Abstract
Female hormone influences on auditory system aging are not completely understood. Because of widespread clinical use of hormone replacement therapy (HRT), it is critical to understand HRT effects on sensory systems. The present study retrospectively analyzed and compared hearing abilities among 124 postmenopausal women taking HRT, treated with estrogen and progestin (E+P; n = 32), estrogen alone (E; n = 30), and a third [non-hormone replacement therapy (NHRT; n = 62)] control group. Subjects were 60–86 years old and were matched for age and health status. All had relatively healthy medical histories and no significant noise exposure, middle-ear problems, or major surgeries. Hearing tests included pure-tone audiometry, tympanometry, distortion-product otoacoustic emissions (DPOAEs), transient otoacoustic emissions, and the hearing-in-noise test (HINT). The HINT tests for speech perception in background noise, the major complaint of hearing-impaired persons. Pure-tone thresholds in both ears were elevated (poorer) for the E+P relative to the E and control groups. For DPOAEs, the E+P group presented with lower (worse) levels than the E and control groups, with significant differences for both ears. For the HINT results, the E+P group had poorer speech perception than the E and control groups across all background noise speaker locations and in quiet. These findings suggest that the presence of P as a component of HRT results in poorer hearing abilities in aged women taking HRT, affecting both the peripheral (ear) and central (brain) auditory systems, and it interferes with the perception of speech in background noise.
Keywords: estrogen, hearing loss, hormone replacement therapy, presbycusis, progesterone
Age-related hearing loss (presbycusis) is the number one communication disorder, and it is one of the top three chronic medical conditions of elderly persons. Because of the widespread prescription of hormone replacement therapy (HRT), it is critical to determine the effects of HRT on sensory systems in postmenopausal females. Sensory function declines with age, yet the effects of HRT on hearing, balance, vision, and the chemical senses are not assessed in HRT drug development.
Estrogen (E) and progestin (P) actions have been linked to key sensory and CNS processes and disorders such as cognition, memory, dementia (Alzheimer's disease), epilepsy, depression, and others. For instance, Rice et al. (1) reported differences between E alone and E+P in rates of cognitive decline, showing E alone as beneficial and the presence of P as detrimental. Shumaker et al. (2) reported that the use of E+P increased the risk for dementia in elderly females. Klaiber et al. (3, 4) demonstrated that the effects of P seem opposed to those of E for mood changes. Stein and Hoffman (5) reported opposite effects of E and P in the treatment of acute brain trauma, attributing to P overall qualities of a neuroprotection agent. Klein et al. (6) suggested a protective effect of E on eye-lens opacities.
The effects of sex hormones on hearing and aging are also controversial and contradictory, suggesting the need for more investigation as to whether HRT is actually beneficial or detrimental to sensory functioning in postmenopausal females. One report claims that the use of contraceptive medications (E+P) leads to a decline in hearing, which the author interpreted in terms of a net masculinization effect by those hormones (7). Some studies suggest a protective effect of E, reporting that estrogen replacement therapy may delay hearing loss in postmenopausal females and that there is an association between low serum E levels and hearing loss (8, 9). Others, investigating hearing problems in Turner's syndrome (10, 11), suggested that the lack of E may influence hearing in those patients and may induce premature aging of the auditory system.
In studies of the central auditory system, postmenopausal females treated with E alone had shorter (better) auditory brainstem response latencies than combined HRT (E+P), suggesting that E could influence neuronal plasticity, metabolic levels of neurotransmitters, or conduction velocities in the auditory nervous system (12, 13). These results are supported by reports of changes in auditory event-related potentials across the menstrual cycle, with the most prominent changes occurring during the luteal phase, when P is at its highest concentration in the cycle (14).
The present investigation aimed to determine the effects of HRT on the auditory system of postmenopausal females by (i) employing a larger number of subjects than most previous investigations, (ii) using a more rigorous battery of classical and state-of-the-art hearing tests assessing both peripheral (ear) and central (brain) auditory systems, and (iii) separating the effects of E alone from the most commonly prescribed form of HRT (E+P).
Results
Pure-Tone Audiometry.
