Abstract
Immunization of newborns against viral infections may be hampered by ineffective CD8+ T cell responses. To characterize the function of CD8+ T lymphocytes in early life, we studied newborns with congenital human cytomegalovirus (HCMV) infection. We demonstrate that HCMV infection in utero leads to the expansion and the differentiation of mature HCMV-specific CD8+ T cells, which have similar characteristics to those detected in adults. High frequencies of HCMV-specific CD8+ T cells were detected by ex vivo tetramer staining as early as after 28 weeks of gestation. During the acute phase of infection, these cells had an early differentiation phenotype (CD28–CD27+CD45RO+, perforinlow), and they acquired a late differentiation phenotype (CD28–CD27-CD45RA+, perforinhigh) during the course of the infection. The differentiated cells showed potent perforin-dependent cytolytic activity and produced antiviral cytokines. The finding of a mature and functional CD8+ T cell response to HCMV suggests that the machinery required to prime such responses is in place during fetal life and could be used to immunize newborns against viral pathogens.
Introduction
The fetus and infant have a high susceptibility to viral infections. A number of viruses, including human cytomegalovirus (HCMV), herpes simplex type 2, respiratory syncytial virus (RSV), and HIV, cause severe or rapidly progressive disease in early life as compared to later life (1–4). Also, young infants infected with hepatitis B are more likely to become chronic carriers than older children or adults (5). It is generally accepted that this increased susceptibility to viral infections is related to the immaturity of the immune system, but the mechanisms involved remain poorly understood (6–8). A better understanding of the immune system in early life is required to develop vaccines protecting young infants from viral infections. CD8+ T lymphocytes play a central role in immunity against viruses by producing cytokines and by killing infected target cells (9, 10). Little is known about the function of CD8+ T cells in early life. CD8+ T cell responses to HIV are infrequently detected in infants infected during the first months of life, whereas potent responses are commonly detected in infected adults (4, 11–13). Cytotoxic CD8+ T cell responses have also been detected in infants infected with RSV, but whether these responses mature with age remains unclear (14, 15).
To gain insight into the function of CD8+ T lymphocytes in early life in humans, we studied newborns with congenital HCMV infection. HCMV is the most common cause of congenital infection, affecting 0.2% of all live births in industrialized countries and up to 3% in developing countries (1). Although HCMV infection causes few symptoms in immunocompetent adults, about 10% of newborns with congenital infection develop symptoms, including cerebral malformations, multiple organ failure, deafness, and mental retardation (1). Another characteristic of congenital HCMV infection is that both symptomatic and asymptomatic children excrete the virus in urine and saliva for prolonged periods of time, up to 5 years after infection. Historical studies suggest that the increased susceptibility of the fetus to HCMV infection could be related to defective cell-mediated immune responses (16, 17).
Following primary infection, HCMV persists throughout life in a latent form in cells of the myeloid lineage (18). In adults, HCMV induces large expansions of a restricted number of CD8+ T cell clones, primarily recognizing the structural protein pp65 and the immediate early protein IE1 (19–22). In contrast with other persistent viruses, including HIV, Epstein-Barr virus (EBV), and hepatitis C virus (HCV), HCMV induces an advanced stage of CD8+ T cell differentiation in adults (22–27). This advanced or “late” stage of differentiation is characterized by the downregulation of the costimulatory molecules CD28 and CD27, the expression of high levels of perforin, correlating with potent cytolytic activity, and a high proportion of memory cells expressing the glycoprotein CD45RA. In this study we show that HCMV infection in fetal life induces the expansion and the differentiation of mature HCMV-specific CD8+ T cells. The frequency, the phenotype, and the effector functions of these cells were similar to those detected in adults.
Methods
Study population.
This study was approved by The Gambia Government/Medical Research Council and the Hôpital Erasme ethical committees. In The Gambia, a prospective study of the immune response to congenital and early postnatal HCMV infection is currently ongoing. After maternal informed consent, cord blood was collected from all enrolled newborns. Congenital HCMV infection was diagnosed by PCR on urine collected during the first week of life. Eight newborns with congenital infection and a group of 15 randomly selected uninfected controls were included in this study. All newborns were asymptomatic at birth, except case no. 5, who had a mild purpura and hepatomegaly that resolved within the first four weeks of life. Adult-type immune response to persistent HCMV infection was studied in five healthy Gambian adult blood bank donors. A prospective cohort study of the immune response to primary HCMV infection during pregnancy is currently being conducted in Belgium. Data obtained from one pregnant women and one unrelated fetus with primary HCMV infection are described here. The woman seroconverted between the 5th and the 11th week of pregnancy, when a blood sample was obtained, and gave birth to an uninfected newborn. HCMV infection in the fetus was diagnosed by PCR and viral culture on amniotic fluid collected at 18 weeks of gestation following maternal HCMV seroconversion. The fetus subsequently developed severe cerebral malformations, and pregnancy was terminated under medical assistance at 28 weeks of gestation. A sample of cord blood (2 ml) was obtained at the time of abortion. Blood obtained from all study subjects was processed within four hours of collection, and PBMCs were stored in liquid nitrogen before analysis, with the exception of the sample obtained from the infected fetus, which was studied immediately after collection. HLA typing was carried out by amplification refractory mutation system PCR using sequence-specific primers as described (28), except in the case of the HCMV-infected fetus, where HLA typing was done by flow cytometry using the anti–HLA-A2 BB7.2 mAb (available from the laboratory facilities at Weatherall Institute ofMolecular Medicine).
HCMV PCR.
