Abstract
Glucocorticoids potentiate the early steps of preadipocyte differentiation and promote obesity in Cushing’s syndrome and during prolonged steroid therapy. We show that glucocorticoids stimulate 3T3 L1 preadipocyte differentiation through a non-transcriptional mechanism mediated through the ligand-binding domain of the glucocorticoid receptor. This enhanced the onset of CCAAT/enhancer binding protein (C/EBPα) expression by potentiating its initial transcriptional activation by C/EBPβ. In the absence of steroid, C/EBPβ associated with a transcriptional corepressor complex containing mSin3A and histone deacetylase 1 (HDAC1), but lacking HDAC2 and RbAp46/48. HDAC1/mSin3A were recruited to the C/EBPα promoter with C/EBPβ and promoted the deacetylation of histone H4. Steroid induced the specific depletion of this corepressor by targeting the HDAC1 within the complex for degradation through the 26S proteasome. Treatment with histone deacetylase inhibitors replaced the effects of steroid treatment on preadipocyte differentiation and C/EBPα expression, while overexpression of HDAC1 abrogated the stimulatory effects of steroid. Recapitulation of the glucocorticoid effect by progestin treatment in the presence of the progesterone receptor ligand-binding domain suggests a conserved mechanism relevant to many aspects of steroid-mediated differentiation.
Keywords: CAAT enhancer binding protein β function/histone deacetylase 1/initiation of preadipocyte differentiation/26S proteasome/steroid hormone action
Introduction
The glucocorticoid receptor (GR) is a ligand-activated nuclear hormone receptor that regulates gene expression primarily through direct interaction with DNA response elements (Mangelsdorf et al., 1995). Glucocorticoids provide an adipogenic stimulus that is most obvious in the truncal obesity of patients with Cushing’s syndrome (Peeke and Chrousos, 1995). Weight gain is also a side-effect of immunosuppressive glucocorticoid therapies (Pijl and Meinders, 1996). In rodents, the weight loss that follows adrenalectomy is prevented by glucocorticoid replacement (Freedman et al., 1986; Sainsbury et al., 2001). However, gene-targeted mice in which GR is compromised for DNA binding are of normal weight and do not display any overt signs of alterations in adipogenesis, suggesting that the effects of glucocorticoids on adipogenesis may be mediated through a non-genomic mechanism (Reichardt et al., 2000a).
The adipocytes that constitute white fat originate from committed precursor cells, which differentiate in response to a series of cues including insulin and inducers of cAMP. In primary preadipocytes and most cell culture models, glucocorticoids strongly potentiate differentiation (Gregoire et al., 1998). The early responses to insulin and cAMP include the transient induction of CCAAT/enhancer binding protein (C/EBP) β and C/EBPδ, and overexpression of C/EBPβ is sufficient to force preadipocyte differentiation in culture (Yeh et al., 1995). In vivo, the stimulatory effect of C/EBPβ activity is complemented by the action of C/EBPδ, with defects in adipogenesis only seen upon ablation of both proteins (Tanaka et al., 1997). In the cellular models, the stimulatory effects of gluco corticoids coincide exactly with the expression of C/EBPβ (Rubin et al., 1978).
Completion of the differentiation program is accomplished by C/EBPα and peroxisome proliferator activated receptor γ (PPARγ) (Freytag et al., 1994; Tontonoz et al., 1994; Hu et al., 1995; Rosen et al., 1999). The regulatory relationship between C/EBPα and PPARγ is complex, involving both auto- and cross-regulatory control (Wu et al., 1999). However, recent studies in PPARγ- and C/EBPα-deficient cells have established that these factors participate in a single pathway of fat cell development. C/EBPα is required for the activation and maintenance of PPARγ expression and the conferment of insulin sensitivity to the mature adipocyte (Rosen et al., 2002).
It is hypothesized that C/EBPβ and C/EBPδ act to initiate C/EBPα and PPARγ expression through C/EBP response elements in the transcriptional control regions of both genes (Christy et al., 1991; Elberg et al., 2000), with recent experiments supporting a preferential role of C/EBPβ in the activation of C/EBPα expression (Elberg et al., 2000; Jiang and Lane, 2000). During preadipocyte differentiation, induction of C/EBPα and PPARγ lags the appearance of C/EBPβ/δ by many hours (Tontonoz et al., 1994; Yeh et al., 1995; Tang and Lane, 1999). This may be partially accounted for by the initial presence of the inhibitory C/EBP family member CHOP, which binds C/EBPβ and δ and restrains their transcriptional activity by inhibiting dimerization and DNA binding (Ron and Habener, 1992; Batchvarova et al., 1995; Tang and Lane, 2000). While stimulating early adipogenic events, glucorticoids do not directly affect the appearance of C/EBPβ or the disappearance of CHOP (Cao et al., 1991).
In present study we report that glucocorticoid stimulation of the early events in preadipocyte differentiation occurs through a non-transcriptional mechanism mediated through the receptor ligand-binding domain (LBD), which suppresses a specific histone deacetylase 1 (HDAC1)/mSin3A copressor complex that otherwise interacts with C/EBPβ to restrain the onset of C/EBPα expression. These results present a molecular basis for the predisposition to weight gain that is observed during prolonged glucocorticoid excess and treatment with the antiepileptic and antidepressant valproate (VPA). They also suggest a mechanism for interaction between GR and C/EBPβ that is likely to be relevant to the effects of glucocorticoids in the immune system and on the hippocampus, and a broader non-transcriptional mechanism of action for GR and other steroid hormone receptors.
Results
The stimulatory effects of steroids on preadipocyte differentiation are mediated through the receptor LBD
Preliminary experiments demonstrating the potential for the GR LBD to enhance C/EBPβ-mediated transcription (Boruk et al., 1998) suggested that the GR LBD might be sufficient to communicate the stimulatory effects of glucocorticoids on preadipocyte differentiation. To test this possibility we examined the effect of a 48 h steroid treatment on the differentiation of 3T3 L1 preadipocytes infected to express the GR LBD from a retroviral vector (Figure 1). The 3T3 L1 cell culture model has been used extensively to study the molecular events governing adipogenesis and in these cells steroid strongly potentiates differentiation in the presence of insulin and 3-isobutyl-1-methylxanthine (MIX), a cAMP phosphodiesterse inhibitor.
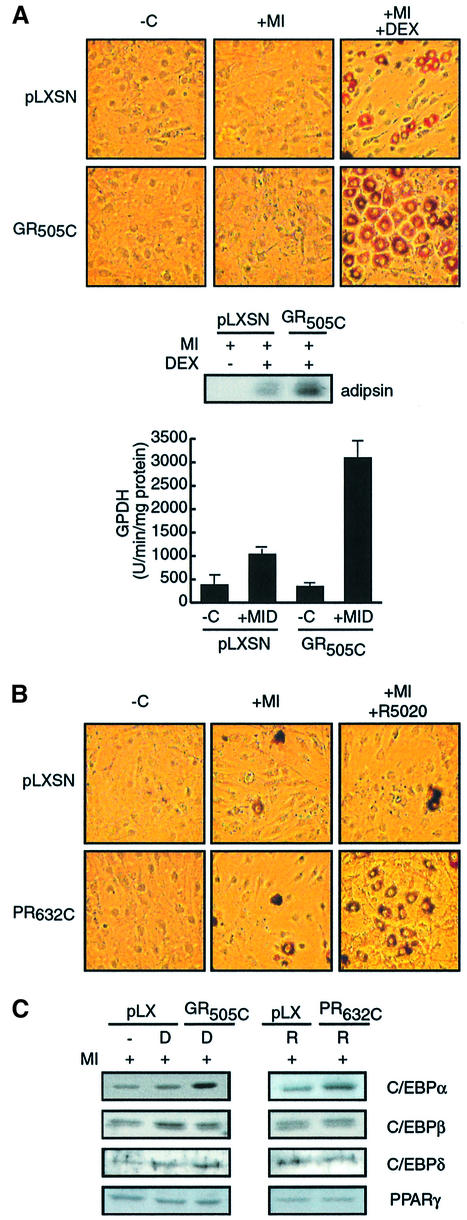
Fig. 1. Enhancement of preadipocyte differentiation is mediated through steroid receptor LBDs. (A) Oil red O staining, adipsin protein levels and GDPH activity of 3T3 L1 cells infected with retrovirus to express amino acids 505–795 of rat GR (GR505C) or control virus (pLXSN) and cultured for 8 days in the presence of MIX and 50 nM insulin (+MI), MIX, insulin and dex (+MID), or in the absence of cocktail (–C). The images displayed are representative of results observed in a minimum of three independent experiments performed in duplicate over a period of several months. For GPDH, 1 mU equals 1 nmol of product/min/mg of protein. (B) Effect of expression of amino acids 632–933 of human PR (PR632C) on 3T3 L1 differentiation as visualized by Oil red O staining. (C) Western analysis of C/EBPβ, δ, α and PPARγ expression in 3T3 L1 cells following 24 h treatment with MIX, insulin and dex (D) or R5020 (R).
