Abstract
During mitosis, the Xenopus chromokinesin Kid (Xkid) provides the polar ejection forces needed at metaphase for chromosome congression, and its degradation is required at anaphase to induce chromosome segregation. Despite the fact that the degradation of Xkid at anaphase seems to be a key regulatory factor to induce chromosome movement to the poles, little is known about the mechanisms controlling this proteolysis. We investigated here the degradation pathway of Xkid. We demonstrate that Xkid is degraded both in vitro and in vivo by APC/Cdc20 and APC/Cdh1. We show that, despite the presence of five putative D-box motifs in its sequence, Xkid is proteolyzed in a D-box-independent manner. We identify a domain within the C terminus of this chromokinesin, with sequence GxEN, whose mutation completely stabilizes this protein by both APC/Cdc20 and APC/Cdh1. Moreover, we show that this degradation sequence acts as a transposable motif and induces the proteolysis of a GST-GXEN fusion protein. Finally, we demonstrate that both a D-box and a GXEN-containing peptides completely block APC-dependent degradation of cyclin B and Xkid, indicating that the GXEN domain might mediate the recognition and association of Xkid with the APC.
During cell division, essential chromosome and microtubule interactions ensure the correct segregation of the chromosomes into two sets. Chromosome alignment and segregation are driven by forces directed both toward and away from the spindle poles. Poleward force is generated at the kinetochore by the kinetochore-associated protein motors XKCM1, CENP-E, and cytoplasmic dynein (24, 31, 34, 39, 41). Force directed away from the pole, termed polar ejection force, acts essentially on the chromosome arms (30) and appears to be mediated by chromosome bound motors or chromokinesins (5, 12). In Drosophila melanogaster the chromokinesin Nod is required for proper alignment of achiasmic chromosomes (1, 45). It is proposed that Nod counterbalances the poleward kinetochore force by the generation of a polar ejection force. Recently, Xkid, a vertebrate homologue of the chromokinesin Nod has been reported to be localized at the chromosome arms. Immunodepletion of Xkid in frog egg extracts induces a misalignment of chromosomes at metaphase, indicating that it is essential for chromosome alignment on the metaphase spindle. It is suggested that Xkid participates in the polar ejection forces acting on the chromosome arms (2, 13).
At the anaphase, chromatids must be separated from each other and move toward the spindle poles. In animal cells, the final stage of anaphase movement may reflect changes in the poleward and polar ejection forces. These changes could include a decrease in the polar ejection force, which would result in a positive kinetochore poleward force inducing the movement of chromatids to the pole. According to this hypothesis, it has been shown that the induction of anaphase in Xenopus egg extracts requires the degradation of Xkid by the anaphase-promoting complex (APC) and the proteasome (13). However, surprisingly, a different degradation timing has been described for human Kid which is still present in anaphase centromeres (37).
The mitotic targets for degradation are selected by the specific ubiquitin ligase, APC. The APC regulates exit from mitosis and events in G1 (44). This E3 is regulated by two different modulators, the Cdc20/Fizzy and Cdh1/Fizzy-Related proteins (10, 21, 23, 35). Both activators transiently interact with the APC in a cell cycle-specific manner. APC/Cdc20 complex mediates the degradation of a number of targets, including securin and cyclin B. This event is essential to mitotic progression (9, 23). Cdh1 protein, on the other hand, mainly controls the progression through the G1 phase by ensuring the complete proteolysis of cyclin B and Cdc20 (26, 38). Substrates recognized by the APC contain destruction signal sequences that target them for degradation. Three different destruction motifs have been described: the destruction box (D-box) with consensus of RXXL (where X is any amino acid) (15), the KEN box (KEN-box) with consensus KEN (26), and the A box (A-box) (8, 22). D-box-containing substrates typically appear to be recognized by either APC/Cdc20 or APC/Cdh1 or by both (4, 26, 32, 42). The KEN-box is believed to be recognized exclusively by APC/Cdh1 (26). Finally, the A-box is required for D-box-dependent degradation of Aurora A by APC/Cdh1 (8, 22).
