Introduction
The ability of cells to respond to extracellular signals relies on a set of mechanisms that are of widespread use in different developmental contexts and are highly conserved among different organisms. One such mechanism is built upon the presence of receptor tyrosine kinase (RTK) molecules in the cell membrane that can be activated by ligands outside the cell and transduce this signal by a well conserved pathway of intracellular molecules to finally elicit different cell responses in terms of morphology and/or gene activation. The Drosophila Torso pathway has been used as one of the model systems to genetically analyse the activity of the RTK signalling pathways. In particular, different studies in this and other systems have allowed identification of the components of these transducing mechanisms and conclusions to be drawn about their interaction. A general conclusion of these experiments is that tyrosine kinase receptors appear to activate a shared group of intracellular effectors, including the Ras/Raf/MAPK cascade. This conclusion has driven many studies to look for the specificity of the different transduction pathways at the events taking place specifically at both ends of the signalling pathways, namely, those leading to the activation of the receptor molecules and those occurring downstream of the phosphorylation cascade. It is the analysis of these events that can help us to understand the great variety of responses that can be elicited by the different RTK signalling pathways. The conserved intracellular mechanisms acting downstream of the Torso receptor have already been reviewed elsewhere and, thus, here we will address specifically the issue of the mechanisms leading to the Torso receptor activation and those responsible for regulating the expression of the Torso pathway target genes.
Activation of the Torso receptor
How to locally activate a widespread receptor?
Transferring positional information from the ovarian cells to the embryo. Torso is a RTK (Sprenger et al., 1989) that is distributed evenly along the embryonic surface at the blastoderm stage (Figure 1C) but its activation occurs only at the poles (Casanova and Struhl, 1989), where it is responsible for the expression of the genes tailless (tll) and huckebein (hkb) (Weigel et al., 1990). These genes will initiate the developmental programmes giving rise to the most anterior and posterior terminal regions of the embryo. It has been known for some time that the restricted activation of the Torso receptor depends critically on the presence of the product of the torso-like (tsl) gene in a subpopulation of follicle cells at each end of the maturing oocyte (Figure 1A) (Stevens et al., 1990). In the absence of Tsl, the receptor is not activated and, conversely, ubiquitous expression of Tsl during oogenesis leads to the general activation of the Torso receptor all over the embryonic surface (Savant-Bhonsale and Montell, 1993; Sprenger et al., 1993; Martin et al., 1994; Furriols et al., 1998). Thus, restricted activation of the Torso receptor at the embryonic surface results from an original unevenness among the ovarian cells that is imposed onto the developing embryo. In addition, the Torso receptor is activated at the embryonic cell surface when the follicle cells no longer surround the embryo. Thus, a mechanism must exist first to transfer the spatial information from the egg chamber to the oocyte and second to ensure that this spatial difference in the follicle cells is transmitted during oogenesis and early embryogenesis until Torso receptor activation takes place. This mechanism appears to be linked to the capacity of the follicle cells to secrete the structural components of the protective shells covering the oocyte: the vitelline membrane and the chorion. The first indication pointing to the link between the eggshell layers and Torso receptor activation came from the genetic characterization of two genetic loci known as fs(1)Nasrat [fs(1)N] and fs(1)polehole [fs(1)ph]. Mutant females for null alleles of both genes lay eggs that collapse, probably due to defects in the vitelline membrane; however, two hypomorphic mutations, one for each gene, do not affect eggshell formation but prevent Torso receptor activation (Degelmann et al., 1990).
