Abstract
The TATA-binding protein (TBP) is a universal transcription factor required for all of the eukaryotic RNA polymerases. In addition to TBP, metazoans commonly express a distantly TBP-related protein referred to as TBP-like protein (TLP/TRF2/TLF). Although the function of TLP in transcriptional regulation is not clear, it is known that TLP is required for embryogenesis and spermiogenesis. In the present study, we investigated the cellular functions of TLP by using TLP knockout chicken DT40 cells. TLP was found to be dispensable for cell growth. Unexpectedly, TLP-null cells exhibited a 20% elevated cell cycle progression rate that was attributed to shortening of the G2 phase. This indicates that TLP functions as a negative regulator of cell growth. Moreover, we found that TLP mainly existed in the cytoplasm and was translocated to the nucleus restrictedly at the G2 phase. Ectopic expression of nuclear localization signal-carrying TLP resulted in an increase (1.5-fold) in the proportion of cells remaining in the G2/M phase and apoptotic state. Notably, TLP-null cells showed an insufficient G2 checkpoint when the cells were exposed to stresses such as UV light and methyl methanesulfonate, and the population of apoptotic cells after stresses decreased to 40%. These phenomena in G2 checkpoint regulation are suggested to be p53 independent because p53 does not function in DT40 cells. Moreover, TLP was transiently translocated to the nucleus shortly (15 min) after stress treatment. The expression of several stress response and cell cycle regulatory genes drifted in a both TLP- and stress-dependent manner. Nucleus-translocating TLP is therefore thought to work by checking cell integrity through its transcription regulatory ability. TLP is considered to be a signal-transducing transcription factor in cell cycle regulation and stress response.
TATA-binding protein (TBP) is a key general transcription factor for all classes of RNA polymerases (RNAPs) in eukaryotes (22). Each RNAP needs a distinct set of general transcription factors for promoter anchoring and transcription initiation. For RNAPII, TFIID, consisting of TBP and several TBP-associated factors, works for transcriptional regulation through association with a specific promoter sequence and for transcriptional activators (33). Recently, variants of the TFIID complex with a distinct promoter recognition specificity have been isolated (14, 27, 65). TBP interacts with the TATA sequence located upstream of the transcription start site to assemble RNAPII and general transcription factors, followed by initiation of transcription (7). TBP has two domains: a variable N-terminal domain and a highly conserved C-terminal core domain. Crystallographic analysis of the core domain has revealed that TBP has a typical saddle-shaped structure (29). This structure confers multiple abilities, such as DNA binding, self-dimerization, and protein-protein interaction, on TBP.
Although plants have more than one TBP-encoding gene (16), vertebrates generally have a single TBP gene. However, two TBP family genes (for TBP-related factor 1 and TBP-like protein) have been identified in metazoans (8, 42). TRF1 has been found only in Drosophila. It functions as a promoter selectivity factor in the nervous system and substitutes for TBP in RNAPII and III transcription (20, 25, 55). In contrast, TLP (also called TRF2 or TLF) has been identified in all of the metazoans examined, although its role in transcriptional regulation remains unknown. Sequence and genomic structure analyses have revealed that TRF1 and TLP have different evolution pathways (9, 52).
TLP bears a 180-amino-acid sequence that resembles the saddle-shaped structure of TBP and displays about 38% identity to the TBP core domain. TLP can bind TFIIA and TFIIB, like TBP, but does not bind to the TATA sequence (40, 42, 48, 57). In vitro experiments have shown that TLP cannot be replaced by TBP for transcription activation (40, 57). However, TLP stimulated transcription from RNAPII promoters like TBP when it was artificially recruited to a promoter (43). Recently, it was reported that TLP enhanced transcription from a core promoter that lacks a TATA sequence in Drosophila (24). We observed that TLP stimulated several TATA-less RNAPII promoters in vivo (43a). It is therefore speculated that TLP works for transcriptional regulation of RNAPII genes that carry a promoter element distinct from the TATA sequence. Suppression of the TLP gene by RNA interference in Caenorhabditis elegans, Xenopus, and zebra fish showed that TLP regulated embryonic development and was required for the expression of a subset of RNAPII genes (10, 28, 41, 61). It is also suggested that TLP is required for correct zygotic transcription as a surrogate for TBP during early embryogenesis. Mouse TLP was found not to be required for embryogenesis but to be essential for spermiogenesis (35, 36, 68). Therefore, although TLP is not essential for cell viability, it seems to have some species-specific functions. Consequently, TLP is thought to be involved in the transcriptional regulation of a particular set of genes required for cell dynamism.
It is generally understood that cell differentiation is associated with cell proliferation and that rapidly proliferating cells do not undergo differentiation (6, 53, 54). Cell proliferation is thought to be controlled by signal transduction pathways that are involved in cell cycle progression, development, differentiation, and apoptosis. Therefore, a protein that regulates cell proliferation can also affect other cell dynamism. For example, p53 tumor suppressor protein affects cell cycle progression, as well as DNA damage response and differentiation (18). The cell cycle is positively regulated by binding of cyclin-dependent kinases to cyclins and subsequent phosphorylation. Progression of the cell cycle is negatively controlled by the binding of cyclin-dependent kinase inhibitors to cyclin-cyclin-dependent kinase complexes (44). These inhibitors of cell cycle progression participate in the differentiation pathway. p21 (WAF1/CIP1), which inhibits the critical G2 regulatory factor cdc2 kinase, is up-regulated during differentiation (5). In addition to typical cell cycle regulatory factors, general transcription factors for RNAPII have been reported to be involved in cell proliferation and differentiation. TFIIA, which interacts with TBP and plays a role as a positive cofactor for TBP-DNA binding, regulates cell cycle progression in yeast (46). TAFII250 and TAFII30 are required for cell cycle progression and differentiation (39, 64). Since it has recently been reported that TBP induces a delay in the G2/M transition (60), it is thought that TLP also participates in cell cycle regulation.
It is therefore important to investigate the ability of TLP to regulate cell proliferation. In this study, we disrupted the TLP gene of chicken DT40 cells to investigate the cellular mechanism of TLP. TLP-null cells were viable but displayed characteristic growth property and stress response phenotypes. TLP prolonged the G2 phase and displayed G2 checkpoint ability. Accordingly, TLP-null cells were more resistant to G2 checkpoint-dependent stress than were wild-type cells. Moreover, TLP was transiently translocated to the nucleus when cells reached the G2 phase and when they were also exposed to stress agents. Expression levels of several cell cycle regulatory genes changed in both stress- and TLP-dependent manners. These results suggest that TLP functions as a transcription factor through nuclear translocation under specific conditions and regulates cell cycle progression and the G2 checkpoint.
MATERIALS AND METHODS
Construction of expression plasmids.
A chicken TLP (cTLP) expression plasmid, FH-TLP, was constructed with the pCI-neo vector (Promega) carrying a FLAG-and-His tag just upstream of the DNA-cloning site. Nuclear localization signal (NLS)-carrying FH-TLP, NLS-TLP, was constructed with oligonucleotides (AATTGGATCCAAAAAAGAAGAGAAAGGTAG and AATTCTACCTTTCTCTTCTTTTTTGGATCC) that contain an NLS of the simian virus 40 T antigen. The NLS was inserted into the EcoRI site just upstream from the tag region.
Cell culture and transfection.
DT40 chicken B lymphoma cells were cultured at 39.5°C in RPMI 1640 medium (Sigma) supplemented with 100 U of penicillin per ml, 100 μg of streptomycin per ml, 10% fetal calf serum, and 1% chicken serum (JRH Bioscience). HeLa and NIH 3T3 cells were maintained in Dulbecco modified Eagle medium (Sigma) supplemented with 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 10% fetal calf serum at 37°C. TLP-deficient DT40 cells (107) were transfected with FH-TLP plasmid (25 μg) by electroporation at 550 V and 25 μF with a Gene Pulser II (Bio-Rad). When wild-type DT40 cells (5 × 105) were transfected with NLS-TLP and FH-TLP, DNA (1 μg) was introduced with Lipofectamine reagent (Invitrogen).
Disruption of the TLP gene of chicken DT40 cells.
The third exons of cTLP of DT40 cells were disrupted by homologous recombination with drug resistance genes. For the first disruption, a histidinol resistance gene was used. Cells (107) were transfected with 25 μg of linearized plasmid by electroporation. Drug selection was performed at 24 h after transfection. After resistant cells were obtained, one representative heterozygous knockout cell line was subjected to secondary disruption by a puromycin or neomycin resistance gene. The concentrations of histidinol, puromycin, and neomycin in media were 1 mg/ml, 0.5 μg/ml, and 2 mg/ml, respectively (31). The recombination efficiency was around 70% throughout the experiments.
Northern blot analysis.