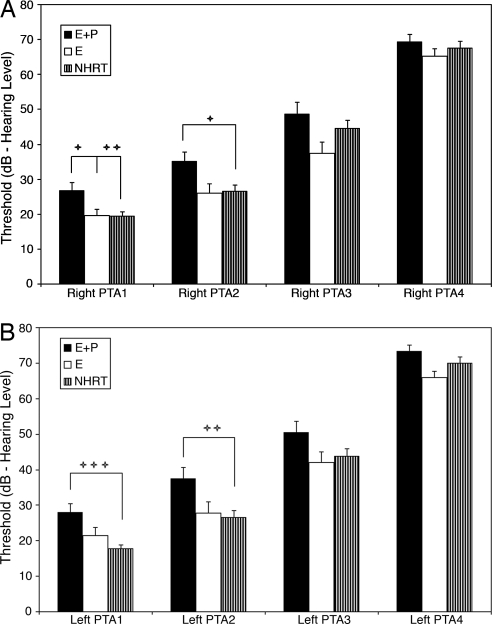
The results are depicted in Fig. 1A (right ear) and B (left ear). The E+P group presented with elevated (poorer) thresholds relative to the E and NHRT groups for all frequencies, especially for the low- and middle-frequency ranges. The main group effect was statistically significant for both ears for PTA1 and PTA2, and the post hoc Bonferroni tests (corrected for multiple comparisons) of the E+P subject group with the other two subject groups were also statistically significant, as shown in Table 1. No statistically significant differences were noted when comparing E with NHRT.
Fig. 1.
Comparisons between E+P × E × NHRT for pure-tone thresholds in the right ear (A) and left ear (B). The E+P group presented with elevated thresholds relative to the E and the NHRT groups at all frequencies, with statistically significant differences for both ears for PTA1 and PTA2, as presented in Table 1. PTA1 represents the average of thresholds for frequencies 0.5, 1, and 2 kHz; PTA2 for 1, 2, and 4 kHz; PTA3 for 4, 8, and 9 kHz; and PTA4 for 10, 11.2, 12.5, and 14 kHz. NHRT, control subjects who did not receive HRT; PTA, pure-tone average. +, P < 0.05; ++, P < 0.01; +++, P < 0.001.
Table 1.
Statistical comparisons (corrected for multiple comparisons) of main effects and individual subject group hearing abilities
| Left ear |
Right ear |
|||
|---|---|---|---|---|
| PTA1 | PTA2 | PTA1 | PTA2 | |
| Main effect | P = 0.0011 | P = 0.01 | P = 0.0046 | P = 0.018 |
| Subject group | F = 7.17 | F = 4.74 | F = 5.62 | F = 4.17 |
| E + P vs. NHRT | P < 0.001 | P < 0.01 | P < 0.01 | P < 0.05 |
| t = 3.78 | t = 3.03 | t = 3.25 | t = 2.74 | |
| E + P vs. E | NS | NS | P < 0.05 | NS |
| t = 2.53 | ||||
ANOVA main effects: degrees of freedom (df) = 2, 121. There were no statistically significant differences between the E and NHRT groups or for PTA3 or PTA4. NS, not statistically significant (0.05 level or better). PTA1 and PTA2 are defined in the Fig. 1 legend.
Distortion-Product (DP) Otoacoustic Emissions (DPOAEs).
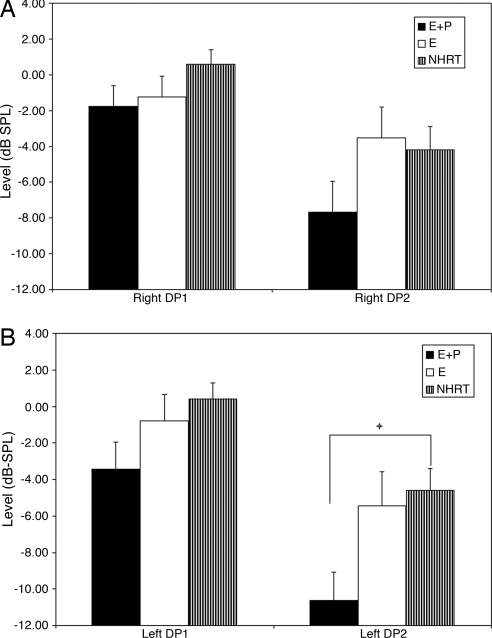
For the right ear (Fig. 2A) and left ear (Fig. 2B), the E+P group showed lower (poorer) levels than the E and NHRT groups. The differences were statistically significant for the left ear at the higher frequencies (DP2; for details, see Fig. 2 legend).