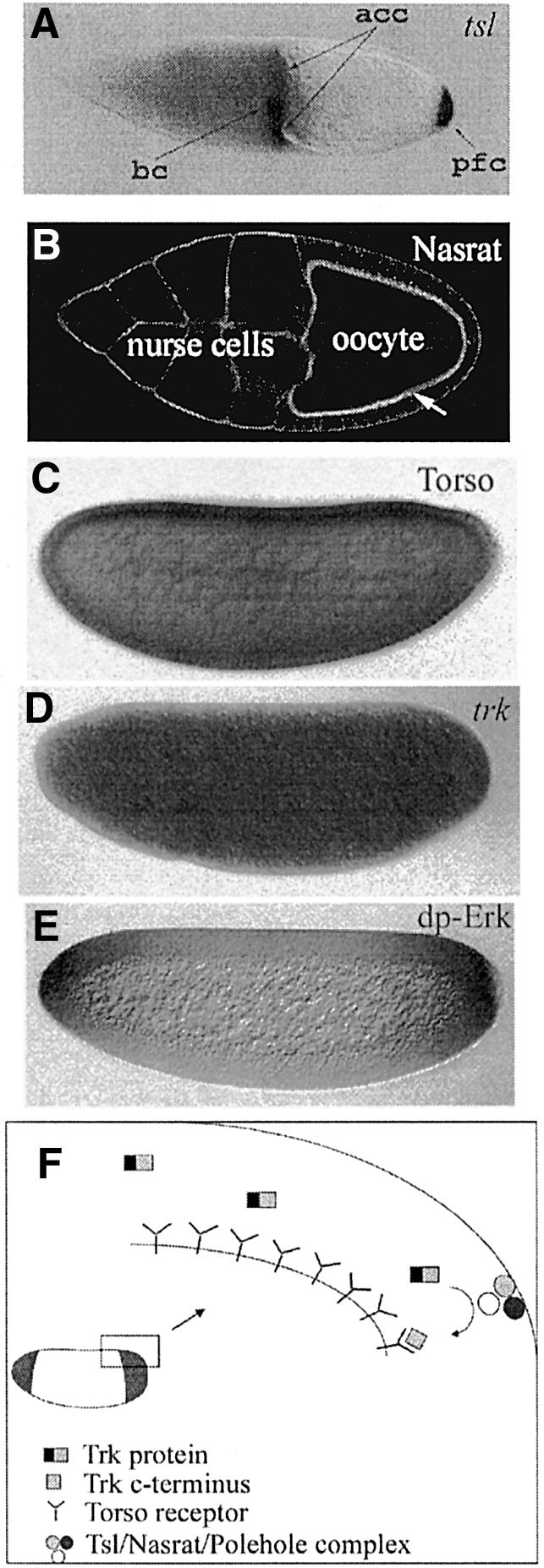
Fig. 1. Steps in Torso receptor activation. (A) The tsl gene is expressed in a subset of follicle cells in the egg chamber at both ends of the oocyte (acc, anterior centripetal cells; bc, border cells; pfc, posterior follicle cells). (B) In an egg chamber (visualized by staining with rhodamine–phalloidin to label the cortical actin), the Nasrat protein accumulates at the oocyte surface (arrow). The Polehole protein displays the same distribution. Nasrat and Polehole proteins are required both for the proper assembly of the eggshell and for the accumulation and/or stabilization of the Tsl product at the poles of the oocyte. (C) At early embryogenesis, the Torso receptor is distributed evenly along the embryonic surface at the blastoderm stages. (D) At the same time, trk mRNA, coding for a secreted growth-factor-like protein, is also distributed uniformly in the embryo. (E) Owing to the restricted localization of the Tsl protein, the Torso receptor will be activated only at the poles, probably by a C-terminal fragment of the trunk protein. As a result, the active diphosphorylated form of Erk (or MAPK), an indicator of the domain of Torso signalling, is restricted to the embryonic poles. (F) Drawing summarizing the model for the activation of the Torso receptor at the embryonic surface. Tsl accumulation is represented at the vitelline membrane, although its precise localization has not yet been determined and the existence of the Nasrat/Polehole/Tsl complex is hypothetical.
The molecular characterization of both genes has provided an indication of how the two processes could be linked (Jiménez et al., 2002). fs(1)N and fs(1)ph are transcribed in the oocyte and their products accumulate at the oocyte surface around stage 10 of oogenesis (Figure 1B). Interestingly, both proteins are mutually required for their cell surface accumulation. At this stage, the oocyte surface and the follicle cell surfaces appear interconnected by many microvilli, and the follicle cells secrete the protein components of the vitelline membrane towards the oocyte surface. There, the Nasrat and Polehole proteins are required for the appropriate cross-linking of vitelline membrane proteins, a step that is critical for correct eggshell formation. On the other hand, accumulation of Nasrat and Polehole proteins at the oocyte cell surface plays an additional role in Torso receptor activation. As mentioned before, expression of the tsl gene in a subpopulation of follicle cells at each end of the maturing oocyte is critical for Torso receptor activation. Also by stage 10 of oogenesis, the Tsl product is detected around the posterior pole of the oocyte, indicating that the protein has been secreted from the follicle cells. (The protein is also probably present around the anterior pole, although this feature is more difficult to assess.) However, in fs(1)N and fs(1)ph mutants, the Tsl protein is hardly detected around the oocyte, indicating a role of both genes in the accumulation and/or stability of the secreted Tsl product. Thus, Nasrat- and Polehole-mediated accumulation of the Tsl product around the poles of the oocyte appears to be the critical element that links the spatial information from the egg chamber to the restricted Torso receptor activation in early embryogenesis (Jiménez et al., 2002).
Delivering a local signal or locally processing a uniform signal? The nature of Tsl as a secreted protein produced in specialized follicle cells at each end of the oocyte prompted the hypothesis that it could act as the ligand for the Torso receptor: deposited by the follicle cells, it would remain tethered to the oocyte surface or to the vitelline membrane during oogenesis and would be made available to the Torso receptor in early embryogenesis. Consistent with this model, Torso is not activated in tsl mutants and, conversely, ectopic expression of tsl all around the oocyte leads to general activation of the Torso receptor.