Ten million cells were washed with phosphate-buffered saline (PBS), and total RNAs were extracted with an RNeasy kit (Qiagen). RNA (10 μg) was separated in a 1% formaldehyde gel and transferred to a Hybond N+ membrane (Amersham). The membrane was then hybridized with a 32P-labeled probe that covers exons 3 to 6 of the cTLP cDNA in hybridization buffer (50% formamide, 5× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 5× Denhardt's reagent [12], 0.1 M sodium phosphate buffer [pH 6.5], 0.5% sodium dodecyl sulfate, 100 μg of yeast RNA per ml) at 42°C overnight. After the membrane was washed, the signal was detected by autoradiography.
Protein extraction and Western blotting analysis.
Ten million cells were lysed in buffer C (50 mM HEPES-KOH [pH 7.8], 420 mM KCl, 0.1 mM EDTA, 5 mM MgCl2, 20% glycerol) supplemented with benzamidine-HCl (1 mM), pepstatin A (1 μg/ml), phenylmethylsulfonyl fluoride (0.5 mM), and leupeptin (1 μg/ml). Following a brief centrifugation, the supernatant fractions were collected as whole-cell extracts. Cytoplasmic and nuclear extracts were prepared as previously described (13). Protein concentrations of the cell extracts were determined with a bicinchoninic acid protein assay kit (Pierce). The cell extracts were lysed in Laemmli buffer. For Western blotting, cell extracts (10 μg) were separated by sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis and then electroblotted onto a nitrocellulose membrane (Millipore). Exogenous FH-TLP and NLS-TLP and endogenous c-fos were detected by an alkaline phosphatase method (Promega) with anti-mouse TLP antiserum (42) and anti-human c-fos polyclonal antiserum (Calbiochem), respectively. Endogenous TLP and β-actin were detected by the enhanced-chemiluminescence system (Amersham) with anti-mouse TLP antiserum and anti-mouse β-actin mouse monoclonal antiserum (Sigma), respectively.
Cell growth inhibitory agents and flow cytometry.
For cell proliferation analysis, 104 cells were seeded into 60-mm-diameter dishes. Cell viability was determined by the trypan blue dye exclusion method, and the number of viable cells was determined with a hemacytometer. To examine UV sensitivity, 5 × 105 cells were irradiated with UV light. Eight hours after irradiation, the viable cells were counted. For determination of MMS sensitivity, 5 × 105 cells were treated with various concentrations of MMS and viable cells were scored after 12 h. For flow cytometry (fluorescence-activated cell sorter [FACS] analysis), cells were washed with PBS, fixed in 70% ethanol at 4°C overnight, and then treated with 100 μg of RNase A (Calbiochem) per ml in PBS for 20 min at room temperature. The cells were stained with propidium iodide (10 μg/ml). Subsequent flow cytometry analysis was performed with a FACScalibur (Becton Dickinson). The proportion of each cell phase was determined with Cell Quest software (Becton Dickinson).
Synchronization of cells.
Cells were synchronized at the G1/S boundary by the double-thymidine block method, with minor modifications. DT40 cells were cultured for 15 h (24 h in the case of NIH 3T3 cells) in medium containing 2.5 mM thymidine. After release in thymidine-free medium for 6 h, 1 mM hydroxyurea was added to the medium and the cells were incubated for 12 h (24 h in the case of NIH 3T3 cells). After release of the blocker in the normal medium, the cells were harvested at the indicated times. To enrich the cell population remaining at early M phase, DT40 cells were cultured in medium containing 2.5 mM thymidine for 15 h (24 h in the case of NIH 3T3 cells) and then maintained for 3 h in the normal medium. In medium containing 0.4 mg of nocodazole per ml, cells were cultured for 5 h (12 h in the case of NIH 3T3 cells). They were released from the block in nocodazole-free medium. The cells were cultured at 37°C in these procedures.
Immunocytochemistry.
Cells were grown on a glass coverslip and fixed with 4% paraformaldehyde in PBS for 6 min. Following blocking with 1% bovine serum albumin in PBS for 40 min, cells were incubated with a primary antibody for 40 min. After washing, the cells were exposed to fluorescein isothiocyanate- and rhodamine-conjugated secondary antibodies (Jackson) at a 1:100 dilution. DNA was stained with 1 μg of 4′,6′-diamidino-2-phenylindole (DAPI; Sigma) per ml. Cells were mounted for microscopy in mounting solution (10 mg of p-phenylenediamine [Wako] per ml in 90% glycerol). Apoptotic cells were detected by DAPI staining. At 18 h after transfection with TLP expression vectors, DT40 cells were cultured for 2 h in medium containing 1 mg of DAPI per ml. These procedures were carried out at 37°C.
RT-PCR.
Total RNAs were extracted from native and stressed DT40 cells and cells derived from them. Reverse transcription (RT) was performed with total RNA (1 μg) with an RNA PCR kit (TaKaRa). Reverse transcripts were amplified by a standard PCR with appropriate primers. The PCR cycle (15 to 25 cycles) of each gene was examined beforehand to obtain optimal band intensities that quantitatively represent the expression level. RT-PCR products (∼500-bp fragments) were separated by 1% agarose gel electrophoresis and stained with ethidium bromide. Band intensity was quantified with ImageMaster 1D software (Amersham Pharmacia Biotech).
Nucleotide sequence accession number.
The nucleotide and amino acid sequences of cTLP appear in the DDBJ/EMBL/GenBank databases under accession number AB024489.
RESULTS
Disruption of the TLP gene results in an increase in the cell cycle progression rate.
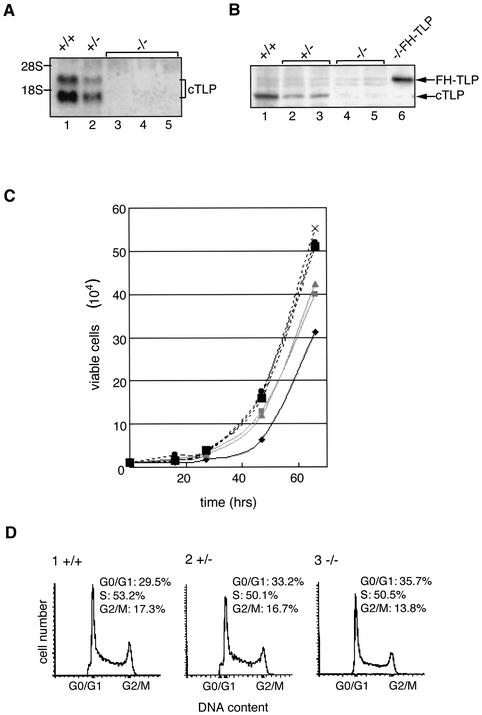
To study the in vivo function of TLP in vertebrate cells, we established TLP knockout cells of chicken DT40 cells (2, 3). DT40 cells propagate rapidly (i.e., spending about 10 h for one generation) in culture and undergo frequent homologous recombination. cTLP, which has 97% identity with its mammalian counterparts, is a single-copy gene located on chromosome 3 (52). The cTLP genes in both alleles were disrupted by replacement with drug resistance genes, and multiple cTLP double-knockout cells were obtained. The established cells contained no detectable cTLP mRNA or protein (Fig. 1A and B). These results indicate that TLP is dispensable for the viability of DT40 cells. The same conclusion has been made for other organisms (10, 28, 35, 41, 61, 68). TBP gene expression was not affected in any strains of TLP-null cells (data not shown). However, we observed several interesting phenotypic changes in TLP-null cells (see below).
FIG. 1.