HCMV was detected in infant urine using a nested PCR method, and products were detected on an ethidium bromide–stained agarose gel as described previously (29). The cutoff of the method is approximately 25 DNA copies/ml. False-positive reactions were controlled for by the inclusion of one negative control per seven test samples, and each run (of 14 test samples) contained positive controls equivalent to 2,500 and 250 DNA copies/ml. Positive diagnoses were confirmed on urine samples collected two weeks later. HCMV was detected in amniotic fluid and fetal tissues by real-time PCR, using primers and probe selected in the pp150 gene and designed by one of us. Sequences of the upstream and downstream primers and probe were: 5′-CTGATGAGGTTTGGGC TTTAA, 5′-TCCGAGGAGTCGTCGTCTT, and 5′-FAM-CAAACTGCAGAGTCA CCGGTCGAA-Tamra. PCR was performed using the TaqMan Universal PCR master mixture and the GenAmp 5700 Sequence Detection System (Perkin Elmer Biosystems, Foster City, California, USA).
Ab’s and peptides.
Anti-CD8 (peridin chlorophyll protein [PerCP]), anti-CD27 (allophycocyanin [APC]), anti-CD38 (APC), anti-CD45RO (APC), anti-CD62-L (APC), anti–TNF-α (APC), anti–IL-2 (APC), anti–IFN-γ (FITC), anti–CD28 (FITC), anti-CD45RA (FITC), anti-CD95 (FITC), anti–HLA-DR (FITC), anti–Bcl-2 (FITC), anti-Ki67 (FITC), anti-perforin (FITC), and anti–granzyme A (FITC) Ab’s were purchased from Becton Dickinson Immunocytometry Systems (San Diego, California, USA). Anti–MIP-1 β (FITC) Ab’s were purchased from R&D Systems Ltd. (Abingdon, United Kingdom). Goat anti-mouse IgG (phycoerythrin [PE]) was purchased from Southern Biotechnology Associates (Birmingham, Alabama, USA). Anti-BV2 and anti-BV23 Ab’s were purchased from Immunotech (Marseille, France). Anti-BV14, anti-BV16, and anti-BV18 Ab’s were obtained from the T cell receptor (TCR) mAb workshop (30). The following HCMV pp65 (UL83) peptides were used: VLGPISGHV (AA 14–22), MLNIPSINV (AA 120–128), and NLVPMVATV (AA 495–503), restricted through HLA-A2 (19, 31); RPHERNGFTV (AA 265–274) and TPRVTGGGAM (AA 417–426), restricted through HLA-B7 (20, 32); IPSINVHHY (AA 123–131) and VFPTKDVAL (AA 187–195), restricted through HLA-B35 (19, 33). We also used the HCMV IE1 (UL123) YILEETSVM (AA 315–323) and HCMV glycoprotein B (UL55) IAGNSAYEYV (AA 619–628) HLA-A2–restricted peptides (34, 35).
TCR CDR3 spectratyping.
CD8+ T cells were positively selected from cord blood mononuclear cells using anti-CD8 MACS beads (Miltenyi Biotec, Auburn, California, USA). Purity of the positively selected population was greater than 95%, as assessed by flow cytometry. Total RNA was extracted by lysis in guanidine thiocyanate buffer (RNA-Bee; Biogenesis Ltd., Poole, United Kingdom), and cDNA was prepared with MMLV reverse transcriptase (Invitrogen Ltd., Paisley, United Kingdom), as previously described (36). The cDNAs were amplified in PCR reactions primed by one of the 24 BV subfamilies and the BC-specific primer as described by Garderet et al. (36). The CDR3 region includes residues 9–106 (37). Amplification products were labeled in an extension, or runoff, reaction using a BC-specific Fam-fluorescent probe (Life Technologies Ltd., Paisley, United Kingdom). Fluorescent runoff products and Tamra-fluorescent DNA weight markers were loaded on sequence gel in an automated sequencer (Perkin Elmer Ltd., Beaconsfield, United Kingdom). CDR3 sizes and fluorescent intensities were analyzed using the Genescan software (Perkin Elmer Ltd.).
HLA-peptide tetrameric complexes.
HLA-peptide tetrameric complexes were synthesized as described previously (25). Briefly, modified HLA heavy-chain molecules containing a sequence encoding the BirA biotinylation enzyme recognition site and β2-microglobulin were synthesized in a prokaryote expression system, purified from inclusion bodies, and allowed to refold with the relevant peptide by dilution. Refolded monomeric complexes were purified by fast-performance liquid chromatography, biotinylated, and combined with PE-labeled streptavidin (Sigma-Aldrich, Gillingham, United Kingdom) at a 4:1 molar ratio to form tetrameric HLA-peptide complexes (hereafter “tetramers”). Tetramers were titrated against PBMCs from HCMV-seropositive adult donors to determine the concentration that induced maximal staining.
Cell surface and intracellular staining.
Cell surface and intracellular staining was conducted on thawed cryopreserved cord blood mononuclear cells or adult PBMCs except for the fetal sample that was studied as fresh whole blood. Cells (105 to 5 × 105) or 150 ml whole blood were stained with titrated tetrameric complexes for 15 minutes at 37°C before addition of a panel of titrated Ab’s directed against surface molecules. Following washing and permeabilization in FACS permeabilzation buffer (Becton Dickinson Immunocytometry Systems), intracellular staining was performed using titrated Ab’s. Cells were then washed and stored in Cell Fix buffer (Becton Dickinson Immunocytometry Systems) at 4°C until analysis. Samples were analyzed on a Becton Dickinson FACScalibur after compensation was checked using freshly stained PBMCs. Intracellular cytokine staining requiring short-term stimulation using cognate peptide was performed as described previously (38).
IFN-γ enzyme-linked immunospot assay.