We demonstrate here that Xkid is proteolyzed both in vitro and in vivo by APC/Cdc20 and APC/Cdh1 in a D-box-independent manner. Moreover, we identify a degradation domain with sequence GXEN, within the C terminus of this chromokinesin, that is required to induce the APC/Cdc20 and APC/Cdh1-dependent degradation of this protein.
MATERIALS AND METHODS
c-DNA cloning of Xkid, immunization procedures, and antibodies.
The oligonucleotides Xkid 5′-ATGGTTCTTACTGGGCCTCTC and 3′-GCTGGCGATGCTGCTCATG were used to amplify the full-length c-DNA from a Xenopus oocyte cDNA library. The PCR product was subcloned blunt into the EcoRV cloning site of pBluescript. For mutagenesis analysis, the Xkid insert was excised from the pBluescript-Xkid by XhoI-XbaI and subcloned in a pCS2+ vector. The glutathione S-transferase (GST)-XKid498-600, GST-ΔD-box(4/5)Xkid498-600, and GST-Δ(GLEN)Xkid498-600 fusion proteins were obtained by a first amplification of the fragment from amino acids 498 to 600 from the wild type, the ΔD-box(1-5) Xkid mutant and the Δ(GLEN)Xkid mutant by PCR and a subsequent subcloning blunt into the SmaI cloning site of the pXen3 vector. For the immunization protocol, pBluescript-Xkid was cut with EcoRI, and the resulting C-terminal insert of Xkid corresponding to amino acids 309 to 651 (XkidΔ309C) was cloned into the EcoRI site of pGEX4T2 vector. The XkidΔ309C-GST fusion protein was expressed in Escherichia coli. Inclusion bodies were prepared and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis according to standard procedures. The fusion protein was electroeluted and used to immunize rabbits. Immune sera was affinity purified on immobilized XkidΔ309C-GST. The Xenopus anti-cyclin B2, anti-Cdc20, anti-CDC27, and anti-Cdh1 antibodies were obtained as previously reported (7, 23). Anti-α-tubulin and the antibodies against the active form of ERK protein (PP-ERK) were obtained from P. Mangeat (Montpellier, France) and New England Biolabs (catalog no. 9106S). Western blots were treated as described by Castro et al. (7).
In vitro production of mRNAs and [35S]methionine-labeled proteins.
mRNAs encoding full-length human cyclin B1, Xenopus EMI1, GST, Cdh1, wild type, Xkid mutants, and fusion proteins were transcribed in vitro with T7 or SP6 polymerase. When used for protein degradation assays, the mRNAs were translated in a rabbit reticulocyte lysate in the presence of [35S]methionine.
Xenopus egg extracts and protein translation and degradation.
Metaphase II-arrested oocyte extracts (CSF extracts) were prepared from unfertilized Xenopus eggs as described in Lorca et al. (23). Protein translation of Xenopus Cdh1 mRNA in interphase extracts was as described previously (6, 11). For protein degradation assays, 1 μl of either 35S-labeled cyclin B or Xkid was incubated at room temperature with 20 μl of CSF or interphase extracts that had been (or not) supplemented with Cdh1 mRNA (1 h before).
Site-directed mutagenesis.
Internal or point mutations of Xkid protein were achieved by using the QuikChange site-directed mutagenesis kit according to the manufacturer's protocol (Stratagene). The sequences of the primers used to generate each mutant can be supplied on request. ΔD-box(1-5) was obtained by sequential mutagenesis with the same primers used for the individual D-box mutations. N-terminal and C-terminal Xkid mutants were obtained by PCR amplification by using the primers at the corresponding amino acid positions. The presence of all of the mutations was confirmed by DNA sequencing of the Xkid cDNAs.
mRNA microinjection in Xenopus oocytes.