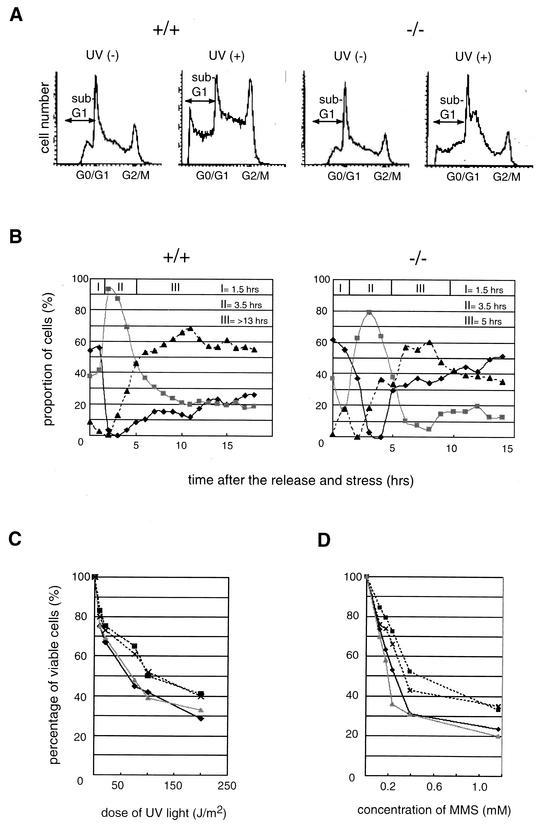
Disruption of the TLP gene and growth rate of DT40 cells. Disruption of the cTLP gene of DT40 cells by homologous recombination was examined by Northern (A) and Western (B) blotting. The positions of endogenous TLP (cTLP) and ectopically expressed FH-TLP are indicated. +/+, +/−, and −/− represent wild-type, heterozygous TLP mutant, and homozygous TLP mutant (TLP-null) cells, respectively. Three strains of TLP-null cells (strains A, B, and C) were examined. Strain A (lane 3 of panel A and lane 4 of panel B) was obtained by neomycin resistance gene-mediated secondary disruption, and strains B (lane 4 of panel A) and C (lane 5 of panels A and B) were obtained by puromycin resistance gene-mediated secondary disruption. −/−FH-TLP, TLP-null (strain C) cells that stably express FH-TLP. (C) The growth rates of cells of several strains were determined by cell counting at the indicated times. Results for individual strains are shown as average numbers of three different dishes. Dark solid line, wild-type cells. Gray solid lines show two lines of heterozygous TLP-disrupted cells: one for lane 2 of panel A and lane 2 of panel B and the other for lane 3 of panel B. Dotted lines indicate three strains of TLP-null cells. Symbols: ▪, strain A; ×, strain B; •, strain C. (D) Flow cytometry of three different types of DT40 cells. Cell numbers are plotted as a fraction of the DNA contents based on intensities of propidium iodide-dependent fluorescence. −/−, TLP-null (strain C) cells. The percentage of each phase is indicated. The positions of representative G0/G1- and G2/M-phase cells are indicated.
Growth profiles of TLP-null cells were analyzed. To exclude effects of the integrated drug resistance genes on acquired properties of TLP-null cells, we used multiple drug-resistant clones (Fig. 1, legend). All strains of TLP-null cells proliferated faster than did wild-type cells (Fig. 1C). The doubling times of wild-type and TLP-null cells were 10 and 8 h, respectively, and heterozygous knockout cells (TLP+/−) exhibited a doubling time that was intermediate between those of wild-type and TLP-null cells (Fig. 1C). The high proliferation rate of TLP-null cells was restored by ectopic expression of TLP (Fig. 2B, part 4). Consistently, it was found that wild-type DT40 cells and mammalian (HeLa and NIH 3T3) cells also exhibited lower growth rates when they overexpressed exogenous TLP (data not shown). Therefore, it was demonstrated that the cell growth rate depended on the level of intracellular TLPs. To characterize the growth profile of TLP-null cells in detail, we analyzed them with a FACS. The proportion of G2/M-phase cells of asynchronously growing TLP-null cells was reduced, while that of G1-phase cells was concomitantly elevated compared with that of wild-type cells (Fig. 1D). These results imply that the high proliferation rate of TLP-null cells is due to the shortened G2/M phase.
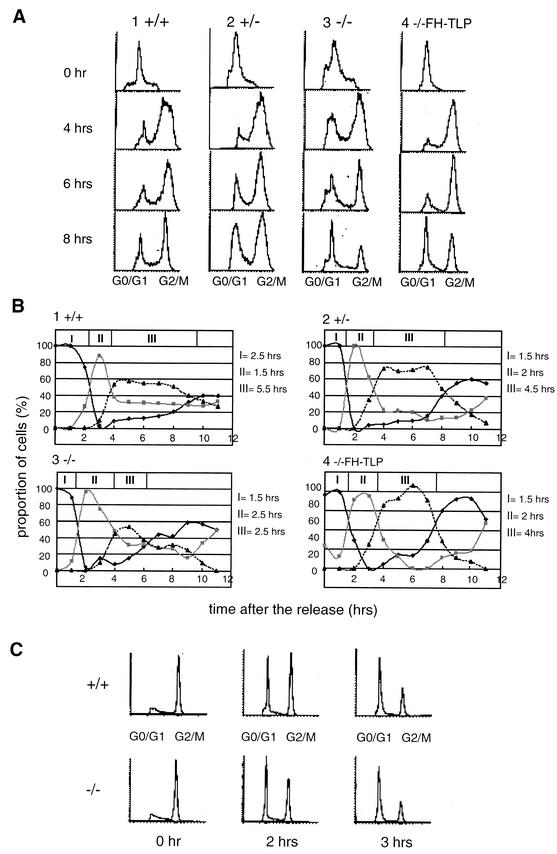
FIG. 2.
Disruption of the TLP gene of DT40 cells results in shortening of the G2 phase. (A) Synchronized and released cells with different cTLP genotypes were analyzed with a FACS. Cells remaining at the G1/S boundary were analyzed at the indicated times after release (0 h). (B) Proportions of three kinds of cell cycle phase at different times after release based on data in panel A are plotted. Dark solid line, G0/G1 phase; gray solid line, S phase; dotted line, G2/M phase; I, period in which G0/G1-phase cells were in the majority (duration from the point of release [0 h] to the time when the proportions of G0/G1-phase and S-phase cells became equal); II, period in which S-phase cells were in the majority (duration from the end of period I to the time when the proportions of S-phase and G2/M-phase cells became equal); III, period in which G2/M-phase cells were in the majority (duration from the end of period II to the time when the proportions of G2/M-phase and G0/G1-phase cells became equal). (C) Cells synchronized in the early M phase were released, and cell cycle phases were analyzed with a FACS.
TLP-null cells have a shortened G2-phase period.
We analyzed the length of cell cycle phases by using synchronized cells. First, cells with different TLP genotypes were maintained in culture medium containing hydroxyurea or thymidine to arrest cells at the G1/S boundary and cell populations were analyzed with a FACS. Most of the cells of all of the cell lines used shifted to the G2/M phase from the S phase at 3.5 to 4 h after release (Fig. 2A and B). Notably, the majority of TLP-null cells remained at the next G1 phase at 7 h after release (Fig. 2B, part 3) whereas the majority of wild-type cells still remained at the G2/M phase (Fig. 2B, part 1). Although period III (G2/M-phase majority time) of wild-type cells lasted 5.5 h (Fig. 2B, part 1), that of TLP-null cells lasted only 2.5 h (Fig. 2B, part 3). Thus, it was thought that TLP-null cells enter the G1 phase from the G2/M phase faster than do wild-type cells. Period III of TLP+/− cells was determined to be 4.5 h, a duration between those of TLP-null and wild-type cells (Fig. 2B, part 2). Moreover, period III of −/− FH-TLP cells was longer (4 h) than that of parental TLP-null cells (Fig. 2B, part 3 versus part 4). These results suggest that TLP prolongs the G2/M phase. The difference between the doubling times of wild-type cells (10 h) and TLP-null cells (8 h) shown in Fig. 1B thus roughly coincides with that shown in Fig. 2A and B. To determine which cell cycle phase, G2 or M, is affected by TLP, we synchronized cells at the early M phase by using nocodazole. Three hours after release, the same proportions of wild-type and TLP-null cells entered the G1 phase from mitosis (Fig. 2C). These results suggest that the rapid proliferation of TLP-null cells is due to shortening of the G2 phase, not the M phase. TLP was thought to regulate the length of the G2 phase.
TLP is translocated to the nucleus in a cell cycle-dependent manner.
