Abstract
The heterochromatin domain at the mat locus of Schizosaccharomyces pombe is bounded by the IR-L and IR-R barriers. A genetic screen for mutations that promote silencing beyond IR-L revealed a novel gene named epe1, encoding a conserved nuclear protein with a jmjC domain. Disruption of epe1 promotes continuous spreading of heterochromatin-associated histone modifications and Swi6 binding to chromatin across heterochromatic barriers. It also enhances position effect variegation at heterochromatic domains, suppresses mutations in silencing genes, and stabilizes the repressed epigenetic state at the mat locus. However, it does not enhance silencing establishment. Our analysis suggests that the jmjC domain is essential for Epe1 activity and that Epe1 counteracts transcriptional silencing by negatively affecting heterochromatin stability. Consistent with this proposition, the meiotic stability of established heterochromatin beyond IR-L is diminished by Epe1 activity, and overexpression of Epe1 disrupts heterochromatin through acetylation of H3-K9 and H3-K14 and methylation of H3-K4. Furthermore, overexpression of Epe1 elevates the rate of chromosome loss. We propose that Epe1 helps control chromatin organization by down-regulating the stability of epigenetic marks that govern heterochromatization.
Epigenetic control of gene expression by clonally inherited higher-order chromatin structures accounts for diverse biological phenomena, such as dosage compensation, imprinting, and position effect variegation (PEV) (26, 44, 45). In addition to transcription regulation, epigenetic effects have an important impact on cellular differentiation, accurate chromosome segregation, recombination, neocentromere maintenance, and mating-type switching in yeast (reviewed in references 1, 18, 24, 25, 28, 37, and 47). Molecular models that explain chromosomal inheritance of epigenetic states assume a long-range memory mechanism that marks imprinted chromosomal domains by self-templating higher-order chromatin structures and covalent modification of DNA (20, 46). Evidence for chromatin modifications in imprinted domains has been obtained through experiments that demonstrate the association of silent chromosomal regions with modified histones and chromatin-associated nonhistone proteins like Saccharomyces cerevisiae SIR3, Drosophila HP1, and Schizosaccharomyces pombe Swi6 (15, 22, 33, 36).
The mat locus of S. pombe shares an extended sequence homology with the centromeric outer repeats (cenH) and provides a useful paradigm for the highly conserved process of heterochromatin assembly and inheritance (17, 18, 25). Constitutive heterochromatin extends along the 15-kb mat2-K-mat3 interval that is bound on its centromere-proximal end by the REII protosilencer (Fig. 1A) (7, 21, 33, 41). Silencing diminishes gradually at the L region as the distance from REII toward the IR-L heterochromatin barrier increases (6). IR-L, like its IR-R homologous counterpart at the centromere-distal end of the heterochromatic domain, is a distinct transition point for Swi6 association with chromatin and histone H3 methylation patterns. Chromatin on the centromere-distal side of IR-L binds Swi6 and is associated with histone H3 Lys-9 methylation, whereas chromatin on its centromere-proximal side is associated with histone H3 Lys-4 methylation (35). The mechanism by which these barriers prevent heterochromatin encroachment toward euchromatic genes is not yet understood (40).
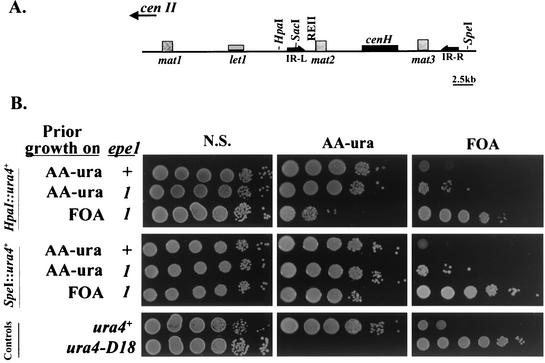
FIG. 1.
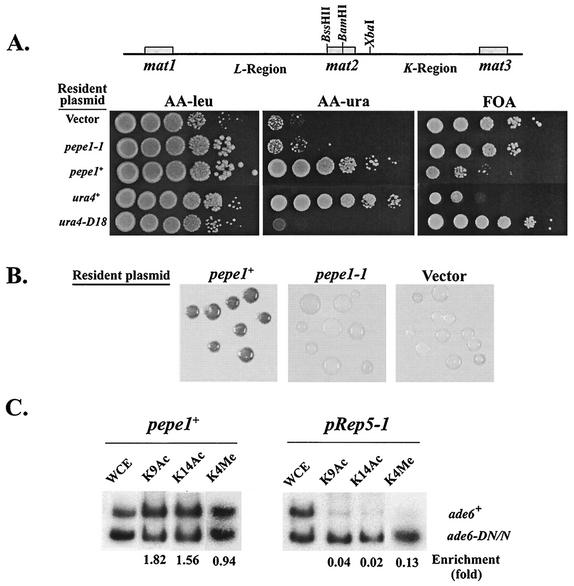
The epe1-1 mutation promotes silencing beyond the heterochromatin barriers at IR-L and IR-R. (A) The mat locus of S. pombe. The mat2-P and mat3-M mating type donor cassettes are located within a heterochromatin domain that is flanked by the IR-L and IR-R barriers. mat2-P and mat3-M are separated from each other by a repressed region named K. mat2-P is separated from the transcriptionally active mat1 by the L region. The locations of the mat cassettes, the essential let1 gene, the cen2 homology (cenH), IR-L, IR-R, and relevant restriction sites are indicated. (B) Cells of strains with a ura4+ insertion at the HpaI site in the L region or at the SpeI site on the centromere-distal side of IR-R were inoculated from colonies on the indicated media into a nonselective liquid medium (YEA) and grown to a density of 2 × 107 cells per ml. The indicated media were spotted with 10-fold serial dilutions of the cultures (N.S., nonselective medium), and plates were incubated at 33°C for 4 days. The epe1+ and epe1-1 strains with a ura4+ insertion at the HpaI site are AP219 and AP2007, respectively. The epe1+ and epe1-1 strains with a ura4+ insertion at the SpeI site are AP876 and AP2050, respectively. The control strains are AP114 (ura4+) and SP1172 (ura4-D18).
To explore conditions that overcome the heterochromatin barrier, we genetically screened for mutations that confer silencing on reporter genes at the centromere-proximal side of IR-L. This screen revealed a novel gene, epe1, which codes for a nuclear protein with a jmjC domain. We show that epe1+ product is an antagonist of heterochromatization and that its inactivation stimulates continuous spreading of heterochromatin-associated histone modifications and Swi6 binding to chromatin across the IR-L barrier. Furthermore, we present evidence that Epe1 negatively controls heterochromatin stability. This work highlights the role of Epe1, and possibly related jmjC domain proteins of higher organisms, in regulating chromatin organization by modulating heterochromatization.
MATERIALS AND METHODS
Strains, plasmids, and genetic procedures.
All strains used in this study and their genotypes are listed in Table 1. Standard genetic crosses and transformation procedures (31) were used in strain construction. For genetic mapping of epe1-1, a strain collection with random chromosomal insertions of ura4+ was constructed by transforming AP2001 with a PCR-generated ura4+ cassette as described before (11). A screen for strains with the ura4+ insertion genetically linked to epe1-1 was performed by crossing isolated clones of the strain collection to AP182 and scoring mat1-P ura4+ epe1-1 recombinants. A strain with a ura4+ insertion within C11E10.09c (UM234) and the ade6 marker on chromosome III was used in genetic crosses for precise localization of epe1-1. To overexpress Epe1, cells were transformed with pRep-1 (30) derivatives expressing epe1+ from an nmt promoter. To overexpress mutated epe1 alleles, cells were transformed with plasmids harboring epe1 with the respective mutations. epe1+ and epe1-1 were constructed by PCR amplification from genomic DNA with the primers 5′-ATCCCTCGAGTCAAAGTGGATTGATGCTC-3′ and 5′-TAGAAGTGCGCTTGTGCTAAATCG-3′. The epe1-Y307A allele was synthesized by in vitro overlap-extension PCR as described previously (27), with the following pairs of primers: 5′-TTGgcGTAAGCTGAGGATCCACC-3′ and 5′-GCGTCGACGATGGATTCCTGGCTTGAATACGT-3′ and 5′-ATCCTCAGCTTACgcCAATATTC-3′ and 5′-TTACACCTAGTATTTTCTCGC-3′ (mutations in internal primers are indicated by lowercase letters). To construct pepe1-Y307A, a BspE1 fragment on pepe1+ was replaced with a homologous fragment carrying the Y307A mutation. The Epe1-green fluorescent protein (GFP) fusion protein was expressed from pepe1-GFP. To construct pepe1-GFP, the epe1+ open reading frame was PCR amplified from genomic DNA with primers that incorporate SalI and AseI restriction sites (5′-ACGCGTCGACAGACTAGCACCTCTGGACCGAAGC-3′ and 5′-ACGCATTAATATGGATTCCTGGCTTGAATACG-3′). A SalI-AseI fragment of the PCR fragment was then ligated to a SalI-NdeI fragment of pRep1-GFP (39). Diploid strains were constructed by mating AP161 and AP182 (epe1+/epe1+), AP161 and AP2002 (epe1+/epe1-1), and AP2002 and AP2003 (epe1-1/epe1-1). Diploids were isolated after 8 to 9 h of incubation on sporulation medium (PM-N) by selection on minimal media with depletion of leucine and histidine (AA −leu −his). Diploidy was checked routinely by scoring tetrads after incubation on sporulation medium.
TABLE 1.