We measured the effect of steroid on 3T3 L1 differentiation at day 8 in three ways: direct visualization of lipid accumulation by Oil red O staining, measurement of the level of adipsin accumulation and the measurement of glycerol-3-phosphate dehydrogenase (GPDH) activity, a key adipogenic enzyme (Wise and Green, 1979) (Figure 1A).
Cells infected with the empty viral vector underwent little differentiation following treatment with insulin and MIX alone under conditions where insulin levels were reduced to 50 nM and serum lots were carefully type matched to focus on the effects of dexamethasone (dex) treatment, although the initial clonal expansion of the cells occurred normally (data not shown). Addition of dex to the treatment regime induced the differentiation of a significant percentage of the cells (20–30%), as demonstrated by development of the characteristic rounded, lipid-laden morphology, the appearance of adipsin and the modest induction of GPDH. The response was steroid dependent and was not observed upon treatment with the glucocorticoid antagonist RU486 (data not shown).
Infection with a retrovirus leading to expression of the GR LBD (GR505C) at approximately twice the level of the endogenous GR (data not shown) did not benefit the response to insulin and MIX (Figure 1A). However, the stimulatory effect of dex was enhanced dramatically, with a strong increase in adipsin expression, a 3-fold increase in GPDH activity, and 60–80% of the cells becoming rounded and staining prominently with Oil red O.
To assess whether the LBD of GR might be sufficient for the stimulatory effect of dex, we repeated our experiment with a viral vector expressing the LBD of the closely related progesterone receptor (PR632C; Figure 1B). In this instance, treatment with 100 nM insulin and MIX alone was sufficient to induce differentiation and lipid accumulation in ∼5% of the cells. Differentiation was not further affected by addition of the synthetic progestin R5020 to 3T3 L1 cells infected with control virus (Figure 1C), as R5020 did not activate the endogenous GR (data not shown). In the presence of the PR LBD, however, R5020 treatment strongly stimulated differentiation, with >40% of the cells displaying altered morphology and prominent Oil red O staining (Figure 1B).
Stimulation of adipogenesis by the GR/PR LBDs correlates with the initial activation of C/EBPα expression by C/EBPβ
As the stimulatory effects of steroid are specific to the onset of the differentiation program, we examined the effect of steroid treatment and expression of the GR and PR LBDs on the initial appearance of early transcription factors in the differentiation cascade (Figure 1C). Consistent with previous results (Cao et al., 1991), the induction of C/EBPβ expression over the first 24 h of stimulation was not significantly influenced by steroid treatment, even in the presence of the GR or PR LBDs. The early response of C/EBPδ and PPARγ was similarly unaffected by steroid. By contrast, dex and R5020 significantly enhanced accumulation of C/EBPα at 24 h in an LBD-dependent manner.
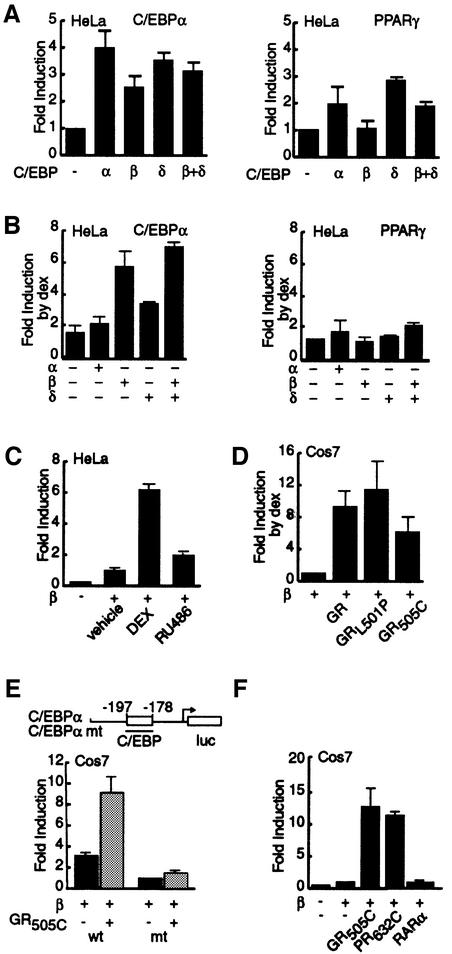
The ability of the GR/PR LBDs to influence transcription from extended regions of C/EBPα and PPARγ promoters was assessed directly in transient transfection assays (Figure 2). In HeLa cells (Figure 2A and B), C/EBPα, C/EBPβ, C/EBPδ and a combination of C/EBPβ and δ induced expression from the C/EBPα promoter between 2.5- and 4-fold (Figure 2A). By contrast, C/EBPβ failed completely to activate transcription from the PPARγ promoter even when expressed at higher levels (data not shown), while C/EBPα and C/EBPδ induced reporter activity 2- to 3-fold. This inability of C/EBPβ to activate transcription from the PPARγ promoter was in agreement with results in 3T3 L1 cells (Elberg et al., 2000).
Fig. 2. GR and PR LBDs potentiate C/EBPβ-dependent C/EBPα expression. (A) Fold induction of luciferase activity from the C/EBPα (–355/+7) and PPARγ (–609/+52) promoters upon ectopic expression of C/EBPα, β and δ in HeLa cells. (B) Fold increase of C/EBPα, β and δ-dependent luciferase activity by dex treatment above the level of activity in (A). (C) Effect of RU486 on the potentiation of C/EBPβ-dependent luciferase activity from the C/EBPα promoter in HeLa cells. (D) Fold increase of C/EBPβ-dependent luciferase activity from the C/EBPα promoter upon dex treatment of Cos7 cells cotransfected with GR, GRL501P or GR505C. (E) Mutation of C/EBP binding site in the C/EBPα promoter abrogates the GR505C-dependent stimulation of C/EBPα transcription in Cos7 cells. The schema delimits the 29 bp mutation that abrogrates the C/EBP response element. (F) Comparison of the potentiation of C/EBPβ-dependent luciferase activity from the C/EBPα promoter by GR505C, PR632C and RARα upon treatment with dex, R5020 and retinoic acid.
Dex treatment had only a very modest stimulatory effect on basal transcription from the C/EBPα promoter and no significant additional effect on expression induced by C/EBPα (Figure 2B). However, in the presence of C/EBPβ and a combination of C/EBPβ and δ, dex treatment enhanced expression from the C/EBPα promoter an additional 5- to 6-fold, while enhancing expression dependent on C/EBPδ alone a more modest 3-fold. Conversely, dex treatment was unable to elicit significant additional activation of the PPARγ promoter by any of the three C/EBPs.
Stimulation of C/EBPβ-induced C/EBPα transcription was agonist dependent, as treatment with RU486 was without effect (Figure 2C). Experiments in Cos7 cells, which lack endogenous GR, verified that the GR LBD was sufficient for potentiation of C/EBPβ-activated C/EBPα transcription and that stimulation by full-length GR was not affected by mutations that abrogated receptor DNA binding (GRL501P; Figure 2D). By contrast, the stimulatory effect of the GR LBD was lost upon mutation of the C/EBP response element in the C/EBPα promoter (Figure 2E).
