol sample (15 mg proteins/ml) was incubated with 30 µl of agarose bead-coupled anti-Cdc2 monoclonal antibody (2 µg/µl; Santa Cruz) or control beads for 3–4 h at 4°C. The beads were removed by centrifugation, and the supernatants were stored at –80°C. Anti-Kid polyclonal antibodies were as described previously (Tokai et al., 1996). Anti-Kid monoclonal antibody was kindly provided by Dr T.Urano, Nagoya University. Monoclonal antibodies against tubulin, cyclin B and Cdc2 were from Santa Cruz, that against Flag (M2) was from Sigma, and that against GFP was from MBL. Anti-PS427 and anti-PT463 antibodies were raised against a synthetic phospho-Ser427 peptide, GCSQKL(pS) PLQKLSSMD, and a phospho-Thr463 peptide, CGEPGAPLLS(pT) PKRERMVLM, respectively, and affinity purified by successive incubation with the immunogens and recombinant GST–KidKD proteins.
Protein analysis
Phosphorylation reactions (30 µl) were carried out in buffer A containing 1 mM ATP or 3 µCi [γ-32P]ATP with purified Cdc2–cyclin B (a gift from Dr T.Kishimoto) or in mitotic cytosols with 1 mM ATP for 30 min at 37°C. Reactions were terminated by adding SDS–PAGE sample buffer. For phosphatase treatment, immunoprecipitated Kid proteins were washed successively in lysis buffer and in bacterial alkaline phosphatase (BAP) buffer (50 mM Tris–HCl pH 9.0 and 1 mM MgCl2) and then incubated with 10 U of BAP (Takara) in the presence or absence of 5 mM EGTA and 5 mM EDTA for 2 h at 37°C. Two-dimensional analysis of tryptic phosphopeptides was performed as previously described (Boyle et al., 1991).
DNA-binding assays
EMSA was carried out as previously described (Zhang and Nonoyama, 1994). A double-stranded DNA fragment corresponding to a part of the previously described probe A (Tokai et al., 1996), 5′-GAAGCTGGGAGTTGCCACTCCCAGACTTGTTGGAATG-3′, was labeled with 32P and used as a probe. GST–Kid proteins were purified from the lysates of M phase-enriched 293T cells that had been transfected with pME18S-GST-Kid. The DNA–protein binding reaction (20 µl) was performed in binding buffer [20 mM Tris–HCl pH 7.5, 50 mM NaCl, 3 mM MgCl2, 1 mM DTT, 0.006% NP-40, 20 mM EDTA, 5% (v/v) glycerol and 50 µg/ml bovine serum albumin (BSA)] at room temperature for 15 min. Each incubation mixture contained 2.5 µl of 0.1% poly(dI–dC), 0.5 µg of radioactive probe and 1 µg of protein. Samples were applied to 5% polyacrylamide gels. After electrophoresis, gels were fixed, dried and visualized by autoradiography. In competition assays, an excess amount of unlabeled probe or anti-Kid antibody was added prior to addition of the labeled probe. For the DNA–cellulose binding assay, Myc-His-tagged Kid proteins were purified from M phase cells transfected with pcDNA3.1-Kid by means of TALON Metal Affinity Resin (Clontech). Kid proteins in binding buffer (50 mM Tris–HCl pH 8.0, 150 mM NaCl, 4 mM MgCl2, 1 mM EDTA, 1 mM DTT and 200 µg/ml BSA) were mixed with double-stranded DNA–cellulose (Sigma) at room temperature for 15 min and centrifuged. Proteins in the pellets and supernatants were separated by SDS–PAGE. Kid proteins were detected by western blotting with anti-Kid or anti-PT463 antibodies.