Oocytes were microinjected with 20 ng of wild-type or mutated Xkid mRNAs or with the same amount of Cdh1 mRNA where indicated. Three oocytes per time point were homogenized in 10 μl of homogenization buffer (20 mM Tris [pH 7.5], 50 mM NaCl, 50 mM NaF, 10 mM β-glycerophosphate, 5 mM Na4P2O7, 1 mM EDTA). After extract centrifugation (13,000 rpm for 3 min at 4°C), the clear supernatant was recovered, and the equivalent volume of one oocyte was used for Western blot analysis. When Xkid mRNA was injected in maturing oocytes, Western blot analysis allowed the selective detection of ectopic Xkid, since, despite the fact that anti-Xkid antibodies detect both endogenous and ectopically expressed Xkid mutated forms, the low endogenous Xkid levels were not visualized at the enhanced chemiluminescence exposure time used to reveal the ectopical mutated Xkid forms.
RESULTS
The Xenopus chromokinesin Kid is first expressed at germinal vesicle breakdown (GVBD) in maturing oocytes and is degraded at the transition from metaphase I to anaphase I and at the metaphase II exit.
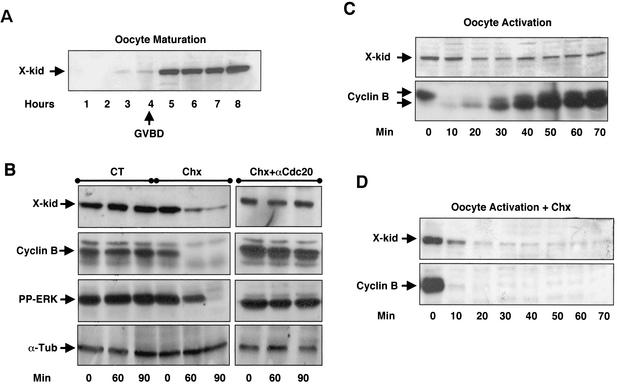
It has been previously reported that the Xenopus chromokinesin Kid is not present in stage VI Xenopus oocytes and that it is clearly detectable in CSF extracts (2). Moreover, previous reports demonstrate that Xkid is degraded at metaphase II exit in CSF extracts (2, 13). To characterize the oocyte expression and degradation patterns of this Xenopus chromokinesin, polyclonal antibodies were raised against a fusion protein containing the last C-terminal 341 amino acids of Xkid. Stage VI oocytes were incubated with progesterone to induce meiotic resumption and, every hour, a mix of three oocytes were homogenized and analyzed by Western blot for the presence or absence of Xkid (Fig. 1A). The homogenized oocytes selected 4, 5, 6, 7, and 8 h after progesterone incubation had all undergone GVBD. Maximal Xkid levels were reached between 4 and 5 h. We then analyzed Xkid degradation pattern in vivo during oocyte maturation and at meiotic exit. To investigate whether Xkid could be degraded at the transition from metaphase I to anaphase I, maturing oocytes were divided into three different groups. The first group was used as a control, and oocytes were homogenized at 30 min (Fig. 1B, CT, 0 min), 90 min (Fig. 1B, CT, 60 min), and 120 min (Fig. 1B, CT, 90 min) post-GVBD. In the second group total protein synthesis was blocked 30 min post-GVBD by the addition of cycloheximide (Chx) to the incubation buffer, and then oocytes were homogenized at 0, 60, and 90 min after Chx treatment (Fig. 1B, Chx). In the third group, anti-Cdc20 antibodies were microinjected into the oocytes to block APC activity 2 h after progesterone addition. At 30 min post-GVBD, Chx was added to the incubation buffer to block total protein synthesis, and oocytes were recovered 0, 60, and 90 min after Chx treatment (Fig. 1B, Chx+αCdc20). Oocyte homogenates were then used for immunoblot analysis with anti-Xkid, anti-cyclin B2, anti-PPERK (the active form of ERK), and anti-α-tubulin antibodies (loading control). As shown in Fig. 1B (Chx), Xkid levels significantly dropped in Chx-treated oocytes compared to nontreated oocytes (CT) concomitantly with the degradation of cyclin B2 and the inactivation of ERK (7). Unlike the decrease of Xkid amount observed in Chx-treated oocytes, Xkid levels remained constant when the APC activity was blocked in these oocytes by microinjection of anti-Cdc20 antibodies prior Chx treatment (Fig. 1B, Chx+αCdc20). In agreement with the findings of Perez et al. (25), these