Abstract
SR proteins constitute a family of splicing factors that play key roles in both constitutive and regulated splicing in metazoan organisms. The proteins are extensively phosphorylated, and kinases capable of phosphorylating them have been identified. However, little is known about how these kinases function, for example, whether they target specific SR proteins or whether the kinases themselves are regulated. Here we describe properties of one such kinase, Clk/Sty, the founding member of the Clk/Sty family of dual-specificity kinases. Clk/Sty is autophosphorylated on both Ser/Thr and Thr residues, and using both direct kinase assays and SR protein-dependent splicing assays, we have analyzed the effects of each type of modification. We find not only that the pattern of phosphorylation on a specific SR protein substrate, ASF/SF2, is modulated by autophosphorylation but also that the ability of Clk/Sty to recognize different SR proteins is influenced by the extent and nature of autophosphorylation. Strikingly, phosphorylation of ASF/SF2 is sensitive to changes in Tyr, but not Ser/Thr, autophosphorylation while that of SC35 displays the opposite pattern. In contrast, phosphorylation of a third SR protein, SRp40, is unaffected by autophosphorylation. We also present biochemical data indicating that as expected for a factor directly involved in splicing control (but in contrast to recent reports), Clk/Sty is found in the nucleus of several different cell types.
Protein phosphorylation and dephosphorylation are required for splicing of pre-mRNA precursors, and a number of proteins are known to undergo phosphorylation and dephosphorylation during the splicing cycle (29, 34, 37). Among these, the SR proteins constitute a major class of proteins that appear to be modified extensively. SR proteins, a family of non-snRNP pre-mRNA splicing factors containing one or two N-terminal RNP-type RNA-binding domains and a C-terminal RS domain, are extensively phosphorylated (14, 17, 55). RS domains consist of multiple consecutive RS/SR dipeptide repeats and differ in length among different SR proteins. Extensive phosphorylation of serines in the RS domain occurs in all SR proteins. This functions both to prevent nonspecific protein-RNA interactions and to modulate protein-protein interactions (3, 54, 62, 63). Phosphorylation must be precisely modulated, as both hyper- and hypophosphorylation have been shown to reduce the overall activity of SR proteins in functional assays (47).
Regulation of SR protein phosphorylation is achieved by a combination of protein phosphatases and kinases (37). Circumstantial evidence suggesting a role for phosphatases was obtained first by using thiophosphorylated proteins and specific phosphatase inhibitors, which were shown to inhibit splicing in nuclear extracts (3, 36, 56, 63). More recently, direct evidence that protein phosphatase 2C-γ is required during early stages of splicing, i.e., formation of spliceosomes, was presented (42). However, the identities of splicing-related target proteins of phosphatase 2C-γ, or any phosphatase, are presently unknown.
Considerably more is known about the protein kinases involved in splicing. For example, a number of kinases have been shown to phosphorylate the RS domains of SR proteins. Among these, the SRPK kinase family phosphorylates serines in the RS domain and they appear to have a strict requirement for an RSR motif (18, 52, 58). However, all SRPK members are predominantly localized to the cytoplasm during interphase (58), suggesting that they control SR protein function in splicing indirectly, for example, by influencing intracellular localization (28, 30, 31). The reported higher activity of SRPK1 (three- to fivefold) during mitosis suggests that its activity is cell cycle dependent, and a partially purified fraction from mitotic cells was shown to hyperphosphorylate SR proteins (18).
The Clk family consists of at least four kinases that are implicated in splicing control. Clk/Sty, the founding member, and Clk2, -3, and -4 can interact with and phosphorylate SR proteins (5, 20, 43). These kinases all display so-called dual specificity, i.e., they are capable of phosphorylating both Ser/Thr and Thr residues (1, 22, 32). Intriguingly, they all contain an N-terminal region enriched in RS dipeptides, as well as a C-terminal kinase domain, with differences between family members primarily lying at the N terminus; the C terminus is highly conserved among all of the members of this family (20, 43). Unlike SR protein RS domains, the Clk RS-rich domains contain a significant number of Arg and Ser amino acids interspersed with a few RS dipeptides as well as Thr. Cells grown in the presence of tyrosine phosphatase inhibitors showed enhanced tyrosine phosphorylation of Clk/Sty, suggesting that Thr autophosphorylation levels are dynamic (11, 13, 43). In vitro phosphorylation assays comparing Clk and SRPK suggest that both kinase families have related but distinct substrate specificities (6).
A number of studies have shown that Clk kinases can modulate the localization and function of SR proteins in the nucleus. Transient overexpression of Clk/Sty disrupts the nuclear speckled pattern of SR proteins and modulates splicing of a cotransfected reporter gene (5, 12). Moreover, Clk/Sty overexpression was also shown to cause loss of structural integrity of interchromatin granule clusters and redistribution of all proteins tested to a diffuse nuclear localization, suggesting an underlying phosphorylation-dependent mechanism in the movement of proteins from nuclear speckles (48). On the other hand, overexpression of a kinase-inactive mutant (ClkR) had a dominant-negative effect, as SR proteins were retained in speckles, probably by interfering with phosphorylation (5, 43, 48). These findings suggest that release of SR proteins from speckles is modulated by phosphorylation and protein-protein interactions (38, 39, 48). Significantly, Doa, the apparent Drosophila homologue of Clk/Sty, is present primarily in the nucleus during development and was shown to modulate splicing of at least some pre-mRNAs by altering the phosphorylation status of SR proteins (10, 64).
The above-cited studies indicate that Clk kinases can influence the subnuclear localization and in vivo activity of SR proteins. We previously showed that SR proteins have reduced activity following hyper- or hypophosphorylation induced by Clk/Sty or ClkR, respectively, in both constitutive and alternative splicing assays (47). Unphosphorylated but not phosphorylated SR proteins were inhibited or were inactive in splicing in the presence of ClkR, and analysis of the proteins' phosphorylation status demonstrated a lower level of phosphorylation. On the other hand, Clk/Sty inhibited splicing by inducing hyperphosphorylation of SR proteins. Together, these results indicate both that Clk/Sty can directly and specifically target SR proteins and that an optimal level of phosphorylation is required for splicing activity (47).
Most studies on SR protein kinases have analyzed the effects of phosphorylation on subcellular localization and on splicing activity, both of which provide a measure of the effect on SR protein function (34, 37). However, questions regarding regulation of SR protein kinase activity, for example by autophosphorylation, in targeting specific SR proteins and/or affecting their activity remain to be addressed. To elucidate possible regulatory mechanisms, we have analyzed the effects of both Ser/Thr and Thr autophosphorylation on Clk/Sty kinase activity and specificity with respect to different SR protein targets. We show that Clk/Sty activity is controlled by autophosphorylation on both Ser/Thr and Thr residues. Not only is the pattern of phosphorylation on a specific SR protein substrate, ASF/SF2, modulated by autophosphorylation, but the ability of the kinase to recognize different SR proteins is also influenced by the extent and nature of Clk/Sty autophosphorylation. Phosphorylation of ASF/SF2 is sensitive to changes in Tyr, but not Ser/Thr, autophosphorylation, while SC35 displays the opposite pattern. SRp40 phosphorylation, on the other hand, is unaffected by Clk/Sty autophosphorylation. We also present biochemical data indicating that Clk/Sty, as expected for a factor directly involved in splicing control, is indeed found in the nucleus of several different cell types. Our results extend the possible mechanisms by which SR proteins, and therefore pre-mRNA splicing, can be controlled.
MATERIALS AND METHODS
Expression and purification of recombinant proteins.
Glutathione transferase (GST)-Clk/Sty (P-Clk/Sty) was purified from Escherichia coli as described previously (47). Tyr-dephosphorylated Clk/Sty (PTP-Clk/Sty) and unphosphorylated Clk/Sty (CIP-Clk/Sty) were prepared by adding 200 U of protein tyrosine phosphatase (PTP) (Boehringer Mannheim) and 40 U of calf intestinal alkaline phosphatase (CIP) (New England Biolabs), respectively, to 400 μg of P-Clk/Sty bound to glutathione beads, and dephosphorylation was carried out according to the manufacturers' instructions. The resin was washed, and proteins were eluted and dialyzed against buffer D (20 mM HEPES [pH 7.9], 100 mM KCl, 0.2 mM EDTA, 20% glycerol, 0.5 mM phenylmethylsulfonyl fluoride). For purification of PY- and PS-Clk/Sty, an antiphosphotyrosine column was prepared by cross-linking anti-phosphotyrosine antibody (Molecular Probes, Eugene, Oreg.) to protein A-agarose, as described previously (21). Purified P-Clk/Sty (500 μg) was added to 0.5 ml of the resin, and the slurry was rocked at 4°C for 1 h. Proteins were eluted with 0.5 ml of 20 mM Tris-Cl (pH 7.9)-150 mM NaCl-1 mM EDTA-60 mM phenyl phosphate. A total of 300 μg of eluted and 150 μg of flowthrough proteins (which essentially contained only tyrosine- and/or Ser/Thr-phosphorylated P-Clk/Sty [designated PY-Clk/Sty and PS-Clk/Sty, respectively]) were obtained and dialyzed against buffer D.