Where sufficient cells were available, IFN-γ enzyme-linked immunospot assay (ELISPOT) was performed according to the manufacturer’s instructions (Mabtech AB, Stockholm, Sweden). Cord blood mononuclear cells were plated in duplicates at 105 cells/well. Peptides (20 μM) or phytohemagglutinin (PHA) (1 μg/ml) were added to appropriate wells, and cells were incubated for 16 hours before development. The number of spots was counted using an automated AID ELISPOT reader (Autoimmun Diagnostika, Strasberg, Germany). The number of spots counted in control wells (no peptide) was subtracted from numbers counted in experimental wells.
Cytotoxicity assay.
Cells from the HLA-A2+ JY lymphoblastoid line were used as target cells in a fresh ex vivo chromium-release assay. JY cells were labeled with 51Cr for one hour, washed, pulsed for one hour with the HCMV pp65495-503 peptide, washed, and aliquoted in microtiter plates (5,000 cells/well). Unpulsed JY cells were used as negative control. CD8+ T cells were positively selected from cord blood mononuclear cells using anti-CD8 MACS beads and added to the target cells in triplicates. A HCMV pp65495-503 peptide-specific CD8+ T cell clone cultured from HLA-A2 HCMV-seropositive donor PBMCs was used as a positive control. Inhibition of perforin-mediated cytotoxicity was obtained by incubating the CD8+ T cells for two hours with 100 nM concanamycin A (CMA; Sigma-Aldrich). The 51Cr release was calculated from the following equation: ([experimental release – spontaneous release]/[maximum release – spontaneous release]) × 100%. Nonspecific killing of unpulsed target cells (2%) was subtracted from that of pulsed target cells.
Statistical analysis.
Expression of activation and differentiation markers by CD8+ T cells in cases and controls were compared using the t test after log transformation. Statistical significance was assessed at the two-sided 0.05 level. Statistical analysis was done using the Stata software (version 6; Stata Corp., College Station, Texas, USA).
Results
HCMV infection in fetal life induces the oligoclonal expansion of differentiated CD8+ T lymphocytes.
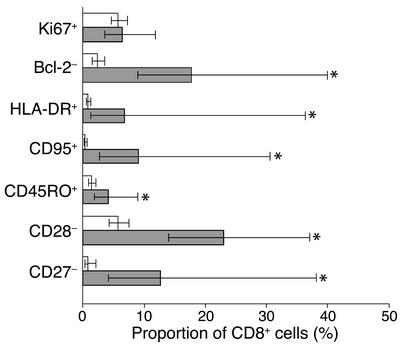
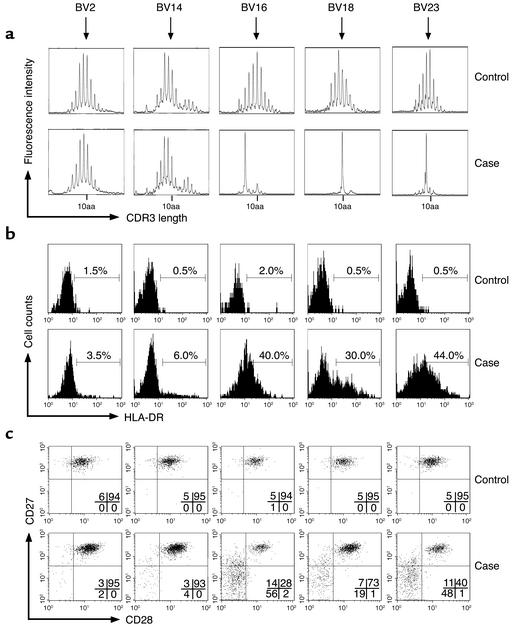
The response of CD8+ T lymphocytes to HCMV infection during fetal life was analyzed in a group of eight newborns diagnosed by screening a cohort of 250 Gambian newborns. HCMV-infected newborns showed high proportions of activated CD8+ T cells, expressing increased levels of HLA-DR and CD95 and decreased levels of Bcl-2, compared with uninfected control newborns (Figure 1). The proportion of CD8+ T cells that were undergoing cell division, as indicated by the expression of high levels of Ki67, were similar in control and infected newborns. HCMV-infected newborns displayed increased proportions of differentiated CD8+ T cells, expressing CD45RO and low levels of CD28 and CD27 (Figure 1). During an antigen-specific response, the expansion of T cell clones can be detected by the analysis of the size of the CDR3 region of the TCR BV chain within a given T cell population. Using the spectratyping method, we studied the influence of HCMV infection during fetal life on the repertoire of CD8+ T cells. Control newborn CD8+ T cells showed a naive repertoire characterized by a Gaussian distribution of TCR CDR3 lengths within all BV families (Figure 2a), as previously described (36). In contrast, newborns with congenital HCMV infection had marked alterations in the distribution of CDR3 lengths of several BV families. In case no. 7, important oligoclonal expansions of CD8+ T cells expressing BV16, BV18, and BV23 were observed (Figure 2a). More discrete alterations of BV6, BV13, and BV24 were detected (data not shown). Spectratypic analysis was also performed on purified CD8+ T cells from case no. 5 and on total PBMCs from case no. 2. Alterations in CDR3 length distribution were found within six BV families in case no. 2 and in 15 BV families in case no. 5 (data not shown). The BV families and the CDR3 lengths involved were different in the three cases analyzed. These alterations of the repertoire within the CD8+ T cell population indicate that HCMV infection in fetal life triggers the expansion of large populations of CD8+ T cells expressing various TCR BV. The expression of markers of activation/differentiation was correlated with the expansion of CD8+ T cells. High proportions of CD8+ T cells from case no. 7 showing oligoclonal expansion by spectratyping (BV16, BV18, and BV23) were HLA-DR+, CD28–, and CD27–, whereas most cells expressing BV2 and BV14 expressed a naive phenotype (HLADR–, CD28+, CD27+) similar to that of control newborns (Figure 2, b and c).