results indicate the presence of a concomitant degradation and neosynthesis of Xkid at the metaphase-I-to-anaphase-I transition. We next analyzed Xkid proteolysis at meiotic exit by activating metaphase II-arrested oocytes with the calcium ionophore A32187. Endogenous levels of Xkid and cyclin B2 in these oocytes were examined at different times postactivation. As expected, endogenous cyclin B2 was completely degraded 10 min after ionophore treatment (Fig. 1C, lower panel). Surprisingly, only a small decrease in the Xkid levels were observed after oocyte activation, indicating either a partial degradation or a concomitant degradation and neosynthesis of this protein (Fig. 1C, upper panel). To discern between these two possibilities, we repeated the same experiment in the presence of the protein synthesis inhibitor Chx. As shown in Fig. 1D, endogenous Xkid importantly decreased at 10 min and was almost completely degraded 20 min after ionophore treatment, although a residual protein level remained. We observed an acceleration of the degradation pattern of endogenous Xkid in Chx-treated oocytes compared to CT (compare Fig. 1C and D). However, we attributed this difference to the variability of the kinetics of activation of distinct batches of oocytes by ionophore treatment. These results indicate that Xkid is degraded both in the transition from metaphase I to anaphase I and at meiotic exit and that, in both cases, in vivo neosynthesis of this chromokinesin rapidly replaces the degraded protein.
FIG. 1.
XKid is first expressed at GVBD in maturing oocytes and is degraded at the transition from metaphase I to anaphase I and at the metaphase II exit. (A) Prophase oocytes were incubated with progesterone, collected in groups of three at the indicated times after progesterone addition and homogenized in homogenization buffer. Lysates were then subjected to Western blot with anti-Xkid antibodies. Arrow indicates the time when all oocytes underwent GVBD. (B) Stage VI oocytes were stimulated to undergo oocyte maturation with progesterone and subdivided in three groups. Oocytes of the first group, used as a control (CT), were homogenized in groups of three at 30 min (0 min), 90 min (60 min), and 120 min (90 min) post-GVBD. In the second group, 30-min post-GVBD, oocytes were incubated with the protein synthesis inhibitor Chx at 100 μg/ml and homogenized at 0, 60, and 90 min after Chx treatment (Chx). In the third group, oocytes were microinjected with 40 ng of anti-Cdc20 antibodies 2 h after progesterone addition and then incubated, 30 min post-GVBD, with 100 μg of Chx (Chx+αCdc20)/ml. Next, groups of three oocytes were homogenized at 0, 30, and 60 min after Chx treatment, and endogenous levels of Xkid (X-kid), cyclin B2 (Cyclin B), active phospho-ERK (PP-ERK) and, as a loading control, α-tubulin (α-Tub) were analyzed by Western blot. (C) Metaphase II-arrested oocytes were activated by the calcium ionophore A32187, homogenized in groups of three, and analyzed at various times after oocyte activation for endogenous Xkid and cyclin B2 levels by Western blotting. The two arrowheads in the anti-cyclin B Western blot represent both the phosphorylated (upper) and the dephosphorylated (lower) forms of cyclin B2. As expected, the dephosphorylated form of this protein is only present at interphase and is completely absent in post-GVBD maturing and metaphase II-arrested oocytes. (D) Similar to panel C except for the addition of 100 μg of Chx/ml in the incubation buffer 1 h before oocyte activation. The equivalent of one oocyte was loaded per lane.