Consistent with our observations in 3T3 L1 cells, the PR LBD was as effective as the GR LBD in potentiating the activation of C/EBPα transcription by C/EBPβ (Figure 2F). Potentiation of C/EBPβ-mediated C/EBPα transcription was not a generic property of nuclear hormone receptors, however, as retinoic acid treatment of cells expressing RARα failed to induce C/EBPα expression in the presence of C/EBPβ.
C/EBPβ and steroidal activation of C/EBPα transcription converge at an mSin3A/HDAC1 corepressor complex
The inability of the GR LBD to interact physically with C/EBPβ (Boruk et al., 1998) pointed to the involvement of intermediary factors with the ability to suppress transcriptional activation by C/EBPβ in the absence of steroid. Recent microarray analysis of preadipocyte differentiation (Soukas et al., 2001) suggested HDAC1 as a potential repressor of C/EBPβ. Our interest in HDAC1 was heightened by reports that the antiepileptic drug VPA acts as a histone deacetylase inhibitor (Gottlicher et al., 2001; Phiel et al., 2001). One of the side-effects of VPA treatment is weight gain reminiscent of that seen upon the clinical application of glucocorticoids (Davis et al., 1994; Jallon and Picard, 2001).
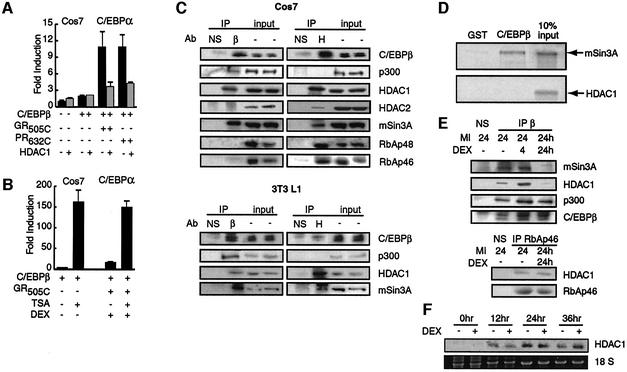
Ectopic expression of HDAC1 in Cos7 cells blocked the C/EBPβ-dependent stimulation of C/EBPα transcription by the GR and PR LBDs (Figure 3A), while having no significant effect in the absence of steroid (data not shown). HDAC1 expression also abrogated the stimulation of C/EBPβ-dependent C/EBPα transcription by endogenous GR in HeLa cells (data not shown). The effect was specific, as transcription of a rous sarcoma virus-driven reporter gene was unaffected by HDAC1 and the GR and PR LBDs (data not shown).
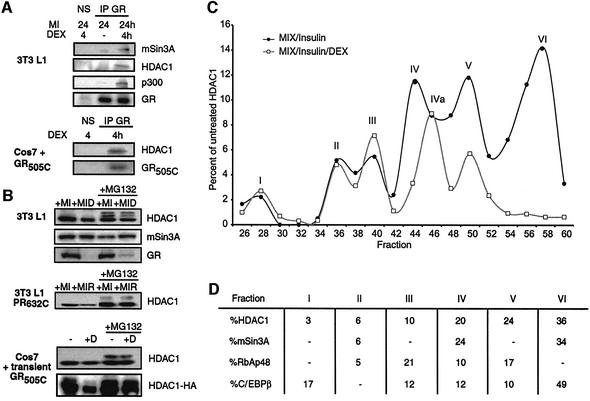
Fig. 3. Interaction of HDAC1 with C/EBPβ is relieved by prolonged steroid treatment. (A) Effect of transiently transfected C/EBPβ, GR505C, PR632C and HDAC1 on the induction of luciferase activity from the C/EBPα promoter in Cos7 cells. (B) Effect of dex and TSA on the fold induction of C/EBPβ-dependent luciferase activity from the C/EBPα promoter in Cos7 cells. (C) Immunoprecipitation of transient transfected C/EBPβ (β) and endogenous HDAC1(H) from Cos7 cells (top) or endogenous C/EBPβ and HDAC1 from 3T3 L1 cells treated for 24 h with MIX and 100 nM insulin (bottom). NS, non-specific type-matched antibody. Immunoprecipitates and 10% of the extracts used for immunoprecipitation (input) were resolved by SDS–PAGE and probed for the presence of the indicated proteins with specific antibodies. (D) GST pull-down assay of the binding of in vitro translated mSin3A and HDAC1 to GSTC/EBPβ and GST. (E) Immunoprecipitations from 3T3 L1 cells performed as described in (C) except that dex was included for the final 4 h or full 24 h of insulin/MIX treatment as indicated. (F) Northern analysis of HDAC1 mRNA following insulin, MIX and dex treatment compared with 18S rRNA.
Treatment of GR LBD-transfected Cos7 cells with the histone deacetylase inhibitor trichostatin A (TSA) dramatically stimulated the induction of C/EBPα transcription by C/EBPβ. Moreover, C/EBPα transcription in the presence of TSA was not stimulated further by dex treatment in Cos7 or HeLa cells (Figure 3B; data not shown).
HDAC1 is a class 1 histone deacetylase that occurs in several corepressor complexes, including the Sin3, NURD/Mi2 and CoRest complexes (You et al., 2001; Narlikar et al., 2002). To define the context in which HDAC1 acted on C/EBPβ, we tested C/EBPβ immunoprecipitates prepared under stringent conditions for components of HDAC1-containing corepressor complexes.
Immunoprecipitates of C/EBPβ expressed in Cos7 cells revealed separate interactions of C/EBPβ with endogenous HDAC1 and p300 (Figure 3C), a coactivator that has been implicated in activation of transcription by C/EBPβ (Mink et al., 1997). mSin3A was also a prominent component of C/EBPβ immunoprecipitates. By contrast, HDAC2, RbAp46 and RbAp48, which also occur in the Sin3 complex, did not appear in C/EBPβ immunoprecipitates, although all were detected upon immunoprecipitation of HDAC1. CoRest and HDAC4 were also not detected with C/EBPβ (data not shown). These data indicated that C/EBPβ interacted with subcomponents of the Sin3 complex, but did not interact with the NURD/Mi2 or CoRest complexes.
We obtained exactly the same result with extracts from 3T3 L1 cells treated with MIX and insulin for 24 h, with C/EBPβ interacting specifically with p300, HDAC1 and mSin3A (Figure 3C). By contrast, we were unable to detect an interaction between C/EBPβ and HDAC2, RbAp46 or RbAp48, which were expressed at relatively low levels in these cells (data not shown).
In vitro binding studies suggested that the interaction between HDAC1 and C/EBPβ was mediated through mSin3A. In vitro translated mSin3A bound strongly to GSTC/EBPβ but was not retained on beads containing GST alone, whereas HDAC1 did not interact significantly with GSTC/EBPβ (Figure 3D).
Strikingly, addition of dex to the 24 h insulin/MIX treatment abrogated the interaction of C/EBPβ with mSin3A and HDAC1 without affecting its interaction with p300 (Figure 3E). This was a long-term effect of dex, as mSin3A/HDAC1 association with C/EBPβ was not significantly affected by addition of dex for only the final 4 h of the insulin/MIX treatment. Dex treatment had no obvious effect on the interaction between HDAC1 and RbAp46 in the same cells (Figure 3E), nor did it suppress HDAC1 transcription (Figure 3F). Indeed, insulin and MIX treatment actually induced the accumulation of HDAC1 mRNA, but this induction was unaffected by dex. Previous analyses have shown that HDAC1 mRNA is subsequently downregulated as cells proceed past 48 h (Soukas et al., 2001).