However, the genetic and molecular characterization of the trunk (trk) gene challenged this model. Mutations in the trk gene had been identified as producing the same phenotype as the torso and tsl mutations (Schüpbach and Wieschaus, 1986a). Like tsl, trk is also required for the activation of the Torso receptor, but in contrast to tsl, the trk gene is required in the oocyte and not in the follicle cells (Schüpbach and Wieschaus, 1986b). One feature of the protein encoded by the trk gene became particularly appealing: its C-terminal domain has an arrangement of cysteines reminiscent of the cystine knot motif found in several growth factors and extracellular ligands. However, while its sequence suggested that trk could encode the ligand of the Torso receptor, its uniform distribution in the oocyte (Figure 1D) seemed difficult to reconcile with the observation that the Torso receptor was only activated at the poles. Yet, a second feature of the Trk protein could provide an explanation for this apparent paradox since it was found that the Trk protein displays putative cleavage sites, suggesting that the protein can be exposed to proteolysis to generate a C-terminal fragment. In addition, a single amino acid substitution in one of the putative cleavage sites of the Trk protein acts as a null mutation, indicating that this site is important for Trk activity in vivo. All these data prompted the hypothesis that the C-terminal fragment of the Trk protein generated by restricted proteolysis only at the poles of the oocyte could act as the ligand of the Torso receptor (Casanova et al., 1995). Unveiling the actual role of Tsl and Trk was not mainly a question of establishing which of the two molecules could act as the bona fide Torso ligand. Instead, solving this issue was important to learn which mechanism was behind the restricted activation of the Torso receptor: either the local deposition of a ligand or the restricted proteolysis of a widespread ligand precursor.
While binding of either Trk or Tsl to the Torso receptor has not been shown, the functional data strongly support the notion that Trk acts as the ligand of Torso. In particular, the C-terminal fragment of the Trk protein can activate the Torso receptor pathway even in fs(1)ph, fs(1)N or tsl mutant backgrounds (Casali and Casanova, 2001). This is in contrast to the observation that ectopic expression of tsl still requires fs(1)ph, fs(1)N and trk function to produce the phenotypes associated with general activation of the Torso receptor (Casanova et al., 1995; Furriols et al., 1998). Altogether, these observations suggest that the control of Torso activation rests on the restricted proteolysis of a widespread ligand precursor (Figure 1F) and indicate an overall similarity with the mechanism of Toll receptor activation in dorsoventral patterning of the Drosophila embryo. However, some differences and puzzles remain. First, no genes encoding for proteases have been found to affect Torso receptor activation, although sequencing of the Drosophila genome has disclosed many new genes coding for proteases. Thus, to validate the model it will be necessary to identify the steps leading to Trk cleavage. Secondly, in the proteolysis model, what is the role of Tsl? At present, it could be argued that Tsl could act as a membrane-bound protein necessary to nucleate an as yet unidentified protease complex, but for the time being this is just a speculation. Clearly, the mode of action of Tsl remains to be elucidated in future experiments.
How to limit the extent of Torso activation?
Role of the receptor in trapping the ligand. We have mentioned before that the Torso receptor, while distributed uniformly over the embryonic surface, is not activated in the middle regions. Indeed, it has been concluded that it is ligand trapping by the Torso receptor at the poles that prevents its further diffusion and impedes Torso activation in the middle body regions (Sprenger and Nüsslein-Volhard, 1992; Casanova and Struhl, 1993). This conclusion was driven by the observation that mutant embryos in which the Torso receptor is only present in the central portion of the body, and not in the poles, display segmentation defects and show terminal structures in the middle of the embryo. These phenotypes are dependent upon the activity of the upstream genes trk and tsl, indicating that they are caused by ligand–receptor interactions. In addition, these phenotypes are suppressed by the presence of mutant receptors that are unable to transduce the signal but which retain an intact extracellular domain and are therefore able to bind the ligand. These observations not only suggested a role for the Torso receptor in limiting the diffusion of the ligand, but also indicated that both the amount of active ligand and the time of ligand production must be critical in controlling the area of Torso receptor activation (Sprenger and Nüsslein-Volhard, 1992; Casanova and Struhl, 1993). Additional observations have revealed that this turns out to be quite a general feature of signalling pathways to restrict the domain of receptor activation in many other scenarios (see, for example, Chen and Struhl, 1996; Hajnal et al., 1997; Briscoe et al., 2001).
Gene regulation by the Torso pathway
Signalling from receptor tyrosine kinases is transduced via a common Ras/Raf/MAPK cascade to regulate the expression of distinct target genes. Thus, the different responses in terms of gene activation are largely dependent on the distinct factors that mediate transcriptional regulation downstream of the RTK cascade. In the following sections, we will address the mechanisms underlying the specificity of gene regulation by the Torso receptor pathway.
How to generate different outputs from a single signal?