Xkid is degraded by APC/Cdc20 and APC/Cdh1 in a D-box-independent pathway.
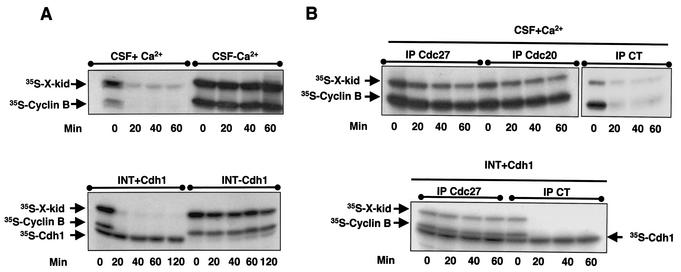
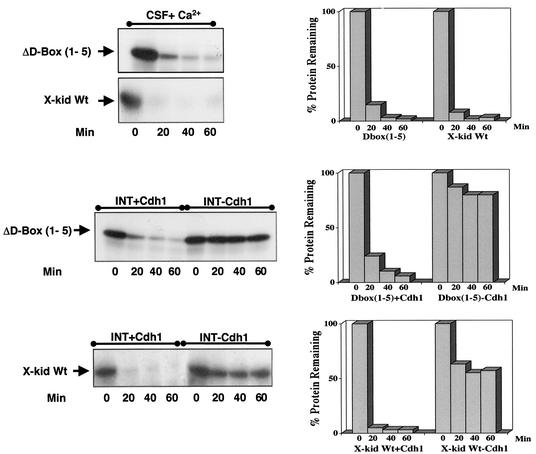
We next addressed the mechanisms regulating Xkid degradation. Proteolysis of proteins, such as cyclins or securins, at mitotic exit is mediated by the APC (9, 23, 44). As described above, this E3 ligase is regulated by two different modulators, the proteins Cdc20 and Cdh1 (10, 21, 23, 35). It has been reported that degradation of Xkid at the transition from mitotic metaphase to anaphase is dependent on APC activity (13). Moreover, Xkid is also degraded in interphase Xenopus egg extracts supplemented with baculovirus-purified human Cdh1 protein (27). To investigate the role of APC/Cdc20 and APC/Cdh1 in Xkid proteolysis, we developed an in vitro degradation assay with either CSF extracts in which APC/Cdc20 was activated by the addition of Ca2+ or interphase Xenopus egg extracts supplemented with a Cdh1 mRNA (Xenopus oocytes are devoid of Cdh1). In these extracts, 35S-radiolabeled Xkid and 35S-radiolabeled cyclin B, as a control, were added, and the levels of these two proteins were then analyzed by autoradiography. As shown in Fig. 2A, the activation of both APC/Cdc20 (upper panel) and APC/Cdh1 (lower panel) induced a complete degradation of Xkid and cyclin B.
FIG. 2.