His-tagged ASF/SF2 and SC35 were purified from Sf9 cells infected with recombinant baculovirus essentially as described previously (47). To purify His-tagged SRp40, an NdeI-BamHI fragment from pET-14b (53) was blunt ended and cloned into the BamHI site (blunt ended) of pFASTBAC (Invitrogen). Recombinant baculoviruses were generated, and His-tagged SRp40 was purified from infected Sf9 cells according to the manufacturer's instructions. Proteins were dialyzed against buffer D. Recombinant His-tagged ASF/SF2 and SRp40 from E. coli were purified as described previously (16, 53).
Protein concentrations and purity were determined visually using Coomassie blue- and silver-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), with bovine serum albumin as the standard.
Antibodies.
Antibodies against Clk/Sty were generated in rabbits using the peptide CRYDHSKTTDSYYL conjugated to keyhole limpet hemocyanin as the antigen. Clk/Sty-specific antibodies were obtained by cross-linking anti-GST antibodies to protein A-agarose beads, which were then cross-linked to GST-ClkR/Sty. Clk/Sty-specific antibodies were eluted with the peptide described above and dialyzed against phosphate-buffered saline.
SDS-PAGE and Western blotting.
Protein samples were routinely boiled for 6 min and fractionated on 10% SDS-PAGE. Proteins were transferred to nitrocellulose, and blots were probed with different antibodies as described previously (27, 62). Proteins were detected using a chemiluminescence kit (Amersham) according to the manufacturer's instructions.
In vitro kinase assays.
In vitro phosphorylation assays were carried out with 5, 20, and 100 ng of P-Clk/Sty and equivalent amounts of PTP-, CIP-, PS-, and PY-Clk/Sty (normalized by kinase activity with myelin basic protein [MBP] as the substrate) with 1 μg of SR protein substrate in the presence of 10 μCi of [γ-32P]ATP and 2 mM ATP, as described previously (5). Briefly, a cocktail of the substrate in kinase buffer was prepared in 45 μl and various amounts of kinase (differing by fivefold) were added to initiate the reaction. Samples were incubated at 30°C for 30 to 45 min, and reactions were stopped by adding 1/2 volume of 3× SDS sample buffer.
In vitro splicing.
Template DNAs were linearized with appropriate restriction enzymes, and splicing substrates were prepared by in vitro transcription as described previously (47). HeLa nuclear and S100 extracts were prepared as described previously (8). Splicing assays in S100 extracts with appropriate SR proteins were performed as described previously (55). For splicing assays in the presence of phosphatase inhibitors, S100 extracts, SR proteins, and polyvinyl alcohol were incubated with 0.01 mM sodium orthovanadate-0.01% H2O2 for 10 min at 30°C before addition of the substrate and kinase (time 0). Splicing reactions were carried out for 80 min and were stopped by addition of proteinase K (250 μg/ml) and deproteinized and precipitated by addition of 2.5 volumes of ethanol. RNA products were fractionated on denaturing PAGE and visualized by autoradiography.
Cell culture and transfections.
The Clk/Sty coding sequence was amplified by PCR using two primers, 5′-GCTATCTAGAAGACATTCAAAGAGAACTTAC-3′ and 5′-ACCCACTTAAAAAGCATACGGGATCCGCAT-3′, and digested with XbaI and BamHI. The product was cloned into the expression vector pCGN with a hemagglutinin (HA) tag (59). Plasmid (4 μg) was used for transfection of 293 cells in 10-cm-diameter plates as described previously (59). Cells were harvested at 48 h posttransfection. Neuro-2A cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 2 mM glutamine, and 1 mM Na pyruvate. NIH 3T3 cells and HeLa cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% bovine calf serum and fetal calf serum, respectively. Cells (4 × 105) were seeded onto 6-well plates. The medium was changed every 2 days.
Cell lysis and fractionation.
Cells were harvested, washed twice with phosphate-buffered saline, and pelleted at 800 × g for 5 min at 4°C. Cells were resuspended in 500 μl of TSM (20 mM Tris-Cl [pH 7.5], 150 mM NaCl, 3 mM MgCl2, 20 mM NaF, 10 mM β-glycerophosphate, 1 mM sodium orthovanadate, 1 mM sodium pyrophosphate and protease inhibitors). Triton X-100 (10%; 25 μl) was added, and the cells were incubated for a further 5 min at 4°C. Nuclei were collected by centrifugation at 1,600 × g for 5 min at 4°C and washed once with TSM. The supernatant constituted the cytoplasmic fraction. All lysates were frozen and stored at −70°C.
RESULTS
Recombinant Clk/Sty purified from E. coli is phosphorylated on Thr and Ser/Thr.
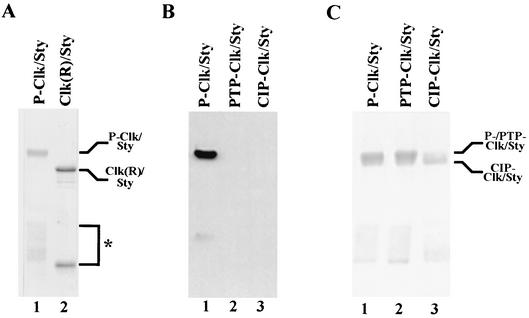
To begin to characterize the possible effects of phosphorylation on Clk/Sty activity, wild-type Clk/Sty (as a GST fusion protein) was purified from E. coli (P-Clk/Sty). P-Clk/Sty migrated at ∼100 kDa, whereas a kinase-inactive mutant, GST-ClkR (which is unphosphorylated), migrated at the expected molecular mass of 84 kDa (47) (Fig. 1A, compare lanes 1 and 2). This difference in apparent molecular mass suggested that Clk/Sty was active in E. coli and underwent autophosphorylation, whereas the kinase-inactive mutant, GST-ClkR, did not. Significantly, P-Clk/Sty purified from E. coli was also phosphorylated on Tyr, as indicated by reactivity to an antiphosphotyrosine antibody (Fig. 1B, lane 1). This reactivity was completely abolished by treatment with PTP (lane 2). Treatment with CIP, a nonspecific phosphatase, also reduced the reactivity of Clk/Sty to the antibody (lane 3), suggesting that CIP dephosphorylated Clk/Sty on both Ser/Thr and Tyr residues. A small extent of phosphorylation was still detected on CIP-Clk/Sty. Complete dephosphorylation of P-Clk/Sty by CIP (as judged by analysis of the repurified proteins) was not possible, perhaps due to inaccessibility of the phosphatase to all phosphorylated residues. Using PTP or CIP to investigate further the extent of autophosphorylation, P-Clk/Sty was dephosphorylated specifically on Tyr or on all residues, respectively, and the proteins were repurified (Fig. 1C). Treatment with PTP to produce PTP-Clk/Sty did not affect gel mobility, suggesting that tyrosine phosphorylation did not affect the mobility of Clk/Sty (lane 2). However, treatment with CIP (to give CIP-Clk/Sty) resulted in increased mobility (lane 3), suggesting that Ser/Thr autophosphorylation is more extensive than Tyr phosphorylation but in any event is reflected in altered gel mobility.
FIG. 1.
Clk/Sty is autophosphorylated on Ser/Thr and Thr. (A) A total of 1 μg each of GST-Clk/Sty (lane 1) and a kinase-inactive mutant (GST-ClkR/Sty; lane 2) was fractionated by 10% SDS-PAGE and stained with Coomassie blue. The species indicated by the asterisk reflects an N-terminal truncation consisting of GST plus an approximately 10-kDa segment of Clk/Sty, which became phosphorylated in the wild-type but not the mutant sample. (B) A total of 0.5 μg of P-, PTP-, and CIP-Clk/Sty (lanes 1 to 3, respectively), was fractionated by 10% SDS-PAGE and stained with Coomassie blue. (C) A total of 20 to 50 ng of P-Clk/Sty (lane 1), PTP-Clk/Sty (lane 2), and CIP-Clk/Sty (lane 3) was fractionated by 10% SDS-PAGE and transferred to nitrocellulose. The blot was probed with antiphosphotyrosine antibody, and proteins were detected by chemiluminescence.