HDAC1 inhibits the onset of C/EBPα expression and preadipocyte differentiation
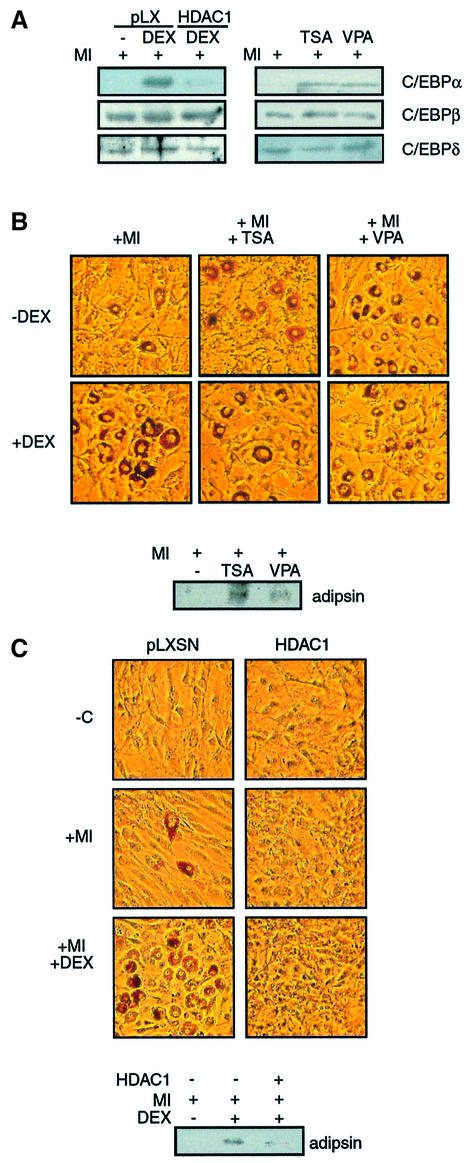
To assess whether regulation of the interaction between mSin3A/HDAC1 and C/EBPβ by dex might be relevant to the stimulation of preadipocyte differentiation, we examined the effect of manipulating HDAC1 on the adipogenic program in 3T3 L1 cells (Figure 4). As for the GR LBD, virally mediated expression of HDAC1 raised HDAC1 levels to approximately twice the level of endogenous protein (data not shown). Infection with virus expressing HDAC1, but not control virus, suppressed the dex-dependent initial accumulation of C/EBPα at 24 h (Figure 4A). Modest suppression of C/EBPδ expression was also observed, but accumulation of C/EBPβ was unaffected. Conversely, addition of TSA and VPA stimulated the accumulation of C/EBPα at 24 h in the absence of dex, without affecting the levels of C/EBPβ or C/EBPδ.
Fig. 4. Histone deacetylases repress preadipocyte differentiation. (A) C/EBPα, β and δ levels at 24 h in whole-cell extracts prepared from 3T3 L1 cells treated with insulin and MIX and dex as indicated, and infected with pLXSN or HDAC1-expressing virus (left) or treated with TSA (400 nM) or valproic acid (VPA; 10 mM, right). (B) Effect of initial 48 h TSA or VPA treatment on Oil red O staining and adipsin expression at day 8 in 3T3 L1 cells stimulated with insulin and MIX (+MI) and dex (+DEX). (C) Effect of virally mediated HDAC1 expression on differentiation of 3T3 L1 cells as revealed by adipsin levels and Oil red O.
Strikingly, treatment of the cells with TSA and VPA for the first 48 h of differentiation strongly stimulated the differentiation of insulin- and MIX-treated cells in the absence of dex, as demonstrated by the expression of adipsin and Oil red O staining of the cells at day 8 (Figure 4B). Moreover, TSA- and VPA-stimulated differentiation was not enhanced further by dex treatment. In an additional parallel to steroid, treatment with TSA/VPA subsequent to the initial 48 h of exposure to insulin and MIX had no effect on differentiation (data not shown). By contrast, viral expression of HDAC1 strongly inhibited preadipocyte differentiation (Figure 4C).
Steroid treatment induces the disappearance of an mSin3A/HDAC1-containing complex that preferentially interacts with C/EBPβ
One possibility suggested by our results was that dex treatment stimulated C/EBPβ transcriptional activity and 3T3 L1 differentiation by directly competing for the mSin3A/HDAC1 complex that interacted with C/EBPβ. Indeed, we observed that both mSin3A and HDAC1 could be detected in co-immunoprecipitates with GR when dex treatment was performed for the final 4 h following the onset of insulin and MIX treatment, and the GR LBD appeared to be sufficient for this interaction (Figure 5A). In an additional similarity to C/EBPβ, GR did not interact with RbAp46/48 or HDAC2 (data not shown). However, 4 h treatment of 3T3 L1 cells with dex failed to significantly compete the interaction of mSin3A/HDAC1 with C/EBPβ (Figure 4F).
Fig. 5. Dex induces selective loss of an HDAC1/mSin3A complex that preferentially interacts with C/EBPβ. (A) Immunoprecipitates from 3T3 L1 cells (top) and Cos7 cells transfected to express the GR LBD (bottom) were prepared as described in Figure 3C except using an antibody to GR (IP GR) and including dex treatment for the final 4 h of the insulin/MIX (MI) treatment. Western analysis of the immunoprecipitates was performed with antibodies to the factors indicated (B) Effect of MG132 (1 µM) on levels of HDAC1, mSin3A and GR in 3T3 L1 cells treated with insulin/MIX and dex for 24 h (top). Effect of 24 h R5020 and MG132 on endogenous HDAC1 levels in Cos7 cells transfected with PR632C (middle) or effect of 24 h dex/MG132 treatment on endogenous and transiently expressed (HDAC1-HA) HDAC1 in Cos7 cells transfected with GR505C. (C) Quantitative display of western analysis of HDAC1 levels in FPLC fractions prepared from 3T3 L1 cells treated with for 24 h with insulin/MIX (dark line) or insulin/MIX/dex (gray line). Curves are standardized against total HDAC1 levels in extracts from untreated cells. Peaks are labelled I–VI, with IVa indicating a shift in the position of peak IV upon dex treatment (D) Quantification of HDAC1, mSin3A, RbAp48 and C/EBPβ levels in fractions from (C) for extract prepared from cells treated with insulin/MIX only. Calculations of percent HDAC1, mSin3A, RbAp48 and C/EBPβ represent areas under the curve for each peak.
Turnover of GR and PR through the 26S proteasome is dependent on the LBD and is accelerated by steroid (Lange et al., 2000; Wallace and Cidlowski, 2001). Analysis of extracts from 3T3 L1 and Cos7 cells showed that the GR/PR LBDs induced a steroid-dependent decrease in the levels of HDAC1 that was prevented by the proteasome inhibitor MG132 (Figure 5B). Twenty-four hour treatment of 3T3 L1 cells with dex resulted in a large decrease in GR levels that was partially rescued by addition of MG132. Concomitantly, dex treatment induced an ∼50% reduction (51.6 ± 5.7%) in the levels of HDAC1 that was prevented by inclusion of MG132 (91.3 ± 6.7% of MG132 treatment alone), but had no obvious effect on the levels of mSin3A. MG132 also induced the appearance of slower migrating forms of HDAC1 that reflect progressive ubiquitylation. Treatment of PR LBD-infected 3T3 L1 cells with R5020 and of Cos7 cells transfected with the GR LBD with dex induced a similar MG132-sensitive decrease in HDAC1 (Figure 5B). The steroid-induced decrease in HDAC1 levels was even more striking for HDAC1 expressed by cotransfection with the GR LBD in Cos7 cells (Figure 5B). Lastly, for 3T3 L1 cells, virally mediated overexpression of HDAC1 maintained the protein levels in dex-treated cells above the endogenous HDAC1 levels in cells treated with MIX and insulin alone (data not shown).
The inability of dex treatment to affect the amount of HDAC1 that immunoprecipitated with RbAp46 (Figure 3E), while decreasing total HDAC1 levels by approximately one-half, suggested that GR might selectively target a specific subpopulation of HDAC1 that interacts preferentially with C/EBPβ. FPLC size fractionation of extracts prepared from 3T3 L1 cells treated with insulin and MIX for 24 h resolved HDAC1 into six components (Figure 5D). Peaks II, IV and VI of HDAC1 contained a proportional amount of mSin3A, while peaks I, III and V lacked mSin3A. By contrast, RbAp48 was enriched in peaks II and V, somewhat under-represented in peaks II and IV, and absent from peaks I and VI. Strikingly, almost 50% of the C/EBPβ in the extract cofractionated with HDAC1 peak VI.