Characteristics of S. pombe strains used in this study
| Strain | mat1 allele | Insertion(s)/deletion(s) | Auxotrophic marker(s) | Other mutation(s) | Source or reference |
|---|---|---|---|---|---|
| AP114 | mat1-M-smt0 | leu1-32 his2 ade6-210 | This study | ||
| AP128 | mat1-PΔ17::LEU2 | ura4-D18 | 7 | ||
| AP129 | mat1-M-smt0 | leu1-32 his2 ura4-D18 | This study | ||
| AP134 | mat1-M-smt0 | L(SacI)::ade6+ | leu1-32 his2 ade6-210 ura4-D18 | clr1-165 | This study |
| AP136 | mat1-M-smt0 | L(SacI)::ade6+ | leu1-32 his2 ade6-210 ura4-D18 | 6 | |
| AP138 | mat1-PΔ17::LEU2 | L(SacI)::ade6+ | leu1-32 his2 ade6-210 ura4-D18 | clr2-760 | This study |
| AP139 | mat1-M-smt0 | L(SacI)::ade6+ | leu1-32 his2 ade6-210 ura4-D18 | swi6-115 | 6 |
| AP147 | mat1-M-smt0 | L(SacI)::ade6+ | leu1-32 his2 ade6-210 ura4-D18 | clr3-735 | This study |
| AP161 | mat1-M-smt0 | L(SacI)::ade6+mat3(EcoRV)::ura4+ | leu1-32 his2 ade6-210 ura4-D18 | 6 | |
| AP182 | mat1-PΔ17::LEU2 | L(SacI)::ade6+ | leu1-32 his2 ade6-210 ura4-D18 | This study | |
| AP208 | mat1-M-smt0 | L(HpaI)::ade6+ | leu1-32 his2 ade6-210 ura4-D18 | This study | |
| AP219 | mat1-M-smt0 | L(HpaI)::ura4+ | leu1-32 his2 ade6-210 ura4-D18 | This study | |
| AP222 | mat1-PΔ17::LEU2 | L(HpaI)::ura4+ | leu1-32 his2 ade6-210 ura4-D18 | This study | |
| AP259 | mat1-M-smt0 | ura4::ade6+-cenH | leu1-32 his2 ade6-210 | 7 | |
| AP264 | mat1-M-smt0 | ura4::ade6+ | leu1-32 his2 ade6-210 | 7 | |
| AP274 | mat1-M-smt0 | ade6::ura4+-cenH | leu1-32 his2 ura4-D18 | This study | |
| AP284 | mat1-M-smt0 | mat2(BamHI)::ade6+REIIΔ K(XbaI)::ura4+ | leu1-32 his2 ade6-DN/N ura4-D18 | This study | |
| AP292 | mat1-M-smt0 | L(PvuII)::ade6+ (HpaI)::ura4+ | leu1-32 his2 ade6-210 ura4-D18 | This study | |
| AP312 | mat1-M-smt0 | L(BssHII)::ade6+K(XbaI)::ura4+ | leu1-32 his2 ade6-210 ura4-D18 | 6 | |
| AP393 | mat1-M-smt0 | mat2(BamHI)::ade6+ | leu1-32 his2 ade6-210 ura4-D18 | This study | |
| AP733 | mat1-M-smt0 | KΔ::ade6+ | leu1-32 his2 ade6-DN/N ura4-D18 | This study | |
| AP836 | mat1-M-smt0 | L(PvuII)::ade6+let1::ura4+ura4::let1+ | leu1-32 his2 ade6-DN/N | This study | |
| AP867 | mat1-M-smt0 | L(SacI)::ade6+ | leu1-32 his2 ade6-210 ura4-D18 | rik1Δ::LEU2 | This study |
| AP869 | mat1-PΔ17::LEU2 | L(SacI)::ade6+ | leu1-32 his2 ade6-210 ura4-D18 | clr6-1 | This study |
| AP870 | mat1-PΔ17::LEU2 | L(SacI)::ade6+ | leu1-32 his2 ade6-210 ura4-D18 | clr3Δ::kanMX6 | This study |
| AP873 | mat1-M-smt0 | L(SacI)::ade6+ | leu1-32 his2 ade6-210 ura4-D18 | clr4Δ::kanMX6 | This study |
| AP874 | h+ | leu1-32 ade6-210 | ch16 | This study | |
| AP876 | mat1-M-smt0 | L(HpaI)::ade6+mat3-M(SpeI)::ura4+ | leu1-32 his2 ade6-210 ura4-D18 | This study | |
| AP877 | mat1-M-smt0 | L(HpaI)::ura4+mat3-M(SpeI)::ade6+ | leu1-32 his2 ade6-210 ura4-D18 | This study | |
| AP880 | mat1-M-smt0 | L(SacI)::ade6+ | leu1-32 his2 ade6-210 ura4-D18 | clr6-1 clr3Δ::kanMX6 | This study |
| AP2001 | mat1-M-smt0 | L(HpaI)::ade6+ | leu1-32 his2 ade6-210 ura4-D18 | epe1-1 | This study |
| AP2002 | mat1-PΔ17:LEU2 | L(SacI)::ade6+ | leu1-32 his2 ade6-210 ura4-D18 | epe1-1 | This study |
| AP2003 | mat1-M-smt0 | L(SacI)::ade6+mat3(EcoRV)::ura4+ | leu1-32 his2 ade6-210 ura4-D18 | epe1-1 | This study |
| AP2005 | mat1-M-smt0 | L(SacI)::ade6+ | leu1-32 his2 ade6-210 ura4-D18 | epe1-1 | This study |
| AP2007 | mat1-M-smt0 | L(HpaI)::ura4+ | leu1-32 his2 ade6-210 ura4-D18 | epe1-1 | This study |
| AP2009 | mat1-PΔ17::LEU2 | L(SacI)::ade6+ | leu1-32 his2 ade6-210 ura4-D18 | epe1-1 clr1-165 | This study |
| AP2012 | mat1-M-smt0 | L(SacI)::ade6+ | leu1-32 his2 ade6-210 ura4-D18 | epe1-1 clr2-760 | This study |
| AP2014 | mat1-PΔ17::LEU2 | L(SacI)::ade6+ | leu1-32 his2 ade6-210 ura4-D18 | epe1-1 swi6-115 | This study |
| AP2022 | mat1-M-smt0 | ura4::ade6+-cenH | leu1-32 his2 ade6-210 | epe1-1 | This study |
| AP2023 | mat1-M-smt0 | ade6::ura4+-cenH | leu1-32 his2 ura4-D18 | epe1-1 | This study |
| AP2026 | mat1-M-smt0 | L(SacI)::ade6+otr1L(dh/BglII)::ura4+oriI | leu1-32 his2 ade6-210 ura4-D18 | epe1-1 | This study |
| AP2027 | mat1-M-smt0 | L(SacI)::ade6+ TM1(NcoI)::ura4+ | leu1-32 his2 ade6-210 ura4-D18 | epe1-1 | This study |
| AP2029 | mat1-M-smt0 | L(PvuII)::ade6+let1::ura4+ura4::let1+ | leu1-32 his2 ade6-DN/N | epe1-1 | This study |
| AP2032 | mat1-M-smt0 | L(SacI)::ade6+ | leu1-32 his2 ade6-210 ura4-D18 | epe1-1 clr4Δ::kanMX6 | This study |
| AP2037 | mat1-PΔ17::LEU2 | L(SacI)::ade6+ | leu1-32 his2 ade6-210 ura4-D18 | epe1-1 cl6-1 | This study |
| AP2038 | mat1-PΔ17::LEU2 | L(SacI)::ade6+ | leu1-32 his2 ade6-210 ura4-D18 | epe1-1 clr3Δ::kanMX6 | This study |
| AP2039 | mat1-M-smt0 | L(SacI)::ade6+ | leu1-32 his2 ade6-210 ura4-D18 | epe1::ura4+ | This study |
| AP2040 | mat1-M-smt0 | L(SacI)::ade6+ | leu1-32 his2 ade6-210 ura4-D18 | epe1::ura4+rik1Δ::LEU2 | This study |
| AP2044 | mat1-M-smt0 | ura4::ade6+ | leu1-32 his2 ade6-210 | epe1-1 | This study |
| AP2045 | mat1-M-smt0 | L(PvuII)::ade6+ (HpaI)::ura4+ | leu1-32 his2 ade6-210 ura4-D18 | This study | |
| AP2050 | mat1-M-smt0 | L(HpaI)::ade6+mat3-M(SpeI)::ura4+ | leu1-32 his2 ade6-210 ura4-D18 | epe1-1 | This study |
| AP2051 | mat1-M-smt0 | L(SacI)::ade6+ | leu1-32 his2 ade6-210 ura4-D18 | epe1-1 clr6-1 clr3Δ::kanMX6 | This study |
| AP2053 | mat1-M-smt0 | L(HpaI)::ura4+mat3-M(SpeI)::ade6+ | leu1-32 his2 ade6-210 ura4-D18 | epe1-1 | This study |
| AP2056 | mat1-M-smt0 | KΔ::ade6+ | leu1-32 his2 ade6-DN/N ura4-D18 | epe1::ura4+ | This study |
| UM234 | mat1-M-smt0 | L(HpaI)::ade6+ | leu1-32 his2 ade6-210 ura4-D18 | C11E10.09c::ura4+epe1-1 | This study |
| SP1172 | mat1-M-smt0 | leu1-32 his2 ade6-210 ura4-D18 | A. J. Klar | ||
| FY312 | h+ | TM1(NcoI)::ura4+ | leu1-32 ade6-210 ura4-D18 | R. C. Allshire | |
| FY988 | h+ | otr1L(dh/BglII)::ura4+oriI | leu1-32 ade6-210 ura4-DS/E | R. C. Allshire |
Culture conditions.
Strains were grown on rich medium (YEA), adenine-deficient rich medium (YE), sporulation medium (PM-N), and minimal medium (AA) supplemented with the appropriate growth requirements (31). Cells were selected for the Ura− phenotype on 5-fluoroorotic acid (FOA) medium, since in this medium, ura4 expression leads to the synthesis of a toxic product from FOA (9). FOA medium was minimal medium supplemented with 1 mg of FOA per ml and 0.1 mg of uracil per ml. Liquid cultures were grown at 30°C, and plates were incubated at the indicated temperature. For scoring Ade phenotypes on YE plates, the standard incubation periods were 4 days at 30°C. To monitor ade6+ expression by cells harboring pIRT1 derivatives, cells were plated on AA −leu medium with a low concentration of adenine (15 mg/liter).
Isolation of epe mutants.
Strain AP208 was mutagenized by 2% ethyl methansulfanate treatment. Cells were washed with water and then plated on YE medium to screen for Ade− (red) candidate clones. The isolated mutants were crossed with AP222, and the respective mat1-P L(HpaI)::ura4+ recombinants were tested for silencing of the ura4+ reporter by plating cells on selective (AA −ura) and counterselective (FOA) media.