Fig. 2.
Histograms showing the comparison between E+P × E × NHRT group results for DP levels. Notice that for both sides [right ear (A) and left ear (B)], the E+P group presented with lower levels than the E and NHRT groups. Statistical significance was found for the left DP2 group main effect: P, 0.017; F, 4.24; df, 2, 121. Bonferroni posttests showed statistical significance for E+P vs. NHRT for the left ear DP2 (+, P < 0.05; t, 2.89). DP1 represents the average for frequencies 1,001, 1,257, 1,587, and 2,002 Hz; and DP2 represents the average for frequencies 3,174, 4,004, 5,042, and 6,748 Hz. DP, distortion-product otoacoustic emission.
Transient Evoked Otoacoustic Emissions (TEOAEs).
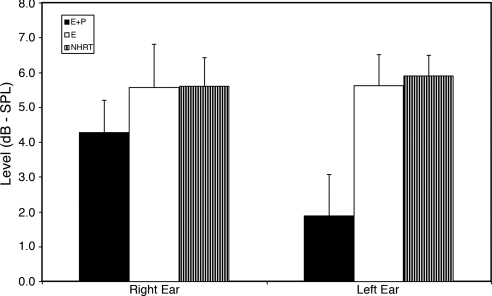
Similar to the DPOAEs, response levels for the TEOAEs (Fig. 3) showed greater damage to the outer-hair cell system for the E+P group, who presented with lower levels vs. E and vs. NHRT, for both ears. Also, like the DPOAEs, greater detrimental effects of the E+P HRT were seen for the left ear relative to the right.
Fig. 3.
Results for TEOAEs. For both ears, the E+P group presented with lower levels than E and NHRT groups, especially in the left ear.
Hearing-in-Noise Test (HINT).
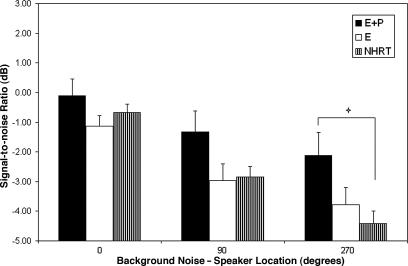
The E+P group presented with decreased recognition of speech in background noise (Fig. 4) across all background-noise speaker locations (for statistical details, see Fig. 4 legend), with the greatest speech-reception performance differences for the E+P vs. NHRT groups for the background-noise speaker located at 270°. Although not depicted in the histogram, the quiet condition was tested, and the E+P group exhibited significantly worse speech perception than the NHRT control group (statistical details in the Fig. 4 legend). As observed in our other tests of auditory function, no significant differences were observed between the E vs. NHRT subject groups.
Fig. 4.
Comparisons for the HINT between E+P × E × NHRT groups. Notice that the E+P group presented with decreased recognition of speech in noise across all background-noise speaker locations. The group main effect was statistically significant for the 270° background-noise speaker position: P, 0.016; F, 4.27; df, 2, 121; Bonferroni posttests showed significant E+P vs. NHRT for the background-noise speaker at 270° (+, P < 0.05; t = 2.92). The HINT quiet condition, although not depicted on the histogram, showed a statistically significant group main effect: P, 0.025; F, 3.83; df, 2, 121; the Bonferroni posttest E+P vs. NHRT was also statistically significant (+, P < 0.05; t= 2.74).
Discussion
The present investigation revealed hearing sensitivity and auditory speech-processing deficits in females taking E+P HRT relative to otherwise healthy age-matched control subjects and females taking E alone. These auditory-processing deficits were manifested at the levels of both the ear (peripheral auditory system) and brain (central auditory system).