Moreover, inclusion of dex with insulin and MIX for 24 h induced the complete loss of HDAC1 from peak VI. HDAC1 peaks I–III were unaffected in their proportion and migration, while peak IV shifted two fractions to the right, and thus was designated IVa. Peak V of HDAC1 was reduced in relative abundance by ∼50%. Repetition of this experiment in HeLa cells showed exactly the same result, six analogous HDAC1-containing peaks with peak VI lost from cells treated with dex (data not shown), illustrating that this effect of steroid is applicable across cell types and occurs independently of the presence of C/EBPβ.
Steroid treatment prevents the recruitment of HDAC1/mSin3A to the C/EBPα promoter
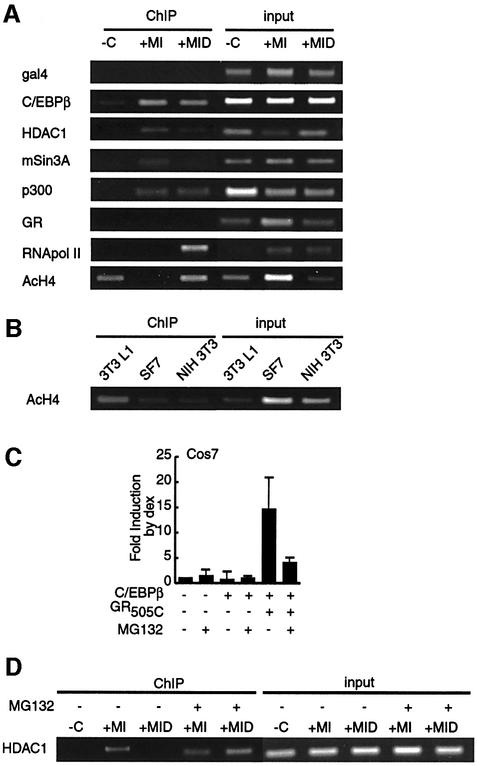
One prediction based on our results was that dex treatment would promote the onset of C/EBPα expression by pre-empting or curtailing the recruitment of mSin3A/HDAC1 to the C/EBPα promoter. Treatment of confluent 3T3 L1 cells with insulin and MIX for 24 h induced the accumulation of C/EBPβ at the C/EBPα promoter, as shown in chromatin immunoprecipitation (ChIP) experiments (Figure 6A), directly implicating C/EBPβ in the initial response of C/EBPα. Similarly, insulin and MIX treatment was sufficient to induce the recruitment of p300 to the C/EBPα promoter, and the accumulation of C/EBPβ and p300 was unaffected by dex treatment. By contrast, we could find no evidence that GR associated with the C/EBPα promoter (Figure 6A), although we have readily detected GR on the mouse mammary tumor virus promoter in similar experiments (data not shown). Nonetheless, the stimulatory effect of steroid on C/EBPα expression at 24 h was reflected by a prominent increase in the presence of RNA polymerase II (PolII) at the C/EBPα promoter.
Fig. 6. MG132 abrogates the effect of dex on recruitment of HDAC1/mSin3A to the C/EBPα promoter. (A) ChIP analysis of the C/EBPα promoter in 3T3 L1 cells treated for 24 h with vehicle (–C), insulin (I), MIX (M) and dex (D) as indicated. Formaldehyde-crosslinked DNA–protein complexes were immunoprecipitated with the antibodies to the proteins indicated and with a pan-acetyl histone H4 antibody (AcH4). DNAs prepared from the extracts employed for the immunoprecipitations were used as input controls (B) ChIP for AcH4 in confluent 3T3 L1, SF7 and NIH 3T3 cells. (C) Effect of MG132 treatment (1 µM) on the dex-dependent fold increase of C/EBPβ-dependent luciferase activity from the C/EBPα promoter in Cos7 cells cotransfected with GR505C. (D) Effect of MG132 treatment (1 µM) on ChIP for HDAC1 on C/EBPα promoter in 3T3 L1 cells performed as in (A).
Prior to adipogenic stimulation, neither mSin3A nor HDAC1 was detected at the C/EBPα promoter. Insulin and MIX treatment induced the accumulation of both factors concomitant with C/EBPβ. However, dex treatment strongly reduced the presence of HDAC1 and mSin3A at 24 h.
Acetylation of histone H4 at the C/EBPα promoter in 3T3 L1 cells correlated inversely with the recruitment of HDAC1/mSin3A. H4 acetylation was readily detected in untreated cells and cells stimulated to differentiate in the presence of dex, but absent from the promoter in cells stimulated by insulin and MIX alone. These results indicate that HDAC1 recruitment to the C/EBPα promoter contributes to active chromatin deacetylation and the inhibition of transcriptional activation by C/EBPβ/p300.
Interestingly, acetylation of the C/EBPα promoter prior to stimulation occurred preferentially in 3T3 L1 cells, as little H4 acetylation was observed in two unrelated fibroblast cell lines (Figure 6B). Thus, H4 acetylation of the C/EBPα promoter in uninduced cells may be one of the factors that determine the commitment of 3T3 L1 cells to the adipocyte lineage and their responsiveness to ectopic overexpression of C/EBPβ (Wu et al., 1995; Yeh et al., 1995).
To seek direct evidence that the role of steroid in potentiating the effects of C/EBPβ on the C/EBPα promoter was linked to the induced degradation of HDAC1 peak VI, we performed two experiments with the proteasome inhibitor MG132. First, in GR LBD-transfected Cos7 cells, addition of MG132 blocked the stimulatory effect of dex on C/EBPβ-mediated C/EBPα reporter gene expression without affecting steroid- independent luciferase activity (Figure 6C). Secondly, addition of MG132 to stimulated 3T3 L1 cells made the insulin/MIX-induced recruitment of HDAC1 and mSin3A to the C/EBPα promoter refractory to dex treatment (Figure 6D).
We conclude that glucocorticoids act to stimulate preadipocyte differentiation by lowering the mSin3A/HDAC1-imparted barrier to activation of C/EBPα transcription by C/EBPβ. This is accomplished by inducing the degradation of an mSin3A/HDAC1-containing corepressor complex that preferentially interacts with and reduces the transcriptional activation potential of C/EBPβ.
Discussion
Potentiation of C/EBPβ activation by GR overcomes an HDAC1-mediated barrier to preadipocyte differentiation
We provide compelling evidence that glucocorticoids potentiate preadipocyte differentiation by specifically targeting a C/EBPβ-interacting HDAC1 corepressor complex that restrains the initial onset of C/EBPα transcription. This was accomplished through an LBD-dependent mechanism that correlated with receptor-induced turnover of an HDAC1-containing complex that preferentially interacted with C/EBPβ. Our results suggest an explanation for the proadipogenic effects of a sustained elevation in circulating glucocorticoids and treatment with the antiepileptic and antidepressant VPA.
The simple size fractionation employed shows that HDAC1 occurs in at least six complexes that differ in the representation of mSin3A and RbAp48. C/EBPβ preferentially cofractionated with HDAC1 in the peak migrating with the smallest molecular size (peak VI), which also contained mSin3A, but lacked RbAp48. The apparent separate interaction of C/EBPβ with p300 and mSin3A/HDAC1 suggests a dynamic competition between the stimulatory effects of histone acetyl transferases and HDAC1 on the C/EBPβ transactivation potential expression. In the absence of steroid, the interactions equilibrated in a manner that favored the HDAC1, promoting deacetylation of histone H4 and restraining recruitment of PolII to the C/EBPα promoter. We have subsequently noted that the C/EBPβ is itself acetylated and this acetylation is attenuated by HDAC1 (data not shown). This suggests that HDAC1 may also regulate the transcriptional regulation potential of C/EBPβ directly.