ChIP.
The chromatin immunoprecipitation (ChIP) procedure was performed with purified anti-Swi6 antiserum, raised against full-length recombinant Swi6 protein or with antibodies against H3 peptides with dimethyl H3-K9, dimethyl H3-K4, acetyl H3-K9, or acetyl H3-K14 (Upstate Biotechnology) as described previously (33, 35).
Photography and microscopy.
Colonies were photographed with overhead illumination, using Kodak Ektachrome slide film, or by a digital Nikon DIX camera. Slides were computer scanned with the Adobe PhotoShop program. Microscopic images were acquired with Nikon microscope and spot image software (Diagnostic Instrument, Sterling Heights, Mich.).
Protein sequence analysis.
A BLAST search (http://www.ncbi.nlm.nih.gov/BLAST) for related proteins was performed with the deduced 948-amino-acid (aa) Epe1 sequence against the nonredundant protein database. Comparison of related amino acid sequences and the display of the resulting alignments were performed with the Pileup procedure of the GCG Wisconsin Package, version 10.2 (Genetics Computer Group, Madison, Wis.) with default parameters. A search for known protein sequence motifs was performed with the Conserved Domain Database (29).
RESULTS
The epe1-1 mutation overcomes the IR-L and IR-R barriers by enhancing heterochromatization.
To screen for mutations that overcome the heterochromatin barrier at IR-L, we mutated cells with an ade6+ insertion beyond the barrier (HpaI; Fig. 1A) and plated survivors on a low-adenine medium (YE), in which Ade− colonies are distinguishable from Ade+ colonies by their red color. To confirm that the Ade− phenotype reflects silencing at the centromere-proximal side of IR-L, mutations were crossed into a strain with a ura4+ insertion at the HpaI site (AP219), and the respective L(HpaI)::ura4+ progeny strains were tested on selective (AA −ura) and counterselective (FOA) media for their Ura phenotype. If PEV extends across IR-L, both Ura+ and Ura− (FOA resistant) colonies are expected. Seventeen independent epe (enhancement of position effect) mutations were isolated, one of which, epe1-1, is described here.
epe1-1 derivatives with an ade6+ insertion at the HpaI site displayed an epigenetic switch between alternative states of ade6+ expression. The alternative phenotypes were clonally inherited, because only a small proportion of the cells from Ade− or Ade+ colonies switched to the opposite phenotype upon replating (Table 2). Likewise, epe1-1 mutants with a ura4+ insertion at the HpaI site yielded colonies on AA −ura or FOA plates, and growth of serial dilutions of cells from colonies on the respective media indicated clonal inheritance of the alternative expression states (Fig. 1B). To test whether a form of epe1-1 also promotes spreading of silencing beyond the IR-R barrier, we tested its effect on expression of ura4+ inserted at the SpeI site (Fig. 1B). Results indicate that the epe1-1 mutation promotes the establishment of heritable repression beyond the IR-R heterochromatin barrier.
TABLE 2.
The epe1-1 mutation enhances PEV and overcomes the IR-L heterochromatin barrier
| Strain | Relevant genotypea | % of plated cells fromb:
|
|||||
|---|---|---|---|---|---|---|---|
| White (Ade+) colonies that were:
|
Red (Ade−) colonies that were:
|
||||||
| Red | Pink | White | Red | Pink | White | ||
| AP208 | epe1+L(HpaI)::ade6+ | <0.01 | <0.01 | 100 | NRc | NR | NR |
| AP2001 | epe1-1 L(HpaI)::ade6+ | 0.7 ± 0.03 | 1.3 ± 0.06 | 98.0 ± 0.12 | 96.0 ± 2.8 | 2.6 ± 1.4 | 1.4 ± 0.9 |
| AP136 | epe1+L(SacI)::ade6+ | <0.05 | 29.1 ± 5.3 | 70.9 ± 5.3 | <0.05 | 81.8 ± 3.9 | 18.1 ± 3.6 |
| AP2005 | epe1-1 L(SacI)::ade6+ | NR | NR | NR | 100 | <0.01 | <0.01 |
| AP2039 | epe1::ura4+L(SacI)::ade6+ | NR | NR | NR | 100 | <0.01 | <0.01 |
| AP259 | epe1+ura4::cenH3.6-ade6+ | <0.1 | 1.8 ± 0.8 | 98.2 ± 0.8 | 24.9 ± 6.1 | 41.3 ± 6.3 | 33.8 ± 3.3 |
| AP2022 | epe1-1 ura4::cenH3.6-ade6+ | 3.8 ± 2.9 | 7.3 ± 3.4 | 88.9 ± 4.5 | 91.5 ± 4.2 | 4.8 ± 2.1 | 3.7 ± 1.8 |
Complete genotypes are presented in Table 1.
Red, pink, and white indicate colonies displaying Ade−, partial ade6 repression, and Ade+ phenotypes on low-adenine medium, respectively.
NR, not relevant.
Mutations of two types, not necessarily mutually exclusive, may induce repression at the centromere-proximal side of IR-L: those that directly impair the heterochromatin barrier and those that enhance PEV, such that the propagating heterochromatin can override the barrier. To determine whether the epe1-1 mutation enhances PEV within the mat2-IR-L interval, we introduced it into a strain with an ade6+ insertion within the SacI site (Fig. 1A) and examined colony color on YE medium. Normally, reporter genes at the SacI site are subjected to PEV, with most colonies exhibiting an Ade+ phenotype, while the rest are partially repressed (light pink colonies on YE medium) (6). In contrast, all colonies of the isogenic epe1-1 mutant (AP2005) exhibited full repression of ade6+ (Table 2), thus indicating that the epe1-1 mutation enhances heterochromatization within the mat2-IR-L interval, to the extent that the propagating heterochromatin overcomes the barrier at IR-L.
epe1-1 enhances PEV within the centromeres' outer repeats and cenH-dependent silencing at an ectopic site.
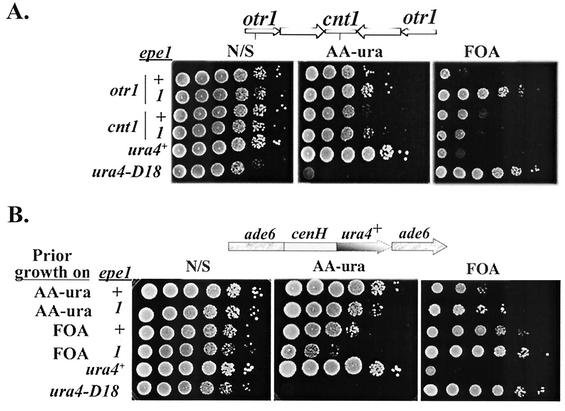
Because of the similarity between heterochromatin assembly mechanisms at the mat locus and the centromeres' outer repeats (2), we explored the possibility that the epe1-1 mutation may also enhance silencing at the centromeres. To this end, 10-fold serial dilutions from cultures of epe1+ and epe1-1 derivatives, with ura4+ insertions within cen1 inner core (cnt1) or outer repeats (otr1), were spotted on selective (AA −ura) and counterselective (FOA) media (Fig. 2A). The epe1-1 mutation enhanced silencing within centromere I outer repeats, but had no detectable effect on silencing within its inner core, in which the chromatin modification mechanism is distinct from that at the outer repeats or at the mat locus (36).
FIG. 2.
epe1-1 enhances PEV at the centromeres and cenH-dependent silencing at an ectopic site. (A) Cells of strains with ura4+ insertions within centromere 1 outer repeats (otr1) or inner core (cnt1) were grown on nonselective (N/S) medium (YEA), subjected to 10-fold serial dilutions, and applied as spots to plates with the indicated media. Because lower temperature enhances repression (3), the effect of epe1 genotype on centromere silencing was measured at 36°C. Vertical lines on the cen1 map indicate ura4+ insertion sites. The strains with a ura4+ insertion within otr1 are FY988 (epe1+) and AP2026 (epe1-1). The strains with a ura4+ insertion within cnt1 are FY312 (epe1+) and AP2027 (epe1-1). (B) Cells of strains with a cenH-ura4+ insertion within ade6 and the indicated epe1 genotype were grown on AA −ura or FOA plates and then transferred to nonselective medium. The indicated media were spotted with 10-fold serial dilutions of the cultures and incubated at 33°C for 4 days. The epe1+ strain is AP274. The epe1-1 strain is AP2023.
The mat silent domain and the centromeres' outer repeats share a 4.3-kb sequence homology (21), and fragments of the shared homology (cenH) promote heterochromatization at an ectopic site (7). To ascertain whether the epe1-1 mutation enhances cenH-mediated heterochromatization, we determined its effect on reporter gene expression in strains with a cenH-ade6+ or cenH-ura4+ insertion at an ectopic site. Consistent with the indication that cenH promotes local heterochromatization (7), both the epe1+ and epe1-1 derivatives, with a cenH-ura4+ insertion at the ectopic site, produced colonies on AA −ura and FOA media. However, the stability of the repressed state, measured by plating serial dilutions of cells from FOAR colonies onto AA −ura and FOA media, was enhanced by the epe1-1 mutation (Fig. 2B). Likewise, the stability of the Ade− epitype in cells of the ura4::cenH-ade6+ strain was enhanced by the epe1-1 mutation (Table 2). This implies that the epe1-1 mutation enhances cenH-mediated heterochromatization and suggests that the epe1+ product plays a general role in the control of chromatin remodeling.
epe1-1 is a recessive nonsense mutation in a gene that encodes a jmjC domain nuclear protein.
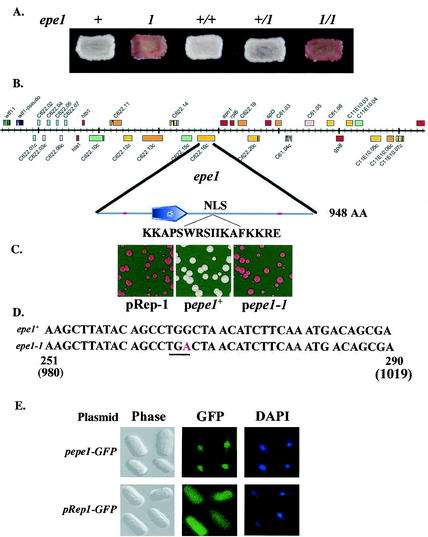
We characterized the epe1-1 mutation in terms of dominance or recessiveness, by constructing an epe1-1/epe1+ diploid with an ade6+ insertion at the SacI site within the L region (Fig. 1A) and comparing its Ade phenotype to that of isogenic homoallelic diploids. The results indicate that epe1-1 is a recessive mutation. The Ade+ phenotype of the heteroallelic strain was similar to that of the epe1+/epe1+ strain, whereas the epe1-1/epe1-1 strain displayed an Ade− phenotype (Fig. 3A).