Autophosphorylation status of Clk/Sty modulates kinase activity in vitro.
The activities of a number of kinases are known to be modulated by autophosphorylation (15, 50). To study the possible effect of autophosphorylation on Clk/Sty kinase activity, the different Clk/Sty derivatives described above were tested in in vitro phosphorylation assays with recombinant SR proteins purified from E. coli. Two assumptions were important parameters in the assays. First, SR proteins purified from E. coli are unphosphorylated. Though SR proteins were extensively phosphorylated in the cells and tissues examined to date, unphosphorylated SR proteins have not been detected (19, 65) and the natural phosphorylation status of SR proteins that are phosphorylated by Clk/Sty (or other SR protein kinases) is not known. Second, the autophosphorylation status of Clk/Sty is assumed to remain constant throughout the assay (i.e., no autophosphorylation of Clk/Sty occurs during the in vitro phosphorylation reaction). Though a limited amount of autophosphorylation may in fact occur during the reaction (unpublished data), the trans reaction, i.e., phosphorylation of the substrate, predominates.
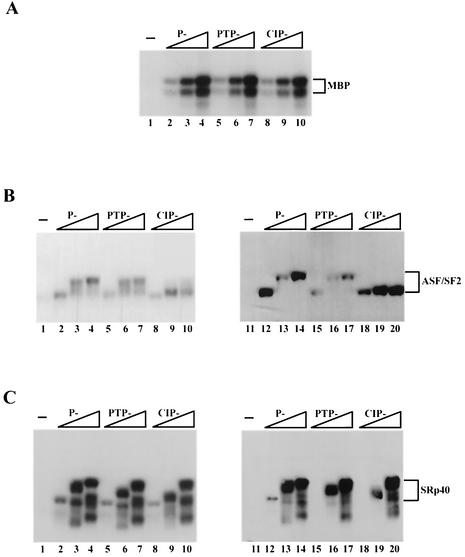
We next measured the kinase activities of repurified P-, PTP-, and CIP-Clk/Sty, first in standard kinase assays with [γ-32P]ATP in which the products were resolved by denaturing PAGE. Importantly, activities of all three kinase preparations with MBP, a likely nonphysiological substrate, were similar (Fig. 2A). However, differences were observed when ASF/SF2 was used as a substrate. Incubation of increasing amounts of P-Clk/Sty with ASF/SF2 resulted in increased incorporation of 32P into forms of ASF/SF2 migrating progressively more slowly (Fig. 2B, lanes 2 to 4). PTP-Clk/Sty catalyzed slightly decreased incorporation of 32P into the slowest-migrating species of ASF/SF2 (lanes 5 to 7). Interestingly, ASF/SF2 phosphorylated by CIP-Clk/Sty (lanes 8 to 10) migrated faster than ASF/SF2 phosphorylated by P- and PTP-Clk/Sty, though the extents of incorporation of 32P were very similar. This suggests that the residues phosphorylated by CIP-Clk/Sty were different from those phosphorylated by the other two forms of the kinase.
FIG. 2.
Autophosphorylation of Clk/Sty modulates kinase activity towards ASF/SF2 but not SRp40. (A) Increasing amounts of P-Clk/Sty (1, 5, and 20 ng) and equivalent amounts of PTP- and CIP-Clk/Sty were incubated with 1 μg of MBP under kinase conditions. Reactions were stopped by adding 1/2 volume of 3× SDS sample buffer, and proteins were fractionated by 10% SDS-PAGE and detected by autoradiography. (B and C) Increasing amounts of P-Clk/Sty (5, 20, and 100 ng) or equivalent amounts of PTP- and CIP-Clk/Sty were added to 1 μg of ASF/SF2 (B) or SRp40 (C) purified from E. coli and incubated for 30 min at 37°C. Reactions were processed as described for panel A except that proteins were transferred to nitrocellulose following PAGE. The extent of γ-32P incorporation was visualized by autoradiography (lanes 1 to 10). Subsequently, blots were incubated with the monoclonal antibody mAb104 (lanes 11 to 20) and reactivity was detected by chemiluminescence.
Phosphorylation patterns were also analyzed by Western blotting with mAb104, which recognizes a phosphoserine epitope in the RS domain of SR proteins (45). In the case of P-Clk/Sty, ASF/SF2 reactivity to mAb104 correlated both with the decrease in mobility and with the extent of incorporation of 32P (Fig. 2B, lanes 12 to 14). With PTP-Clk/Sty, however, the decreased reactivity observed with the slowest-migrating form of ASF/SF2 confirmed that the extent of phosphorylation was indeed less than that catalyzed by P-Clk/Sty (lanes 15 to 17). Strikingly, CIP-Clk/Sty activity measured by reactivity to mAb104 was again distinct, indicating significant phosphorylation but no change in gel mobility (lanes 18 to 20). These results strongly support the conclusion that dephosphorylated Clk/Sty can phosphorylate the RS domain of ASF/SF2 as efficiently as the phosphorylated forms but that the pattern is distinct. They also indicate that the extent of phosphorylation (as measured by 32P incorporation or reactivity to mAb104) need not necessarily correlate with decreased ASF/SF2 gel mobility (compare lanes 14 and 20).
To determine whether the effects of differential autophosphorylation on kinase activity observed with ASF/SF2 were a general feature among SR proteins, another SR protein, SRp40, was used as the substrate under similar assay conditions. All three derivatives of Clk/Sty phosphorylated SRp40 equally well, as evaluated by levels of 32P incorporation, gel mobility, and mAb104 reactivity (Fig. 2C). That additional slower-migrating forms were detected suggests the presence of either heterogenously phosphorylated forms of SRp40 and/or incomplete translated forms. However, these results indicate that differences in autophosphorylation do not influence the ability of Clk/Sty to phosphorylate all SR proteins.
P-Clk/Sty is a mixture of differentially Ser/Thr- and Tyr-autophosphorylated forms.
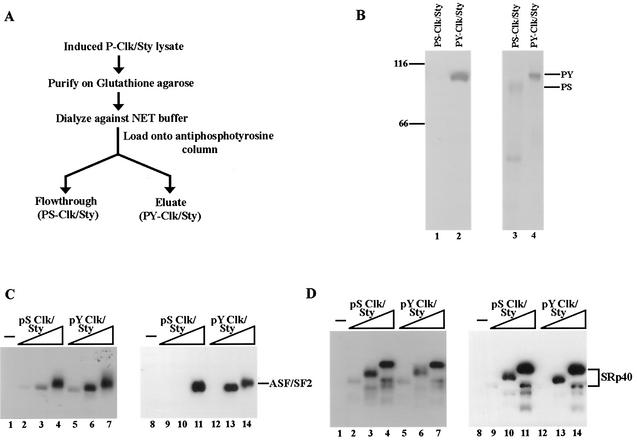
The lack of difference in gel mobility observed after Tyr dephosphorylation of P-Clk/Sty (Fig. 1C) suggested that P-Clk/Sty is a mixture of Ser/Thr- and Tyr-autophosphorylated forms. Moreover, the diffuse nature of the P-Clk/Sty band found by SDS-PAGE, in contrast with the sharp band detected by Western blotting with the antiphosphotyrosine antibody, also suggested the presence of a mixed population of Clk/Sty containing or lacking Tyr autophosphorylation. To determine whether this is the case, and if so, to investigate the properties of these two forms of the kinase, a purification procedure was devised to separate the two populations of Clk/Sty into which purified P-Clk/Sty was fractionated on an antiphosphotyrosine column (Fig. 3A). Clk/Sty phosphorylated only on Ser/Thr should not bind the column and thus accumulate in the flowthrough fraction (PS-Clk/Sty), while the Tyr-phosphorylated population (PY-Clk/Sty) should bind and be specifically eluted with phenyl phosphate (see Materials and Methods). Aliquots of the two fractions obtained were resolved by SDS-PAGE and analyzed first by Western blotting with antiphosphotyrosine antibody (Fig. 3B, lanes 1 and 2). Reactivity only to PY-Clk/Sty (lane 2) and not to PS-Clk/Sty (lane 1) indicated that the column successfully retained all of the Tyr-phosphorylated Clk/Sty. The gel was then subjected to Coomassie staining, which revealed that a significant fraction of P-Clk/Sty (∼70%) indeed lacked Tyr autophosphorylation. PY-Clk/Sty migrated at a position similar to that of P-Clk/Sty, suggesting that PY-Clk/Sty was extensively phosphorylated on Ser/Thr as well as Tyr. It is conceivable that a basal level of Ser/Thr phosphorylation is required for Tyr autophosphorylation. In contrast, PS-Clk/Sty migrated more rapidly, indicating that it was underphosphorylated on Ser/Thr as well as lacking Thr phosphorylation.