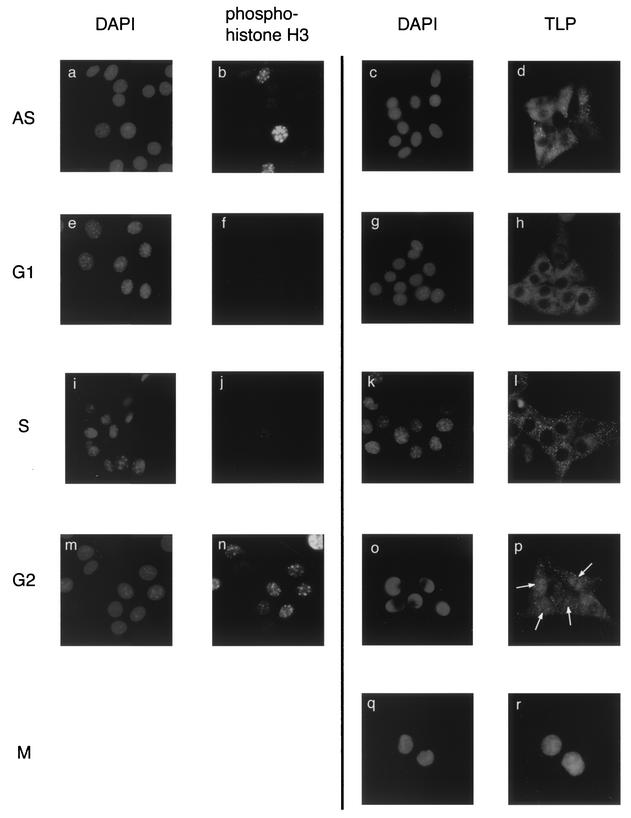
Many lines of evidence suggest that TLP is a transcription factor. We also found that TLP can act as an unconventional transcriptional activator (43). However, unexpectedly, most (more than 90%) of the TLPs were observed in the cytoplasm of asynchronized NIH 3T3 cells (Fig. 3d) and DT40 cells (Fig. 4B, cTLP; see also Fig. 6A, lane 1 versus lane 6). We examined the localization of TLP and G2-phase-specific phosphorylated histone H3 (21) in synchronized NIH 3T3 cells (Fig. 3). On the basis of phospho-histone H3 staining patterns, the cell populations were confirmed to be synchronized at objective phases (Fig. 3e to r). G1- and S-phase cells, which did not exhibit phospho-histone H3 in their nuclei (Fig. 3f and j), contained TLP in the cytoplasm (Fig. 3 h and l), while cells remaining at the G2 phase showed a nuclear staining pattern with both phospho-histone H3 and TLP (Fig. 3n and p, respectively). On the basis of the results of cell number counting, proportions of cytoplasmic TLP in the G1- and S-phase cells and nuclear TLP in the G2-phase cells were estimated to be more than 90 and 70%, respectively (Fig. 3h, l, and p). At the G2 phase, phospho-histone H3 showed a characteristic dot staining pattern (Fig. 3n). At the M phase, both phospho-histone H3 (data not shown) and TLP (Fig. 3r) were present relatively homogeneously in cells. Eventually, cytoplasmic TLP was found to be translocated to the nucleus at the G2 phase.
FIG. 3.
Cell cycle-specific nuclear translocation of cytoplasmic TLP. NIH 3T3 cells synchronistically staying at the G1/S boundary were released and cultured for 4, 8, and 12 h to prepare S-, G2-, and M-phase cell populations, respectively. For preparation of G1-phase cells, cells were synchronized at the early M phase and subsequently cultured for 4 h after release. AS, asynchronized cells. Cells were stained with DAPI, anti-phospho-histone H3, and anti-TLP antibody. Cell populations used for DAPI and anti-phospho-histone H3 staining were identical for the AS, G1, S, and G2 phases. Cells remaining in the M phase showed a homogeneous staining pattern with phospho-histone H3 (not shown). Among five cells remaining in the G2 phase, four nuclei (arrows) were stained with anti-TLP antibody (o and p). The microscopic images were taken at a magnification of ×640.
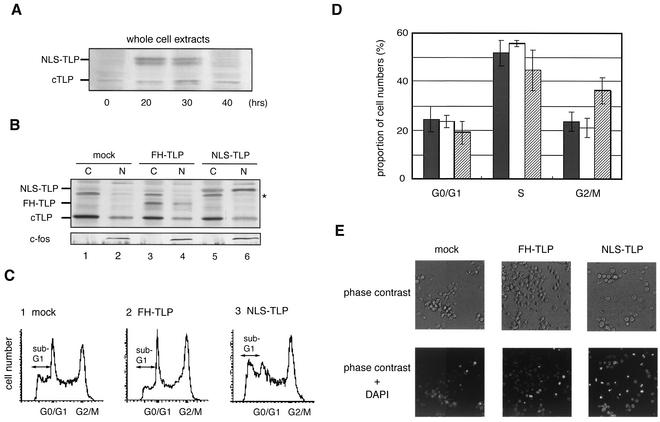
FIG. 4.
NLS-TLP increases the population of cells remaining in the G2 phase and apoptotic cells. (A) Ectopic expression of NLS-TLP proteins was examined by Western blotting. Wild-type cells transfected with the NLS-TLP plasmid were incubated for the indicated times, and amounts of endogenous (cTLP) and exogenous (NLS-TLP) TLP in whole-cell extracts were determined. (B) Nuclear localization of exogenous FH-TLP and NLS-TLP. Twenty hours after transfection, amounts of endogenous TLP (cTLP), c-fos, and exogenous FH-TLP (lanes 3 and 4) and NLS-TLP (lanes 5 and 6) in cytoplasmic (C) and nuclear (N) extracts were determined by Western blotting. Cytoplasmic and nuclear extracts were applied to 1/10 and 3/4 of the total extracts from the same number of cells (approximately 106), respectively. Mock, empty expression vector-transfected cells; FH-TLP and NLS-TLP, wild-type cells transfected with expression plasmids for FH-TLP and NLS-TLP, respectively. An asterisk indicates a nonspecific band. (C) Shift in cell cycle phases elicited by NLS-TLP overexpression. Twenty hours after transfection, the cell cycle profile of each cell batch was analyzed with a FACS. Positions of the sub-G1 region (apoptotic cell population) are indicated. (D) Histogram of results shown in panel C. Solid columns, mock-transfected cells; open columns, cells overexpressing FH-TLP; hatched columns, cells overexpressing NLS-TLP. Averages and standard deviations were calculated from four independent experiments. (E) Detection of apoptotic cells by DAPI staining. At 18 h after transfection, cells were cultured in medium containing DAPI. The microscopic images were taken at an original magnification of ×400.
FIG. 6.
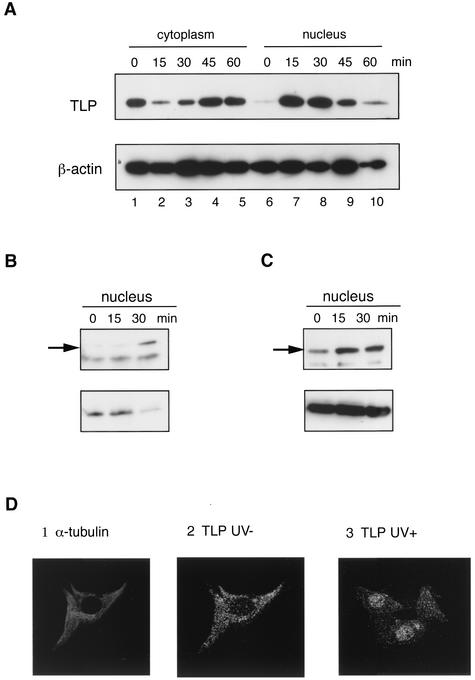
TLP is translocated to the nucleus when cells are exposed to stress agents. (A) UV-irradiated DT40 cells (100 J/m2) were harvested at the indicated times after irradiation, and TLP and β-actin in the nuclear and cytoplasmic extracts were analyzed by Western blotting. NIH 3T3 (B) and HeLa (C) cells were exposed to UV light (100 J/m2), and the nuclear extracts were analyzed as described for panel A. Cytoplasmic and nuclear extracts were applied to 1/10 and 3/4 of the total extracts from the same number of cells (approximately 106), respectively. (D) NIH 3T3 cells exposed (panel 3) or not exposed (panels 1 and 2) to UV light (100 J/m2) were harvested 15 min after irradiation, and the localization of TLP (panels 2 and 3) and α-tubulin (panel 1) was analyzed immunocytochemically.
TLP translocated compulsorily to the nucleus induces G2 delay and apoptosis.
Next, we addressed the question of whether TLP actively induces a G2 delay. Unlike TLPs of lower animals, those of vertebrates do not have a conventional NLS. We ectopically expressed artificial NLS-TLP in DT40 cells. The expression levels of NLS-TLP (Fig. 4A) and parental FH-TLP (data not shown) in cells were about 1.5 times greater than that of endogenous TLP at 20 h after transfection. About 30% of NLS-TLPs were localized in the nucleus (Fig. 4B, lane 5 versus lane 6), while most (more than 90%) of the FH-TLPs remained in the cytoplasm (Fig. 4B, lane 3 versus lane 4). Our cell fractionation method was confirmed to be relevant by Western blotting with an anti-c-fos antibody against reference nuclear protein c-fos (59) (Fig. 4B, bottom). Accordingly, the amounts of nucleus-localizing TLPs were estimated to be three- to fivefold greater than those in mock-transfected cells. As had been expected, the size of the G2/M population in NLS-TLP-expressing cells was 1.5-fold greater than that in wild-type cells at 20 h after transfection (Fig. 4C, part 3, and D), and such an increase in the G2/M population was not observed in FH-TLP-expressing cells (Fig. 4C, part 2, and D). Furthermore, there was a significant increase (about twofold) in apoptotic cells in NLS-TLP-expressing cells compared with those in FH-TLP-expressing cells and mock-transfected cells (Fig. 4C, sub-G1 groups, and E, DAPI-stained cells). Consequently, in the nucleus, TLP was found to actively work to induce a G2 delay and apoptosis.
TLP functions for the G2/M checkpoint and induces apoptosis.