FIG. 3.
epe1-1 is a recessive nonsense mutation in a gene encoding a jmjC domain nuclear protein. (A) Cells of haploid and diploid strains with an ade6+ insertions at the SacI site in the L region (Fig. 1A) were patched onto a YE plate and photographed after 4 days at 30°C. The haploid strains are AP161 (epe1+) and AP2002 (epe1-1). The diploid strains were constructed and maintained as described in Materials and Methods. (B) Genetic analysis and complementation testing identified epe1 as the C622.16C open reading frame on chromosome III. The location of epe1 on a 70-kb fragment of chromosome III and the locations of the jmjC domain and the putative nuclear localization signal (NLS) on Epe1 are indicated. (C) A complementation test. Cells of an epe1-1 mutant with an ade6+ insertion at the L(SacI) site (AP2005) were transformed with pRep-1 (30) or with its derivatives, containing the C622.16C sequence of an epe1+ strain (pepe1+), an epe1-1 mutant (pepe1-1), or an epe1 sequence with a Y307A mutation (pepe1-Y307A). Transformants were plated on leucine-depleted low-adenine medium and incubated at 30°C. (D) Comparative sequence analysis reveals that epe1-1 is a G-to-A transition mutation within the jmjC domain of the gene. This mutation replaced a TGG tryptophan codon with a TGA stop codon (underlined) at position 996 of the gene. Numbers indicate nucleotide locations with respect to the beginning of the jmjC domain (positions 251 to 290), and the beginning of the open reading frame (positions 980 to 1019). (E) Subcellular localization of Epe1. Cells of an epe1-1 mutant (AP2005) harboring a plasmid encoding an Epe1-GFP fusion protein (pepe1-GFP) or the vector plasmid (pRep1-GFP) were examined by phase microscopy (Phase), fluorescence microscopy to localize the fusion protein or GFP (GFP), and DAPI staining of DNA (DAPI).
To map the epe1-1 mutation, we tested isolated clones of a strain collection with random chromosomal insertions of ura4+ for genetic linkage of the ura4+ marker to epe1. Employing this approach and additional crosses with chromosomal markers, epe1-1 was located at about 40 centimorgans distal to ade6 on chromosome III (Fig. 3B). Candidate genes of the identified chromosomal region were isolated by PCR from epe1+ and epe1-1 strains and tested for complementation of the epe1-1 mutation. This was accomplished by transforming cells with an epe1-1 mutation and an ade6+ insertion at the SacI site (AP2001) by plasmids expressing the respective candidate genes and screening for alleviation of ade6+ repression. Of the three genes tested, only one, designated C622.16C by the S. pombe Genome Project (48), complemented the epe1-1 mutation. Transformed epe1-1 mutants harboring the gene from epe1+ cells produced Ade+ colonies on YE medium. Yet, transformants harboring the same gene, but from an epe1-1 mutant, displayed an Ade− phenotype (Fig. 3C). The complementing gene, tentatively named epe1, encodes a putative 948-aa protein (NP_588188) with a predicted jmjC domain (38) near its N terminus (aa 287 to 385; Fig. 4). A search of the database revealed a potential nuclear localization signal at positions 608 to 624 (Fig. 3B), but other known motives were not detected. Comparative sequence analysis of the gene from epe1+ and epe1-1 strains indicated a G-to-A transition within the jmjC domain of the mutated gene. This mutation replaced a TGG tryptophan codon with a TGA stop codon at position 996 of the gene, thus yielding a putative truncated protein of 331 aa (Fig. 3D).
FIG. 4.
Epe1 is a conserved protein. Alignment of Epe1 with the six most-related protein sequences. The sequences and accession numbers are as follows: S. pombe NP_588188 (aa 138 to 473), A. gambiae EAA01048 (aa 29 to 371), D. melanogaster AAF54335 (aa 73 to 417), H. sapiens BAA76848 (aa 41 to 368); M. musculus AAD21792 (aa 89 to 431), Caenorhabditis elegans AAL02526 (aa 1 to 313), S. cerevisiae AAB64586 (aa 130 to 474), and the jmjC domain consensus sequence (pfam02373). Comparison and alignment were performed with the Pileup procedure of the GCG Wisconsin Package, version 10.2, with default parameters. Solid boxes represent identical amino acids, dark gray boxes indicate a high degree of similarity, and light gray boxes indicate weak similarity. Dotted lines indicate gaps introduced to maximize alignment.
To establish that C622.16C is the wild-type allele of epe1, the C622.16C sequence was disrupted by a ura4+ insertion upstream to the jmjC domain, and the mutant (AP2039) was tested for viability and ade6+ expression from the SacI site at the L region. As expected, the phenotype of the strain with the ura4+ insertion in C622.16C was indistinguishable from that of the epe1-1 mutant (Table 2). To confirm that the ura4+ insertion is genetically linked to epe1-1, we crossed AP2039 to an epe1-1 mutant (AP2002) and performed random spore analysis of the progeny. Because both parent strains had an ade6+ insertion at the L(SacI) site, segregation of an epe1+ allele would have generated white (Ade+) or light pink recombinants. More than 104 colonies were examined on YE medium, and all were Ade− (red). This further substantiates the proposition that C622.16C is indeed the wild-type allele of epe1-1. The recessive nature of the epe1-1 mutation, together with results of the complementation experiment suggest that epe1-1 and epe1::ura4+ are loss-of-function mutations.
To test whether the jmjC domain is essential for epe1+ activity, we introduced a double-base substitution that replaced the conserved tyrosine at position 307 in the jmjC domain on pepe1+ with alanine. We then tested the mutated gene for complementation of the epe1-1 allele. Unlike epe1+, the epe1-Y307A allele did not complement the epe1-1 mutation (Fig. 3C), thus indicating that an intact jmjC domain is essential for Epe1 activity.
To confirm that epe1+ codes for a nuclear protein, we fused epe1+ to a GFP tag and expressed the chimera protein from an nmt promoter in an epe1-1 mutant. The fusion protein rescued the mutant phenotype, and fluorescence microscopy revealed an intense fluorescent signal that colocalized Epe1-GFP with the 4′,6′-diamidino-2-phenylindole (DAPI)-stained nucleus (Fig. 3E). The nuclear localization of Epe1 is consistent with the proposed role for this protein in the control of chromatin modification.
Epe1 is a conserved protein.
The 948-aa-long sequence (NP_588188) of Epe1 was subjected to a BLAST search (5) against the protein nonredundant database. BLAST finds the Epe1 sequence significantly similar to those of proteins of Anopheles gambiae, Drosophila melanogaster, Homo sapiens, Mus musculus, Caenorhabditis elegans, and S. cerevisiae—all containing the jmjC domain. As can be clearly seen (Fig. 4), the conservation among these proteins stretches beyond the shared jmjC domain consensus. Except for S. pombe Epe1 and the C. elegans jmjC domain protein, the other related proteins have, in addition to the shared jmjC domain, domains that imply DNA binding and/or protein-protein interaction. The D. melanogaster, H. sapiens, and A. gambiae gene products harbor a CXXC zinc finger and an F-box domain. The H. sapiens, M. musculus, and S. cerevisiae gene products have a PHD zinc finger domain.
Meiotic stability of epe1-1-induced repression beyond IR-L.
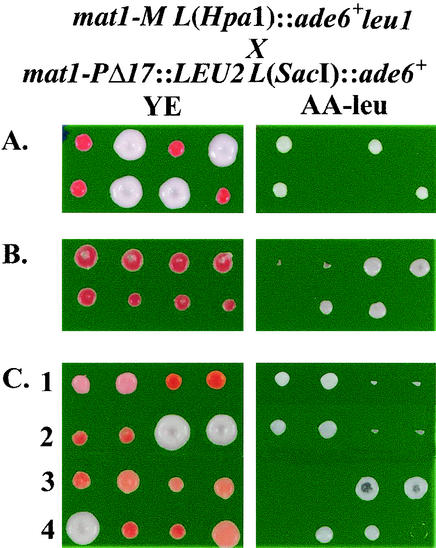
Clonal stability of the induced repression at the HpaI site in epe1 mutants suggests an epigenetic switch at the centromere-proximal side of IR-L. Because alternative epigenetic states are often meiotically stable (20, 42), we asked whether the induced repression across the IR-L barrier would survive meiosis. To this end, we crossed epe1-1 mat1-M cells with an ade6+ insertion at the HpaI site with mat1-P epe1-1 cells with an ade6+ insertion at the SacI site and subjected the progeny to tetrad analysis. In this cross, a LEU2+ marker, genetically linked to mat1-P, distinguishes between the alternative mat1 alleles. The pattern of marker segregation in meiosis indicates that the alternative states of ade6+ expression at the HpaI site were stably inherited in cis. With the mat1-M parent displaying an Ade+ epitype, all mat1-M progeny colonies (Leu−) were Ade+ (Fig. 5A), and with the mat1-M parent displaying an Ade− epitype (Fig. 5B), most mat1-M progeny colonies (68 of 70) were Ade− (Table 3).
FIG. 5.
The mutated epe1 allele is required for meiotic stability of the repressed state beyond the IR-L barrier. Segregation of the mat1-M and mat1-P alleles is followed in all three crosses by the linkage of mat1-P to LEU2. (A and B) Both parent strains in crosses A and B are epe1-1. The mat1-M parent displayed an Ade+ epitype (white) in cross A and an Ade− epitype (red) in cross B. Cosegregation of the expressed (A) or repressed (B) epialleles of L(HpaI)::ade6+ with the genetically linked mat1-M allele indicates that the respective epialleles are inherited in cis. (C) The meiotic stability of the repressed epiallele of L(HpaI)::ade6+ depends on the maintenance of the epe1-1 allele. The mat1-P parent in this cross is epe1+, and the mat1-M parent is epe1-1, displaying an Ade− epitype. Examples of different segregation patterns of crosses in which mat1-M cosegregated with the epe1-1 allele (rows 1 and 3) or with the epe1+ allele (rows 2 and 4) are presented. Segregation of the different alleles and epialleles in all three crosses is presented in Table 3.