FIG. 3.
P-Clk/Sty is a mixture of Tyr- and Ser/Thr-autophosphorylated forms. (A) Purification scheme of Tyr (PY-Clk/Sty) and Ser/Thr (PS-Clk/Sty) autophosphorylated forms. P-Clk/Sty was initially purified on a glutathione Sepharose column as described by Prasad et al. (47). It was then dialyzed and fractionated on an antiphosphotyrosine column. The flowthrough consisted of Clk/Sty phosphorylated exclusively on Ser/Thr (PS-Clk/Sty), and Tyr autophosphorylated Clk/Sty (PY-Clk/Sty) was eluted specifically with phenyl phosphate. (B) Clk/Sty is a mixture of differentially phosphorylated forms. PY-Clk/Sty (lane 1) and PS-Clk/Sty (lane 2) were fractionated by 10% SDS-PAGE and transferred to nitrocellulose. Blots were processed as described for Fig. 1. (C and D) Increasing amounts of PY-Clk/Sty (lanes 2 to 4) or PS-Clk/Sty (lanes 5 to 7) were incubated with recombinant ASF/SF2 (C) or SRp40 (D) purified from E. coli. Reactions were processed as described for Fig. 2.
To compare the kinase activities of PS-Clk/Sty and PY-Clk/Sty, assays similar to those described for Fig. 2 were used. PS-Clk/Sty and PY-Clk/Sty activities were first normalized with MBP as a substrate and were again found to have similar activities (results not shown). Next, ASF/SF2 and SRp40 purified from E. coli were incubated with increasing amounts of PS- and PY-Clk/Sty in the presence of [γ-32P]ATP (Fig. 3C and D, lanes 1 to 7). PY-Clk/Sty phosphorylated ASF/SF2 more efficiently than did PS-Clk/Sty (Fig. 3C). The difference between PY- and PS-Clk/Sty was most apparent at the intermediate concentration (compare lanes 2 and 3 with 5 and 6), but in any event, the results demonstrate that the activity of Tyr-autophosphorylated Clk/Sty towards ASF/SF2 was enhanced relative to that of the form lacking Tyr autophosphorylation. Significantly, the extent of phosphorylation measured by mAb104 reactivity (lanes 8 to 14) was identical to that observed with 32P incorporation. In contrast, SRp40 was phosphorylated equivalently by PS- and PY-Clk/Sty (Fig. 3D). The absence of any difference in either 32P incorporation or mAb104 reactivity with SRp40 as a substrate suggests that Clk/Sty activity was not generally regulated by Thr autophosphorylation. Coupled with the lack of any significant difference between P-, PTP-, and CIP-Clk/Sty activities towards SRp40 and the similarity of this response to MBP phosphorylation, it is conceivable that SRp40 is not a specific substrate of Clk/Sty which is regulated by autophosphorylation. In any event, these experiments indicate that the extent of Clk/Sty autophosphorylation can regulate its activity towards specific substrates.
P-Clk/Sty is dephosphorylated on Thr under splicing conditions in vitro.
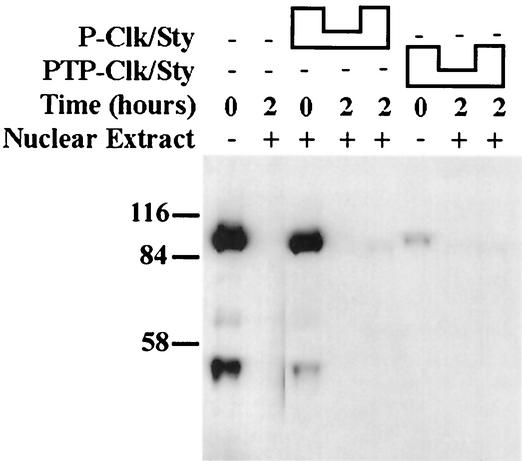
A number of SR and other proteins undergo phosphorylation and dephosphorylation cycles during the course of splicing (3, 56, 58, 62), and it is possible that Clk/Sty phosphorylation can also change during a splicing reaction. Therefore, before testing the effect of autophosphorylation on Clk/Sty activity in the modulation of splicing, we examined whether Clk/Sty undergoes phosphorylation and dephosphorylation in HeLa extracts. P-Clk/Sty was added to nuclear (Fig. 4) or S100 (data not shown) extracts and incubated under splicing conditions. Significantly reduced reactivity to the antiphosphotyrosine antibody was detected, suggesting that a Tyr phosphatase activity capable of dephosphorylating Clk/Sty was active in the extracts. Dephosphorylation was complete at the earliest time tested (18 min; results not shown), indicating that the phosphatase acts rapidly. Blots were stripped and reprobed with anti-GST antibody, which confirmed the presence of Clk/Sty at all time points (data not shown). These observations lend further credence to the possibility that Clk/Sty activity is regulated at the level of autophosphorylation.
FIG. 4.
Clk/Sty is dephosphorylated on Tyr during splicing in nuclear extracts. A total of 20 and 100 ng of P-Clk/Sty (first lane and third through fifth lanes) or PTP-Clk/Sty (sixth through eighth lanes) was added to nuclear extracts (second through fifth, seventh, and eighth lanes) under splicing conditions. Splicing reactions were allowed to proceed for the indicated times. Proteins were precipitated with 10% trichloroacetic acid and washed with acetone, and proteins were fractionated on 10% SDS-PAGE. Western blots were probed with antiphosphotyrosine antibody, and proteins were detected by chemiluminescence.
PY- and PS-Clk/Sty differentially affect splicing activated by ASF/SF2 and SRp40.
Next, using the well-characterized pre-mRNA β-globin substrate as previously described (47), we determined the effects of the various kinase preparations on splicing activity. However, because of the rapid Thr dephosphorylation of P-Clk/Sty, we first developed conditions in which Clk/Sty phosphorylation could be maintained (by specific phosphatase inhibitors) without significantly affecting splicing efficiency (see Materials and Methods). A panel of phosphatase inhibitors was initially tested, either singly or in different combinations, such that activation of splicing in S100 extracts complemented with SR proteins was not affected and Thr autophosphorylation of P-Clk/Sty was maintained. The presence of these phosphatase inhibitors in fact allowed for slightly increased Tyr autophosphorylation of PTP- and CIP-Clk/Sty during the course of the splicing reaction (data not shown and see below).
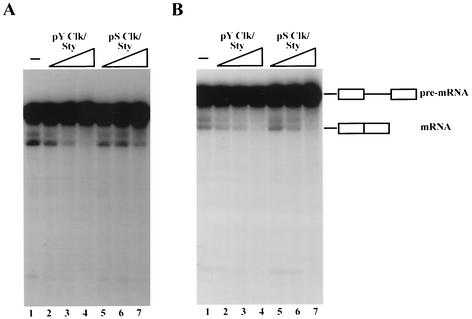
We next used the phosphatase-inactivated splicing extracts to examine the effects of PS- and PY-Clk/Sty on splicing activated by individual SR proteins. Given the results of the kinase assays described above, we hypothesized that PY-Clk/Sty would inhibit ASF/SF2-activated splicing more effectively than PS-Clk/Sty while both would similarly affect SRp40-activated splicing. As predicted, PY-Clk/Sty inhibited ASF/SF2-activated splicing (Fig. 5A, lanes 2 to 4) more efficiently than did PS-Clk/Sty (three- to fourfold; lanes 5 to 7), suggesting that Thr autophosphorylation of Clk/Sty enhanced kinase activity and inhibited splicing via hyperphosphorylation of ASF/SF2. PS-Clk/Sty also inhibited ASF/SF2-activated splicing but only at the highest concentration tested (Fig. 5A, compare lanes 1 and 7). These observations are in good agreement with the in vitro phosphorylation assays, in which PS-Clk/Sty activity was lower than PY-Clk/Sty activity by at least a factor of four. Also in agreement with the in vitro phosphorylation assays, inhibition of SRp40-activated splicing was not affected by the autophosphorylation status of Clk/Sty; splicing was inhibited equivalently by PY- and PS-Clk/Sty (Fig. 5B, compare lanes 2 to 4 and 5 to 7). This suggests that the two SR proteins are differentially sensitive to changes in Clk/Sty phosphorylation status.
FIG. 5.