A basic question in trying to understand the role of signalling pathways in development is how a single signal can be transduced into different cell responses in terms of gene activation. This is also the case for the Torso receptor since its signalling pathway is responsible for at least the activation of two genes in different but overlapping domains at the posterior pole of the embryo: while tll is expressed in all the posterior end of the embryo, hkb is activated only in its most terminal part (Figure 2C and D) (Pignoni et al., 1990; Weigel et al., 1990; Brönner and Jäckle, 1991). A first hint indicating that a difference in the ‘amount’ of signalling could be responsible for the distinct domains of tll and hkb expression came from experiments suggesting that variation in the number of activated Torso receptors correlated with differential gene expression. In particular, mutant conditions that allow a gradual increase in the amount of Torso receptor molecules at the cell surface or the levels of Trk gave rise only to tll expression when these levels were low and to the additional expression of hkb when these levels where higher. Thus, higher levels of Torso signalling would induce both tll and hkb expression, while low levels would only be able to give rise to tll expression (Furriols et al., 1996). Subsequent experiments have demonstrated that this holds true at the different levels of the transducing cascade that have been investigated (Greenwood and Struhl, 1997; Ghiglione et al., 1999). All these observations prompted a model with the following features: (i) the tll and hkb promoters would be differentially responsive to the same combination of transcription factors; and (ii) the activity of these transcription factors would depend on phosphorylation by the Ras/Raf/MAPK cascade. According to this model, different signalling thresholds would be translated into different levels of activity of transcription factors, which ultimately will establish the different domains of tll and hkb gene expression. We will come to this model again later. Finally, other experiments have shown that induction of tll and hkb expression by the Torso pathway can be blocked by interactions with other transcription factors acting in the central region of the embryo. These latter observations have prompted the suggestion that these interactions may act as a reinforcing mechanism and contribute to specifying the correct domains of tll and hkb in wild-type embryos (Casanova and Struhl, 1989; Casanova et al., 1994; Greenwood and Struhl, 1997).
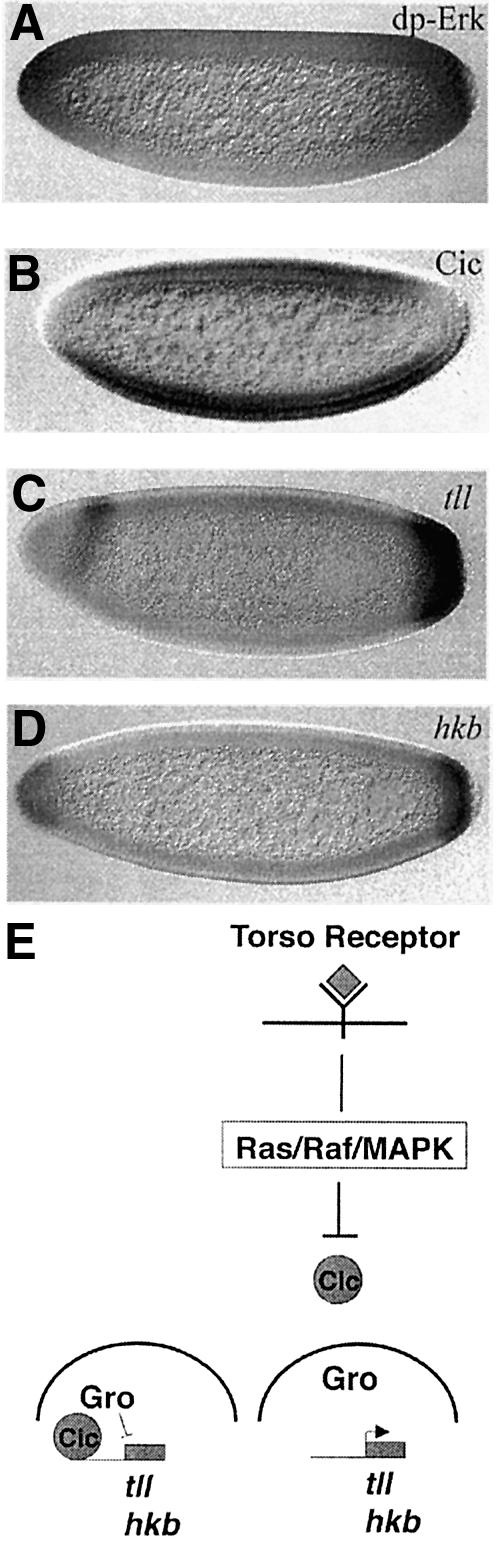
Fig. 2. Gene regulation by the Torso pathway. (A) The domains of Torso signalling are restricted to the embryonic poles, as indicated by an antibody that specifically recognizes the active diphosphorylated form of Erk (or MAPK). (B) As a consequence, the Cic protein is excluded from the nuclei at the terminal regions. (C and D) Relief of the repression by the Cic–Gro complex allows expression of tll and hkb in different but overlapping domains at the embryonic poles. Different signalling thresholds are responsible for the distinct domains of tll and hkb. (E) Schematic representation of the mechanism of gene regulation by relief of repression by the Torso pathway. In the absence of Torso signalling, Cic–Gro complexes repress tll and hkb transcription. However, upon activation of the Torso pathway at the poles, Cic is excluded from the nuclei and the Cic–Gro complexes do not form, allowing transcription of tll and hkb.