Figure 1.
Phenotype of total CD8+ T cells from control and HCMV-infected newborns. Expression of markers of activation (Ki67, Bcl-2, HLA-DR, CD95) and differentiation (CD45RO, CD28, CD27) by CD8+ T cells of ten controls (white bars) and eight cases (gray bars). Data presented are geometric means and 95% confidence interval. *P < 0.05.
Figure 2.
TCR BV spectratypic analysis of CD8+ T lymphocytes and phenotypic analysis of BV-specific CD8+ T cells from control and HCMV-infected newborns. (a) Spectratypic analysis. CDR3 length distribution was Gaussian for all BV families of control newborn no. 9 (shows five representative families), indicating a naive CD8+ T cell repertoire. Similar results were obtained in control newborns no. 8 and 12. In contrast, alterations in CDR3 length distribution were detected in BV16, BV18, and BV23 of case no. 7, indicating oligoclonal expansions of CD8+ T cells. (b and c) Phenotypic analysis. Expression of HLA-DR, CD27, and CD28 by BV2, BV14, BV16, BV18, and BV23 CD8+ T cells of control no. 9 and case no. 7. High proportions of CD8+ T lymphocytes from HCMV-infected newborns had a phenotype of activated (b) and differentiated (c) cells. These alterations were primarily observed in the BV families that included oligoclonal expansions in the spectratypic analysis (BV16, BV18, and BV23).
Newborn HCMV-specific CD8+ T cells have a late differentiation phenotype.
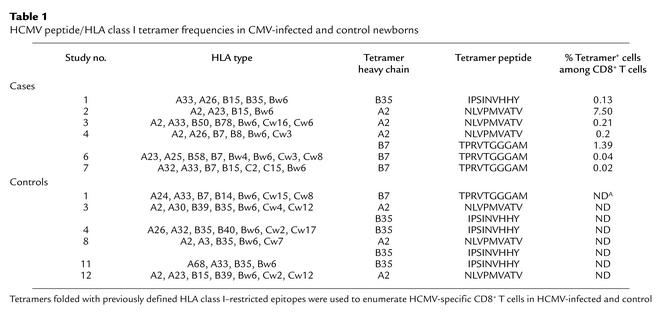
The above results strongly suggest that in utero HCMV infection triggers the activation and the differentiation of antigen-specific CD8+ T cells. This was investigated using tetramers refolded with immunodominant HCMV peptides. Among the eight infected newborns, six expressed HLA alleles (A2, B7, or B35) allowing us to perform tetramer analysis (Table 1). Four out of the six cases had detectable ex vivo frequencies of HCMV–tetramer-positive CD8+ T cells, and high frequencies (1.39 and 7.5%) were detected in two of them. All controls had frequencies below 0.01%. The peptide specificity of the tetramer staining was proved by the absence of binding of an HLA-A2 tetramer containing a BMLF1 EBV peptide (GLCTLVAML) by CD8+ T cells from the HLA-A2+ cases (data not shown).
Table 1.
HCMV peptide/HLA class I tetramer frequencies in CMV-infected and control newborns
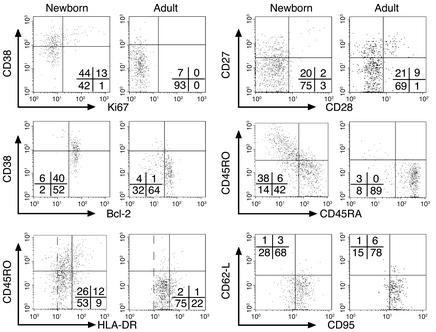
Newborn HCMV-specific CD8+ T cells expressed increased levels of HLA-DR and CD95 and displayed low levels of the lymph node homing receptor CD62-L (Figure 3), in keeping with the fact that they were antigen experienced. The majority of these cells displayed a resting phenotype characterized by the expression of relatively low levels of the activation markers CD38 and Ki67, as well as high levels of Bcl-2. This phenotype was similar to that observed in immune adults and indicated that newborn HCMV-specific CD8+ T cells had already gone beyond the acute stage of activation and acquired a phenotype resembling that of memory cells (23). This concept was further supported by the pattern of expression of markers of differentiation because, as observed in immune adults, the majority of newborn cells displayed low levels of CD28 and CD27 and a large proportion of them expressed the marker CD45RA. In adults, HCMV-specific memory cells revert from CD45RO+ to CD45RA+ during the chronic phase of the infection (26). Taken together, these data indicate that following in utero HCMV infection newborn CD8+ T cells are able to expand and acquire a late stage of differentiation.
Figure 3.
Phenotype of HCMV-specific CD8+ T cells from HCMV-infected newborns and immune adults. Expression of markers of activation (Ki67, Bcl-2, CD38, HLA-DR, CD62-L, CD95) and differentiation (CD45RA, CD45RO, CD28, CD27) by HCMV-specific CD8+ T cells (gated using the relevant tetramers) of case no. 2 and a representative adult. Three levels of HLA-DR expression are shown: negative, low, and high. HCMV-specific CD8+ T cells from infected newborns had a late differentiation phenotype similar to that of adults. Similar results were obtained in cases no. 1, 3, and 4, and in four other immune adults.
HCMV-specific CD8+ T cells are functionally active in newborns, display perforin-dependent cytotoxicity, and produce antiviral cytokines.