PY- and PS-Clk/Sty differentially affect splicing activated by distinct SR proteins. Increasing amounts (8, 20, and 50 ng) of PY-Clk/Sty (lanes 2 to 4) and PS-Clk/Sty (lanes 5 to 7) were added to splicing reactions performed with S100 extracts in the presence of phosphatase inhibitors and ASF/SF2 (A) and SRp40 (B). Reaction mixtures were deproteinized after 80 min, and RNAs were precipitated with ethanol. RNAs were fractionated by 5% denaturing PAGE and visualized by autoradiography.
Clk/Sty, Ser/Thr, and Tyr autophosphorylation can independently influence substrate specificity.
Intriguing differences were observed between the preparations of Clk/Sty in which Tyr phosphorylation was removed by specific phosphatase treatment (PTP-Clk/Sty; Fig. 1) or by biochemical separation (PS-Clk/Sty; Fig. 3B). While Tyr phosphorylation was undetectable in each case, the gel mobility of PTP-Clk/Sty was identical to that of fully phosphorylated P-Clk/Sty but the mobility of PS-Clk/Sty was significantly greater than that of P-Clk/Sty. This indicates that the fraction of kinase naturally lacking Thr phosphorylation was also underphosphorylated on Ser/Thr. Consistent with this, CIP treatment of Clk/Sty, which removed both Thr and Ser/Thr phosphorylation, resulted in enhanced gel mobility (Fig. 1). We therefore wished to test the effects of P-, PTP-, and CIP-Clk/Sty on splicing and to compare the results not only with the activities of the three derivatives in kinase assays but also with the effects of PY- and PS-Clk/Sty on splicing. To this end, we again used the phosphatase-inhibited splicing assay, in this case with ASF/SF2 and another canonical SR protein, SC35, both purified from baculovirus-infected insect cells.
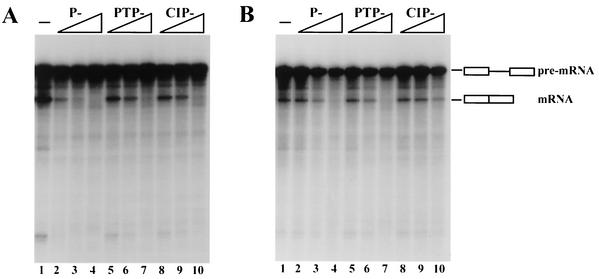
We first tested the effects of the three kinases on ASF/SF2-activated splicing (Fig. 6A). As expected, P-Clk/Sty effectively inhibited splicing. PTP-Clk/Sty also inhibited splicing but about fivefold less efficiently (compare lanes 2 and 3 with 5 and 6). This correlates well not only with the difference in kinase activity (Fig. 2) but also with the difference in splicing inhibition observed with PY- and PS-Clk/Sty (Fig. 5A). The results suggest that the apparent difference in Ser/Thr phosphorylation between PS-Clk/Sty and PTP-Clk/Sty does not affect activity towards ASF/SF2 and that the reduced activity is thus determined entirely by the extent of Tyr phosphorylation. Consistent with this, CIP-Clk/Sty inhibited splicing to the same extent as PTP-Clk/Sty (compare lanes 5 to 7 and 8 to 10).
FIG. 6.
Ser/Thr and Tyr autophosphorylation independently influence substrate specificity. Increasing amounts (8, 20, and 50 ng) of P-Clk/Sty (lanes 2 to 4), PTP-Clk/Sty (lanes 5 to 7), and CIP-Clk/Sty (lanes 8 to 10) were added to splicing reactions performed with S100 extracts in the presence of phosphatase inhibitors and ASF/SF2 (A) or SC35 (B). Reaction mixtures were deproteinized after 80 min, and RNAs were precipitated with ethanol. RNAs were fractionated by 5% denaturing PAGE and visualized by autoradiography.
Strikingly different results were obtained when the ability of the three kinase preparations to inhibit SC35-activated splicing was tested (Fig. 6B). In this case, PTP-Clk/Sty inhibited splicing to nearly the same extent as P-Clk/Sty (compare lanes 2 to 4 and 5 to 7). These results suggest that in this case, Ser/Thr phosphorylation, not Thr phosphorylation, determines the activity of Clk/Sty towards SC35. Providing strong support for this idea, CIP-Clk/Sty (like PS-Clk/Sty) was much less efficient at inhibiting SC35-mediated splicing (compare lanes 5 to 7 and 8 to 10). Together, these results suggest that both Ser/Thr and Tyr phosphorylation can influence Clk/Sty activity but in a substrate-dependent manner.
Exogenous and endogenous Clk/Sty SR protein kinases are present in the nucleus.
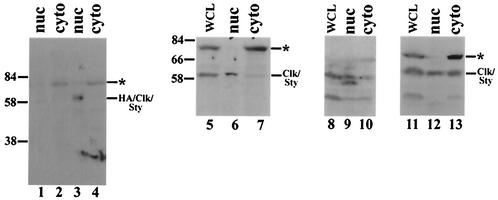
An important assumption behind our experiments is that Clk/Sty participates actively in the splicing process and as such should be localized to the nucleus. Surprisingly, however, a recent report has suggested that both endogenous and exogenous, overexpressed Clk/Sty SR protein kinases are localized predominantly in the cytoplasm of PC12 and NIH 3T3 cells, respectively, as determined by indirect immunofluorescence (35). This is in contrast with earlier observations using similar methods in which both overexpressed wild-type and kinase-inactive Clk/Sty SR protein kinases localized exclusively in the nucleus (5, 11). More recently, transiently expressed GFP fusions of Clk/Sty and ClkR were also found to be present exclusively in the nucleus (48). To examine this issue biochemically, Clk/Sty was first overexpressed in 293 cells and nuclear and cytoplasmic fractions were prepared. As antibodies against Clk/Sty have generally been difficult to generate, perhaps reflecting the fact that the kinase is highly homologous across species, we generated an antipeptide antiserum against a sequence from the N terminus of Clk/Sty and affinity purified the anti-Clk/Sty antibodies (see Materials and Methods). Western blot analysis with these antibodies indicated that the overexpressed kinase was present as a diffuse band, implying the presence of multiple phosphorylated forms (Fig. 7, lane 3). More importantly, Clk/Sty was present mainly, if not exclusively, in the nuclear fraction (compare lanes 3 and 4). (The very faint species at ∼60 kDa in mock-transfected cells [lane 1], which, as expected, was very weak relative to the overexpressed HA-tagged form, likely corresponds to endogenous Clk/Sty, which also appears to be nuclear. The larger, cytoplasmic species is discussed below.)
FIG. 7.
Exogenous and endogenous Clk/Sty SR protein kinases are present in the nucleus. For detection of exogenously expressed Clk/Sty, human 293 cells on 10-cm-diameter plates were either mock transfected (lanes 1 and 2) or transfected with HA-Clk/Sty (lanes 3 and 4). Cells were collected at 48 h posttransfection, and nuclear (lanes 1 and 3) and cytoplasmic (lanes 2 and 4) fractions were prepared. Protein samples were boiled in sample buffer and fractionated by 10% SDS-PAGE before being transferred to nitrocellulose. Blots were probed with anti-Clk/Sty antibody, and proteins were detected by chemiluminescence. For detection of endogenous Clk/Sty, Neuro 2A (lanes 5 to 7), NIH 3T3 (lanes 8 to 10), and HeLa (lanes 11 to 13) cells were harvested and subcellular fractionation was performed as described above. Whole-cell lysates (lanes 5, 8, and 11), nuclear fractions (lanes 6, 9, and 12), and cytoplasmic fractions (lanes 7, 10, and 13) were resolved by 10% SDS-PAGE and transferred to nitrocellulose. Blots were probed with anti-Clk/Sty antibody, and proteins were detected by chemiluminescence. The asterisks denote a possible cytoplasmic isoform (see text).