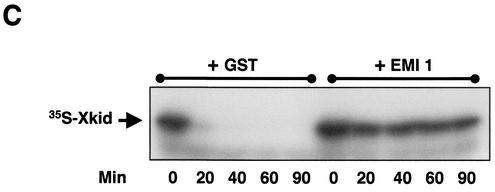
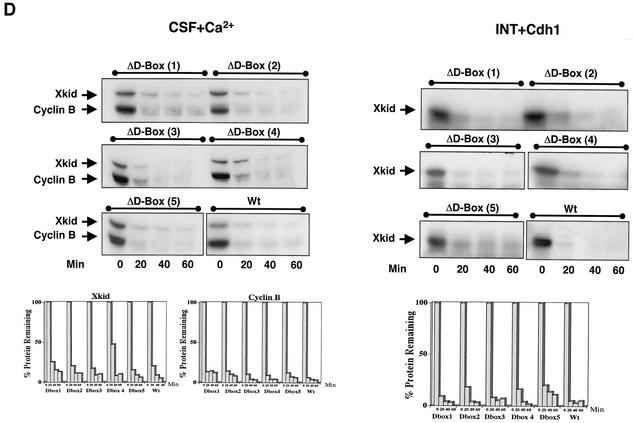
Xkid is degraded by APC/Cdc20 and APC/Cdh1 in a D-box-independent pathway. (A) In the upper panel, CSF extract (20 μl) was supplemented with 1 μl of both 35S-labeled cyclin B and Xkid. Where indicated, the extract was then activated by the addition of 0.5 mM CaCl2 (CSF+Ca2+). Samples (2 μl) were taken at different times, and Xkid and cyclin B levels were analyzed by autoradiography. In the lower panel, Cdh1 mRNA was mixed in interphase extracts where indicated (INT+Cdh1) in the presence of 35S-labeled methionine. One hour later, 1 μl of both in vitro-translated 35S-labeled Xkid and 35S-labeled cyclin B was added. Samples (2 μl) were then taken at different times, and Xkid, cyclin B, and Cdh1 levels were analyzed by autoradiography. (B) In the upper panel, CSF extract (50 μl) was first depleted with 5 μl of either α-Cdc27 antibodies (IP Cdc27), α-Cdc20 antibodies (IP Cdc20), or control antibodies (IP CT) and then supplemented with 1 μl of 35S-labeled Xkid and the same volume of 35S-labeled cyclin B. Subsequently, the samples were activated by the addition of 0.5 mM CaCl2. Samples (2 μl) were then collected at the indicated times after calcium addition, and the levels of Xkid and cyclin B were analyzed by autoradiography. In the lower panel, interphase extract (50 μl) was first depleted with (5 μl) of α-Cdc27 antibodies (IP Cdc27) or the same volume of control antibodies (IP CT) and complemented with Cdh1 mRNA in the presence of 35S-labeled methionine. One hour later, 1 μl of 35S-labeled Xkid and the same volume of 35S-labeled cyclin B were added, and 2-μl samples were taken at different times for analysis of the levels of Xkid, cyclin B, and Cdh1 proteins by autoradiography. (C) Interphase extract was first supplemented with either the mRNA for the APC inhibitor EMI1 or the GST mRNA as a control, and after a 1-h incubation, with the Cdh1 mRNA. One hour later, 1 μl of 35S-labeled Xkid was mixed, and 2-μl samples were collected to analyze the 35S-labeled Xkid levels. (D) CSF extracts (CSF+Ca2+, left panel) were first supplemented with 1 μl of 35S-labeled cyclin B and the wild-type (Wt) or one of five D-box [D-box(1) to D-box(5)] 35S-labeled Xkid mutants and subsequently activated by the addition of calcium. The degradation pattern of these different Xkid mutants and of cyclin B were then analyzed at different times after calcium activation by autoradiography. Interphase extracts (INT+Cdh1, right panel) were first supplemented with Cdh1 mRNA, and 1 h later they were mixed with the wild type or the different radiolabeled D-box mutants of Xkid. The Xkid protein levels were analyzed by autoradiography. The amount of Xkid and cyclin B remaining at each proteolysis assay is quantified by densitometry and plotted with respect to the amount at time zero.
We further investigated the role of APC/Cdc20 in Xkid degradation by immunodepleting in CSF extracts either the APC activator Cdc20 or the APC subunit CDC27 before Ca2+ addition. Neither Xkid nor cyclin B proteolysis was detected when immunodepletion was performed with anti-Cdc27 or anti-Cdc20 antibodies compared with control antibodies (Fig. 2B, upper panel), indicating, as expected, that the degradation of Xkid is mediated by APC/Cdc20 in metaphase II exit. We also investigated in more detail, in interphase extracts, the role of APC/Cdh1 in Xkid proteolysis by either immunodepleting Cdc27 (Fig. 2B, lower panel) or by adding to the extract the mRNA for the APC inhibitor EMI1 (Fig. 2C) (28, 29). When Cdc27 was immunodepleted in interphase egg extracts before the addition of the Cdh1 mRNA neither Xkid, nor cyclin B was degraded compared with control immunodepletion (Fig. 2B, lower panel). Similarly, the presence of the APC inhibitor EMI1 in Cdh1-containing interphase egg extracts completely blocked Xkid degradation, indicating that the proteolysis of this protein induced by Cdh1 in interphase egg extracts is mediated by the APC (Fig. 2C). Thus, in our in vitro assays, Xkid proteolysis may be mediated by both APC/Cdc20 and APC/Cdh1 complexes.