Eukaryotic cells commonly have a checkpoint mechanism specific for each cell cycle phase, enabling the cells to prevent and repair DNA damage at different stages, depending on the agent causing the damage. The above-described results imply that TLP acts as a G2 checkpoint factor. We investigated the role of TLP in the DNA damage response elicited by UV light and methyl methanesulfonate (MMS). UV- and MMS-induced DNA repairs occur predominantly at the G2 checkpoint (45, 47). We analyzed the proportions of cell phases in irradiated TLP-null and wild-type cells with a FACS. In agreement with previous observations (4, 56), the proportions of G2/M and G1 phases of wild-type cells had increased and decreased at 8 h after UV irradiation, respectively (Fig. 5A, +/+). However, the proportions of these two phases of TLP-null cells did not change significantly (Fig. 5A, −/−). Furthermore, the percentage of apoptotic TLP-null cells reached only 45% of that of wild-type cells (sub-G1 group cells in Fig. 5A, UV+). The same phenomenon was observed in MMS-treated cells (data not shown). These results suggest that TLP is responsible for the G2 delay and apoptosis of stressed cells. It was thought that a significant proportion of G2-phase TLP-null cells escaped from the G2 checkpoint mechanism and entered the G1 phase even if they had been exposed to stresses because the G2 phase of TLP-null cells is too short for the G2 checkpoint to function sufficiently. The G1/S boundary-synchronized cells were treated with MMS, and the populations of G2/M phase-escaping cells and G1 phase arrival cells were analyzed with a FACS. Consistent with the results shown in Fig. 1, wild-type and TLP-null cells were in the G2 phase (period III) as a major population at 5 h after release (Fig. 5B, dotted line). Only 25% of the wild-type cells were in the next G1 phase, even at 18 h after release and later (Fig. 5B, +/+, solid line). However, more than 40% of the TLP-null cells reached the next G1 phase and the majority remained at the G1 phase 10 h after release (Fig. 5B, −/−, solid line), even though period III of TLP-null cells under the stressed condition (5 h) was twofold longer than that under the nonstressed condition (2.5 h) (Fig. 5B, −/−, versus 2B, part 3). In contrast to a small percentage (less than 5%) of nonstressed TLP-null cells remaining at the G2 phase (Fig. 2B, part 3), about 35% of the stressed TLP-null cells still remained at the G2 phase even when the majority of cells were in the G1 phase at 14 h after release (Fig. 5B, −/−). The accumulation of TLP-null cells remaining at the G2 phase is thought to be due to a TLP-independent G2 checkpoint mechanism (see Discussion). These results suggest that a significant part of the G2 checkpoint in DT40 cells is conducted by TLP.
FIG. 5.
TLP is required for G2 checkpoint regulation. (A) Proportions of cell cycle phases following UV irradiation. Eight hours after UV irradiation (75 J/m2) [UV (+)], wild-type cells (+/+) and TLP-null strain C cells (−/−) were analyzed with a FACS. UV (−), without irradiation. (B) Changes in proportions of cell cycle phases after MMS exposure. G1/S boundary-synchronized cells were released and exposed to MMS (0.2 mM). Cells were then analyzed with a FACS. Dark solid line, G0/G1 phase; gray solid line, S phase; dotted line, G2/M phase. Three periods (I, II, and III) during which cells of each phase were in the majority were determined as described in the legend to Fig. 2B. Dose-response curves of viable DT40 cells following UV irradiation (C) and MMS exposure (D) are shown. Results are shown as average numbers of two separate dishes in each assay. Wild-type cells (dark solid line), −/− FH-TLP cells (gray solid line), and two lines of TLP-null cells (dotted lines) (strains A [▪] and C [×]) were examined.
Next, we examined the proportions of cells that had undergone apoptotic stresses. The number of viable wild-type cells was drastically reduced by UV irradiation (Fig. 5C, solid line). However, TLP-null cells were more tolerant to UV than were wild-type cells (Fig. 5C, dotted lines). The UV dose required to kill TLP-null cells was about twofold greater than that required to kill wild-type cells (Fig. 5C, solid line versus dotted lines). Likewise, TLP-null cells appeared to be more tolerant (1.5 times) to MMS treatment than were wild-type cells (Fig. 4D). The tolerance of −/− FH-TLP cells to UV and MMS was the same as that of wild-type cells (Fig. 5C and D, gray line versus solid line). These results suggest that TLP functions as a G2 checkpoint factor against UV and MMS and enhances an apoptosis pathway in the G2 phase. Since p53 does not function in DT40 cells (56, 66), the above phenomenon is thought to occur in a p53-independent manner.
We examined whether TLP is translocated to the nuclei of stress-exposed cells. Surprisingly, a high concentration of TLP was detected conversely in nuclear extracts shortly (15 to 30 min) after UV irradiation (Fig. 6A, lanes 2 and 3 versus lanes 7 and 8). The net amounts of TLP proteins in a cell did not change (data not shown). Afterward, the level of nuclear TLPs rapidly decreased and cytoplasmic TLP again became dominant within 1 h after UV irradiation. The same results were obtained with HeLa and NIH 3T3 cells (Fig. 6B and C). These phenomena were confirmed by immunocytochemical analysis of NIH 3T3 cells because TLPs in native and UV-irradiated cells were mostly localized in the cytoplasm and nucleus, respectively (Fig. 6D). The same results were obtained when cells were treated with MMS (data not shown). The localization pattern of TBP was not altered by stresses (data not shown). TLP was clearly demonstrated to behave as a nucleus-cytoplasm shuttle in accordance with stress exposure.
Identification of TLP-dependent and stress-responsive genes.
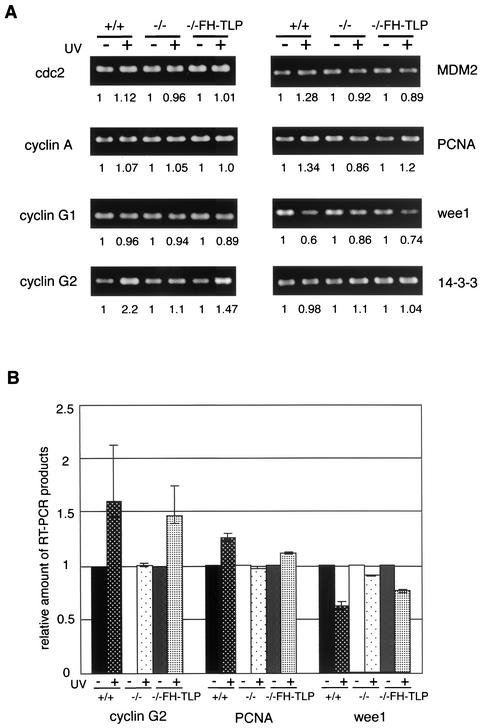
To determine the potential downstream target genes of TLP responsible for cell cycle and checkpoint regulation, we analyzed mRNA levels of 18 genes by RT-PCR. To observe the direct effect of nuclear TLP and to amplify changes in TLP-dependent transcripts, RNAs were prepared from cells shortly (1 h) after UV exposure. It was found that the amounts of mRNAs of the cyclin G2, PCNA, and wee1 genes, which are related to cell cycle progression and checkpoint (26, 49, 58), were significantly altered in irradiated wild-type cells (Fig. 7, +/+). The expression levels of the cyclin G2 and PCNA genes were increased (by 1.8- and 1.3-fold, respectively) by UV irradiation, whereas wee1 showed a reduced expression level (∼0.6-fold). Changes in these three genes were negligible in irradiated TLP-null cells (Fig. 7, −/−), and the expression levels were almost restored in −/− FH-TLP cells (Fig. 7, −/−FH-TLP). We reproducibly obtained the same results in multiple experiments. TLP is therefore thought to be involved in the expression of these genes. Changes in the expression levels could not be observed under the nonstressed condition (Fig. 7A, no-UV condition), suggesting that the basal levels of these genes are governed in a TLP-independent manner and that TLP plays a regulatory role.
FIG. 7.
Changes in mRNA expression levels of UV-irradiated cells. (A) Expression of genes related to checkpoint and cell cycle regulation was examined by RT-PCR. RNAs were prepared from three kinds of DT40 cells, wild-type (+/+), TLP-null (−/−; strain C), and −/− FH-TLP cells, 1 h after (+) or without (−) UV irradiation (100 J/m2), and RT-PCR products were detected. Results of representative genes are shown. The values at the bottom of each panel represent relative amounts of mRNAs in UV-irradiated cells with respect to those in nonirradiated cells of each strain. (B) Histogram of results of three genes (cyclin G2, PCNA, wee1) whose transcript levels were changed significantly by UV irradiation. Averages and standard deviations were obtained from five independent experiments.
DISCUSSION
TLP negatively regulates the cell proliferation rate.