To determine whether endogenous Clk/Sty was also present in the nucleus, subcellular fractionation and Western blotting of several cell lines were performed. A neuroblastoma cell line, Neuro 2A (N2A), was analyzed first. While Clk/Sty was detected in most cell lines tested (data not shown and see below), N2A cells displayed the highest levels. Western blot analysis of the fractionated extracts again demonstrated the presence (predominantly in the nuclear fraction) of a species of the size (∼60 kDa) predicted for Clk/Sty (Fig. 7, compare lanes 4 and 5). We next examined two other well-characterized cell lines, NIH 3T3 and HeLa. While Northern blot analysis suggests that Clk/Sty is expressed in HeLa cells (58), using Western blots, Menegay et al. were unable to detect Clk/Sty in NIH 3T3 cells (35). A subcellular fractionation and Western blot procedure similar to that used with N2A cells was employed to analyze Clk/Sty expression and localization in these cells (lanes 8 to 13). Two points are apparent from the results. First, Clk/Sty was detected in both cell lines in both nuclear and cytoplasmic fractions. This establishes that Clk/Sty is present in the nuclei of both cell types and possibly the cytoplasm as well, although in this case nuclear leakage cannot be ruled out. Second, the two cell lines showed apparently distinct patterns of Clk/Sty phosphorylation. Clk/Sty was present as multiple forms in NIH 3T3 cell nuclei (lane 9), while the cytoplasmic fraction contained only the slower-migrating (presumably hyperphosphorylated) form (lane 10), suggesting that Clk/Sty is differentially modified in the nucleus and cytoplasm. On the other hand, HeLa cells showed only one form, which was almost equally distributed between the nuclear and cytoplasmic fractions (compare lanes 12 and 13). Intriguingly, all of the cell lines (and especially the N2A, HeLa, and 293 cells) also displayed a higher-molecular-weight species of ∼80,000 that was exclusively cytoplasmic. Although we do not know the identity of this species, this pattern is remarkably similar to that detected in Drosophila for the Clk/Sty homologue Doa (64). Western blotting of extracts from all developmental stages revealed not only the predicted 55-kDa isoform, which was largely nuclear, but also an unexpected 105-kDa isoform that was exclusively cytoplasmic.
Whatever the significance of the various cytoplasmic forms, our results provide important evidence that a significant fraction of Clk/Sty is indeed nuclear and thus able to participate actively in splicing control. Additional experiments, with more sensitive reagents, would be important for determination of the extent and consequences of Clk/Sty autophosphorylation in vivo. But together, our results have established autophosphorylation as a potentially important mechanism for controlling Clk/Sty activity.
DISCUSSION
The SR proteins in mammals constitute a family of about a dozen proteins that share related RS domains, which are known to be phosphorylated extensively on multiple serines. While Clk/Sty and related kinases that phosphorylate RS domains have been characterized, until now it has been unknown whether any of these kinases display substrate specificity, i.e., whether they can differentially phosphorylate individual SR proteins. It was also unknown whether the activity of such kinases could be regulated, an especially interesting question with respect to the Clk/Sty kinases because they themselves contain RS-like domains and are also known to be autophosphorylated on tyrosines. We have described here biochemical data that address both of these questions, showing that the phosphorylation status of Clk/Sty affects its activity and that these effects are substrate specific. Significantly, we have also provided evidence that Ser/Thr and Tyr phosphorylation can each influence Clk/Sty activity in substrate-specific ways. Our experiments also provided biochemical evidence that consistent with a direct role in splicing, Clk/Sty indeed localizes to the nucleus. Below we discuss how these properties of Clk/Sty relate both to other protein kinases and to the mechanism and regulation of pre-mRNA splicing.
A number of protein kinases are known to be regulated by autophosphorylation. These include, for example, the well-studied receptor protein tyrosine kinases (RPTKs), where dimerization induced by ligand binding induces transautophosphorylation (24, 50). Membrane-associated Ser/Thr kinases can also be activated by similar dimerization-induced autophosphorylation. For example, the interleukin-1 receptor-associated kinase/Pelle kinases associate with Toll-like receptors and become autophosphorylated in response to ligand binding and receptor multimerization (33, 51). The complex Ca2+/calmodulin-dependent kinase II is activated by autophosphorylation, which results in a change of properties, including generation of Ca2+/calmodulin-independent activity (23). Finally, an especially relevant group of kinases is that of the mitogen-activated protein kinases because, like Clk/Sty, they are dual-specificity kinases (4). Although most frequently activated by signaling cascades that appear not to involve autophosphorylation, at least one of these kinases, p38α, can be activated by a distinct mechanism that involves autophosphorylation induced by binding of a specific activating protein, TAB1 (15).
There are also instances in which changes in the pattern of autophosphorylation of specific kinases can have more qualitative effects on substrate specificity. For example, the colony-stimulating factor 1 RPTK is phosphorylated on multiple tyrosines, and mutations altering different subsets of these residues differentially affect recognition of distinct SH2 domain-containing substrates (24). Similar results were obtained with fibroblast growth factor receptor 1, another RPTK (7). An autophosphorylated form of the Src-related Lyn kinase displays increased activity towards peptide substrates but reduced activity towards protein substrates relative to the unphosphorylated version, conceivably suggesting differences in substrate specificity (9). The activity of GSK-3β, another dual-specificity kinase, was shown to be activated by Thr autophosphorylation and inhibited by Ser/Thr autophosphorylation (60). To our knowledge, however, ours is the first study to show that changes in autophosphorylation patterns can both have qualitative effects on phosphorylation of a specific substrate and change specificity towards distinct substrates such that one is favored by Tyr and another is favored by Ser/Thr autophosphorylation.
Our studies have provided considerable evidence that the activity of Clk/Sty can be modulated by changes in its autophosphorylation pattern, but a key question is whether this happens in vivo and, if so, what factors might control autophosphorylation. At least two lines of evidence suggest that Clk/Sty phosphorylation statuses can differ and have functional consequences in vivo. First, the Clk/Sty family member Clk2 has been shown to be differentially phosphorylated in vivo and its subnuclear localization can be affected by altering its phosphorylation status with kinase inhibitors or mutation of a conserved phosphorylation site, Ser-141 (44). Second, our Western blot analyses revealed differences in Clk/Sty gel mobility, which likely reflect at least in part different phosphorylation levels between different cell types and nuclear and cytoplasmic fractions (see also below). However, additional work is required to document specific changes and indeed, beyond the Ser-141 site mentioned above, specific Clk/Sty phosphorylation sites have not yet been determined.
Clk/Sty autophosphorylation levels may be modulated by mechanisms utilized by other kinases. For example, Clk/Sty has been shown to have the ability to dimerize in vitro (11) and, as with the RPTKs and other kinases, it is possible that dimerization facilitates transautophosphorylation. However, interactions between the Clk/Sty C-terminal catalytic domain and N-terminal regulatory domain were not detected in a yeast two-hybrid screening and the isolated catalytic region could not phosphorylate the N-terminal region in vitro (46), providing some evidence against a transphosphorylation mechanism. Another possibility is that interaction with another protein(s) influences Clk/Sty autophosphorylation, similar to the mechanism described above for the mitogen-activated protein kinase p38α. Such an interaction would likely involve the RS domain of Clk/Sty. RS domains are well known to mediate protein-protein interactions (27, 61), and the Clk/Sty RS-rich region is also necessary (but not sufficient) to mediate interactions with SR proteins (5, 46). RS domain phosphorylation has significant, protein-specific effects on protein-protein interactions (62, 63). Indeed, a novel RS domain containing CLASP, a protein that interacts with unphosphorylated (i.e., kinase-inactive) but not phosphorylated Clk4, was recently described (26). Such interactions could affect kinase activity and autophosphorylation in a number of ways, for example, by facilitating or interfering with recognition of specific residues or by directly influencing the structure and activity of the catalytic domain (25). Consistent with this, the Clk/Sty N-terminal region has been shown to have a generally negative effect on catalytic activity (35). It is likely that similar phosphorylation-mediated changes in protein-protein interactions underlie the changes in substrate specificity we have described here.
Important issues affecting the possible physiological function(s) of Clk/Sty kinases include their tissue distribution and subcellular localization. The lack of antibodies recognizing these proteins have limited such studies, but Northern analyses have suggested that Clk/Sty is widely expressed and present at various levels in most tissue and cell lines analyzed (43, 58). Also, as described above, a number of studies with transiently overexpressed epitope-tagged Clk/Sty have suggested that the kinase is predominantly nuclear. These studies have been taken as evidence supporting the involvement of SR proteins in splicing. It was thus surprising that a recent study suggested that Clk/Sty was expressed in a very limited number of cells and tissues and when expressed was almost entirely cytoplasmic (35). In contrast, our studies suggest that Clk/Sty is expressed in several cell types and that a large fraction is nuclear. The reason(s) for this discrepancy is unclear. One possibility is that the antibodies used by Menegay et al. preferentially recognize a hyperphosphorylated form of Clk/Sty. Hyperphosphorylation of SR and related proteins has been associated with cytoplasmic localization (2, 49). Furthermore, our biochemical analyses suggest that the apparent cytoplasmic form of Clk/Sty detected in NIH 3T3 cells is hyperphosphorylated. We also detected a significantly larger species that may correspond to a novel isoform of Clk/Sty and which was exclusively cytoplasmic. This form may be analogous to a high-molecular-weight species detected in Drosophila which was suggested to arise by alternative splicing rather than phosphorylation (64). It is conceivable that this was the form detected by Menegay et al. In any event, the data together now suggest that a significant fraction of Clk/Sty is indeed nuclear, although another fraction is likely localized in the cytoplasm, perhaps depending on cell or tissue type. The function of these cytoplasmic forms is not known. However, one cytoplasmic target of Clk/Sty has been suggested previously (41), and it is also possible that intracellular localization provides a mechanism for regulating the role of the kinase in splicing, perhaps analogous to the mechanism proposed to control hnRNP A1 activity in response to osmotic stress (57).