As GR and the GR LBD alone were also observed to interact physically with mSin3A/HDAC1, it may have been expected that cross-talk between GR and C/EBPβ would have involved either the formation of a ternary complex mediated through mSin3A/HDAC1 or the titration of mSin3A/HDAC1 from C/EBPβ by direct competition. Cross-talk through ternary complex formation has been described previously for the alteration of AP1 and NFκB signaling by glucocorticoids (Jonat et al., 1990; Schule et al., 1990; Yang-Yen et al., 1990; Heck et al., 1997), while competition for HDAC1 has recently been described in myogenesis (Puri et al., 2001). Furthermore, it is interesting to note that GR has also recently been shown to promote the recruitment of HDAC2 to DNA-bound NFκB (Ito et al., 2000).
However, at the physiological and near physiological levels of GR and C/EBPβ employed in our experiments, we have failed to detect the formation of a ternary complex between GR and C/EBPβ in solution, or the recruitment of GR to the C/EBPα promoter. Furthermore, little effect of steroid was observed on the mSin3A/HDAC1–C/EBPβ interaction after 4 h, a time that would normally be expected to be more than sufficient for re-equilibration of interactions in a competition mechanism. In cells lacking CHOP, fast-acting TSA and VPA treatments had a much stronger effect on C/EBPβ transactivation than slower-acting steroid. Thus, our data support a competition model for the titration of mSin3A/HDAC1 from C/EBPβ by GR that results in an orderly transition of C/EBPβ in stimulated preadipocytes from a CHOP-associated form unable to bind DNA, to a promoter-bound form restrained through interaction with HDAC1 that becomes fully active as HDAC1 is titrated through GR-facilitated proteosomal degradation.
Recent work has shown that steroid receptors are degraded through the 26S proteasome in a manner that is accelerated by steroid treatment and is dependent on determinants within the receptor LBD. Evidence is also emerging that steroid receptors act to facilitate proteasomal degradation of other factors. For example, there is a convincing relationship between the targeting of GR and p53 to the proteasome (Sengupta and Wasylyk, 2001).
In our experiments, the effect of steroid on C/EBPβ-induced transcription and on the displacement of HDAC1 and mSin3A from the C/EBPα promoter was abrogated completely by proteasome inhibitor. Under physiological conditions, steroid treatment decreased total HDAC1 levels over a period of 24 h by ∼50% in an MG132-sensitive manner without affecting HDAC1 mRNA levels. Underlying this relatively modest effect on total HDAC1 levels was the elimination of a specific HDAC1-containing complex that preferentially associated with C/EBPβ. The FPLC peak containing this complex accounted for one-third of the HDAC1 in extracts prepared from untreated cells and its steroid-induced elimination accounted for two-thirds of the total effect of steroid on overall HDAC1 levels. Furthermore, co-immunoprecipitation experiments indicated that GR, like C/EBPβ, preferentially interacted with what is probably the same subcomplex of HDAC1 containing mSin3A, but lacking RbAp46/48.
As the broad effect of proteasome inhibitors blurred the separation of HDAC1 by size fractionation (data not shown), it is not yet possible to tell whether the loss of HDAC1 peak VI results directly from targeted degradation or whether the interaction with steroid receptor leads to an initial disruption of the complex that is followed thereafter by separate targeting of free HDAC1 to the proteasome.
Broader implications of the modulation of HDAC1 by steroid
GR influences a variety of cellular processes in which C/EBPβ also plays an essential role. For example, glucocorticoids potentiate transcriptional activation by C/EBPβ (NF-IL6) in the immune system (Nishio et al., 1993), and both factors play important roles in inflammatory responses and influence macrophage and granulocyte differentiation (Lloberas et al., 1998; Nerlov et al., 1998). Recent work with GRdim mice suggests that glucocorticoid-mediated repression of inflammatory responses is independent of GR DNA binding (Reichardt et al., 2000b). Thus, some of the effects of glucocorticoids in the immune system may be related to the downregulation of HDAC1.
Glucocorticoids also play an important role in the hippocampus in promoting the development of long-term memory (Kim and Yoon, 1998; Roozendaal, 2000; McGaugh and Roozendaal, 2002; Sousa and Almeida, 2002). The recent implication that C/EBPβ is involved in the consolidation of memory (Taubenfeld et al., 2001) suggests a potential role for downregulation of HDAC1 in this process. Furthermore, decreases in long-term memory in aged animals show a correlation with the decreased responsiveness of GR (Murphy et al., 2002).
Steroid-dependent targeting of HDAC1 was also observed for PR. C/EBPβ is known to have an important role in progesterone-dependent lobuloalveolar development (Seagroves et al., 2000). Furthermore, as weight gain is a normal side-effect of pregnancy, it is possible that progestins may contribute directly to potentiating the adipogenesis that is seen during this time. Given the high degree of conservation within the LBDs of steroid receptors, it will also be interesting to assess whether other steroid receptors have similar effects on HDAC1. Specific modulation of HDAC1 complexes by steroid hormone receptors also has the potential to impact on a broad variety of gene regulatory events that are indirectly regulated by steroids beyond C/EBPβ.
Materials and methods
Constructs and infection
pTL2PR632C expresses amino acids 632–933 of human PR. GR, HDAC1, RARα, C/EBP and the wild-type C/EBPα-luciferase reporter gene (–350/+7), have been described previously (Legraverend et al., 1993; Yeh et al., 1995; Boruk et al., 1998). A C/EBPα promoter mutant (–199 to –163) was made by site-directed mutagenesis. Viral expression for GR505–795, PR632–933 and HDAC1 was accomplished in pLXSN (Clontech). Retrovirus was generated in Phoenix Ampho cells (ATCC). Fifty percent confluent 3T3 L1 cells were infected with 1 ml of viral supernatant in the presence of 4 µg/µl polybrene (Sigma) and selected for 10 days.
3T3 L1 differentiation
Cells were maintained in DMEM containing 1.0 g/l glucose and 10% calf serum in 10% CO2. Calf serum lots were type matched for consistent responses to hormonal cocktail. Two-day post-confluent preadipocytes (day 0) were treated with 50–100 nM insulin, 500 µM MIX and 250 nM dex, 250 nM of the synthetic progestin R5020, 400 nM TSA or 10 mM VPA as indicated for 48 h. Cells were then cultured in DMEM with 10% calf serum and insulin for 6 days. GPDH assays and Oil red O staining were as described previously (Wise and Green, 1979; Schwarz et al., 1997). Phase-contrast photomicrographs are representative of a minimum of three experiments performed in duplicate.
Transient transfection
Cos7 and HeLa cells were transfected using Lipofectamine™ (Invitrogen) and ExGen 500™ (MBI Fermentas), respectively. Two hundred nanograms of reporter DNA, 200–400 ng of C/EBPα, β, δ-expressing cDNAs, 100 ng of GR/PR- and RARα-encoding plasmids and 300 ng of HDAC1 plasmid were used. Following transfection, cells were cultured in phenol red-free DMEM with 10% charcoal-stripped FBS (Wisent) and treated with dex (10–6 M), RU486 (10–6 M), R5020 (10–6 M), TSA (160 nM), MG132 (1 µM) or vehicle for 20 h. Three-fold scale-up was used for protein analysis. Luciferase assays were performed by standard assay. Error represents the standard error of the mean of a minimum of three experiments performed in duplicate. Transfection efficiency was monitored by cotransfection of an RSV-β-gal plasmid.
Analysis of protein–protein binding
Whole-cell extracts were prepared from cells treated as described. For western blots, 50–100 µg of extracts were probed with antibodies [C/EBPβ C-19, C/EBPα 14AA, C/EBPδ C-22, adipsin P-16, GR P-20, anti-HDAC1 H-11 (Santa Cruz) and PPARγ (ABR)] and visualized by chemiluminescence (NEN). For co-immunoprecipitation, extracts were incubated with antibody-conjugated protein A–Sepharose beads for 2 h. Buffers for immunoprecipitation of GR included 20 mM sodium molybdate. Precipitates were washed with 30 mM HEPES pH 7.5, 300 mM NaCl, 5 mM EDTA, 0.5% Triton X-100, 0.5% sodium deoxycholate and 0.2 mM DTT, separated by SDS–PAGE, and probed with antibodies to C/EBPβ, RbAp46 N-19, HDAC1 (Affinity Bioreagents), HDAC2 C-8 and RbAp48 (Santa Cruz). For in vitro binding, GST and GST-C/EBPβ were prepared in E.coli BL21 (Boruk et al., 1998). In vitro translated 35S-labeled mSin3A or HDAC1 was incubated with 1 µg of GST proteins for 2 h in 0.6× lysis buffer + 0.1% NP-40. Following extensive washings, binding was visualized by PhosphorImager (Molecular Dynamics). Results shown are representative of a minimum of three independent experiments.