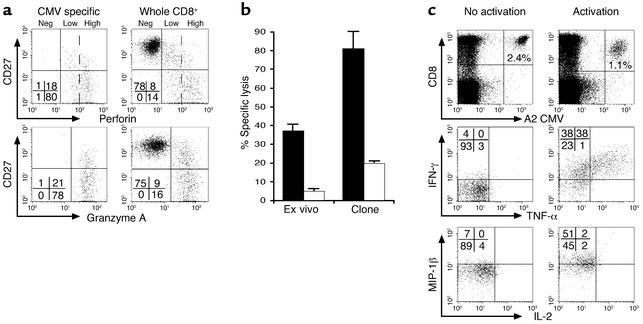
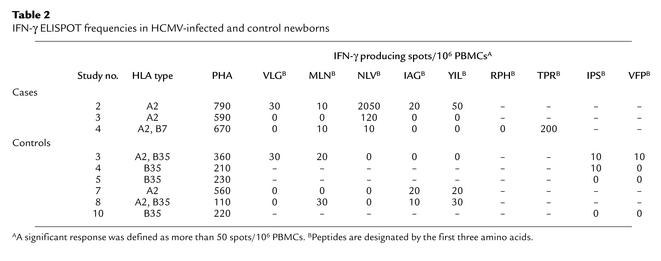
To evaluate the effector function of newborn HCMV-specific cells, the cytolytic capacity of newborn cells was first analyzed by ex vivo intracellular staining of perforin and granzyme A without in vitro stimulation. In keeping with the late differentiation phenotype, granzyme A and high levels of perforin were detected in HCMV-specific cells as well as in a large proportion of total CD8+ T cells (Figure 4a). The expression of high levels of perforin was associated with a potent cytolytic activity of newborn cells: in an ex vivo killing assay, purified CD8+ T cells, including 7.5% of tetramer-positive cells, performed significant specific lysis of peptide-pulsed target cells (Figure 4b). This cytolytic activity was dependent on perforin because it was inhibited by preincubation of CD8+ T cells with CMA. This potent cytolytic activity was associated with the production of antiviral cytokines. Cytokine production by tetramer-positive cells was analyzed following short-term stimulation with relevant peptide. The majority of cells produced IFN-γ and MIP-1β, and a high proportion of them also produced TNF-α (Figure 4c). In contrast, no production of IL-2 was detected, as described previously in HCMV-immune adults (39). ELISPOT analysis confirmed the capacity of newborn cells to produce IFN-γ in response to the peptides used for the tetramer analysis (Table 2).
Figure 4.
Cytolytic activity and antiviral cytokine production by HCMV-specific CD8+ T cells in HCMV-infected newborns. (a) Expression of perforin and granzyme A by total and HCMV-specific CD8+ T cells from case 2. HCMV-specific CD8+ T cells expressed high levels of perforin and granzyme A. Three levels of perforin expression are shown: negative, low, and high. Similar results were obtained in cases no. 1, 3, and 4. (b) Ex vivo cytolytic activity of HCMV tetramer+ CD8+ T cells from case no. 2. Purified CD8+ T lymphocytes including 7.5% of pp65495-503 peptide-specific cells were incubated with peptide-loaded JY cells at 5:1 tetramer positive cell/target ratio (black bars). An HLA-A2-restricted pp65495-503 peptide-specific clone was used as a positive control at a similar effector/target ratio. HCMV-specific CD8+ T cells had potent cytolytic activity that was dependent on perforin, as demonstrated by inhibition with CMA (white bars). (c) HLA-A2/pp65495-503 tetramer (A2 CMV)+ CD8+ T cells from case no. 2 produce IFN-γ, TNF-α, and MIP-1β, but no IL-2 following short-term peptide restimulation.
Table 2.
IFN-γ ELISPOT frequencies in HCMV-infected and control newborns
Activation and expansion of HCMV-specific CD8+ T cells during fetal life.
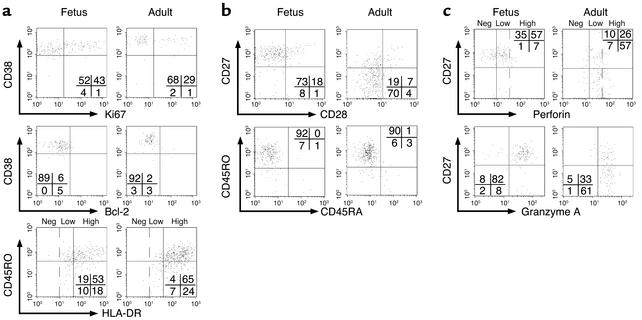
The expansions of mature and functional HCMV-specific CD8+ T cells described above were detected at birth, and it is unclear how early during fetal life they could have developed. To address this question, we studied a 28-week-old fetus with symptomatic HCMV infection at the time of medically assisted abortion. Because little was known about the phenotype of HCMV-specific cells during the acute phase of the infection in adults, we studied an unrelated pregnant woman with primary HCMV infection and compared her response to that of the infected fetus. Two percent of fetal CD8+ T cells and 5% of maternal cells were tetramer positive (data not shown). Fetal cells displayed a phenotype of acute activation with high levels of HLA-DR, a large proportion of cells expressing high levels of Ki67 and low levels of Bcl-2 (Figure 5a), contrasting with the resting population observed in newborns (Figure 3). Adult cells displayed a similar phenotype; the expression of high levels of CD38 further supported their state of acute activation. The acute activation phenotype of fetal cells was associated with an earlier stage of differentiation, with most cells expressing low levels of CD28 but still expressing CD27 and CD45RO (Figure 5b). Adult cells were at a more advanced stage of differentiation because they had already downregulated CD27. The majority of fetal and adult cells were perforinlow and expressed high levels of granzyme A (Figure 5c). The detection of lower levels of perforin, as compared with newborn T cells, is compatible with an earlier stage of differentiation (23).
Figure 5.