We now know that alternative splicing occurs frequently in metazoan systems, and present estimates suggest that expression of nearly 50% of human genes involves alternative splicing (40). It is therefore becoming apparent that splicing regulation provides an important avenue for gene control. As with transcription, there are multiple mechanisms by which splicing can be regulated, and combinatorial interactions involving SR proteins appear to be especially significant. Consistent with playing a role in splicing control, the distribution of individual SR proteins in different tissues and cells differs significantly (65). Given that the phosphorylation status of these proteins strongly affects their activity (reference 47 and references therein), the fact that families of SR protein kinases, such as the Clk kinases, exist and themselves exhibit tissue specificity in their distribution (43) further extends the possibilities for combinatorial regulation of splicing. We have demonstrated here that the activity and substrate specificity of Clk/Sty can be modulated by qualitative and quantitative changes in autophosphorylation. While future studies would be important to understand the precise patterns of autophosphorylation involved and how they are utilized in living cells, our experiments have added another layer of complexity to the network of interactions capable of controlling splicing of mRNA precursors.
REFERENCES
- 1.Ben-David, Y., K. Letwin, L. Tannock, A. Bernstein, and T. Pawson. 1991. A mammalian protein kinase with potential for serine/threonine and tyrosine phosphorylation is related to cell cycle regulators. EMBO J. 10:317-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caceres, J. F., G. R. Screaton, and A. R. Krainer. 1998. A specific subset of SR proteins shuttles continuously between the nucleus and the cytoplasm. Genes Dev. 12:55-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao, W., S. F. Jamison, and M. A. Garcia-Blanco. 1997. Both phosphorylation and dephosphorylation of ASF/SF2 are required for pre-mRNA splicing in vitro. RNA 3:1456-1467. [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37-40. [DOI] [PubMed] [Google Scholar]
- 5.Colwill, K., T. Pawson, B. Andrews, J. Prasad, J. L. Manley, J. C. Bell, and P. I. Duncan. 1996. The Clk/Sty protein kinase phosphorylates SR splicing factors and regulates their intranuclear distribution. EMBO J. 15:265-275. [PMC free article] [PubMed] [Google Scholar]
- 6.Colwill, K., L. L. Feng, J. M. Yeakley, G. D. Gish, J. F. Caceres, T. Pawson, and X. D. Fu. 1996. SRPK1 and Clk/Sty protein kinases show distinct substrate specificities for serine/arginine-rich splicing factors. J. Biol. Chem. 271:24569-24575. [DOI] [PubMed] [Google Scholar]
- 7.Dell'Era, P., M. Mohammadi, and M. Presta. 1999. Different tyrosine autophosphorylation requirements in fibroblast growth factor receptor-1 mediate urokinase-type plasminogen activator induction and mitogenesis. Mol. Biol. Cell 10:23-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dignam, J. D., R. M. Lebovitz, and R. G. Roeder. 1983. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11:1475-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donella-Deana, A., L. Cesaro, M. Ruzzene, A. M. Brunati, O. Marin, and L. A. Pinna. 1998. Spontaneous autophosphorylation of Lyn tyrosine kinase at both its activation segment and C-terminal tail confers altered substrate specificity. Biochemistry 37:1438-1446. [DOI] [PubMed] [Google Scholar]
- 10.Du, C., M. E. McGuffin, B. Dauwalder, L. Rabinow, and W. Mattox. 1998. Protein phosphorylation plays an essential role in the regulation of alternative splicing and sex determination in Drosophila. Mol. Cell 2:741-750. [DOI] [PubMed] [Google Scholar]
- 11.Duncan, P. I., B. W. Howell, R. M. Marius, S. Drmanic, E. M. Douville, and J. C. Bell. 1995. Alternative splicing of STY, a nuclear dual specificity kinase. J. Biol. Chem. 270:21524-21531. [DOI] [PubMed] [Google Scholar]
- 12.Duncan, P. I., D. F. Stojdl, R. M. Marius, and J. C. Bell. 1997. In vivo regulation of alternative pre-mRNA splicing by the Clk1 protein kinase. Mol. Cell. Biol. 17:5996-6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duncan, P. I., D. F. Stojdl, R. M. Marius, K. H. Scheit, and J. C. Bell. 1998. The Clk2 and Clk3 dual-specificity protein kinases regulate the intranuclear distribution of SR proteins and influence pre-mRNA splicing. Exp. Cell. Res. 241:300-308. [DOI] [PubMed] [Google Scholar]
- 14.Fu, X. D. 1995. The superfamily of arginine/serine-rich splicing factors. RNA 1:663-680. [PMC free article] [PubMed] [Google Scholar]
- 15.Ge, B., H. Gram, F. Di Padova, B. Huang, L. New, R. J. Ulevitch, Y. Luo, and J. Han. 2002. MAPKK-independent activation of p38α mediated by TAB1-dependent autophosphorylation of p38α. Science 295:1291-1294. [DOI] [PubMed] [Google Scholar]
- 16.Ge, H., P. Zuo, and J. L. Manley. 1991. Primary structure of the human splicing factor ASF reveals similarities with Drosophila regulators. Cell 66:373-382. [DOI] [PubMed] [Google Scholar]
- 17.Graveley, B. R. 2000. Sorting out the complexity of SR protein functions. RNA 6:1197-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gui, J. F., W. S. Lane, and X. D. Fu. 1994. A serine kinase regulates intracellular localization of splicing factors in the cell cycle. Nature 369:678-682. [DOI] [PubMed] [Google Scholar]
- 19.Hanamura, A., J. F. Caceres, A. Mayeda, B. R. Franza, Jr., and A. R. Krainer. 1998. Regulated tissue-specific expression of antagonistic pre-mRNA splicing factors. RNA 4:430-444. [PMC free article] [PubMed] [Google Scholar]
- 20.Hanes, J., H. von der Kammer, J. Klaudiny, and K. H. Scheit. 1994. Characterization by cDNA cloning of two new human protein kinases. Evidence by sequence comparison of a new family of mammalian protein kinases. J. Mol. Biol. 244:665-672. [DOI] [PubMed] [Google Scholar]
- 21.Harlow, E., and D. Lane. 1998. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 22.Howell, B. W., D. E. Afar, J. Lew, E. M. Douville, P. L. Icely, D. A. Gray, and J. C. Bell. 1991. STY, a tyrosine-phosphorylating enzyme with sequence homology to serine/threonine kinases. Mol. Cell. Biol. 11:568-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hudmon, A., and H. Schulman. 2002. Neuronal Ca2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu. Rev. Biochem. 71:473-510. [DOI] [PubMed] [Google Scholar]
- 24.Hunter, T. 1998. The phosphorylation of proteins on tyrosine: its role in cell growth and disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 353:583-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huse, M., and J. Kuriyan. 2002. The conformational plasticity of protein kinases. Cell 109:275-282. [DOI] [PubMed] [Google Scholar]
- 26.Katsu, R., H. Onogi, K. Wada, Y. Kawaguchi, and M. Hagiwara. 2002. Novel SR-related protein Clasp specifically interacts with inactivated Clk4 and induces the exon EB inclusion of Clk. J. Biol. Chem. 277:44220-44228. [DOI] [PubMed] [Google Scholar]
- 27.Kohtz, J. D., S. F. Jamison, C. L. Will, P. Zuo, R. Luhrmann, M. A. Garcia-Blanco, and J. L. Manley. 1994. Protein-protein interactions and 5′-splice-site recognition in mammalian mRNA precursors. Nature 368:119-124. [DOI] [PubMed] [Google Scholar]
- 28.Koizumi, J., Y. Okamoto, H. Onogi, A. Mayeda, A. R. Krainer, and M. Hagiwara. 1999. The subcellular localization of SF2/ASF is regulated by direct interaction with SR protein kinases (SRPKs). J. Biol. Chem. 274:11125-11131. [DOI] [PubMed] [Google Scholar]
- 29.Kojima, T., T. Zama, K. Wada, H. Onogi, and M. Hagiwara. 2001. Cloning of human PRP4 reveals interaction with Clk1. J. Biol. Chem. 276:32247-32256. [DOI] [PubMed] [Google Scholar]