Sex steroids have some similarities to neurotrophins for regulating cell death, neuronal migration, neurogenesis, as well as neurotransmitter plasticity; and their signaling events involve interactions between nuclear hormone receptors and interactions with neurotrophin and neurotransmitter signal-transduction pathways (15). For example, some of these pathways are related to the γ-aminobutyric acid (GABA) receptors in the brain. Neuroactive steroids are capable of altering the chemosensitivity of nerve-terminal membranes by enhancing GABA inhibition, and the sensitivity of the GABAA synaptic receptors provides a pathway for the sex steroid to regulate secretion from neurosecretory neurons (16). For example, E can decrease the number of synaptic vesicles adjacent to the presynaptic membrane of certain inhibitory synapses (17). E decreases the GABAB receptor-mediated autoinhibition of the GABAergic preoptic area as well as the ability of those neurons to synthesize GABA, therefore increasing neuron excitability (18).
In contrast, P can potentiate GABA receptor activation by a nongenomic mechanism that may involve actions at the plasma membrane (19). Allopregnanolone, a P metabolite, is a positive modulator of GABA, increasing inhibitory chloride ion conductance and therefore decreasing neuronal excitability (20).
In addition to the individual actions of the sex steroids on their respective receptors, some studies have reported a coadjuvant effect of those hormones, in which the presence of E up-regulated the expression of P in the brain (21), to the point that some even consider E as a priming requisite for P-mediated actions in the normal brain (22).
The results of the present study are in agreement with some previous investigations reporting that the presence of P, either as a component of HRT or during the normal menstrual cycle (luteal phase), relates to poorer hearing relative to E alone or during the follicular phase of menses (8, 9, 12–14). However, the present investigation did not confirm the hypothesis that E alone has a protective effect on the auditory system (10, 11, 23, 24) because no differences were observed between the hearing abilities of females taking E relative to those who had never taken HRT (i.e., NHRT).
GABA changes in the brainstem auditory system, including the inferior colliculus, an important auditory midbrain structure, have been linked to age-related hearing loss, i.e., presbycusis (25–28). Some of the changes reported by Caspary and coworkers (25) included decreased numbers of GABA-immunoreactive neurons as well as declines in concentrations and release of GABA, decreased glutamic acid decarboxylase (a GABA-synthesis enzyme) activity, decreased GABAB receptor binding, decreased numbers of presynaptic terminals; and subtle GABAA receptor-binding changes. Considering the presence of GABA and its receptors in the ear and brainstem hearing pathways and the actions that female sex steroids can have on them, these changes represent a possible biochemical mechanism for the actions of HRT in accelerating auditory-processing declines in the aged auditory system.
Summary and Conclusions
The present investigation clarifies some of the controversy concerning side effects, including actions on sensory systems, of HRT in postmenopausal females. P, as a component of HRT, negatively affects the ear and parts of the CNS used for hearing, whereas E does not. Sensory declines in elderly women, in this case exacerbated by P, can significantly interfere with communication abilities, including speech and hearing, professional and economic productivity, family relations, and quality of life. Therefore, increased and improved sensory testing in drug and medication development for our elderly population is warranted to prevent medication-related acceleration of sensory declines and their socioeconomic sequelae.
Materials and Methods
The methods were similar to those of our previous basic and clinical research investigations of age-related hearing loss (29–33).
Subjects.
Volunteer subjects were recruited and paid for participation in this study of presbycusis and its comorbidities. Excluded from this study were individuals who had been treated with ototoxic medications; who had serious health problems, neurological conditions, Ménière's disease, or labyrinthitis; who failed cognition-screening tests (mini-mental state examination); who were current/heavy smokers; or who had conductive-hearing loss, history of noise damage, and/or audiograms signifying noise damage or poor speech-discrimination scores (80% or less). In the experimental groups, subjects had been treated with E+P or only E, whereas subjects in the control group did not receive hormonal therapy (NHRT). The majority of subjects in experimental group E consisted of females who had had a hysterectomy. The length of hormonal treatment varied from 5 to 35 years. Data were obtained from a total of 124 subjects (E+P, n = 32; E, n = 30; and NHRT, n = 62), matched by age (60–86 years). All human-subject procedures were approved by the University of Rochester Research Subjects Review Board and are consistent with the principles of the Declaration of Helsinki, including written informed consent from each subject.