Torso signalling regulates gene expression by relief of repression
Rather than regulating tll and hkb expression by direct activation, the Torso signalling pathway functions by antagonizing at the poles a repressor present throughout the embryo. The first evidence for this mechanism was obtained from analysis of the tll promoter (Liaw et al., 1995). First, a minimal regulatory region was identified in the tll promoter that was sufficient to reproduce the pattern of expression of the tll gene. Secondly, this regulatory region was found to respond to Torso activation since it did not drive expression at the poles in the absence of the Torso receptor, while it caused general expression when the Torso receptor was ectopically activated all over the embryo. Finally, it was found that this region of the tll promoter bore some specific regulatory elements required for restricting tll expression at the embryonic poles; deletion or mutation of these elements caused uniform expression of the tll gene resembling what was found in mutations that caused activation of the Torso receptor all over the embryo. The presence of these elements, termed torso response elements or tor-RE, suggested the following model. In the central region of the embryo, some repressor factors would interact with the tor-RE and block tll transcription. However, at the poles, Torso signalling would modify the repressor factors; as a result, they would be inactivated, allowing tll transcription (Liaw et al., 1995).
In an independent approach, it was found that early embryos lacking groucho (gro) activity displayed ectopic tll and hkb expression. This indicated that the Gro protein, a well-know corepressor, was also required to confine tll and hkb expression to the poles of the embryo, and strengthened the model that tll and hkb transcription were activated by relief of repression (Paroush et al., 1997). In particular, in the absence of Gro-mediated repression, tll and hkb are expressed even in the absence of Torso signalling. According to these observations, Gro could be part of the repressor complex antagonized at the poles by Torso signalling. The mode of action of Gro has been analysed and reviewed extensively, and thus it will not be discussed here; instead, we will specifically address the role of Gro as an effector of the Torso transduction pathway. One feature of Gro, a protein highly conserved during evolution, is that it does not bind directly to DNA but is recruited to different promoters by other DNA-binding proteins. Depending on its partners, Gro participates in the repression of a great variety of target genes. The observation that other Gro-mediated transcriptional repressor processes were active in the early embryo and were not inhibited by Torso signalling suggested that Gro itself was not the direct target for the Torso pathway. Thus, it was hypothesized that an additional protein would be required both to target Gro to the tll and hkb promoters and as a target of Torso signalling regulation (Paroush et al., 1997).
The Capicua protein, a sensor for Torso signalling
The product of the gene capicua (cic) appears to fulfil all these requirements (Jiménez et al., 2000). cic mutations were identified because they give rise to embryos with expanded domains of tll and hkb expression very similar to those found in gro mutant embryos. Molecular characterization of the cic gene showed that it encodes a protein with a DNA-binding domain of the HMG-box class. In addition, Cic was shown to interact with Gro in vitro. Finally, while cic RNA is uniformly distributed over the embryo, the Cic protein is present in the nuclei of the central region of the embryo but excluded from the nuclei at both poles (Figure 2B). Moreover, downregulation of Cic at the poles is due to Torso signalling, as Cic is present in the nuclei at the poles in mutants impairing Torso signalling. Altogether, these data support the notion that Cic could act as a DNA-binding protein recruiting Gro to the tll and hkb promoters, and behave as the regulatory element connecting Torso signalling to transcriptional gene regulation (Figure 2E). In support of this idea, the Cic protein contains many putative MAPK phosphorylation sites (Jiménez et al., 2000).
We have dealt before with the capacity of the Torso pathway to generate more than one response from a single signal and how it was suggested that different signalling thresholds could be translated into different levels of activity of transcription factors. Interestingly, Cic protein, due to its downregulation by the Torso pathway, displays a graded distribution at the nuclei of the poles, being minimal at the tips. This distribution explains how Cic-mediated repression could antagonize differentially the activation of tll and hkb, thus providing a link between the ‘amount’ of Torso signalling and the generation of distinct domains of gene expression.
Obviously, many features of this model remain to be substantiated. To begin with, the nature of the mechanism of Cic downregulation itself. Moreover, several studies suggest that many more elements might participate in the repressor complex regulating tll and hkb. In particular, the characterization of the tor-RE sequences was used to purify and identify putative candidates for tor-RE binding proteins. By this approach, GAGA (the product of the Trithorax-like gene) and NTF-1 (the product of the grainyhead gene) were picked as proteins able to bind the tll promoter in vitro (Liaw et al., 1995). Also, a similar approach identified Tramtrack69 as binding to other sequences in the same tll promoter (Chen et al., 2002). While mutations in any of these genes do not produce a phenotype as strong as that of gro or cic, they probably contribute to the overall transcriptional regulation of tll and hkb, suggesting that these proteins could also be part of a large repressor complex. Similarly to Gro, Cic is also conserved in many organisms across evolution, suggesting that they could participate in repressor complexes acting in different systems.