RNA analysis
Total RNA from 3T3 L1 cells treated for 24 h with MIX and insulin in the presence or absence of dex was prepared by RNeasy® RNA isolation kit (Qiagen). HDAC1 mRNA was detected by northern blotting of 10 µg of RNA with a 311 bp region of human HDAC1 (amino acids 321–425).
FPLC separation of HDAC1-containing complexes
Whole-cell extracts (1–4 mg) in TEDG buffer were separated over Superose HR300. Fractions were TCA precipitated, separated by SDS–PAGE and analyzed by western blotting. Band intensities were calculated for every second fraction and compared with standard curves of column inputs. Results are represented as a percent of total intensity of HDAC1 in whole-cell extracts from untreated cells.
ChIP
ChIPs were performed essentially as described previously (Yahata et al., 2001). 3T3 L1 cells were treated as indicated for 24 h and treated with 1% formaldehyde at 20°C for 10 min. After harvesting in PBS and buffer I (0.25% Triton X-100, 10 mM EDTA, 0.5 mM EGTA and 10 mM HEPES pH 6.5), cell pellets were resuspended and sonicated. Supernatants (13 000 g) were diluted and incubated with antibodies to gal4, C/EBPβ C-19, mSin3A AK-11, HDAC1 C-19, p300 N-15, RNA polII N-20 and acetyl-H4 (Upstate Biotechnology) at 4°C overnight, and precipitated using a protein A–Sepharose slurry with 2 µg of sheared salmon sperm DNA. Precipitates were washed for 10 min at 4°C in TSE I, TSE II, buffer III and twice in TE, then extracted three times in 100 µl of 1% SDS, 0.1 M NaHCO3. Eluates were kept at 65°C overnight to reverse cross-links. DNA was purified by Qiaquick PCR purification kit™ (Qiagen) and amplified by PCR using the following primers for the C/EBPα promoter: –334 and –118, –108 and +17. Results shown are representative of a minimum of three independent experiments.
Acknowledgments
Acknowledgements
We thank S.Soubeyrand and A.Sorisky for advice, Drs S.K.McKnight, X.-J.Yang, P.Antonson and M.McBurney for plasmids, and G.Nolan for the Phoenix Ampho cells. This work was supported by grants from the Canadian Institutes of Health Research. N.W.-B. was supported by an Ontario Graduate Studentship in Science and Technology, while R.J.G.H. is an Investigator of the Canadian Institutes of Health Research.
References
- Batchvarova N., Wang,X.Z. and Ron,D. (1995) Inhibition of adipogenesis by the stress-induced protein CHOP (Gadd153). EMBO J., 14, 4654–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boruk M., Savory,J.G. and Hache,R.J. (1998) AF-2-dependent potentiation of CCAAT enhancer binding protein β-mediated transcriptional activation by glucocorticoid receptor. Mol. Endocrinol., 12, 1749–1763. [DOI] [PubMed] [Google Scholar]
- Cao Z., Umek,R.M. and McKnight,S.L. (1991) Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev., 5, 1538–1552. [DOI] [PubMed] [Google Scholar]
- Christy R.J., Kaestner,K.H., Geiman,D.E. and Lane,M.D. (1991) CCAAT/enhancer binding protein gene promoter: binding of nuclear factors during differentiation of 3T3-L1 preadipocytes. Proc. Natl Acad. Sci. USA, 88, 2593–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R., Peters,D.H. and McTavish,D. (1994) Valproic acid. A reappraisal of its pharmacological properties and clinical efficacy in epilepsy. Drugs, 47, 332–372. [PubMed] [Google Scholar]
- Elberg G., Gimble,J.M. and Tsai,S.Y. (2000) Modulation of the murine peroxisome proliferator-activated receptor γ2 promoter activity by CCAAT/enhancer-binding proteins. J. Biol. Chem., 275, 27815–27822. [DOI] [PubMed] [Google Scholar]
- Freedman M.R., Horwitz,B.A. and Stern,J.S. (1986) Effect of adrenalectomy and glucocorticoid replacement on development of obesity. Am. J. Physiol., 250, R595–R607. [DOI] [PubMed] [Google Scholar]
- Freytag S.O., Paielli,D.L. and Gilbert,J.D. (1994) Ectopic expression of the CCAAT/enhancer-binding protein α promotes the adipogenic program in a variety of mouse fibroblastic cells. Genes Dev., 8, 1654–1663. [DOI] [PubMed] [Google Scholar]
- Gottlicher M. et al. (2001) Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J., 20, 6969–6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire F.M., Smas,C.M. and Sul,H.S. (1998) Understanding adipocyte differentiation. Physiol. Rev., 78, 783–809. [DOI] [PubMed] [Google Scholar]
- Heck S., Bender,K., Kullmann,M., Gottlicher,M., Herrlich,P. and Cato,A.C. (1997) IκBα-independent downregulation of NF-κB activity by glucocorticoid receptor. EMBO J., 16, 4698–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu E., Tontonoz,P. and Spiegelman,B.M. (1995) Transdifferentiation of myoblasts by the adipogenic transcription factors PPARγ and C/EBPα. Proc. Natl Acad. Sci. USA, 92, 9856–9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Barnes,P.J. and Adcock,I.M. (2000) Glucocorticoid receptor recruitment of histone deacetylase 2 inhibits interleukin-1β-induced histone H4 acetylation on lysines 8 and 12. Mol. Cell. Biol., 20, 6891–6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jallon P. and Picard,F. (2001) Bodyweight gain and anticonvulsants: a comparative review. Drug Saf., 24, 969–978. [DOI] [PubMed] [Google Scholar]
- Jiang M.S. and Lane,M.D. (2000) Sequential repression and activation of the CCAAT enhancer-binding protein-α (C/EBPα) gene during adipogenesis. Proc. Natl Acad. Sci. USA, 97, 12519–12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonat C., Rahmsdorf,H.J., Park,K.K., Cato,A.C., Gebel,S., Ponta,H. and Herrlich,P. (1990) Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell, 62, 1189–1204. [DOI] [PubMed] [Google Scholar]
- Kim J.J. and Yoon,K.S. (1998) Stress: metaplastic effects in the hippocampus. Trends Neurosci., 21, 505–509. [DOI] [PubMed] [Google Scholar]
- Lange C.A., Shen,T. and Horwitz,K.B. (2000) Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc. Natl Acad. Sci. USA, 97, 1032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legraverend C., Antonson,P., Flodby,P. and Xanthopoulos,K.G. (1993) High level activity of the mouse CCAAT/enhancer binding protein (C/EBPα) gene promoter involves autoregulation and several ubiquitous transcription factors. Nucleic Acids Res., 21, 1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloberas J., Soler,C. and Celada,A. (1998) Dexamethasone enhances macrophage colony stimulating factor- and granulocyte macrophage colony stimulating factor-stimulated proliferation of bone marrow-derived macrophages. Int. Immunol., 10, 593–599. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D.J. et al. (1995) The nuclear receptor superfamily: the second decade. Cell, 83, 835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh J.L. and Roozendaal,B. (2002) Role of adrenal stress hormones in forming lasting memories in the brain. Curr. Opin. Neurobiol., 12, 205–210. [DOI] [PubMed] [Google Scholar]
- Mink S., Haenig,B. and Klempnauer,K.H. (1997) Interaction and functional collaboration of p300 and C/EBPβ. Mol. Cell. Biol., 17, 6609–6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E.K., Spencer,R.L., Sipe,K.J. and Herman,J.P. (2002) Decrements in nuclear glucocorticoid receptor (GR) protein levels and DNA binding in aged rat hippocampus. Endocrinology, 143, 1362–1370. [DOI] [PubMed] [Google Scholar]