ATPase assays
ATPase assays were performed according to published methods (Kodama et al., 1986). GST–Kid-del.DB proteins (wild type, T463A, T463E and T134N) were purified from E.coli. Myc-His-tagged Kid proteins (wild type and T463A) were purified from 293T cells synchronized in S phase or in M phase. Kid proteins (0.1 µM) in potassium acetate buffer (10 mM PIPES-KOH pH 6.8, 4 mM MgCl2, 100 or 200 mM K-acetate, 1 mM EGTA, 2 mM DTT) were incubated at 25°C with 1 mM Mg-ATP in the presence or absence of taxol-stabilized MTs (1 mg/ml). The rate of ATPase activity was determined by measuring the amount of inorganic phosphates in the reaction mixture with malachite green after 1, 2 and 4 min incubation.
MT co-sedimentation assay
GST–Kid-del.DB and GST–D2 proteins were purified from E.coli, and Myc-His-tagged wild-type and T463A mutant Kid proteins were purified from lysates of 293T cells transfected with pcDNA3.1-Kid by means of TALON Metal Affinity Resin (Clontech). Pre-centrifuged Kid proteins were incubated with 10 µg of taxol-stabilized MTs at 25°C for 5 min in a potassium acetate buffer (10 mM PIPES–KOH pH 6.8, 4 mM MgCl2, 50–200 mM K-acetate, 1 mM EGTA, 0.5% Triton X-100, 2 mM DTT) with or without 10 mM ATP or 3 mM AMP-PNP. The MTs and associated proteins were pelleted by centrifugation at 100 000 g for 10 min. Proteins in the pellets and supernatants were separated by SDS–PAGE. Tubulin was detected by Coomassie blue staining, and Kid proteins were detected by western blotting with anti-GST, anti-Kid or anti-PT463 antibodies.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank T.Kishimoto for providing us with purified Cdc2–cyclin B complex, T.Kitamura for the retrovirus expression system, T.Urano for anti-Kid monoclonal antibody, K.Umezono for mammalian GFP fusion protein expression vector, T.Tezuka for advice on phosphopeptide mapping, Y.Takeuchi and Y.Horiuchi for help in this study, and the Human Genome Center, Institute of Medical Science, for the sequence analysis package of the Genetics Computer Group (GCG). This work was supported in part by a grant for Advanced Cancer Research from the Ministry of Education, Science, Sports and Culture of Japan and from the Organization for Pharmaceutical Safety and Research of Japan.
References
- Andersen S.S. (2000) Spindle assembly and the art of regulating microtubule dynamics by MAPs and stathmin/Op18. Trends Cell Biol., 10, 261–267. [DOI] [PubMed] [Google Scholar]
- Andersen S.S., Buendia,B., Dominguez,J.E., Sawyer,A. and Karsenti,E. (1994) Effect on microtubule dynamics of XMAP230, a microtubule-associated protein present in Xenopus laevis eggs and dividing cells. J. Cell Biol., 127, 1289–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonio C., Ferby,I., Wilhelm,H., Jones,M., Karsenti,E., Nebreda,A.R. and Vernos,I. (2000) Xkid, a chromokinesin required for chromosome alignment on the metaphase plate. Cell, 102, 425–435. [DOI] [PubMed] [Google Scholar]
- Bischoff J.R. and Plowman,G.D. (1999) The Aurora/Ipl1p kinase family: regulators of chromosome segregation and cytokinesis. Trends Cell Biol., 9, 454–459. [DOI] [PubMed] [Google Scholar]
- Blangy A., Lane,H.A., d’Herin,P., Harper,M., Kress,M. and Nigg,E.A. (1995) Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell, 83, 1159–1169. [DOI] [PubMed] [Google Scholar]
- Blangy A., Chaussepied,P. and Nigg,E.A. (1998) Rigor-type mutation in the kinesin-related protein HsEg5 changes its subcellular localization and induces microtubule bundling. Cell Motil. Cytoskeleton, 40, 174–182. [DOI] [PubMed] [Google Scholar]