In this study, we analyzed cellular functions of TLP by generation of TLP knockout cells. The general transcription factors are thought to be essential for cell functions because they are commonly required for transcriptional initiation. Contrary to the TBP gene, the present study with TLP-null cells clearly showed that TLP is not an essential factor for cell viability. This finding is consistent with results of TLP knockout and knockdown analyses of various kinds of eukaryotes even though TLP is required for development and differentiation (10, 28, 35, 36, 41, 61, 68).
To elucidate the in vivo function of TLP more precisely, we examined the growth properties of TLP-null cells. TLP-null cells showed a greater rate of proliferation than did wild-type cells, due to the shortened G2 phase, because the length of period III of TLP-null cells was only half of that for wild-type cells (Fig. 2B). Because −/− FH-TLP cells prolonged only the G2 phase (Fig. 2B), the low growth rate of −/− FH-TLP cells shown in Fig. 1C was not thought to be due to simple growth inhibition by the overexpression of exogenous proteins. Hence, this phenotype was dependent on the TLP expression level (Fig. 2A and B). Moreover, compulsorily nucleus-localized NLS-TLP actively increased the proportion of G2-phase cells (Fig. 4). It was concluded that TLP negatively regulates G2/M progression. Multiple general transcription factors, such as TFIIA, TFIIH, and TAFIIs, have been reported to participate in cell cycle regulation (39, 46, 51, 64). It has also been reported that heterozygous disruption of the TBP gene results in a delay in the G2/M transition (60). Hence, these two TBP family proteins have related but opposite roles in the G2/M transition; i.e., TBP accelerates the onset of M phase, while TLP prolongs the G2 phase.
TLP has been demonstrated to be required for embryogenesis of C. elegans and Xenopus and spermiogenesis in mice. It is widely recognized that differentiation is dependent on cell growth status. Our findings are consistent with those observations because it is generally accepted that differentiation is coupled with growth arrest. Consistently, growth factors such as FGF-2 and TGF-β and cell cycle checkpoint factors such as ATM and p53 are known to induce differentiation (1, 30, 37, 38). TLP may contribute to differentiation through negative cell growth regulation. Expression of TBP family genes is differently stimulated during differentiation and development; i.e., TBP is constantly expressed, whereas TLP is expressed at restricted stages (36). These differences in expression patterns may provide the specificity of subsequently transcribed genes and determine differentiation timing.
TLP regulates the G2 checkpoint.
TLP-null cells increased in the cell population escaping from the G2 phase compared with wild-type cells when cells were treated with G2 checkpoint-targeted stress agents (Fig. 5B). Moreover, TLP-null cells were more resistant to the stresses than were wild-type cells (Fig. 5C and D). The shorter G2 length of TLP-null cells compared with that of wild-type cells may result in an incomplete checkpoint and decreased numbers of apoptotic cells. Ectopic expression of NLS-carrying TLP resulted in an increase in G2 phase-remaining and apoptotic cell populations (Fig. 4). Therefore, TLP is suggested to conduct the G2 checkpoint regulation. However, the contribution of TLP seems to be limited in the whole checkpoint system governed by several major checkpoint factors, such as p53 and chk2 (11, 23). Since stress treatment also caused apoptotic death in some populations of TLP-null cells even though the rate of apoptotic cell death decreased compared to that of wild-type cells (Fig. 5C and D), a part of the G2 checkpoint is thought to be governed in a TLP-independent manner. Since p53 does not function in DT40 cells (56, 66), TLP most likely regulates the G2 checkpoint in a p53-independent pathway. It is widely known that p53, a central checkpoint factor, strongly induces stress-mediated G1 arrest and subsequent apoptosis. Stress tolerance of TLP-null cells was thought to appear because of a deficiency in functional p53 and a shortened G2 phase, where a checkpoint regulation mechanism should work. We also observed that TLP was translocated to the nucleus in response to stresses in NIH 3T3 cells, which have the functional p53 gene (Fig. 6). Hence, TLP is thought to function as a G2 checkpoint factor of other cells in which p53 normally works.
We discovered that TLP was transiently translocated to the nucleus in response to stresses. Thus, TLP can be regarded as a sensor and a transducer for checkpoint induction signals. Our finding that a basal transcription factor-related protein is directly involved in stress-related signal transduction is new. TLP probably functions in the nucleus as a transcription factor for cell cycle progression and the G2 checkpoint, presumably not as a direct checkpoint factor. This speculation is based on the data in Fig. 7 showing that expression of the cyclin G2, PCNA, and wee1 genes was affected when TLP was concentrated in the nucleus. Cyclin G2 is a negative regulator of cell cycle progression (15, 26). PCNA is also known as a G2 damage-dependent checkpoint regulator, and overexpression of PCNA results in G2 arrest (58, 63). Wee1 regulates the G2/M transition and is downregulated by DNA damage signals (32, 49). These factors work upstream of G2/M transition and checkpoint decision factors. It is thought that slight induction of these upstream regulatory proteins greatly influences the regulation of downstream proteins. Moreover, it has been reported that the expression level of these upstream regulatory factors drifted in response to stresses (15, 32, 63). Thus, altered expression of the cell cycle regulation genes shown in Fig. 7 is suggested to affect cell dynamics. We measured the gene expression levels 1 h after stress treatment to focus on the direct effect of nuclear TLP. TLP may directly participate in the regulation of these genes because it was translocated 15 min after stress treatment (Fig. 6). Recently, it has also been reported that TLP activates transcription from the TATA-less promoter of the PCNA gene in Drosophila (24). It is probable that the cyclin G2 and wee1 genes are also direct targets of TLP because these genes possess a TATA-less promoter, like the PCNA gene. Our results imply that TLP controls the expression of cell growth-, checkpoint-, and apoptosis-related genes.
It has been reported that TBP in stressed cells leaves off transcriptional regulation and preferentially binds to injured DNAs for repair (62). TBP family genes seem to play a common role in the defense of cells against stress damage through two different pathways; i.e., TBP directly participates in DNA repair, and TLP mediates cell cycle arrest and apoptosis as a transcription factor.
TLP is translocated to the nucleus in response to cell cycle phase and stress stimuli.
Transcription factors, which are ready for a quick response in transcriptional regulation, are basically localized in the nucleus. Nevertheless, we detected TLP in the cytoplasm with several assays (Fig. 3 and 6). TLP was thus concluded to be a cytoplasmic protein. It is noteworthy that vertebrate TLPs do not possess an NLS, unlike TBP and TLPs of lower animals. By using synchronized cells, we demonstrated that TLP was translocated to the nucleus at the G2 phase (Fig. 3). Moreover, we observed nuclear translocation of TLP in response to stress stimuli (Fig. 6). In every case, transiently nucleus-translocated TLP quickly returned to the cytoplasm. TLP was demonstrated to behave as a nucleus-cytoplasm shuttle protein.
We identified the potential downstream genes that were induced under the condition in which TLP was localized in the nucleus (Fig. 7). Knockout mouse experiments showed that TLP was expressed at a high level in the nuclei of round spermatids (36). Hence, the concentration of TLP in the nucleus seems to be associated with activation of TLP functions. Nuclear translocation is a critical event for certain transcription factors to acquire regulation ability in cells. p53 and NF-κB possess functional activity via nuclear localization upon receiving intracellular activation signals (17, 50). The STAT and SMAD families are directly activated by a membrane-bound receptor, followed by nuclear translocation (34, 67). In general, cytoplasmic proteins enter the nucleus through nuclear-pore complexes containing importins α and β and Ran GTPase that recognize NLSs (19). The mechanism that regulates the nuclear translocation of TLP is not clear because vertebrate TLPs do not have an NLS. Nuclear translocation of TLP is probably governed by other regulatory factors.
Is TLP function a transcription factor?
On the basis of its structural similarity to TBP, TLP is thought to be a transcription factor. In in vivo reporter assays, TLP activated transcription from model promoters when TLP was artificially recruited to a proximal promoter element (43). Moreover, TLP ectopically expressed in cells stimulated some promoters (43a). Several studies with knockout animals have revealed TLP-sensitive potential genes (35, 61, 68). It is thus thought that TLP regulates the transcription of certain genes by a novel mechanism distinct from that of TBP. The results of the present study support this assumption. TLP usually exists in the cytoplasm in an inactive form. It is speculated that when cells are exposed to particular signals derived from stress agents, cell cycle (G2 phase), and probably differentiation, TLP is transiently translocated to the nucleus upon receiving signals and temporarily regulates the transcription of specific target genes. Therefore, both TBP family proteins may act as transcription factors in distinct ways; i.e., TBP constantly remains in the nucleus and directs transcription initiation ubiquitously, whereas TLP temporarily or transiently modulates the expression of a limited set of genes under specific conditions.