Middle-Ear Testing.
Before data collection, tympanometric measures (Grason–Stadler GSI 33 middle-ear analyzer; Viasys, Conshohocken, PA), bone-conduction audiometry (GSI 61), and tympanometry were used to rule out middle-ear diseases.
Pure-Tone Audiometry.
Pure-tone absolute thresholds were obtained in a soundproof room (GSI 61 audiometer, 0.25–8 kHz) with E-A-R insert earphones (Aearo, Indianapolis, IN) for speech frequencies. For ultra-high frequencies (8–14 kHz) Sennheiser HDA 200 headphones (Buckinghamshire, U.K) were used.
Otoacoustic Emissions.
The otoacoustic-emissions procedures consisted of sending broad-band or pure-tone stimuli into the inner ear and thereby evoking a response in the form of an echo from the cochlear outer-hair cells. The ILO88 system (Otodynamics, Hatfield, U.K.) for TEOAEs and the ILO92 system for DPOAEs were used. All measurements were obtained in a sound room with the subject seated in an armchair throughout the test session of ≈30 min. A standard ILO92 DPOAE probe was positioned in the subject's ear canal either by a foam E-A-R-type ear tip or by an individually fitted ear mold.
The TEOAE stimulus was a click with a broad-frequency spectrum delivered at a level of 84 dB spl. DPOAE measures employ primary (f1) and secondary (f2) pure-tone stimuli. Because of the active nonlinearity of the inner ear, the two-tone combinations presented simultaneously generate a third tone called the distortion product. Thus, DPOAEs reflect specific frequency responses of the inner ear contrasted with an overall response that characterizes the TEOAE. For DPOAE measures, the ratio of f2 to f1 was fixed at 1.22. The stimulus levels were held constant at L1 = 70 dB spl and L2 = 60 dB spl. The 2f1–f2 DPOAE level as a function of frequency was recorded in the 1- to 6-kHz range in three steps per octave. DPs were considered to be present when they were at least 3 dB above the noise level (34). DP software (Otodynamics) recorded the levels found in 12 adjacent frequencies and set the significance levels for the DPOAEs at two standard deviations above the mean noise level (for 95% confidence).
HINT.
Sentences and the spectrally shaped speech noise from the HINT (35) compact disc were digitized with an AP-2 array processor [Tucker–Davis Technologies (TDT), Alachua, FL], transduced (D/A converter; TDT), and attenuated [PA 4 (TDT) and Grason–Stadler GSI 16] for use in the speech-in-quiet and speech-in-noise tasks. The HINT speech stimuli consisted of four lists of 20 sentences and a practice list of 10 sentences.
The subject was seated 1 m equidistant from three loudspeakers in a double-walled sound booth (RE243; Acoustic Systems, Austin, TX). The 0° azimuth loudspeaker was directly in front of the subject, the 90° azimuth speaker was to the right of the subject, and the 270° azimuth speaker was to the left. Speech was presented at 0° azimuth in quiet (Q) and in each of the following three noise conditions: (i) in 65 dBA of noise located at 0° azimuth (N0); (ii) at 90° azimuth (N90); and (iii) at 270° azimuth (N270). An adaptive procedure (36) without feedback was used to determine the 50% point on the psychometric function required for speech-recognition thresholds. In the noise conditions, noise onset preceded each sentence by 1 s and was turned off 1 s after each sentence was completed. The calculation of the sentence speech-reception threshold in quiet or signal-to-noise ratio necessary for 50% sentence recognition in noise was based on averaging the presentation levels of sentences 4–20 for each test list.
Data Analyses and Statistics.