Phenotype of HCMV-specific CD8+ T cells in a fetus and an adult with acute HCMV infection. Expression of markers of (a) activation (Ki67, Bcl-2, CD38, HLA-DR), (b) differentiation (CD45RA, CD45RO, CD28, CD27), and (c) cytolytic activity (perforin, granzyme A) by HCMV-specific CD8+ T cells from a 28-week-old fetus and from a pregnant women (adult) with acute HCMV infection. Three levels of HLA-DR and perforin expression are shown: negative (neg), low, and high. HCMV-specific CD8+ T cells from the fetus had an intermediate differentiation phenotype.
Discussion
In this study we demonstrate that human fetal CD8+ T lymphocytes can expand, differentiate, and acquire effector functions during a viral infection and that this response has very similar characteristics to that of adults. The earliest response we could measure was at 28 weeks of gestation, but mature responses may be able to develop even earlier during gestation. In addition to the information regarding the priming of CD8+ T cell responses during fetal life, our study provides insights in the CD8+ T cell response to HCMV more generally. Due to the largely asymptomatic nature of HCMV infection in adults, relatively little is known about the primary immune response to this virus. Data we obtained in the 28-week-old fetus and in the pregnant women indicate that during the acute phase of HCMV infection, CD8+ T cells have a phenotype rather similar to that induced by other persistent viruses, including HIV, EBV, and HCV, which is characterized by a significant proportion of highly activated cells in cycle, the downregulation of Bcl-2, some downregulation of CD28, and the expression of CD45RO (23). Later, during the course of the infection, HCMV-specific fetal CD8+ T cells acquire a late differentiation phenotype similar to that observed in adults, which is characterized by the upregulation of Bcl-2, the downregulation of CD27, the re-expression of CD45RA, and the expression of high levels of perforin and granzymes (22–27). These differentiated fetal CD8+ T cells have potent perforin-dependent cytolytic activity and produce antiviral cytokines. Similar observations were recently made by Gamadia et al. in adult kidney-transplanted patients with primary HCMV infection (40).
The mature CD8+ T cell response to HCMV in early life contrasts with the low response induced by HIV in young infants (4, 11–13). This difference is reminiscent of the situation in adults where HCMV induces a more advanced stage of CD8+ T cell differentiation than HIV (23, 24). HCMV could be particularly efficient at promoting the activation and differentiation of CD8+ T cells, either directly or through its interaction with DCs or CD4+ T cells. Data reported recently by Hermann et al. in newborns with congenital Trypanosoma cruzi infection indicate that HCMV is not the only pathogen stimulating a potent CD8+ T lymphocyte response during fetal life (41). The understanding of the mechanisms involved would help in the design of vaccines protecting newborns against viral infections. T lymphocytes differentiate early during human embryogenesis. By 14 weeks of gestation, single positive thymocytes can be detected in the thymus, and CD8+ and CD4+ T lymphocytes emigrate in the fetal liver and spleen (6). Functional studies using cord blood lymphocytes stimulated with polyclonal activators or allogeneic cells showed reduced cytolytic activity (6). These results suggest that CD8+ T lymphocytes are immature at birth. This apparent immaturity, however, may be at least partly related to the absence of memory and effector cells in the neonatal CD8+ T cell pool. Whether a similar deficiency would characterize antigen-specific responses is currently unknown. We observed recently that neonatal CD8+ T cells specific for a melan-A peptide can be primed by cord blood DCs and can acquire effector functions similar to those of adult cells (ref. 42; and Salio et al., manuscript submitted for publication). These in vitro results indicate that neonatal CD8+ T lymphocytes and DCs can acquire mature effector functions, in keeping with our in vivo observation of newborns with congenital HCMV infection.
The newborns enrolled in this study were asymptomatic. It is likely that the mature CD8+ T cell response we detected participated in the control of HCMV replication, although other cell types such as NK cells were probably also involved (43). The fetus we studied had severe pathology despite a potent CD8+ T cell response. Further studies of a larger number of cases are required to understand the role of CD8+ T lymphocytes in the pathogenesis of symptomatic congenital HCMV infection and their relation to viral load. An important characteristic of congenital HCMV infection is the persistent excretion of the virus. This may represent an ideal equilibrium between the pathogen and the host, allowing the host to survive while promoting transmission of the pathogen in the community. In the mouse, systemic control of MCMV replication is dependent on CD8+ T cells, whereas viral replication in salivary glands is under the control of CD4+ T lymphocytes (44). Epidemiological data indicate that termination of HCMV excretion in children is associated with an increased proliferative response to the virus (17). Studies are required to characterize the response of CD4+ T cells to fetal HCMV infection.
We conclude that the development of a mature and fully functional CD8+ T lymphocyte response to HCMV in utero suggests that the machinery required to prime such responses is in place early in life and could be used to immunize newborns against viral infections.
Acknowledgments
We are grateful to Tim Rostron, Assan Jaye, and Louis-Marie Yindom for HLA typing; Pauline Kaye for data management; Kati Di Gleria for peptide synthesis; Momodou Jobe for technical assistance; Omar Badjie, Saihou Bob, Janko Camara, Isatou Drammeh, Sarjo Sanneh, Mamadi Sidibeh, and Ebrima Touray for help in field work; and Tao Dong for providing the HCMV-specific T cell clone. This work was funded by the Medical Research Council United Kingdom, Cancer Research United Kingdom, and GlaxoSmithKline Biologicals, Rixensart, Belgium.
Footnotes
See the related Commentary beginning on page 1645.
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: human cytomegalovirus (HCMV); respiratory syncytial virus (RSV); Epstein-Barr virus (EBV); hepatitis C virus (HCV); allophycocyanin (APC); phycoerythrin (PE); T cell receptor (TCR); enzyme-linked immunospot assay (ELISPOT); phytohemagglutinin (PHA); concanamycin A (CMA).