Acknowledgments
We thank S. Takeda for the gift of DT40 cells and A. Dutta, M. Miura, and H. Abe for reading the manuscript. We also thank T. Aoki, T. Ohbayashi, and T. Wakamatsu for valuable discussions throughout this study.
Part of this work was supported by the CREST Japan Science and Technology Corporation.
REFERENCES
- 1.Almog, N., and V. Rotter. 1997. Involvement of p53 in cell differentiation and development. Biochim. Biophys. Acta 1333:F1-F27. [DOI] [PubMed] [Google Scholar]
- 2.Baba, T. W., B. P. Giroir, and E. H. Humphries. 1985. Cell lines derived from avian lymphomas exhibit two distinct phenotypes. Virology 144:139-151. [DOI] [PubMed] [Google Scholar]
- 3.Buerstedde, J. M., and S. Takeda. 1991. Increased ratio of targeted to random integration after transfection of chicken B cell lines. Cell 67:179-188. [DOI] [PubMed] [Google Scholar]
- 4.Bunz, F., A. Dutriaux, C. Lengauer, T. Waldman, S. Zhou, J. P. Brown, J. M. Sedivy, K. W. Kinzler, and B. Vogelstein. 1998. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282:1497-1501. [DOI] [PubMed] [Google Scholar]
- 5.Choi, Y. H., W. H. Lee, K. Y. Park, and L. Zhang. 2000. p53-independent induction of p21 (WAF1/CIP1), reduction of cyclin B1 and G2/M arrest by the isoflavone genistein in human prostate carcinoma cells. Jpn. J. Cancer Res. 91:164-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coffman, F. D., and G. P. Studzinski. 1999. Differentiation-related mechanisms which suppress DNA replication. Exp. Cell Res. 248:58-73. [DOI] [PubMed] [Google Scholar]
- 7.Colgan, J., and J. L. Manley. 1992. TFIID can be rate limiting in vivo for TATA-containing, but not TATA-lacking, RNA polymerase II promoters. Genes Dev. 6:304-315. [DOI] [PubMed] [Google Scholar]
- 8.Crowley, T. E., T. Hoey, J. K. Liu, Y. N. Jan, L. Y. Jan, and R. Tjian. 1993. A new factor related to TATA-binding protein has highly restricted expression patterns in Drosophila. Nature 361:557-561. [DOI] [PubMed] [Google Scholar]
- 9.Dantonel, J. C., J. M. Wurtz, O. Poch, D. Moras, and L. Tora. 1999. The TBP-like factor: an alternative transcription factor in metazoa? Trends Biochem. Sci. 24:335-339. [DOI] [PubMed] [Google Scholar]
- 10.Dantonel, J. C., S. Quintin, L. Lakatos, M. Labouesse, and L. Tora. 2000. TBP-like factor is required for embryonic RNA polymerase ll transcription in C. elegans. Mol. Cell 6:715-722. [DOI] [PubMed] [Google Scholar]
- 11.Deng, C., P. Zhang, J. W. Harper, S. J. Elledge, and P. Leder. 1995. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 82:675-684. [DOI] [PubMed] [Google Scholar]
- 12.Denhardt, D. T. 1966. A membrane-filter technique for the detection of complementary DNA. Biochem. Biophys. Res. Commun. 23:641-646. [DOI] [PubMed] [Google Scholar]
- 13.Dignam, J. D., R. M. Lebovitz, and R. G. Roeder. 1983. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11:1475-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dikstein, R., S. Zhou, and R. Tjian. 1996. Human TAFII 105 is a cell type-specific TFIID subunit related to hTAFII130. Cell 87:137-146. [DOI] [PubMed] [Google Scholar]
- 15.Gajate, C., F. An, and F. Mollinedo. 2002. Differential cytostatic and apoptotic effects of ecteinascidin-743 in cancer cells. Transcription-dependent cell cycle arrest and transcription-independent JNK and mitochondrial mediated apoptosis. J. Biol. Chem. 277:41580-41589. [DOI] [PubMed] [Google Scholar]
- 16.Gasch, A., A. Hoffmann, M. Horikoshi, R. G. Roeder, and N. H. Chua. 1990. Arabidopsis thaliana contains two genes for TFIID. Nature 346:390-394. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh, S., and M. Karin. 2002. Missing pieces in the NF-κB puzzle. Cell 109:S81-S96. [DOI] [PubMed] [Google Scholar]
- 18.Giaccia, A. J., and M. B. Kastan. 1998. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 12:2973-2983. [DOI] [PubMed] [Google Scholar]
- 19.Gorlich, D. 1998. Transport into and out of the cell nucleus. EMBO J. 17:2721-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen, S. K., S. Takada, R. H. Jacobson, J. T. Lis, and R. Tjian. 1997. Transcription properties of a cell type-specific TATA-binding protein, TRF. Cell 91:71-83. [DOI] [PubMed] [Google Scholar]
- 21.Hendzel, M. J., Y. Wei, M. A. Mancini, A. Van Hooser, T. Ranalli, B. R. Brinkley, D. P. Bazett-Jones, and C. D. Allis. 1997. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106:348-360. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez, N. 1993. TBP, a universal eukaryotic transcription factor? Genes Dev. 7:1291-1308. [DOI] [PubMed] [Google Scholar]
- 23.Hirao, A., Y. Y. Kong, S. Matsuoka, A. Wakeham, J. Ruland, H. Yoshida, D. Liu, S. J. Elledge, and T. W. Mak. 2000. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science 287:1824-1827. [DOI] [PubMed] [Google Scholar]
- 24.Hochheimer, A., S. Zhou, S. Zheng, M. C. Holmes, and R. Tjian. 2002. TRF2 associates with DREF and directs promoter-selective gene expression in Drosophila. Nature 420:439-445. [DOI] [PubMed] [Google Scholar]
- 25.Holmes, M. C., and R. Tjian. 2000. Promoter-selective properties of the TBP-related factor TRF1. Science 288:867-870. [DOI] [PubMed] [Google Scholar]
- 26.Horne, M. C., K. L. Donaldson, G. L. Goolsby, D. Tran, M. Mulheisen, J. W. Hell, and A. F. Wahl. 1997. Cyclin G2 is up-regulated during growth inhibition and B cell antigen receptor-mediated cell cycle arrest. J. Biol. Chem. 272:12650-12661. [DOI] [PubMed] [Google Scholar]
- 27.Jacq, X., C. Brou, Y. Lutz, I. Davidson, P. Chambon, and L. Tora. 1994. Human TAFII30 is present in a distinct TFIID complex and is required for transcriptional activation by the estrogen receptor. Cell 79:107-117. [DOI] [PubMed] [Google Scholar]
- 28.Kaltenbach, L., M. A. Horner, J. H. Rothman, and S. E. Mango. 2000. The TBP-like factor CeTLF is required to activate RNA polymerase II transcription during C. elegans embryogenesis. Mol. Cell 6:705-713. [DOI] [PubMed] [Google Scholar]
- 29.Kim, J. L., D. B. Nikolov, and S. K. Burley. 1993. Co-crystal structure of TBP recognizing the minor groove of a TATA element. Nature 365:520-527. [DOI] [PubMed] [Google Scholar]
- 30.Knittweis, J. 1998. An ataxia telangiectasia model: inefficient cell differentiation and possible reversal by serine protease inhibitors, tumor necrosis factor inhibitors, dexamethasone, and glutathione enhancers. Med. Hypotheses 51:53-57. [DOI] [PubMed] [Google Scholar]
- 31.Lahti, J. M. 1999. Use of gene knockouts in cultured cells to study apoptosis. Methods 17:305-312. [DOI] [PubMed] [Google Scholar]
- 32.Leach, S. D., C. D. Scatena, C. J. Keefer, H. A. Goodman, S. Y. Song, L. Yang, and J. A. Pietenpol. 1998. Negative regulation of Wee1 expression and Cdc2 phosphorylation during p53-mediated growth arrest and apoptosis. Cancer Res. 58:3231-3236. [PubMed] [Google Scholar]
- 33.Lee, T. I., and R. A. Young. 1998. Regulation of gene expression by TBP-associated proteins. Genes Dev. 12:1398-1408. [DOI] [PubMed] [Google Scholar]
- 34.Leonard, W. J., and J. J. O'Shea. 1998. Jaks and STATs: biological implications. Annu. Rev. Immunol. 16:293-322. [DOI] [PubMed] [Google Scholar]
- 35.Martianov, I., G. M. Fimia, A. Dierich, M. Parvinen, P. Sassone-Corsi, and I. Davidson. 2001. Late arrest of spermiogenesis and germ cell apoptosis in mice lacking the TBP-like TLF/TRF2 gene. Mol. Cell 7:509-515. [DOI] [PubMed] [Google Scholar]
- 36.Martianov, I., S. Brancorsini, A. Gansmuller, M. Parvinen, I. Davidson, and P. Sassone-Corsi. 2002. Distinct functions of TBP and TLF/TRF2 during spermatogenesis: requirement of TLF for heterochromatic chromocenter formation in haploid round spermatids. Development 129:945-955. [DOI] [PubMed] [Google Scholar]