- Narlikar G.J., Fan,H.Y. and Kingston,R.E. (2002) Cooperation between complexes that regulate chromatin structure and transcription. Cell, 108, 475–487. [DOI] [PubMed] [Google Scholar]
- Nerlov C., McNagny,K.M., Doderlein,G., Kowenz-Leutz,E. and Graf,T. (1998) Distinct C/EBP functions are required for eosinophil lineage commitment and maturation. Genes Dev., 12, 2413–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio Y., Isshiki,H., Kishimoto,T. and Akira,S. (1993) A nuclear factor for interleukin-6 expression (NF-IL6) and the glucocorticoid receptor synergistically activate transcription of the rat α1-acid glycoprotein gene via direct protein–protein interaction. Mol. Cell. Biol., 13, 1854–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeke P.M. and Chrousos,G.P. (1995) Hypercortisolism and obesity. Ann. N. Y. Acad. Sci., 771, 665–676. [DOI] [PubMed] [Google Scholar]
- Phiel C.J., Zhang,F., Huang,E.Y., Guenther,M.G., Lazar,M.A. and Klein,P.S. (2001) Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem., 276, 36734–36741. [DOI] [PubMed] [Google Scholar]
- Pijl H. and Meinders,A.E. (1996) Bodyweight change as an adverse effect of drug treatment. Mechanisms and management. Drug Saf., 14, 329–342. [DOI] [PubMed] [Google Scholar]
- Puri P.L. et al. (2001) Class I histone deacetylases sequentially interact with MyoD and pRb during skeletal myogenesis. Mol. Cell, 8, 885–897. [DOI] [PubMed] [Google Scholar]
- Reichardt H.M., Tronche,F., Bauer,A. and Schutz,G. (2000a) Molecular genetic analysis of glucocorticoid signaling using the Cre/loxP system. Biol. Chem., 381, 961–964. [DOI] [PubMed] [Google Scholar]
- Reichardt H.M., Tuckermann,J.P., Bauer,A. and Schutz,G. (2000b) Molecular genetic dissection of glucocorticoid receptor function in vivo. Z. Rheumatol., 59(Suppl. 2:II), 1–5. [DOI] [PubMed] [Google Scholar]
- Ron D. and Habener,J.F. (1992) CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev., 6, 439–453. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. (2000) 1999 Curt P. Richter award. Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology, 25, 213–238. [DOI] [PubMed] [Google Scholar]
- Rosen E.D., Sarraf,P., Troy,A.E., Bradwin,G., Moore,K., Milstone,D.S., Spiegelman,B.M. and Mortensen,R.M. (1999) PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell, 4, 611–617. [DOI] [PubMed] [Google Scholar]
- Rosen E.D., Hsu,C.H., Wang,X., Sakai,S., Freeman,M.W., Gonzalez,F.J. and Spiegelman,B.M. (2002) C/EBPα induces adipogenesis through PPARγ: a unified pathway. Genes Dev., 16, 22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin C.S., Hirsch,A., Fung,C. and Rosen,O.M. (1978) Development of hormone receptors and hormonal responsiveness in vitro. Insulin receptors and insulin sensitivity in the preadipocyte and adipocyte forms of 3T3-L1 cells. J. Biol. Chem., 253, 7570–7578. [PubMed] [Google Scholar]
- Sainsbury A., Wilks,D. and Cooney,G.J. (2001) Central but not peripheral glucocorticoid infusion in adrenalectomized male rats increases basal and substrate-induced insulinemia through a parasympathetic pathway. Obes. Res., 9, 274–281. [DOI] [PubMed] [Google Scholar]
- Schule R., Rangarajan,P., Kliewer,S., Ransone,L.J., Bolado,J., Yang,N., Verma,I.M. and Evans,R.M. (1990) Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell, 62, 1217–1226. [DOI] [PubMed] [Google Scholar]
- Schwarz E.J., Reginato,M.J., Shao,D., Krakow,S.L. and Lazar,M.A. (1997) Retinoic acid blocks adipogenesis by inhibiting C/EBPβ-mediated transcription. Mol. Cell. Biol., 17, 1552–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagroves T.N., Lydon,J.P., Hovey,R.C., Vonderhaar,B.K. and Rosen,J.M. (2000) C/EBPβ (CCAAT/enhancer binding protein) controls cell fate determination during mammary gland development. Mol. Endocrinol., 14, 359–368. [DOI] [PubMed] [Google Scholar]
- Sengupta S. and Wasylyk,B. (2001) Ligand-dependent interaction of the glucocorticoid receptor with p53 enhances their degradation by Hdm2. Genes Dev., 15, 2367–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soukas A., Socci,N.D., Saatkamp,B.D., Novelli,S. and Friedman,J.M. (2001) Distinct transcriptional profiles of adipogenesis in vivo and in vitro. J. Biol. Chem., 276, 34167–34174. [DOI] [PubMed] [Google Scholar]
- Sousa N. and Almeida,O.F. (2002) Corticosteroids: sculptors of the hippocampal formation. Rev. Neurosci., 13, 59–84. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Yoshida,N., Kishimoto,T. and Akira,S. (1997) Defective adipocyte differentiation in mice lacking the C/EBPβ and/or C/EBPδ gene. EMBO J., 16, 7432–7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q.Q. and Lane,M.D. (1999) Activation and centromeric localization of CCAAT/enhancer-binding proteins during the mitotic clonal expansion of adipocyte differentiation. Genes Dev., 13, 2231–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q.Q. and Lane,M.D. (2000) Role of C/EBP homologous protein (CHOP-10) in the programmed activation of CCAAT/enhancer-binding protein-β during adipogenesis. Proc. Natl Acad. Sci. USA, 97, 12446–12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenfeld S.M., Milekic,M.H., Monti,B. and Alberini,C.M. (2001) The consolidation of new but not reactivated memory requires hippocampal C/EBPβ. Nat. Neurosci., 4, 813–818. [DOI] [PubMed] [Google Scholar]
- Tontonoz P., Hu,E. and Spiegelman,B.M. (1994) Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell, 79, 1147–1156. [DOI] [PubMed] [Google Scholar]
- Wallace A.D. and Cidlowski,J.A. (2001) Proteasome-mediated glucocorticoid receptor degradation restricts transcriptional signaling by glucocorticoids. J. Biol. Chem., 276, 42714–42721. [DOI] [PubMed] [Google Scholar]
- Wise L.S. and Green,H. (1979) Participation of one isozyme of cytosolic glycerophosphate dehydrogenase in the adipose conversion of 3T3 cells. J. Biol. Chem., 254, 273–275. [PubMed] [Google Scholar]
- Wu Z., Xie,Y., Bucher,N.L. and Farmer,S.R. (1995) Conditional ectopic expression of C/EBPβ in NIH-3T3 cells induces PPARγ and stimulates adipogenesis. Genes Dev., 9, 2350–2363. [DOI] [PubMed] [Google Scholar]
- Wu Z., Rosen,E.D., Brun,R., Hauser,S., Adelmant,G., Troy,A.E., McKeon,C., Darlington,G.J. and Spiegelman,B.M. (1999) Cross-regulation of C/EBPα and PPARγ controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol. Cell, 3, 151–158. [DOI] [PubMed] [Google Scholar]
- Yahata T. et al. (2001) Selective coactivation of estrogen-dependent transcription by CITED1 CBP/p300-binding protein. Genes Dev., 15, 2598–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang-Yen H.F., Chambard,J.C., Sun,Y.L., Smeal,T., Schmidt,T.J., Drouin,J. and Karin,M. (1990) Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein–protein interaction. Cell, 62, 1205–1215. [DOI] [PubMed] [Google Scholar]
- Yeh W.C., Cao,Z., Classon,M. and McKnight,S.L. (1995) Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev., 9, 168–181. [DOI] [PubMed] [Google Scholar]
- You A., Tong,J.K., Grozinger,C.M. and Schreiber,S.L. (2001) CoREST is an integral component of the CoREST–human histone deacetylase complex. Proc. Natl Acad. Sci. USA, 98, 1454–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]