- Boyle W.J., van der Geer,P. and Hunter,T. (1991) Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol., 201, 110–149. [DOI] [PubMed] [Google Scholar]
- Cassimeris L. (1999) Accessory protein regulation of microtubule dynamics throughout the cell cycle. Curr. Opin. Cell Biol., 11, 134–141. [DOI] [PubMed] [Google Scholar]
- Drewes G., Ebneth,A., Preuss,U., Mandelkow,E.M. and Mandelkow,E. (1997) MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell, 89, 297–308. [DOI] [PubMed] [Google Scholar]
- Endow S.A. and Higuchi,H. (2000) A mutant of the motor protein kinesin that moves in both directions on microtubules. Nature, 406, 913–916. [DOI] [PubMed] [Google Scholar]
- Funabiki H. and Murray,A.W. (2000) The Xenopus chromokinesin Xkid is essential for metaphase chromosome alignment and must be degraded to allow anaphase chromosome movement. Cell, 102, 411–424. [DOI] [PubMed] [Google Scholar]
- Germani A., Bruzzoni-Giovanelli,H., Fellous,A., Gisselbrecht,S., Varin-Blank,N. and Calvo,F. (2000) SIAH-1 interacts with α-tubulin and degrades the kinesin Kid by the proteasome pathway during mitosis. Oncogene, 19, 5997–6006. [DOI] [PubMed] [Google Scholar]
- Giet R., Uzbekov,R., Cubizolles,F., Le Guellec,K. and Prigent,C. (1999) The Xenopus laevis aurora-related protein kinase pEg2 associates with and phosphorylates the kinesin-related protein XlEg5. J. Biol. Chem., 274, 15005–15013. [DOI] [PubMed] [Google Scholar]
- Glover D.M., Hagan,I.M. and Tavares,A.A. (1998) Polo-like kinases: a team that plays throughout mitosis. Genes Dev., 12, 3777–3787. [DOI] [PubMed] [Google Scholar]
- Heald R. (2000) Motor function in the mitotic spindle. Cell, 102, 399–402. [DOI] [PubMed] [Google Scholar]
- Kennelly P.J. and Krebs,E.G. (1991) Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J. Biol. Chem., 266, 15555–15558. [PubMed] [Google Scholar]
- Kodama T., Fukui,K. and Kometani,K. (1986) The initial phosphate burst in ATP hydrolysis by myosin and subfragment-1 as studied by a modified malachite green method for determination of inorganic phosphate. J. Biochem., 99, 1465–1472. [DOI] [PubMed] [Google Scholar]
- Levesque A.A. and Compton,D.A. (2001) The chromokinesin Kid is necessary for chromosome arm orientation and oscillation, but not congression, on mitotic spindles. J. Cell Biol., 154, 1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H., Li,G. and Yen,T.J. (1994) Mitotic regulation of microtubule cross-linking activity of CENP-E kinetochore protein. Science, 265, 394–398. [DOI] [PubMed] [Google Scholar]
- Lu K.P. and Hunter,T. (1995) The NIMA kinase: a mitotic regulator in Aspergillus nidulans and vertebrate cells. Prog. Cell Cycle Res., 1, 187–205. [DOI] [PubMed] [Google Scholar]
- Matthies H.J., Baskin,R.J. and Hawley,R.S. (2001) Orphan kinesin NOD lacks motile properties but does possess a microtubule-stimulated ATPase activity. Mol. Biol. Cell, 12, 4000–4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meluh P.B. and Rose,M.D. (1990) KAR3, a kinesin-related gene required for yeast nuclear fusion. Cell, 60, 1029–1041. [DOI] [PubMed] [Google Scholar]
- Morita S., Kojima,T. and Kitamura,T. (2000) Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther., 7, 1063–1066 [DOI] [PubMed] [Google Scholar]
- Mountain V. and Compton,D.A. (2000) Dissecting the role of molecular motors in the mitotic spindle. Anat. Rec., 261, 14–24. [DOI] [PubMed] [Google Scholar]