Because it has been previously described that the amino-terminal fragment of cyclin B, which contains the D-box sequence, blocks Xkid proteolysis (13), we next investigated whether the degradation of this protein could be mediated by a D-box motif. Xkid contains five putative D-box sequences at amino acids 69 to 72, 141 to 144, 455 to 458, 509 to 512, and 573 to 576. We tested whether these sequences specified Xkid degradation by using site-directed mutagenesis to convert the arginine residue of the RXXL of each D-box sequence to alanine. The stability of the resulting constructs was assessed in CSF+Ca2+ and in Cdh1 mRNA-supplemented interphase egg extracts. Surprisingly, except for the small delay observed in the proteolysis pattern of the ΔD-box(4) mutant, the point mutation of the individual D-box mutants had no effect on Xkid degradation (Fig. 2D) either in CSF+Ca2+ (left panel) or in Cdh1-supplemented (right panel) extracts. These results suggest that Xkid may be degraded in a D-box-independent manner. However, to eliminate a possible cooperative effect among the five D-box motifs, we developed a mutant form of Xkid wherein the five D-box sequences were mutated. The quintuple D-box(1-5) mutant only slightly slows down Xkid degradation, indicating that the proteolysis of this protein by either APC/Cdc20 or APC/Cdh1 is not mediated by a D-box motif (Fig. 3). Since Xkid protein sequence has no putative KEN-box or A-box motifs, Xkid might be proteolyzed in a D-box/KEN-box/A-box-independent pathway.
FIG. 3.
Xkid is degraded in a D-box-independent pathway. CSF (CSF+Ca2+) and interphase extracts (INT+Cdh1 and INT-Cdh1) were treated as in Fig. 2D except for the addition of either a radiolabeled wild-type Xkid (X-kid Wt) or a Xkid protein in which the five D-box sequences were point mutated [ΔD-box(1-5)]. The amount of Xkid remaining with time is quantified from the autoradiographs.
Identification of the Xkid sequence necessary for its degradation.
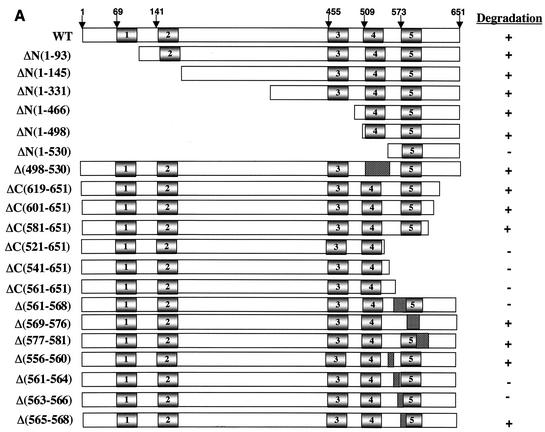
In order to elucidate whether Xkid harbors a motif that could act as a destruction signal, a series of amino- and carboxyl-terminal deletion mutants of Xkid were constructed. Figure 4A shows the portion of the coding sequence remaining in each mutant. The position of the putative D-box motifs are indicated with shaded rectangles. When internal sequences were deleted, the position of these sequences was shown as punctuated rectangles. The stability of each mutant was measured in CSF+Ca2+ and Cdh1-containing interphase extracts.
FIG. 4.