- 30.Lai, M. C., R. I. Lin, and W. Y. Tarn. 2001. Transportin-SR2 mediates nuclear import of phosphorylated SR proteins. Proc. Natl. Acad. Sci. USA 98:10154-10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lai, M. C., R. I. Lin, S. Y. Huang, C. W. Tsai, and W. Y. Tarn. 2000. A human importin-beta family protein, transportin-SR2, interacts with the phosphorylated RS domain of SR proteins. J. Biol. Chem. 275:7950-7957. [DOI] [PubMed] [Google Scholar]
- 32.Lee, K., C. Du, M. Horn, and L. Rabinow. 1996. Activity and autophosphorylation of LAMMER protein kinases. J. Biol. Chem. 271:27299-27303. [DOI] [PubMed] [Google Scholar]
- 33.Li, S., A. Strelow, E. J. Fontana, and H. Wesche. 2002. IRAK-4: a noel member of the IRAK family with the properties of an IRAK-kinase. Proc. Natl. Acad. Sci. USA 99:5567-5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manley, J. L., and R. Tacke. 1996. SR proteins and splicing control. Genes Dev. 10:1569-1579. [DOI] [PubMed] [Google Scholar]
- 35.Menegay, H. J., M. P. Myers, F. M. Moeslein, and G. E. Landreth. 2000. Biochemical characterization and localization of the dual specificity kinase CLK1. J. Cell Sci. 113:3241-3253. [DOI] [PubMed] [Google Scholar]
- 36.Mermoud, J. E., P. T. Cohen, and A. I. Lamond. 1994. Regulation of mammalian spliceosome assembly by a protein phosphorylation mechanism. EMBO J. 13:5679-5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Misteli, T. 1999. RNA splicing: what has phosphorylation got to do with it? Curr. Biol. 9:R198-R200. [DOI] [PubMed] [Google Scholar]
- 38.Misteli, T., J. F. Caceres, and D. L. Spector. 1997. The dynamics of a pre-mRNA splicing factor in living cells. Nature 387:523-527. [DOI] [PubMed] [Google Scholar]
- 39.Misteli, T., J. F. Caceres, J. Q. Clement, A. R. Krainer, M. F. Wilkinson, and D. L. Spector. 1998. Serine phosphorylation of SR proteins is required for their recruitment to sites of transcription in vivo. J. Cell Biol. 143:297-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Modrek, B., A. Resch, C. Grasso, and C. Lee. 2001. Genome-wide detection of alternative splicing in expressed sequences of human genes. Nucleic Acids Res. 29:2850-2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moeslein, F. M., M. P. Myers, and G. E. Landreth. 1999. The CLK family kinases, CLK1 and CLK2, phosphorylate and activate the tyrosine phosphatase, PTP-1B. J. Biol. Chem. 274:26697-26704. [DOI] [PubMed] [Google Scholar]
- 42.Murray, M. V., R. Kobayashi, and A. R. Krainer. 1999. The type 2C Ser/Thr phosphatase PP2Cγ is a pre-mRNA splicing factor. Genes Dev. 13:87-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nayler, O., S. Stamm, and A. Ullrich. 1997. Characterization and comparison of four serine- and arginine-rich (SR) protein kinases. Biochem. J. 326:693-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nayler, O., F. Schnorrer, S. Stamm, and A. Ullrich. 1998. The cellular localization of the murine serine/arginine-rich protein kinase CLK2 is regulated by serine 141 autophosphorylation. J. Biol. Chem. 273:34341-34348. [DOI] [PubMed] [Google Scholar]
- 45.Neugebauer, K. M., J. A. Stolk, and M. B. Roth. 1995. A conserved epitope on a subset of SR proteins defines a larger family of pre-mRNA splicing factors. J. Cell Biol. 129:899-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nikolakaki, E., C. Du, J. Lai, T. Giannakouros, L. Cantley, and L. Rabinow. 2002. Phosphorylation by LAMMER protein kinases: determination of a consensus site, identification of in vitro substrates, and implications for substrate preferences. Biochemistry 41:2055-2066. [DOI] [PubMed] [Google Scholar]
- 47.Prasad, J., K. Colwill, T. Pawson, and J. L. Manley. 1999. The protein kinase Clk/Sty directly modulates SR protein activity: both hyper- and hypophosphorylation inhibit splicing. Mol. Cell. Biol. 19:6991-7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sacco-Bubulya, P., and D. L. Spector. 2002. Disassembly of interchromatin granule clusters alters the coordination of transcription and pre-mRNA splicing. J. Cell Biol. 156:425-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanford, J. R., and J. P. Bruzik. 2001. Regulation of SR protein localization during development. Proc. Natl. Acad. Sci. USA 98:10184-10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schlessinger, J. 2000. Cell signaling by receptor tyrosine kinases. Cell 103:211-225. [DOI] [PubMed] [Google Scholar]
- 51.Shen, B., and J. L. Manley. 2002. Pelle kinase is activated by autophosphorylation during Toll signaling in Drosophila. Development 129:1925-1933. [DOI] [PubMed] [Google Scholar]
- 52.Stojdl, D. F., and J. C. Bell. 1999. SR protein kinases: the splice of life. Biochem. Cell Biol. 77:293-298. [PubMed] [Google Scholar]
- 53.Tacke, R., Y. Chen, and J. L. Manley. 1997. Sequence-specific RNA binding by an SR protein requires RS domain phosphorylation: creation of an SRp40-specific splicing enhancer. Proc. Natl. Acad. Sci. USA 94:1148-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tacke, R., M. Tohyama, S. Ogawa, and J. L. Manley. 1998. Human Tra2 proteins are sequence-specific activators of pre-mRNA splicing. Cell 93:139-148. [DOI] [PubMed] [Google Scholar]
- 55.Tacke, R., and J. L. Manley. 1999. Determinants of SR protein specificity. Curr. Opin. Cell Biol. 11:358-362. [DOI] [PubMed] [Google Scholar]
- 56.Tazi, J., U. Kornstadt, F. Rossi, P. Jeanteur, G. Cathala, C. Brunel, and R. Luhrmann. 1993. Thiophosphorylation of U1-70K protein inhibits pre-mRNA splicing. Nature 363:283-286. [DOI] [PubMed] [Google Scholar]
- 57.van der Houven van Oordt, W., M. T. Diaz-Meco, J. Lozano, A. R. Krainer, J. Moscat, and J. F. Caceres. 2000. The MKK(3/6)-p38-signaling cascade alters the subcellular distribution of hnRNP A1 and modulates alternative splicing regulation. J. Cell Biol. 149:307-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang, H. Y., W. Lin, J. A. Dyck, J. M. Yeakley, Z. Songyang, L. C. Cantley, and X. D. Fu. 1998. SRPK2: a differentially expressed SR protein-specific kinase involved in mediating the interaction and localization of pre-mRNA splicing factors in mammalian cells. J. Cell Biol. 140:737-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang, J., and J. L. Manley. 1995. Overexpression of the SR proteins ASF/SF2 and SC35 influences alternative splicing in vivo in diverse ways. RNA 1:335-346. [PMC free article] [PubMed] [Google Scholar]
- 60.Wang, Q. M., C. J. Fiol, A. A. DePaoli-Roach, and P. J. Roach. 1994. Glycogen synthase kinase-3 beta is a dual specificity kinase differentially regulated by tyrosine and serine/threonine phosphorylation. J. Biol. Chem. 269:14566-14574. [PubMed] [Google Scholar]
- 61.Wu, J. Y., and T. Maniatis. 1993. Specific interactions between proteins implicated in splice site selection and regulated alternative splicing. Cell 75:1061-1070. [DOI] [PubMed] [Google Scholar]
- 62.Xiao, S. H., and J. L. Manley. 1997. Phosphorylation of the ASF/SF2 RS domain affects both protein-protein and protein-RNA interactions and is necessary for splicing. Genes Dev. 11:334-344. [DOI] [PubMed] [Google Scholar]
- 63.Xiao, S. H., and J. L. Manley. 1998. Phosphorylation-dephosphorylation differentially affects activities of splicing factor ASF/SF2. EMBO J. 17:6359-6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yun, B., K. Lee, R. Farka, C. Hitte, and L. Rabinow. 2000. The LAMMER protein kinase encoded by the Doa locus of Drosophila is required in both somatic and germline cells and is expressed as both nuclear and cytoplasmic isoforms throughout development. Genetics 156:749-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zahler, A. M., K. M. Neugebauer, W. S. Lane, and M. B. Roth. 1993. Distinct functions of SR proteins in alternative pre-mRNA splicing. Science 260:219-222. [DOI] [PubMed] [Google Scholar]