TABLE 3.
Meiotic stability of silencing beyond the IR-L barrier in epe1-1 mutants
| Crossa | epe1 genotype of mat1-M progeny | No. (%) of mat1-M progeny with Ade phenotype:
|
|||
|---|---|---|---|---|---|
| Total | Red (Ade−) | Pink | White (Ade+) | ||
| mat1-M epe1-1 × mat1-P epe1-1 | epe1-1 | 70 | 68 (97) | None | 2 (3) |
| mat1-M epe1-1 × mat1-P epe1+ | epe1-1 | 24 | 16 (80) | 4 (20) | None |
| epe1+ | 20 | None | 8 (33) | 16 (66) | |
mat1-M parents have an ade6+ insertion at the HpaI site, and mating cells were of Ade− (red) clones. mat1-P parents have an ade6+ insertion at the SacI site. (See Table 1 for detailed genotypes.)
To examine whether the mutated epe1 allele is required for inheritance of the repressed state, we performed a similar cross as in Fig. 5B, except that the mat1-P parent was epe1+. Because the epe1-1 mutation markedly enhanced repression of ade6+ at the SacI site, repression alleviation in the mat1-M progeny that lost the epe1-1 allele to the mat1-P parent would yield tetrads with two Ade+ Leu− (white) and two Ade− Leu+ (red) colonies. In contrast, inheritance of the repressed state by the mat1-M epe1+ progeny would yield tetrads with four red colonies. Most mat1-M colonies that regained the epe1+ allele in meiosis acquired an Ade+ phenotype, because none of these colonies displayed an Ade− phenotype, and only one-third displayed partial ade6+ repression (Fig. 5C and Table 3). More than 95% of the cells in the partially repressed clones yielded Ade+ colonies upon replating (data not shown). We note that in this cross, silencing in the mat1-M progeny that maintained the epe1-1 allele was less stably inherited than in the cross in which both parents were epe1-1 (Table 3). This may be explained by the temporal exposure to a functional Epe1 in the heterozygote. Altogether, these results indicate that maintenance of the mutated allele is required for stable inheritance of the repressed state in meiosis.
Overexpression of Epe1 disrupts heterochromatin structure at the mat locus.
Given that epe1 mutations enhance heterochromatization, we sought to determine whether overexpression of Epe1 would have the opposite effect. Cells with a ura4+ insertion within the silent K region (XbaI) and an ade6+ insertion at the junction between mat2 and REII (BssHII) were transformed by a plasmid expressing epe1+ from an nmt promoter (pepe1+) and plated on a selective medium with a low concentration of adenine. Control transformants harbored the vector plasmid or a plasmid expressing epe1-1 (pepe1-1). Consistent with earlier results (6), ade6+ at the BssHII site was repressed at a normal epe1+ gene dosage, because more than 99% of the colonies harboring the control plasmids displayed an Ade− (red) phenotype. In contrast, all colonies harboring the epe1+-expressing plasmid displayed an Ade+ (white) phenotype (data not shown). To confirm that overexpression of Epe1 suppresses PEV within the silent domain, we monitored ura4+ expression from the K region (XbaI) by applying spots of serial dilutions on uracil-deficient and FOA media (Fig. 6A). The results clearly indicate that overexpression of Epe1 impairs silencing within the K region. Cells of the control cultures grew well on FOA medium and poorly on uracil-depleted medium, whereas cells that overexpress epe1+ grew poorly on FOA medium and well on uracil-depleted medium.
FIG. 6.
Overexpression of Epe1 disrupts heterochromatin structure at the mat silent domain. (A) Cultures of AP312 cells [K(XbaI)::ura4+] harboring the indicated plasmids were subjected to 10-fold serial dilutions and applied as spots to leucine-depleted minimal medium (AA −leu) and on the same medium with uracil also depleted (AA −ura) or supplemented with FOA. Plates were incubated at 33°C for 5 days before photography. (B) Colonies of AP312 cells harboring the indicated plasmids were replica plated from leucine-depleted minimal medium onto leucine-depleted sporulation medium and stained with iodine vapor following 3 days of incubation at 30°C. (C) ChIP analysis of extracts from cells with an ade6+ insertion within mat2 (AP284), harboring the indicated plasmids, with antibodies against acetylated H3-K9 (K9Ac), acetylated H3-K14 (K14Ac), and dimethylated H3-K4 (K4Me). The locations of the electrophoretically separated ade6+ and ade6-DN/N PCR products are indicated. Enrichment values (ade6+/ade6-DN-N) were normalized for the ratio in whole-cell extract (WCE).
To examine whether overexpression of Epe1 also alleviates repression of mat2-P, we replica plated colonies of transformed cells onto sporulation medium and monitored haploid meiosis. Because mat2-P expression in heterothallic mat1-M cells induces haploid meiosis, and spores, but not vegetative cells, contain a starch component, iodine-stained colonies indicate mat2 expression (10). The staining pattern (Fig. 6B) and microscopic examination (data not shown) indicated alleviation of silencing at mat2-P in cells that overexpress epe1+.
We next examined the effect of overexpression of Epe1 on histone H3 modification within the silent domain of the mat locus. This was accomplished by ChIP analysis of whole-cell extracts with antibodies specific to acetylated H3-K9, acetylated H3-K14, and dimethylated H3-K4. The immunoprecipitated DNA was subjected to PCR analysis with primers that generate products of different lengths from an ade6+ reporter gene within mat2-P (BamHI) and the ade6-DN/N allele at the endogenous ade6 locus (15). Comparison between clones that overexpress epe1+ and control clones indicates enrichment for all three histone markers in clones that overexpress epe1+ (Fig. 6C). Because these markers are commonly associated with euchromatic domains, the observed changes in the pattern of histone modification indicate that overexpression of Epe1 disrupts heterochromatin structure within the mat silent domain.
Overexpression of Epe1 impairs centromere function.
Mutations that impair heterochromatization at the centromere outer repeats disrupt faithful chromosome segregation (4, 14). To ascertain whether overexpression of Epe1 has a similar effect on centromere function, we transformed a strain harboring the artificial minichromosome ch16 by pepe1+ and monitored chromosome stability by scoring half-sectored colonies on low-adenine plates. Because the ade6-216 allele on ch16 complements the chromosomal ade6-210 allele to generate an Ade+ phenotype, chromosome loss frequency may be ascertained by scoring half-sectored colonies on a YE medium (4). Consistent with the proposition that Epe1 counteracts heterochromatization, overexpression of Epe1 enhanced the loss of the ch16 minichromosome (Table 4).
TABLE 4.
Effect of overexpression of Epe1 on stability of the ch16 minichromosome
| Strain/plasmid | No. of half-sectored colonies/total colonies | % Loss/cell division |
|---|---|---|
| AP874/pRep1 | 2/3,115, 5/5,355, 3/4,984, 3/6,116 | 0.06 |
| AP874/pepe1+ | 35/4,237, 25/5,815, 18/5,118, 22/6,833 | 0.45 |
epe1 genotype affects heterochromatin stability rather than heterochromatin establishment.
Epe1 may antagonize heterochromatization by inhibiting heterochromatin assembly and/or affecting heterochromatin stability. In an attempt to resolve these possibilities, we determined the effect of epe1 mutations and overexpression of Epe1 on the stability of the alternative Ade epitypes in a KΔ::ade6+ background. Because of the deletion of the centromere sequence homology (cenH), heterochromatin establishment within the mat locus of this strain occurs at low frequency. However, because cenH is not required for silencing maintenance and inheritance, the alternative epitypes are clonally stable (20, 42). Thus, if Epe1 affects silencing establishment, epe1 mutation would enhance conversion of Ade+ to Ade−, as observed in cells that overexpress Swi6 (33). On the other hand, if Epe1 negatively controls the stability of the repressed state, the frequency of conversion from Ade− to Ade+ would be lowered by epe1 mutations and enhanced by overexpression of Epe1. Our results are consistent with the latter possibility (Table 5). An epe1-1 mutation enhanced the stability of the repressed state but did not increase the frequency of conversion from Ade+ to Ade−. Furthermore, transformation of cells displaying an Ade− epitype by an epe1+-expressing plasmid disrupted ade6+ repression in all transformants. These data suggest that Epe1 modulates silencing by negatively affecting heterochromatin stability rather than by inhibiting heterochromatin establishment.
TABLE 5.
Effect of epe1 genotype on silencing establishment and stability in KΔ mutants
| Strain | Relevant genotypea | % of plated or transformed cells fromb:
|
|||||
|---|---|---|---|---|---|---|---|
| White (Ade+) colonies that were:
|
Red (Ade−) colonies that were:
|
||||||
| Red | Pink | White | Red | Pink | White | ||
| AP733 | epe1+KΔ::ade6+ | 4.0 ± 1.1 | 2.6 ± 0.8 | 93.4 ± 1.9 | 94.5 ± 1.2 | 3.0 ± 0.5 | 2.5 ± 1.0 |
| AP2056 | epe1-1 KΔ::ade6+ | 1.8 ± 0.6 | 1.6 ± 0.6 | 96.6 ± 1.9 | 100 | <0.05 | <0.05 |
| AP733/pepe1+c | epe1+KΔ::ade6+/ppepe1+ | <0.05 | <0.05 | 100 | <0.05 | <0.05 | 100 |
Complete genotypes are presented in Table 1.
Red, pink, and white indicate colonies displaying Ade−, partial ade6 repression, and Ade+ phenotypes on low-adenine medium, respectively.
Ade+ or Ade− cells were transformed by promoter pepe1 and plated on AA −leu medium with 15 mg of adenine per liter.
Inactivation of Epe1 suppresses mutations in silencing genes.