Integrating information from two different transduction pathways
Indeed, both Gro and Cic are also required for Dorsal-mediated repression, another repressor event taking place simultaneously in the early embryo (Dubnicoff et al., 1997; Jiménez et al., 2000). Dorsal is a bifunctional transcription factor that accumulates at the nuclei of the ventral part of the embryo and acts both as a repressor and an activator of transcription (Jiang et al., 1993; Kirov et al., 1993). Cic does not participate in the latter and is specifically required for Dorsal-mediated repression, although the effects on dorsoventral patterning of removing Cic appear to be milder than those caused by removal of Gro. Similarly, other factors such as Dri and Cut also seem to contribute to switch Dorsal to a repressor (Valentine et al., 1998; Häder et al., 2000). These data reinforce the suggestion that Cic functions in association with Gro and other factors in a big repressor complex.
Furthermore, the dual role of Cic in terminal and dorsal repression offers an explanation for the molecular nature of the interaction between the transduction pathways involved in both patterning events. As mentioned before, Dorsal accumulates in the nuclei at the ventral part of the embryo and its localization relies on a complex mechanism elicited by activation of the Toll receptor at the embryonic surface (Roth et al., 1989; Rushlow et al., 1989; Steward, 1989). It was already known that Torso signalling altered dorsoventral patterning at the embryonic poles (Casanova, 1991; Rusch and Levine, 1994) and the observation that Dorsal-mediated repression is impaired at the poles by downregulation of Cic could account for the interaction between these two transduction pathways.
Cic-mediated repression in other RTK signalling pathways
Not only do Cic and Gro participate in two different repression processes in the early embryo, but they also mediate repression in other unrelated events such as wing vein development (Roch et al., 2002) and cell follicle patterning (Goff et al., 2001). In this case, however, it is the EGFR signalling that leads to downregulation of Cic. In addition, in wing vein patterning, gro mutations display a similar phenotype to that of cic mutations (de Celis and Ruíz-Gómez, 1995). These observations suggest that EGFR signalling can also induce activation of some of its target genes by inhibiting Cic/Gro-mediated repression. While Cic does not seem to act in all the developmental processes mediated by the Ras/Raf/MAPK cascade, these data indicate that Cic and Gro could be part of a conserved repressor complex downregulated by different RTK pathways in different cellular contexts.
Need for activation
The model put forward for the regulation of tll and hkb implies that one or more transcription factors must exist to activate their expression when Torso signalling abolishes transcriptional repression. However, nothing is known yet about the nature of these factors. The broad expression of tll in the absence of repression suggests that this transcriptional activator(s) could be ubiquitously distributed in the embryo. Alternatively, different factors could be required for tll and hkb activation in distinct regions of the embryo. Recently, the STAT transcription factor has been implicated in the positive regulation of tll, but only under circumstances where specific mutations render the Torso receptor constitutively active (Li et al., 2002). Clearly, identification of the transcriptional factors required for normal tll activation will be necessary to fully understand how its activation can be antagonized by Cic/Gro-mediated repression.
Conclusion
Analysis of the in and out of Torso signalling has unveiled a variety of molecular mechanisms, such as localized proteolysis of a widespread ligand precursor, restriction of ligand diffusion by receptor trapping, variation of the number of activated receptors as a means to generate different signalling outcomes and regulation of gene expression by signalling-induced relief of repression. Different combinations of these mechanisms are likely to operate in several RTK pathways and contribute to their specificity in cell differentiation and cell proliferation.
Acknowledgments
Acknowledgements
We thank all past and present members of the laboratory who have contributed to the work presented in this review, especially Andreu Casali and Gerardo Jiménez. We are grateful to Josep Casacuberta, Gerardo Jiménez, Marta Llimargas and Ernesto Sánchez-Herrero for critical reading of the manuscript. Work in our laboratory has been funded by grants from the Spanish government and supported by the Generalitat de Catalunya.