References
- 1.Stagno, S. 2001. Cytomegalovirus. In Infectious Diseases of the fetus and newborn infant. J.S. Remington and J.O. Klein, editors. W.B. Saunders Company. Philadelphia, Pennsylvania, USA. 389–424.
- 2.Arvin, A., and Whitley, R.J. 2001. Herpes simplex virus infections. In Infectious Diseases of the fetus and newborn infant. J.S. Remington and J.O. Klein, editors. W.B. Saunders Company. Philadelphia, Pennsylvania, USA. 425–446.
- 3.Hall CB. Respiratory syncytial virus and parainfluenza virus. N. Engl. J. Med. 2001;344:1917–1928. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- 4.Goulder PJ, Jeena P, Tudor-Williams G, Burchett S. Paediatric HIV infection: correlates of protective immunity and global perspectives in prevention and management. Br. Med. Bull. 2001;58:89–108. doi: 10.1093/bmb/58.1.89. [DOI] [PubMed] [Google Scholar]
- 5.Mahoney, F.J., and Kane, M. 1999. Hepatitis B vaccine. In Vaccines. S.A. Plotkin and W.A. Orenstein, editors. W.B. Saunders Company. Philadelphia, Pennsylvania, USA. 158–182.
- 6.Lewis, D.B., and Wilson, C.B. 2001. Developmental immunology and role of host defenses in fetal and neonatal susceptibility to infection. In Infectious diseases of the fetus and newborn infant. J.S. Remington and J.O. Klein, editors. W. B. Saunders Company. Philadelphia, Pennsylvania, USA. 25–138.
- 7.Siegrist CA. Neonatal and early life vaccinology. Vaccine. 2001;14:3331–3346. doi: 10.1016/s0264-410x(01)00028-7. [DOI] [PubMed] [Google Scholar]
- 8.Adkins B. T-cell function in newborn mice and humans. Immunol. Today. 1999;220:330–335. doi: 10.1016/s0167-5699(99)01473-5. [DOI] [PubMed] [Google Scholar]
- 9.Guidotti LG, Chisari FV. Noncytolytic control of viral infections by the innate and adaptive immune responses. Annu. Rev. Immunol. 2001;19:65–91. doi: 10.1146/annurev.immunol.19.1.65. [DOI] [PubMed] [Google Scholar]
- 10.Barouch DH, Letvin NL. CD8+ cytotoxic T lymphocyte responses to lentiviruses and herpesviruses. Curr. Opin. Immunol. 2001;13:479–482. doi: 10.1016/s0952-7915(00)00244-2. [DOI] [PubMed] [Google Scholar]
- 11.Luzuriaga K, et al. HIV-1-specific cytotoxic T lymphocyte responses in the first year of life. J. Immunol. 1995;154:433–443. [PubMed] [Google Scholar]
- 12.Scott ZA, et al. Infrequent detection of HIV-1-specific, but not cytomegalovirus-specific, CD8(+) T cell responses in young HIV-1-infected infants. J. Immunol. 2001;167:7134–7140. doi: 10.4049/jimmunol.167.12.7134. [DOI] [PubMed] [Google Scholar]
- 13.Wasik TJ, et al. Diminished HIV-specific CTL activity is associated with lower type 1 and enhanced type 2 responses to HIV-specific peptides during perinatal HIV infection. J. Immunol. 1997;158:6029–6036. [PubMed] [Google Scholar]
- 14.Issacs D, Bangham CRM, McMichael AJ. Cell-mediated cytotoxic response to respiratory syncytial virus in infants with bronchiolitis. Lancet. 1987;2:769–771. doi: 10.1016/s0140-6736(87)92502-5. [DOI] [PubMed] [Google Scholar]
- 15.Mbawuike IN, et al. HLA-restricted CD8+ cytotoxic T lymphocyte, interferon-γ, and interleukin-4 responses to respiratory syncytial virus infection in infants and children. J. Infect. Dis. 2001;183:687–696. doi: 10.1086/318815. [DOI] [PubMed] [Google Scholar]
- 16.Gehrz RC, Marker SC, Knorr SO, Kalis JM, Balfour HH. Specific cell-mediated immune defect in active cytomegalovirus infection of young children and their mothers. Lancet. 1977;2:844–847. doi: 10.1016/s0140-6736(77)90782-6. [DOI] [PubMed] [Google Scholar]
- 17.Pass RF, Stagno S, Britt WJ, Alford CA. Specific cell-mediated immunity and the natural history of congenital infection with cytomegalovirus. J. Infect. Dis. 1983;148:953–961. doi: 10.1093/infdis/148.6.953. [DOI] [PubMed] [Google Scholar]
- 18.Taylor-Wiedeman J, Sissons JG, Borysiewicz LK, Sinclair JH. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J. Gen. Virol. 1991;72:2059–2064. doi: 10.1099/0022-1317-72-9-2059. [DOI] [PubMed] [Google Scholar]
- 19.Wills MR, et al. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J. Virol. 1996;70:7569–7579. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weekes MP, Wills MR, Mynard K, Carmichael AJ, Sissons JG. The memory cytotoxic T-lymphocyte (CTL) response to human cytomegalovirus infection contains individual peptide-specific CTL clones that have undergone extensive expansion in vivo. J. Virol. 1999;73:2099–2108. doi: 10.1128/jvi.73.3.2099-2108.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddehase MJ. The immunogenicity of human and murine cytomegaloviruses. Curr. Opin. Immunol. 2000;12:390–396. doi: 10.1016/s0952-7915(00)00106-0. [DOI] [PubMed] [Google Scholar]