- 37.Massague, J., S. W. Blain, and R. S. Lo. 2000. TGFβ signaling in growth control, cancer, and heritable disorders. Cell 103:295-309. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto, T., I. Turesson, M. Book, P. Gerwins, and L. Claesson-Welsh. 2002. p38 MAP kinase negatively regulates endothelial cell survival, proliferation, and differentiation in FGF-2-stimulated angiogenesis. J. Cell Biol. 156:149-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Metzger, D., E. Scheer, A. Soldatov, and L. Tora. 1999. Mammalian TAF(II)30 is required for cell cycle progression and specific cellular differentiation programmes. EMBO J. 18:4823-4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore, P. A., J. Ozer, M. Salunek, G. Jan, D. Zerby, S. Campbell, and P. M. Lieberman. 1999. A human TATA binding protein-related protein with altered DNA binding specificity inhibits transcription from multiple promoters and activators. Mol. Cell. Biol. 19:7610-7620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Müller, F., L. Lakatos, J. C. Dantonel, U. Strahle, and L. Tora. 2001. TBP is not universally required for zygotic RNA polymerase II transcription in zebrafish. Curr. Biol. 11:282-287. [DOI] [PubMed] [Google Scholar]
- 42.Ohbayashi, T., Y. Makino, and T. Tamura. 1999. Identification of a mouse TBP-like protein (TLP) distantly related to the Drosophila TBP-related factor. Nucleic Acids Res. 27:750-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohbayashi, T., M. Shimada, T. Nakadai, and T. Tamura. 2001. TBP-like protein (TLP/TLF/TRF2) artificially recruited to a promoter stimulates basal transcription in vivo. Biochem. Biophys. Res. Commun. 285:616-622. [DOI] [PubMed] [Google Scholar]
- 43a.Ohbayashi, T., M. Shimada, T. Nakadai, T. Wada, H. Handa, and T. Tamura. 2003. Vertebrate TBP-like protein (TLP/TRF2/TLF) stimulates TATA-less terminal deoxynucleotidyl transferase promoters in a transient reporter assay, and TFIIA-binding capacity of TLP is required for this function. Nucleic Acids Res. 31:2127-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olashaw, N., and W. J. Pledger. 2002. Paradigms of growth control: relation to Cdk activation. Sci. STKE 2002:RE7.. [DOI] [PubMed] [Google Scholar]
- 45.Orren, D. K., L. N. Petersen, and V. A. Bohr. 1995. A UV-responsive G2 checkpoint in rodent cells. Mol. Cell. Biol. 15:3722-3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ozer, J., L. E. Lezina, J. Ewing, S. Audi, and P. M. Lieberman. 1998. Association of transcription factor IIA with TATA binding protein is required for transcriptional activation of a subset of promoters and cell cycle progression in Saccharomyces cerevisiae. Mol. Cell. Biol. 18:2559-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petersen, L. N., D. K. Orren, and V. A. Bohr. 1995. Gene-specific and strand-specific DNA repair in the G1 and G2 phases of the cell cycle. Mol. Cell. Biol. 15:3731-3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rabenstein, M. D., S. Zhou, J. T. Lis, and R. Tjian. 1999. TATA box-binding protein (TBP)-related factor 2 (TRF2), a third member of the TBP family. Proc. Natl. Acad. Sci. USA 96:4791-4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raleigh, J. M., and M. J. O'Connell. 2000. The G2 DNA damage checkpoint targets both Wee1 and Cdc25. J. Cell Sci. 113:1727-1736. [DOI] [PubMed] [Google Scholar]
- 50.Scotto, C., C. Delphin, J. C. Deloulme, and J. Baudier. 1999. Concerted regulation of wild-type p53 nuclear accumulation and activation by S100B and calcium-dependent protein kinase C. Mol. Cell. Biol. 19:7168-7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seroz, T., J. R. Hwang, Y. Moncollin, and J. M. Egly. 1995. TFIIH: a link between transcription, DNA repair and cell cycle regulation. Curr. Opin. Genet. Dev. 5:217-221. [DOI] [PubMed] [Google Scholar]
- 52.Shimada, M., T. Ohbayashi, M. Ishida, T. Nakadai, Y. Makino, T. Aoki, T. Kawata, T. Suzuki, Y. Matsuda, and T. Tamura. 1999. Analysis of the chicken TBP-like protein (tlp) gene: evidence for a striking conservation of vertebrate TLPs and for a close relationship between vertebrate tbp and tlp genes. Nucleic Acids Res. 27:3146-3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steinman, R. A. 2002. Cell cycle regulators and hematopoiesis. Oncogene 21:3403-3413. [DOI] [PubMed] [Google Scholar]
- 54.Studzinski, G. P., and L. E. Harrison. 1999. Differentiation-related changes in the cell cycle traverse. Int. Rev. Cytol. 189:1-58. [DOI] [PubMed] [Google Scholar]
- 55.Takada, S., J. T. Lis, S. Zhou, and R. Tjian. 2000. A TRF1:BRF complex directs Drosophila RNA polymerase III transcription. Cell 101:459-469. [DOI] [PubMed] [Google Scholar]
- 56.Takao, N., H. Kato, R. Mori, C. Morrison, E. Sonada, X. Sun, H. Shimizu, K. Yoshioka, S. Takeda, and K. Yamamoto. 1999. Disruption of ATM in p53-null cells causes multiple functional abnormalities in cellular response to ionizing radiation. Oncogene 18:7002-7009. [DOI] [PubMed] [Google Scholar]
- 57.Teichmann, M., Z. Wang, E. Martinez, A. Tjernberg, D. Zhang, F. Vollmer, B. T. Chait, and R. G. Roeder. 1999. Human TATA-binding protein-related factor-2 (hTRF2) stably associates with hTFIIA in HeLa cells. Proc. Natl. Acad. Sci. USA 96:13720-13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tournier, S., D. Leroy, F. Goubin, B. Ducommun, and J. S. Hyams. 1996. Heterologous expression of the human cyclin-dependent kinase inhibitor p21cip1in the fission yeast Schizosaccharomyces pombe reveals a role for PCNA in the chk1+cell cycle checkpoint pathway. Mol. Biol. Cell 7:651-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tratner, I., and I. M. Verma. 1991. Identification of a nuclear targeting sequence in the Fos protein. Oncogene 6:2049-2053. [PubMed] [Google Scholar]
- 60.Um, M., J. Yamauchi, S. Kato, and J. L. Manley. 2001. Heterozygous disruption of the TATA-binding protein gene in DT40 cells causes reduced cdc25B phosphatase expression and delayed mitosis. Mol. Cell. Biol. 21:2435-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Veenstra, G. J., D. L. Weeks, and A. P. Wolffe. 2000. Distinct roles for TBP and TBP-like factor in early embryonic gene transcription in Xenopus. Science 290:2312-2315. [DOI] [PubMed] [Google Scholar]
- 62.Vichi, P., F. Coin, J. P. Renaud, W. Vermeulen, J. H. Hoeijmakers, D. Moras, and J. M. Egly. 1997. Cisplatin- and UV-damaged DNA lure the basal transcription factor TFIID/TBP. EMBO J. 16:7444-7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waseem, N. H., K. Labib, P. Nurse, and D. P. Lane. 1992. Isolation and analysis of the fission yeast gene encoding polymerase delta accessory protein PCNA. EMBO J. 11:5111-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wassarman, D. A., N. Aoyagi, L. A. Pile, and E. M. Schlag. 2000. TAF250 is required for multiple developmental events in Drosophila. Proc. Natl. Acad. Sci. USA 97:1154-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wieczorek, E., M. Brand, X. Jacq, and L. Tora. 1998. Function of TAF(II)-containing complex without TBP in transcription by RNA polymerase II. Nature 393:187-191. [DOI] [PubMed] [Google Scholar]
- 66.Winding, P., and M. W. Berchtold. 2001. The chicken B cell line DT40: a novel tool for gene disruption experiments. J. Immunol. Methods 249:1-16. [DOI] [PubMed] [Google Scholar]
- 67.Wrana, J. L., and L. Attisano. 2000. The Smad pathway. Cytokine Growth Factor Rev. 11:5-13. [DOI] [PubMed] [Google Scholar]
- 68.Zhang, D., T. L. Penttila, P. L. Morris, M. Teichmann, and R. G. Roeder. 2001. Spermiogenesis deficiency in mice lacking the Trf2 gene. Science 292:1153-1155. [DOI] [PubMed] [Google Scholar]