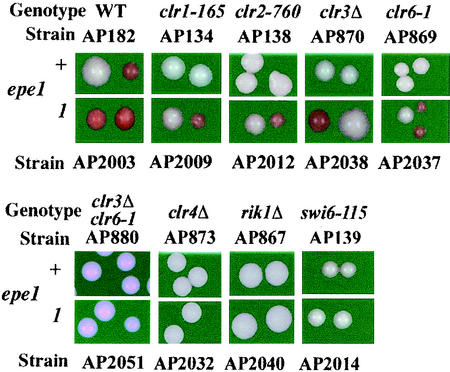
Because Epe1 counteracts heterochromatization, we asked whether its inactivation would suppress mutations in genes that encode heterochromatin assembly proteins. To explore this possibility, we constructed epe1+ and epe1-1 derivatives with an ade6+ insertion at the L(SacI) site (Fig. 1A) on the background of mutations in silencing genes. Cells of the respective strains were plated on YE medium to compare the expression state of ade6+ in the double mutants to that of the corresponding single mutants (Fig. 7). These results indicate that epe1-1 suppresses mutations in clr1, clr2, clr3, and clr6 genes, but not in swi6, rik1, or clr4 genes.
FIG. 7.
epe1-1 suppresses mutations in silencing genes. Cells of epe1+ and epe1-1 derivatives with the indicated silencing gene mutations were plated on YE medium and incubated for 3 days at 30°C. The occurrence of Ade− (red) colonies in spreads of epe1-1 derivatives indicates suppression of the respective mutations by epe1-1. WT, wild type.
Suppression of clr3 or clr6 mutations by epe1-1 may reflect histone deacetylase (HDAC)-independent heterochromatization. However, because Clr3 and Clr6 display overlapping activities (8, 19), it is plausible that in the epe1-1 background, either one of the two HDACs is sufficient to promote heterochromatization. To test this possibility we examined ade6+ expression from the L::SacI site in a clr3Δ clr6-1 epe1-1 triple mutant. The consistent Ade+ phenotype of this strain indicates that HDAC activity is required for heterochromatization in epe1 mutants. However, unlike in epe1+ derivatives, either Clr3 or Clr6 activity is sufficient.
Heterochromatin propagation along the L region in epe1-1 mutants.
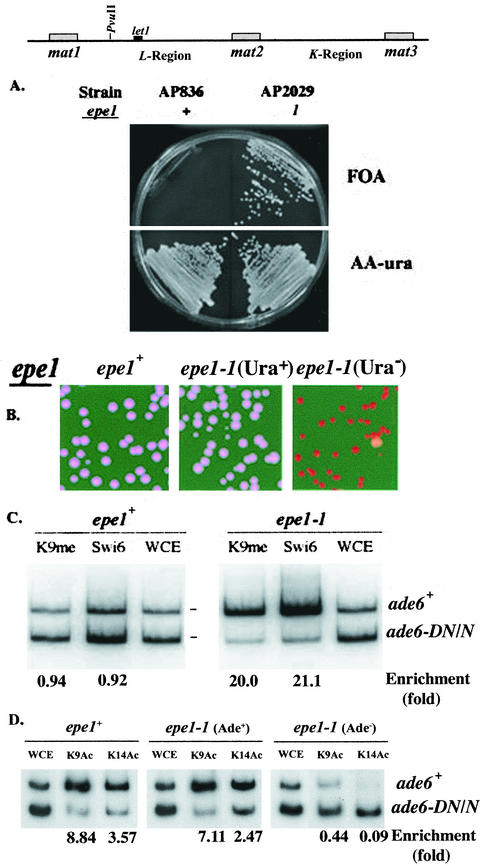
The epe1 mutations were isolated in a screen for mutations that promote silencing at the euchromatic side of IR-L. However, because heterochromatin propagation toward the essential let1+ gene causes growth defects (35), the induced repression could not be monitored beyond let1. To overcome this problem, we monitored heterochromatin spreading along the L region in strains with the let1+ gene translocated to the ura4 locus on chromosome III. Derivatives of this strain with a ura4+ insertion within the endogenous let1 locus and an ade6+ insertion at the PvuII site were used to examine the effect of the epe1-1 mutation on silencing along the L region. As anticipated, the epe1+ derivative was FOA sensitive, and the epe1-1 mutant grew on both AA −ura and FOA media (Fig. 8A). To test whether silencing in the FOAR clones extended all the way to the PvuII site, cells were examined for their Ade phenotype. More than 99% of the cells from FOAR colonies and less than 0.1% of the cells from Ura+ colonies produced Ade− colonies on YE medium. (Fig. 8B). Selection for FOA resistance in an isogenic strain, but with the let1+ gene at its endogenous locus and a ura4+ insertion at the HpaI site (AP292), yielded Ura− Ade+ clones exclusively (data not shown). These results indicate that in the absence of selection against silencing of let1+, once heterochromatin overrides the IR-L barrier, it spreads along the L region.
FIG. 8.
Silencing at the L region of an epe1-1 mutant. (A) Cells of strains with a ura4+ insertion within let1, an ade6+ insertion at the L(PvuII) site, and the indicated epe1 allele were spread on FOA and AA −ura plates and incubated at 33°C for 3 days. (B) Cells of strains with a ura4+ insertion within let1, an ade6+ insertion at the L(PvuII) site, and the indicated epe1 allele from AA −ura (Ura+) or FOA (Ura−) medium were spread on YE plates and incubated for 3 days at 30°C. (C) ChIP analysis of extracts from an epe1+ (AP836) strain or a Ura− clone of an epe1-1 (AP2029) mutant. Antibodies are specific for Swi6 (Swi6) and dimethylated H3-K9 (K9Me), and PCR primers amplify ade6 sequences. The locations of the electrophoretically separated ade6+ and ade6-DN/N PCR products are indicated. Enrichment values (ade6+/ade6-DN-N) were normalized for the ratio in whole-cell extract (WCE). (D) ChIP analysis of extracts from an epe1+ strain and Ade+ or Ade− clone of an epe1-1 mutant, with antibodies against acetylated H3-K9 (K9Ac) and acetylated H3-K14 (K14Ac). Experimental conditions are as described for panel C.
To confirm that the Ade− phenotype of the FOAR clones reflects chromatin modification, we examined by ChIP analysis the association of two heterochromatin markers, Swi6 and H3-K9methyl, with the ade6+ reporter gene at the PvuII site (Fig. 8C). Comparison between the epe1+ strain and an Ade− clone of the epe1-1 mutant indicated enrichment for the two markers at the L(PvuII) locus of the epe1-1 mutant. Thus, repression of ade6+ in the Ura− clones involves heterochromatization at the centromere-proximal end of the L region.
Deacetylation of K9 and K14 on histone H3 is functional upstream to methylation of H3-K9 by Clr4 in the heterochromatin assembly pathway (17). To examine the effect of the epe1 genotype on the acetylation state of histone H3 at the L(PvuII) site, we performed ChIP analysis with the appropriate antibodies and ade6 primers as described above. The results show no significant differences in the acetylation states at the L(PvuII) site between the epe1+ strain and Ura+ clones of the epe1-1 mutant. In contrast, histone H3 was hypoacetylated in clones selected for ura4+ repression at the let1 locus (Fig. 8D).
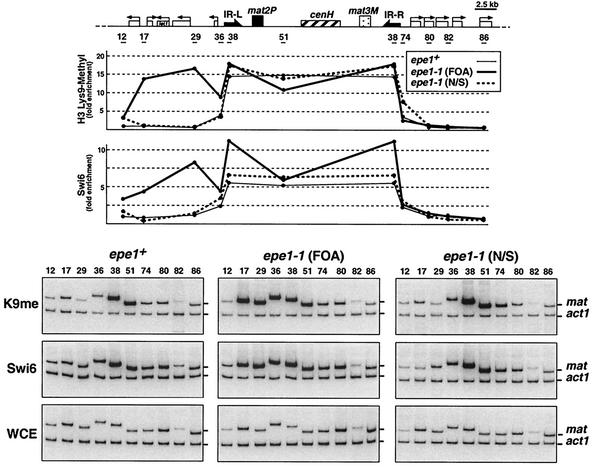
To determine whether heterochromatin in the Ura− clones spreads continuously, we subjected the entire length of the mat region to ChIP analysis with antibodies to Swi6 and methylated H3-K9 by using multiplex PCR with mat locus and act1 primers (35) (Fig. 9). The results indicate continuous spreading of heterochromatin across the IR-L barrier and along the L region in clones of epe1-1 mutants that were selected for FOA resistance. However, spreading of heterochromatin in epe1-1 mutants that were not selected for silencing at the let1 locus was indistinguishable from that in epe1+ cells. Furthermore, heterochromatin spreading beyond the barrier was observed only at the centromere-proximal side of IR-L, where the ura4+ gene, used for counterselection of expression, is located. Transition of H3-K9 methylation and Swi6 association with chromatin at the opposite end of the silent domain occurred abruptly at the IR-R locus, regardless of the epe1 genotype or the ura4 epitype of the epe1-1 mutant. These observations suggest that the propagating heterochromatin in epe1-1 mutants overrides either one of the two barriers in a stochastic manner without affecting the other. Thus, selection for a repressed ura4+ at the L(HpaI) site revealed only clones with an impaired IR-L barrier. To test this proposition, we constructed epe1-1 strains with ura4+ insertions beyond either one of the two barriers (HpaI or SpeI) and an ade6+ reporter gene beyond the opposite barrier. We then selected for Ura+ or Ura− (FOA resistant) clones and examined the expression state of ade6+ at the other end of the silent domain by plating cells on YE medium (Table 6). Consistent with our proposition, silencing of ura4+ beyond either one of the two barriers had no detectable effect on the state of ade6+ expression beyond the other barrier. These results indicate that the propagating heterochromatin in epe1 mutants overcomes either one of the two barriers in a stochastic and independent manner to establish a clonally inherited state of repression on its euchromatic side.
FIG. 9.