References
- Briscoe J., Chen,Y., Jessell,T.M. and Struhl,G. (2001) A Hedgehog-insensitive form of Patched provides evidence for direct long-range morphogen activity of Sonic hedgehog in the neural tube. Mol. Cell, 7, 1279–1291. [DOI] [PubMed] [Google Scholar]
- Brönner G. and Jäckle,H. (1991) Control and function of terminal gap gene activity in the posterior pole region of the Drosophila embryo. Mech. Dev., 35, 205–211. [DOI] [PubMed] [Google Scholar]
- Casali A. and Casanova,J. (2001) The spatial control of Torso RTK activation: a C-terminal fragment of the Trunk protein acts as a signal for Torso receptor in the Drosophila embryo. Development, 128, 1709–1715. [DOI] [PubMed] [Google Scholar]
- Casanova J. (1991) Interaction between torso and dorsal, two elements of different transduction pathways in the Drosophila embryo. Mech. Dev., 36, 41–45. [DOI] [PubMed] [Google Scholar]
- Casanova J. and Struhl,G. (1989) Localized surface activity of torso, a receptor tyrosine kinase, specifies terminal body pattern in Drosophila. Genes Dev., 3, 2025–2038. [DOI] [PubMed] [Google Scholar]
- Casanova J. and Struhl,G. (1993) The torso receptor localizes as well as transduces the spatial signal specifying terminal body pattern in Drosophila. Nature, 362, 152–155. [DOI] [PubMed] [Google Scholar]
- Casanova J., Llimargas,M., Greenwood,S. and Struhl,G. (1994) An oncogenic form of human raf can specify terminal body pattern in Drosophila. Mech. Dev., 48, 59–64. [DOI] [PubMed] [Google Scholar]
- Casanova J., Furriols,M., McCormick,C.A. and Struhl,G. (1995) Similarities between trunk and spätzle, putative extracellular ligands specifying body pattern in Drosophila. Genes Dev., 9, 2539–2544. [DOI] [PubMed] [Google Scholar]
- Chen Y. and Struhl,G. (1996) Dual roles for Patched in sequestering and transducing Hedgehog. Cell, 87, 553–563. [DOI] [PubMed] [Google Scholar]
- Chen Y.-J., Chinag,C.-S., Weng,L.-C., Lengyel,J.A. and Liaw,G.-J. (2002) Tramtrack 69 is required for the early repression of tailless expression. Mech. Dev., 116, 75–83. [DOI] [PubMed] [Google Scholar]
- de Celis J.F. and Ruíz-Gómez,M. (1995) groucho and hedgehog regulate engrailed expression in the anterior compartment of the Drosophila wing. Development, 121, 3467–3476. [DOI] [PubMed] [Google Scholar]
- Degelmann A., Hardy,P.A. and Mahowald,A.P. (1990) Genetic analysis of two female-sterile loci affecting eggshell integrity and embryonic pattern formation in Drosophila melanogaster. Genetics, 126, 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnicoff T., Valentine,S.A., Chen,G., Shi,T., Lengyel,J.A., Paroush,Z. and Courey,A.J. (1997) Conversion of Dorsal from an activator to a repressor by the global corepressor Groucho. Genes Dev., 11, 2952–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furriols M., Sprenger,F. and Casanova J. (1996) Variation in the number of activated Torso receptors correlates with differential gene expression. Development, 122, 2313–2317. [DOI] [PubMed] [Google Scholar]
- Furriols M., Casali,A. and Casanova,J. (1998) Dissecting the mechanism of torso receptor activation. Mech. Dev., 70, 111–118. [DOI] [PubMed] [Google Scholar]
- Ghiglione C., Perrimon,N. and Perkins,L.A. (1999) Quantitative variations in the level of MAPK activity control patterning of the embryonic termini in Drosophila. Dev. Biol., 205, 181–193. [DOI] [PubMed] [Google Scholar]
- Goff D.J., Nilson,L.A. and Morisato,D. (2001) Establishment of dorsal–ventral polarity of the Drosophila egg requires capicua action in ovarian follicle cells. Development, 128, 4553–4562. [DOI] [PubMed] [Google Scholar]
- Greenwood S. and Struhl,G. (1997) Different levels of Ras activity can specify distinct transcriptional and morphological consquences in early Drosophila embryos. Development, 124, 4879–4886. [DOI] [PubMed] [Google Scholar]
- Häder T., Wainwright,D., Shandala,T., Saint,R., Taubert,H., Brönner,G. and Jäckle,H. (2000) Receptor tyrosine kinase signaling regulates different modes of Groucho-dependent control of Dorsal. Curr. Biol., 10, 51–54. [DOI] [PubMed] [Google Scholar]
- Hajnal A., Whitfield,C.W. and Kim,S.K. (1997) Inhibition of Caenorhabditis elegans vulval induction by gap-1 and by let-23 receptor tyrosine kinase. Genes Dev., 11, 2715–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Cai,H., Zhou,Q. and Levine,M. (1993) Conversion of a dorsal-dependent silencer into an enhancer: evidence for dorsal corepressors. EMBO J., 12, 3201–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez G., Guichet,A., Ephrussi,A. and Casanova,J. (2000) Relief of gene repression by Torso RTK signaling: role of capicua in Drosophila terminal and dorsoventral patterning. Genes Dev., 14, 224–231. [PMC free article] [PubMed] [Google Scholar]