The HINT, conducted in the free field (binaural/spatial processing), relied on both ears. All other tests were monaural, and they were presented to each ear independently; therefore, data for the two ears were analyzed separately. For statistical analysis, individual frequency responses to some hearing tests, such as audiograms (PTA1, PTA2, PTA3, PTA4) and DPOAEs (DP1-low, DP2-high), were grouped together for determining overall effects between subject groups. The frequencies represented for these groupings are specified in the figure legends. Data were analyzed with one-way ANOVA for each hearing test. Statistical significance of the main effects was obtained; and post hoc Bonferroni pairwise comparisons, power-corrected for repeated pairwise testing, were performed to assess the significance of differences between specific subject groups (Prism Version 4; GraphPad, San Diego, CA). Error bars in the figures represent the S.E.M.
Acknowledgments
This work was supported by National Institutes of Health Grants P01 AG09524 from the National Institute on Aging and P30 DC05409 from the National Institute on Deafness and Communication Disorders and by the International Center for Hearing and Speech Research (Rochester, NY) (to R.D.F. and D.R.F.).
Abbreviations
- DP
distortion product
- DPOAEs
distortion-product otoacoustic emissions
- E
estrogen
- HINT
hearing-in-noise test
- HRT
hormone replacement therapy
- NHRT
non-hormone replacement therapy
- P
progestin
- PTA
pure-tone average
- spl
sound pressure level
- TEOAEs
transient evoked otoacoustic emissions.
Footnotes
The authors declare no conflict of interest.
References
- 1.Rice MM, Graves AB, McCurry SM, Gibbons LE, Bowen JD, McCormick WC, Larson EB. Arch Intern Med. 2000;160:1641–1649. doi: 10.1001/archinte.160.11.1641. [DOI] [PubMed] [Google Scholar]
- 2.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, III, Assaf AR, Jackson RD. J Am Med Assoc. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 3.Klaiber EL, Broverman DM, Vogel W, Peterson LG, Snyder MB. Psychoneuroendocrinology. 1996;21:575–592. doi: 10.1016/s0306-4530(96)00023-6. [DOI] [PubMed] [Google Scholar]
- 4.Klaiber EL, Broverman DM, Vogel W, Peterson LG, Snyder MB. Psychoneuroendocrinology. 1997;22:549–558. doi: 10.1016/s0306-4530(97)00043-7. [DOI] [PubMed] [Google Scholar]
- 5.Stein DG, Hoffman SW. Pediatric Rehab. 2003;6:13–22. doi: 10.1080/1363849031000095279. [DOI] [PubMed] [Google Scholar]
- 6.Klein BE, Klein R, Ritter LL. Arch Ophthalmol. 1994;112:85–91. doi: 10.1001/archopht.1994.01090130095025. [DOI] [PubMed] [Google Scholar]
- 7.McFadden D. Arch Sexual Behav. 2002;31:99–111. doi: 10.1023/a:1014087319682. [DOI] [PubMed] [Google Scholar]
- 8.Kilicdag EB, Yavuz H, Bagis T, Tarim E, Erikan AN, Kazanci F. Am J Obstet Gynecol. 2004;190:77–82. doi: 10.1016/j.ajog.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Kim SH, Kang BM, Chae HD, Kim CH. Obstet Gynecol. 2002;99:726–730. doi: 10.1016/s0029-7844(02)01963-4. [DOI] [PubMed] [Google Scholar]
- 10.Hultcrantz M, Sylven L, Brog E. Hear Res. 1994;76:127–132. doi: 10.1016/0378-5955(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 11.Hultcrantz M. Acta Oto-Laryngol. 2003;123:253–257. doi: 10.1080/00016480310001097. [DOI] [PubMed] [Google Scholar]
- 12.Caruso S, Maiolino L, Rugolo S, Intelisano G, Farina M, Cocuzza S, Serra A. Hum Reprod. 2003;18:85–89. doi: 10.1093/humrep/deg003. [DOI] [PubMed] [Google Scholar]