Heterochromatin spreads continuously along the L region in epe1-1 mutants. Association of Swi6 and H3-K9methyl with chromatin along the mat locus was determined by ChIP analysis with antibodies to Swi6 (Swi6) and dimethylated H3-K9 (K9me). DNA isolated from cross-linked chromatin, immunoprecipitated with the respective antibodies, or whole-cell extracts (WCE) was subjected to multiplex PCR to amplify the fragments indicated by horizontal bars on the mat locus map and an act1 fragment serving as internal control. Cell extracts were from epe1+ (AP836) and epe1-1 (AP2029) strains with a ura4+ insertion within let1 and an ade6+ insertion at the L(PvuII) site. Extracts of two AP2029 clones were analyzed. One was selected for a Ura− epitype on FOA medium (FOA). The nonselected clone (N/S) was mainly Ura+. Enrichment with respect to the mat/act1 DNA ratio in whole-cell extract at each of the indicated sites along the mat locus was determined as described previously (35).
TABLE 6.
Silencing is established in epe1-1 mutants beyond the IR-L or IR-R barrier, without affecting the expression state beyond the other barrier
| Strain | Relevant genotypea | Ura epitype | Distribution of Ade epitypes (%)b
|
||
|---|---|---|---|---|---|
| Red | Pink | White | |||
| AP2050 | epe1-1 L(HpaI)::ade6+mat3-M (SpeI)::ura4+ | Ura− | 0.6 ± 0.2 | 1.5 ± 0.4 | 97.9 ± 1.2 |
| Ura+ | 1.3 ± 0.8 | 1.2 ± 0.7 | 97.5 ± 1.7 | ||
| AP2053 | epe1-1 L(HpaI)::ura4+mat3-M (SpeI)::ade6+ | Ura− | 2.4 ± 0.7 | 3.6 ± 1.5 | 94.0 ± 3.2 |
| Ura+ | 3.0 ± 0.84 | 3.3 ± 1.3 | 93.7 ± 1.1 | ||
Complete genotypes are presented in Table 1. HpaI is located beyond IR-L. SpeI is located beyond IR-R. (See Fig. 1 for locations of reporter gene insertion sites.)
Red, pink, and white indicate colonies displaying Ade−, partial ade6 repression, and Ade+ phenotypes on low-adenine medium, respectively.
DISCUSSION
By screening for mutations that promote silencing propagation across a heterochromatin barrier, we identified a novel gene, epe1, that encodes a phylogenetically conserved nuclear protein containing a jmjC domain. Inactivation of Epe1 enhances heterochromatization and suppresses mutations in silencing genes. Conversely, overexpression of Epe1 disrupts heterochromatin organization and centromere function. The effect of epe1 genotype on the frequency of epitype switching in the KΔ::ade6+ background and on the meiotic stability of a repressed state beyond the IR-L barrier suggests that Epe1 negatively controls heterochromatin stability. The results of complementation experiments indicate that the jmjC domain is essential for Epe1 activity.
epe1 encodes a jmjC domain protein that modulates heterochromatization.
More than 190 proteins, including human hairless, an Rb-binding protein, and members of the jumonji family of transcription factors, share the jmjC domain (http://www.ncbi.nlm.nih.gov/BLAST). The secondary structure of this domain predicts enzymatic activity, and its frequent association with DNA binding motifs and putative chromatin modification activities suggest a role in regulating the integrity of chromatin structure (12). However, the enzymatic or biological activity of this domain remains enigmatic. The observation that a single amino acid substitution within the jmjC domain (Y307A) abolishes the ability of a cloned epe1 gene to complement the nonsense epe1-1 mutation (Fig. 3C) indicates that the jmjC domain is essential for Epe1 activity. Unlike many of the jmjC domain-containing proteins, Epe1 does not have additional recognizable motifs that imply a specific biological activity. Nevertheless, the phenotypic manifestations of epe1 mutations and overexpression of Epe1 indicate a role for this protein in the control of chromatin structure. Most significantly, inactivation of Epe1 enhances PEV at the mat locus and the centromeres, as well as cenH-mediated silencing at an ectopic site. In contrast, overexpression of Epe1 impairs silencing within the mat locus and disrupts centromere functions. These results, together with the observation that epe1-1 suppresses mutations in silencing genes, suggest that Epe1 counteracts heterochromatization. The nuclear localization of Epe1 (Fig. 3D) is consistent with a role for this protein in chromatin modification.
How does Epe1 counteract heterochromatization? Several lines of evidence indicate that heterochromatin assembly involves sequential modifications of the histone H3 amino-terminal tail. HDACs cooperate with Rik1 and Clr4 histone methyltransferase to establish a histone code for Swi6 localization to heterochromatic loci. Swi6 binds to the methylated H3-K9 and subsequently recruits Clr4 to the growing end of the heterochromatin structure (17, 32). At least two additional proteins, namely Clr1 and Clr2, participate heterochromatin assembly (13, 16, 41, 43), yet the role of these proteins is not fully understood. The ability of epe1 mutations to suppress clr3 or clr6 but not clr4 or swi6 mutations suggests genetic interaction of epe1+ with the heterochromatin assembly pathway upstream to Clr4-mediated methylation of H3-K9. Thus, inactivation of Epe1 may partially alleviate the dependence of the Clr4-catalyzed methylation reaction on preceding histone modification activities, but downstream events require methylated H3-K9. Another possibility is that Epe1 negatively controls heterochromatin stability at the periphery of the mat silent domain. A role for Epe1 in controlling heterochromatin stability is suggested by the effect of epe1 mutations on the expression state beyond the heterochromatin barriers in the mat locus. Silencing beyond the IR-L and IR-R barriers is established stochastically at a very low frequency. Yet, once established, it is stably inherited through mitosis and meiosis. Furthermore, meiotic stability of the repressed state depends on maintenance of the mutated epe1 allele (Fig. 5 and Table 3). Another indication that Epe1 affects heterochromatin stability, rather than heterochromatin establishment, emerges from the effect of the epe1 genotype on epitype switching in a KΔ::ade6+ background. Normally, the alternative Ade epitypes in this background are clonally stable, with a frequency of switching between the alternative epitypes of 1 to 4%. An epe1 mutation has little or no effect on the frequency of conversion of Ade+ to Ade−, yet it lowers the frequency of conversion of Ade− to Ade+. Furthermore, overexpression of Epe1 in Ade− clones of this strain abolishes ade6+ repression (Table 5). These data support the proposition that Epe1 affects chromatin remodeling by negatively regulating the stability of the heterochromatic state. The effect of overexpression of Epe1 on the pattern of histone H3 modifications (Fig. 6) suggests that Epe1 promotes an activity that reverses epigenetic marks that govern heterochromatization. Yet, the nature this activity remains to be discovered.
The Clr4/Swi6 chromatin remodeling system, like the homologous SUV39H/HP1 system in mammals, was first implicated in heterochromatin assembly. However, a recent study indicates that Clr4/Swi6-mediated chromatin modification is also involved in the control of euchromatic gene expression (23). Furhermore, SUV39H1 and HP1 are recruited by the Rb/E2F complex for transient repression of E2F-responsive promoters (34). Transient repression of euchromatic genes by SUV39H1/Clr4-catalyzed methylation of H3-K9 implies that the repressive effect of the H3-K9methyl mark is reversible. However, an enzymatic system that reverse or suppress this biochemically stable mark is yet unknown. If Epe1 is functional in a euchromatic context, it may provide a link to a mechanism that reverse Clr4/Swi6- or SUV39H1/HP1-promoted nucleosomal silencing.
Heterochromatin propagation across the barrier alters an epigenetic state at IR-L.
An epe1-1 mutation enhances PEV at the REII-IR-L interval and promotes heterochromatin propagation across the IR-L barrier. While PEV enhancement at the REII-IR-L interval affects the entire cell population, repression beyond IR-L is established at a very low frequency (Table 2). Nevertheless, once established, silencing across IR-L is clonally inherited. The stochastic nature of silencing establishment and its stable inheritance suggest a binary mode of PEV enhancer action. Normally, the barrier at IR-L blocks heterochromatin propagation, and enhancement of heterochromatization by epe1 mutations has no detectable effect on barrier activity in most cell lines (35, 40) (Table 2). However, in the rare event of overriding the barrier by the propagating heterochromatin, a heritable state of repression is established across the barrier. This suggests that barrier activity is associated with an epigenetic state that blocks heterochromatin propagation, but if heterochromatin overrides the barrier, an alternative state is established and clonally inherited. The nature of the barrier at IR-L and IR-R is not yet understood. Local recruitment of histone modification activities that counteract heterochromatization is an attractive possibility. However, recruitment of protein complexes that act via other mechanisms is also conceivable. Such putative complexes may be stably inherited in most cell lines, even under conditions of enhanced heterochromatization. However, in the event of IR-L occupation by heterochromatin, reassembly of the boundary complex is inhibited by the inaccessibility of the assembly locus to DNA binding proteins. Considering the indications that Epe1 down-regulates heterochromatin stability, we postulate that the propagating heterochromatin at the mat silent domain overrides the IR-L or IR-R barrier stochastically, regardless of the epe1 genotype. Yet, only in the absence of Epe1 activity is the heterochromatic state perpetually maintained outside the IR-L-IR-R interval.
ADDENDUM IN PROOF
The current InterPro (http://www.ebi.ac.uk/interpro/) protein database identifies the glycosylhydrolase family 5 signature as a putative motif in Epel (aa 489 to 499).
Acknowledgments
We are grateful to Zeev Paroush for useful comments on the manuscript and Robin Allshire, Amar Klar, Susan Forsburg, Olaf Nielson, Genevieve Thon, Paul Young, and Mitsuhiro Yanagida for S. pombe strains and plasmids.
This research was supported by grants from the Israel Science Foundation (N157/00-1 to A.C.) and National Institutes of Health (GM59772 to S.I.S.G). N.A. was supported by a fellowship to minority students from the Israeli Ministry of Science.