- Jiménez G., González-Reyes,A. and Casanova,J. (2002) Cell surface proteins Nasrat and Polehole stabilize the Torso-like extracellular determinant in Drosophila oogenesis. Genes Dev., 16, 913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov N., Zhelnin,L., Shah,J. and Rushlow,C. (1993) Conversion of a silencer into an enhancer: evidence for a co-repressor in dorsal-mediated repression in Drosophila. EMBO J., 12, 3193–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.X., Agaisse,H., Mathey-Prevot,B. and Perrimon,N. (2002) Differential requirement for STAT by gain-of-function and wild-type receptor tyrosine kinase Torso in Drosophila. Development, 129, 4241–4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw G., Rudolph,K.M., Huang,J.-D., Dubnicoff,T., Courey,A.J. and Lengyel,J.A. (1995) The torso response element binds GAGA and NTF-1/Elf-1 and regulates tailless by relief of repression. Genes Dev., 9, 3163–3176. [DOI] [PubMed] [Google Scholar]
- Martin J.-R., Raibaud,A. and Ollo,R. (1994) Terminal pattern elements in Drosophila embryo induced by the torso-like protein. Nature, 367, 741–745. [DOI] [PubMed] [Google Scholar]
- Paroush Z., Wainwright,S.M. and Ish-Horowicz,D. (1997) Torso signalling regulates terminal patterning in Drosophila by antagonising Groucho-mediated repression. Development, 124, 3827–3834. [DOI] [PubMed] [Google Scholar]
- Pignoni F., Baldarelli,R.M., Steingrímsson,E., Diaz,R.J., Patapoutian,A., Merriam,J.R. and Lengyel,J.A. (1990) The Drosophila gene tailless is expressed at the embryonic termini and is a member of the steroid receptor superfamily. Cell, 62, 151–163. [DOI] [PubMed] [Google Scholar]
- Roch F., Jiménez,G. and Casanova,J. (2002) EGFR signalling inhibits Capicua-dependent repression during specification of Drosophila wing veins. Development, 129, 993–1002. [DOI] [PubMed] [Google Scholar]
- Roth S., Stein,D. and Nüsslein-Volhard,C. (1989) A gradient of nuclear localization of the dorsal protein determines dorsoventral pattern in the Drosophila embryo. Cell, 59, 1189–1202. [DOI] [PubMed] [Google Scholar]
- Rusch J. and Levine,M. (1994) Regulation of the dorsal morphogen by the Toll and torso signaling pathways: a receptor tyrosine kinase selectively masks transcriptional repression. Genes Dev., 8, 1247–1257. [DOI] [PubMed] [Google Scholar]
- Rushlow C.A., Han,D., Manley,J.S. and Levine,M. (1989) The graded distribution of the dorsal morphogen is initiated by selective nuclear transport in Drosophila. Cell, 59, 1165–1177. [DOI] [PubMed] [Google Scholar]
- Savant-Bhonsale S. and Montell,D.J. (1993) torso-like encodes the localized determinant of Drosophila terminal pattern formation. Genes Dev., 7, 2548–2555. [DOI] [PubMed] [Google Scholar]
- Schüpbach T. and Wieschaus,E. (1986a) Maternal-effect mutations altering the anterior–posterior pattern of the Drosophila embryo. Roux’s Arch. Dev. Biol., 195, 302–317. [DOI] [PubMed] [Google Scholar]
- Schüpbach T. and Wieschaus,E. (1986b) Germ-line autonomy of maternal-effect mutations altering the embryonic body pattern of Drosophila. Dev. Biol., 113, 443–448. [DOI] [PubMed] [Google Scholar]
- Sprenger F. and Nüsslein-Volhard,C. (1992) Torso receptor activity is regulated by a diffusible ligand produced at the extracellular terminal regions of the Drosophila egg. Cell, 71, 987–1001. [DOI] [PubMed] [Google Scholar]
- Sprenger F., Stevens,L.M. and Nüsslein-Volhard,C. (1989) The Drosophila gene torso encodes a putative receptor tyrosine kinase. Nature, 338, 478–483. [DOI] [PubMed] [Google Scholar]
- Sprenger F., Trosclair,M.M. and Morrison,D.K. (1993) Biochemical analysis of Torso and D-Raf during Drosophila embryogenesis: implications for terminal signal transduction. Mol. Cell. Biol., 13, 1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens L.M., Fronhöfer,H.G., Klinger,M. and Nüsslein-Volhard,C. (1990) Localized requirement for torso-like expression in follicle cells for development of the terminal anlagen of the Drosophila embryo. Nature, 346, 660–663. [DOI] [PubMed] [Google Scholar]
- Steward R. (1989) Relocalization of the dorsal protein from the cytoplasm to the nucleus correlates with its function. Cell, 59, 1179–1188. [DOI] [PubMed] [Google Scholar]
- Valentine S.A., Chen,G., Shandala,T., Fernández,J., Mische,S., Saint,R. and Courey,A.J. (1998) Dorsal-mediated repression requires the formation of a multiprotein repression complex at the ventral silencer. Mol. Cell. Biol., 18, 6584–6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D., Jürgens,G., Klinger,M. and Jäckle,H. (1990) Two gap genes mediate maternal terminal pattern formation in Drosophila. Science, 248, 495–498. [DOI] [PubMed] [Google Scholar]