- 13.Caruso S, Maiolino L, Agnello A, Garozzo A, Di Mari L, Serra A. Fertil Steril. 2003;79:556–561. doi: 10.1016/s0015-0282(02)04763-5. [DOI] [PubMed] [Google Scholar]
- 14.Walpurger V, Pietrowsky R, Kirscbaum C, Wolf OT. Horm Behav. 2004;46:600–606. doi: 10.1016/j.yhbeh.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Simerly RB. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- 16.Zhang SJ, Jackson MB. J Neuroendocrinol. 1994;50:533–538. doi: 10.1111/j.1365-2826.1994.tb00616.x. [DOI] [PubMed] [Google Scholar]
- 17.Ledoux VA, Woolley CS. J Neurosci. 2005;25:971–976. doi: 10.1523/JNEUROSCI.3489-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner EJ, Ronnekleiv OK, Bosch MA, Kelly MJ. J Neurosci. 2001;21:2085–2093. doi: 10.1523/JNEUROSCI.21-06-02085.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeBold JF, Frye CA. Horm Behav. 1994;28:445–453. doi: 10.1006/hbeh.1994.1042. [DOI] [PubMed] [Google Scholar]
- 20.Rogawski MA. Ann Neurol. 2003;53:288–291. doi: 10.1002/ana.10534. [DOI] [PubMed] [Google Scholar]
- 21.Moffatt CA, Rissman EF, Shupnik MA, Blaustein JD. J Neurosci. 1998;18:9556–9563. doi: 10.1523/JNEUROSCI.18-22-09556.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tischkau SA, Ramirez VD. Proc Natl Acad Sci USA. 1993;90:1285–1289. doi: 10.1073/pnas.90.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stenberg AE, Wang H, Sahlin L, Hultcrantz M. Hear Res. 1999;136:29–34. doi: 10.1016/s0378-5955(99)00098-2. [DOI] [PubMed] [Google Scholar]
- 24.Stenberg AE, Wang H, Fish J, III, Schrott-Fisher A, Sahlin L, Hultcrantz M. Hear Res. 2001;157:87–92. doi: 10.1016/s0378-5955(01)00280-5. [DOI] [PubMed] [Google Scholar]
- 25.Caspary DM, Milbrandt JC, Helfert RH. Exp Gerontol. 1995;30:349–360. doi: 10.1016/0531-5565(94)00052-5. [DOI] [PubMed] [Google Scholar]
- 26.Frisina RD, Walton JP. In: Handbook of Mouse Auditory Research: From Behavior to Molecular Biology. Willott JP, editor. Boca Raton, FL: CRC; 2001. pp. 339–379. [Google Scholar]
- 27.Frisina RD. In: Functional Neurobiology of Aging. Hof PR, Mobbs CV, editors. San Diego: Academic; 2001. pp. 531–547. [Google Scholar]
- 28.Frisina RD, Rajan R. In: The Inferior Colliculus. Winer J, Schreiner C, editors. New York: Springer; 2005. pp. 559–584. [Google Scholar]
- 29.Frisina DR, Frisina RD. Hear Res. 1997;106:95–104. doi: 10.1016/s0378-5955(97)00006-3. [DOI] [PubMed] [Google Scholar]
- 30.Kim SH, Frisina DR, Frisina RD. Audiol Neurotol. 2002;7:348–357. doi: 10.1159/000066159. [DOI] [PubMed] [Google Scholar]
- 31.Tadros SF, Frisina ST, Mapes F, Frisina DR, Frisina RD. Hear Res. 2005;209:10–18. doi: 10.1016/j.heares.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Tadros SF, Frisina ST, Mapes F, Kim S, Frisina DR, Frisina RD. Audiol Neurootol. 2005;10:44–52. doi: 10.1159/000082307. [DOI] [PubMed] [Google Scholar]
- 33.Frisina ST, Mapes F, Kim S, Frisina DR, Frisina RD. Hear Res. 2006;211:103–113. doi: 10.1016/j.heares.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lonsbury-Martin BL, Harris FP, Stagner BB, Hawkins MD, Martin GK. Ann Otol Rhinol Laryngol Suppl. 1990;147:3–14. [PubMed] [Google Scholar]
- 35.Nilsson M, Soli SD, Sullivan JA. J Acoust Soc Am. 1994;95:1085–1099. doi: 10.1121/1.408469. [DOI] [PubMed] [Google Scholar]
- 36.Levitt H. J Acoust Soc Am. 1971;49(Suppl 2):467. [PubMed] [Google Scholar]