- Nakata T. and Hirokawa,N. (1995) Point mutation of adenosine triphosphate-binding motif generated rigor kinesin that selectively blocks anterograde lysosome membrane transport. J. Cell Biol., 131, 1039–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E.A. (1995) Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. BioEssays, 17, 471–480. [DOI] [PubMed] [Google Scholar]
- Nigg E.A. (1998) Polo-like kinases: positive regulators of cell division from start to finish. Curr. Opin. Cell Biol., 10, 776–783. [DOI] [PubMed] [Google Scholar]
- Nigg E.A., Blangy,A. and Lane,H.A. (1996) Dynamic changes in nuclear architecture during mitosis: on the role of protein phosphorylation in spindle assembly and chromosome segregation. Exp. Cell Res., 229, 174–180. [DOI] [PubMed] [Google Scholar]
- Ohi R. and Gould,K.L. (1999) Regulating the onset of mitosis. Curr. Opin. Cell Biol., 11, 267–273. [DOI] [PubMed] [Google Scholar]
- Onishi M. et al. (1996) Applications of retrovirus-mediated expression cloning. Exp. Hematol., 24, 324–329. [PubMed] [Google Scholar]
- Osmani S.A., Pu,R.T. and Morris,N.R. (1988) Mitotic induction and maintenance by overexpression of a G2-specific gene that encodes a potential protein kinase. Cell, 53, 237–244. [DOI] [PubMed] [Google Scholar]
- Rasooly R.S., New,C.M., Zhang,P., Hawley,R.S. and Baker,B.S. (1991) The lethal(1)TW-6cs mutation of Drosophila melanogaster is a dominant antimorphic allele of nod and is associated with a single base change in the putative ATP-binding domain. Genetics, 129, 409–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romberg L., Pierce,D.W. and Vale,R.D. (1998) Role of the kinesin neck region in processive microtubule-based motility. J. Cell Biol., 140, 1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin K.E. and Mitchison,T.J. (1995) Mutations in the kinesin-like protein Eg5 disrupting localization to the mitotic spindle. Proc. Natl Acad. Sci. USA, 92, 4289–4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp D.J., McDonald,K.L., Brown,H.M., Matthies,H.J., Walczak,C., Vale,R.D., Mitchison,T.J. and Scholey,J.M. (1999) The bipolar kinesin, KLP61F, cross-links microtubules within interpolar microtubule bundles of Drosophila embryonic mitotic spindles. J. Cell Biol., 144, 125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp D.J., Rogers,G.C. and Scholey,J.M. (2000) Microtubule motors in mitosis. Nature, 407, 41–47. [DOI] [PubMed] [Google Scholar]
- Tokai N., Fujimoto-Nishiyama,A., Toyoshima,Y., Yonemura,S., Tsukita,S., Inoue,J. and Yamamota,T. (1996) Kid, a novel kinesin-like DNA binding protein, is localized to chromosomes and the mitotic spindle. EMBO J., 15, 457–467. [PMC free article] [PubMed] [Google Scholar]
- Verde F., Labbe,J.C., Doree,M. and Karsenti,E. (1990) Regulation of microtubule dynamics by cdc2 protein kinase in cell-free extracts of Xenopus eggs. Nature, 343, 233–238. [DOI] [PubMed] [Google Scholar]
- Westendorf J.M., Rao,P.N. and Gerace,L. (1994) Cloning of cDNAs for M-phase phosphoproteins recognized by the MPM2 monoclonal antibody and determination of the phosphorylated epitope. Proc. Natl Acad. Sci. USA, 91, 714–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima J., Edamatsu,M., Watai-Nishii,J., Tokai-Nisizumi,N., Yamamoto,T. and Toyoshima,Y.Y. (2003) The human chromokinesin Kid is a plus-end directed microtubule-based motor. EMBO J., 22, 1067–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. and Nonoyama,M. (1994) The cellular proteins that bind specifically to the Epstein–Barr virus origin of plasmid DNA replication belong to a gene family. Proc. Natl Acad. Sci. USA, 91, 2843–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]