- 22.Khan N, et al. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J. Immunol. 2002;169:1984–1992. doi: 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- 23.Appay V, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 24.Champagne P, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–111. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 25.Gillespie GM, et al. Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8(+) T lymphocytes in healthy seropositive donors. J. Virol. 2000;74:8140–8150. doi: 10.1128/jvi.74.17.8140-8150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wills MR, et al. Identification of naive or antigen-experienced human CD8(+) T cells by expression of costimulation and chemokine receptors: analysis of the human cytomegalovirus-specific CD8(+) T cell response. J. Immunol. 2002;168:5455–5464. doi: 10.4049/jimmunol.168.11.5455. [DOI] [PubMed] [Google Scholar]
- 27.Tomiyama H, Matsuda T, Takigushi M. Differentiation of human CD8(+) T cells from a memory to memory/effector phenotype. J. Immunol. 2002;168:5538–5550. doi: 10.4049/jimmunol.168.11.5538. [DOI] [PubMed] [Google Scholar]
- 28.Bunce M, et al. Phototyping: comprehensive DNA typing for HLA-A, B, C, DRB1, DRB3, DRB4, DRB5 and DQB1 by PCR with 144 primer mixes utilizing sequence-specific primers (PCR-SSP) Tissue Antigens. 1995;46:355–367. doi: 10.1111/j.1399-0039.1995.tb03127.x. [DOI] [PubMed] [Google Scholar]
- 29.Preiser W, et al. Evaluation of diagnostic methods for the detection of cytomegalovirus in recipients of allogeneic stem cell transplants. J. Clin. Virol. 2001;20:59–70. doi: 10.1016/s1386-6532(00)00156-6. [DOI] [PubMed] [Google Scholar]
- 30.Posnett DN, Romagné F, Necker A, Kotzin BL, Sekaly RP. First human TCR monoclonal antibody workshop. The Immunologist. 1996;4:5–9. [Google Scholar]
- 31.Solache A, et al. Identification of three HLA-A*0201-restricted cytotoxic T cell epitopes in the cytomegalovirus protein pp65 that are conserved between eight strains of the virus. J. Immunol. 1999;163:5512–5518. [PubMed] [Google Scholar]
- 32.Kern F, et al. Target structures of the CD8(+)-T-cell response to human cytomegalovirus: the 72-kilodalton major immediate-early protein revisited. J. Virol. 1999;73:8179–8184. doi: 10.1128/jvi.73.10.8179-8184.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gavin MA, Gilbert MJ, Riddell SR, Greenberg PD, Bevan MJ. Alkali hydrolysis of recombinant proteins allows for the rapid identification of class I MHC-restricted CTL epitopes. J. Immunol. 1993;151:3971–3980. [PubMed] [Google Scholar]
- 34.Retiere C, et al. Generation of cytomegalovirus-specific human T-lymphocyte clones by using autologous B-lymphoblastoid cells with stable expression of pp65 or IE1 proteins: a tool to study the fine specificity of the antiviral response. J. Virol. 2000;74:3948–3952. doi: 10.1128/jvi.74.9.3948-3952.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Utz U, Koenig S, Coligan JE, Biddison WE. Presentation of three different viral peptides, HTLV-1 Tax, HCMV gB, and influenza virus M1, is determined by common structural features of the HLA-A2.1 molecule. J. Immunol. 1992;149:214–221. [PubMed] [Google Scholar]
- 36.Garderet L, et al. The umbilical cord blood alphabeta T-cell repertoire: characteristics of polyclonal and naive but completely formed repertoire. Blood. 1998;91:340–346. [PubMed] [Google Scholar]
- 37.Even J, et al. T-cell repertoires in healthy and diseased human tissues analysed by T-cell receptor beta-chain CDR3 size determination: evidence for oligoclonal expansions in tumours and inflammatory diseases. Res. Immunol. 1995;146:65–80. doi: 10.1016/0923-2494(96)80240-9. [DOI] [PubMed] [Google Scholar]
- 38.Appay V, et al. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 2000;192:63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandberg JK, Fast NM, Nixon DF. Functional heterogeneity of cytokines and cytolytic effector molecules in human CD8+ T lymphocytes. J. Immunol. 2001;167:181–187. doi: 10.4049/jimmunol.167.1.181. [DOI] [PubMed] [Google Scholar]
- 40.Gamadia LE, et al. Primary immune response to human cytomegalovirus. A critical role for IFN-γ producing CD4+ T cells in protection against CMV disease. Blood. 2002;101:2686–2692. doi: 10.1182/blood-2002-08-2502. [DOI] [PubMed] [Google Scholar]
- 41.Hermann E, et al. Human fetuses are able to mount an adultlike CD8 T-cell response. Blood. 2002;100:2153–2158. [PubMed] [Google Scholar]
- 42.Salio M, et al. Mature dendritic cells prime functionally superior melan-A-specific CD8+ lymphocytes as compared with nonprofessional APC. J. Immunol. 2001;167:1188–1197. doi: 10.4049/jimmunol.167.3.1188. [DOI] [PubMed] [Google Scholar]
- 43.Krmpotic A, et al. MCMV glycoprotein gp40 confers virus resistance to CD8+ T cells and NK cells in vivo. Nat. Immunol. 2002;3:529–535. doi: 10.1038/ni799. [DOI] [PubMed] [Google Scholar]
- 44.Jonjic S, Mutter W, Weiland F, Reddehase MJ, Koszinowski UH. Site-restricted persistent cytomegalovirus infection after selective long-term depletion of CD4+ T lymphocytes. J. Exp. Med. 1989;169:1199–1212. doi: 10.1084/jem.169.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]