REFERENCES
- 1.Allshire, R. C. 1997. Centromeres, checkpoints and chromatid cohesion. Curr. Opin. Genet. Dev. 7:264-275. [DOI] [PubMed] [Google Scholar]
- 2.Allshire, R. C. 1996. Transcriptional silencing in the fission yeast: a manifestation of higher order chromosome structure and functions, p. 443-466. In V. E. A. Russo, R. A. Martienssen, and A. D. Riggs (ed.), Epigenetic mechanisms of gene regulation. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 3.Allshire, R. C., J.-P. Javerzat, N. J. Redhead, and G. Cranston. 1994. Position effect variegation at fission yeast centromeres. Cell 76:157-169. [DOI] [PubMed] [Google Scholar]
- 4.Allshire, R. C., E. R. Nimmo, E. Ekwall, J. P. Javerzat, and G. Cranston. 1995. Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev. 9:218-233. [DOI] [PubMed] [Google Scholar]
- 5.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayoub, N., I. Goldshmidt, and A. Cohen. 1999. Position effect variegation at the mating-type locus of fission yeast: a cis-acting element inhibits covariegated expression of genes at the silent and expressed domains. Genetics 152:495-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayoub, N., I. Goldshmidt, R. Lyakhovetsky, and A. Cohen. 2000. A fission yeast repression element cooperates with centromere-like sequences and defines a mat silent domain boundary. Genetics 156:983-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjerling, P., R. A. Silverstein, G. Thon, A. Caudy, S. Grewal, and K. Ekwall. 2002. Functional divergence between histone deacetylases in fission yeast by distinct cellular localization and in vivo specificity. Mol. Cell. Biol. 22:2170-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boeke, J. D., J. Trueheart, G. Natsoulis, and G. R. Fink. 1987. 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol. 154:164-175. [DOI] [PubMed] [Google Scholar]
- 10.Bresch, C., G. Muller, and R. Egel. 1968. Genes involved in meiosis and sporulation of a yeast. Mol. Gen. Genet. 102:301-306. [DOI] [PubMed] [Google Scholar]
- 11.Chua, G., L. Taricani, W. Stangle, and P. G. Young. 2000. Insertional mutagenesis based on illegitimate recombination in Schizosaccharomyces pombe. Nucleic Acids Res. 28:E53.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clissold, P. M., and C. P. Ponting. 2001. JmjC: cupin metalloenzyme-like domains in jumonji, hairless and phospholipase A2β. Trends Biochem. Sci. 26:7-9. [DOI] [PubMed] [Google Scholar]
- 13.Egel, R., M. Willer, and O. Nielsen. 1989. Unblocking of meiotic crossing-over between the silent mating-type cassettes of fission yeast, conditioned by the recessive, pleiotropic mutant rik1. Curr. Genet. 15:407-410. [Google Scholar]
- 14.Ekwall, K., E. R. Nimmo, J.-P. Javerzat, B. Borgstrom, R. Egel, G. Cranston, and R. Allshire. 1996. Mutations in the fission yeast silencing factors clr4+ and rik1+ disrupt the localisation of the chromo domain protein Swi6p and impair centromere function. J. Cell Sci. 109:2637-2648. [DOI] [PubMed] [Google Scholar]
- 15.Ekwall, K., T. Olsson, B. M. Turner, G. Cranston, and R. C. Allshire. 1997. Transient inhibition of histone deacetylation alters the structural and functional imprint at fission yeast centromeres. Cell 91:1021-1032. [DOI] [PubMed] [Google Scholar]
- 16.Ekwall, K., and T. Ruusala. 1994. Mutations in rik1, clr2, clr3 and clr4 genes asymmetrically derepress the silent mating-type loci in fission yeast. Genetics 136:53-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grewal, S. I., and S. C. Elgin. 2002. Heterochromatin: new possibilities for the inheritance of structure. Curr. Opin. Genet. Dev. 12:178-187. [DOI] [PubMed] [Google Scholar]
- 18.Grewal, S. I. S. 2000. Transcriptional silencing in fission yeast. J. Cell. Physiol. 184:311-318. [DOI] [PubMed] [Google Scholar]
- 19.Grewal, S. I. S., M. J. Bonaduce, and A. J. S. Klar. 1998. Histone deacetylase homologs regulate epigenetic inheritance of transcriptional silencing and chromosome segregation in fission yeast. Genetics 150:563-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grewal, S. I. S., and A. J. S. Klar. 1996. Chromosomal inheritance of epigenetic states in fission yeast during mitosis and meiosis. Cell 86:95-101. [DOI] [PubMed] [Google Scholar]
- 21.Grewal, S. I. S., and A. J. S. Klar. 1997. A recombinationally repressed region between mat2 and mat3 loci shares homology to centromeric repeats and regulates directionality of mating-type switching in fission yeast. Genetics 146:1221-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hecht, A., S. Strahl-Bolsinger, and M. Grunstein. 1996. Spreading of transcriptional repressor Sir3 from telomeric heterochromatin. Nature 383:92-96. [DOI] [PubMed] [Google Scholar]
- 23.Ivanova, A. V., M. J. Bonaduce, S. V. Ivanov, and A. J. S. Klar. 1998. The chromo and SET domains of the Clr4 protein are essential for silencing in fission yeast. Nat. Genet. 19:192-195. [DOI] [PubMed] [Google Scholar]
- 24.Karpen, G. H., and R. C. Allshire. 1997. The case for epigenetic effect on centromere identity and function. Trends Genet. 13:489-496. [DOI] [PubMed] [Google Scholar]
- 25.Klar, A. J. S., A. V. Ivanova, J. Z. Dalgaard, M. J. Bonaduce, and S. I. S. Grewal. 1998. Multiple epigenetic events regulate mating-type switching of fission yeast, p. 87-103. In Epigenetics. Novartis Foundation Symposium 214. Wiley, Chichester, United Kingdom. [DOI] [PubMed]
- 26.Lee, J. T., and R. Jaenisch. 1997. The (epi)genetic control of mammalian X-chromosome inactivation. Curr. Opin. Genet. Dev. 7:274-280. [DOI] [PubMed] [Google Scholar]
- 27.Ling, M. M., and B. H. Robinson. 1997. Approaches to DNA mutagenesis: an overview. Anal. Biochem. 254:157-178. [DOI] [PubMed] [Google Scholar]
- 28.Lyko, F., and R. Paro. 1999. Chromosomal elements confering epigenetic inheritance. Bioessays 21:824-832. [DOI] [PubMed] [Google Scholar]
- 29.Marchler-Bauer, A., A. R. Panchenko, B. A. Shoemaker, P. A. Thiessen, L. Y. Geer, and S. H. Bryant. 2002. CDD: a database of conserved domain alignments with links to domain three-dimensional structure. Nucleic Acids Res. 30:281-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maundrell, K. 1993. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene 123:127-130. [DOI] [PubMed] [Google Scholar]
- 31.Moreno, S., A. J. S. Klar, and P. Nurse. 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194:795-823. [DOI] [PubMed] [Google Scholar]
- 32.Nakayama, J., J. C. Rice, B. D. Strahl, C. D. Allis, and S. I. Grewal. 2001. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science 292:110-113. [DOI] [PubMed] [Google Scholar]
- 33.Nakayama, J.-I., A. J. S. Klar, and S. I. S. Grewal. 2000. A chromodomain protein, Swi6, performs imprinting functions in fission yeast during mitosis and meiosis. Cell 101:307-317. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen, S. J., R. Schneider, U. M. Bauer, A. J. Bannister, A. Morrison, D. O'Carroll, R. Firestein, M. Cleary, T. Jenuwein, R. E. Herrera, and T. Kouzarides. 2001. Rb targets histone H3 methylation and HP1 to promoters Nature 412:561-565. [DOI] [PubMed] [Google Scholar]
- 35.Noma, K., C. D. Allis, and S. I. Grewal. 2001. Transitions in distinct histone H3 methylation patterns at the heterochromatin domain boundaries. Science 293:1150-1155. [DOI] [PubMed] [Google Scholar]
- 36.Partridge, J. F., B. Borgstrom, and R. C. Allshire. 2000. Distinct protein interaction domains and protein spreading in a complex centromere. Genes Dev. 14:783-791. [PMC free article] [PubMed] [Google Scholar]
- 37.Reik, W., and J. Walter. 1998. Imprinting mechanisms in mammals. Curr. Opin. Genet. Dev. 8:154-164. [DOI] [PubMed] [Google Scholar]
- 38.Takeuchi, T., Y. Yamazaki, Y. Katoh-Fukui, R. Tsuchiya, S. Kondo, J. Motoyama, and T. Higashinakagawa. 1995. Gene trap capture of a novel mouse gene, jumonji, required for neural tube formation. Genes Dev. 9:1211-1222. [DOI] [PubMed] [Google Scholar]
- 39.Taricani, L., M. L. Tejada, and P. G. Young. 2002. The fission yeast ES2 homologue, Bis1, interacts with the Ish1 stress-responsive nuclear envelope protein. J. Biol. Chem. 277:10562-10572. [DOI] [PubMed] [Google Scholar]
- 40.Thon, G., P. Bjerling, C. M. Bunner, and J. Verhein-Hansen. 2002. Expression-state boundaries in the mating-type region of fission yeast. Genetics 161:611-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thon, G., A. Cohen, and A. J. S. Klar. 1994. Three additional linkage groups that repress transcription and meiotic recombination in the mating-type region of Schizosaccharomyces pombe. Genetics 138:29-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thon, G., and T. Friis. 1997. Epigenetic inheritance of trancriptional silencing and switching competence in fission yeast. Genetics 145:685-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thon, G., and A. J. S. Klar. 1992. The clr1 locus regulates the expression of the cryptic mating-type loci of fission yeast. Genetics 131:287-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wallrath, L. L. 1998. Unfolding the mysteries of heterochromatin. Curr. Opin. Genet. Dev. 8:147-153. [DOI] [PubMed] [Google Scholar]
- 45.Weiler, K. S., and B. T. Wakimoto. 1995. Heterochromatin and gene expression in Drosophila. Annu. Rev. Genet. 29:577-605. [DOI] [PubMed] [Google Scholar]
- 46.Wolffe, A. P. 1996. Genomic imprinting: causes and consequences, p. 49-70. In R. Ohlsson, K. Hall, and M. Ritzen (ed.), Epigenetic inheritance: the chromatin connection. Cambridge University Press, Cambridge, United Kingdom.
- 47.Wolffe, A. P., and M. A. Matzke. 1999. Epigenetics: regulation through repression. Science 286:481-486. [DOI] [PubMed] [Google Scholar]
- 48.Wood, V., et al. 2002. The genome sequence of Schizosaccharomyces pombe. Nature 415:871-880. [DOI] [PubMed] [Google Scholar]