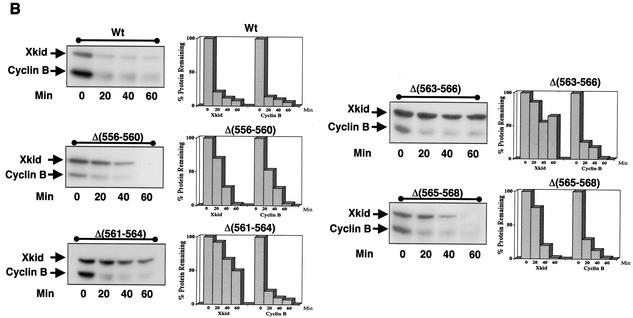
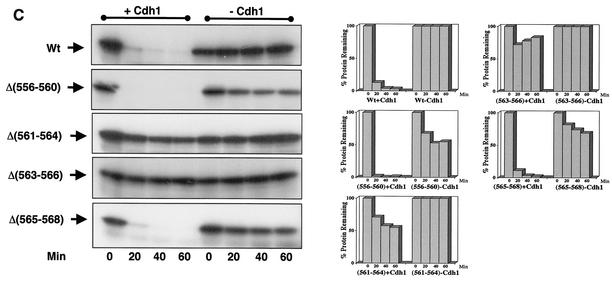
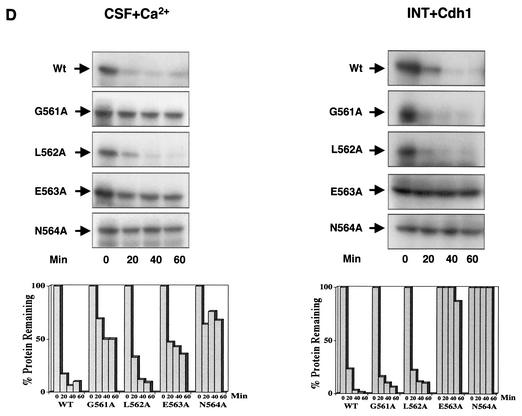
Xkid is degraded by a new GXEN proteolysis signal. (A) Schematic representation of the wild-type Xkid sequence (WT) and the different mutants. N, N terminus; C, C terminus. The numbers in brackets show the deleted amino acids. Where internal sequences were deleted, they are depicted by hatched rectangles. All mutants of Xkid were translated in vitro and incubated in CSF extracts activated by calcium- or with Cdh1-containing interphase extracts to measure protein stability. Results of this stability are summarized on the right-hand side. (B) Degradation pattern of the wild type and the mutants Δ(556-560), Δ(561-564), Δ(563-566), and Δ(565-568) of Xkid in activated CSF extracts (CSF+Ca2+). (C) Degradation pattern of the wild type and the Δ(556-560), Δ(561-564), Δ(563-566), and Δ(565-568) Xkid mutants in Cdh1-containing interphase extracts (INT+Cdh1). (D) Degradation pattern of the wild type (Wt) or the G561A, L562A, E563A, and N564A Xkid point mutants in activated CSF extracts (CSF+Ca2+) and in Cdh1-containing interphase egg extracts (INT+Cdh1). The Xkid protein levels remaining in the time course of every degradation assay were quantified by densitometry and are plotted with respect to the amount at time zero.
All of the N-terminal deleted forms of Xkid underwent degradation in both CSF+Ca2+ and Cdh1-containing interphase extracts except for the mutant ΔN(1-530) in which only the last 121 amino acids were left. Thus, since ΔN(1-498) is normally degraded, the lack of proteolysis observed in this mutant can be due to the presence of a destruction sequence between amino acids 498 and 530. In order to test this possibility, we developed a mutant form of Xkid lacking amino acids from 498 to 530 [Fig. 4A, Δ(498-530)]. This mutant was degraded normally in both CSF+Ca2+ and in Cdh1-containing interphase extracts (data not shown), indicating that sequence from 498 to 530 of Xkid does not include a degradation signal. We cannot exclude the possibility that one of the lysines present at positions 521, 523, and 524 is required for Xkid ubiquitination and degradation; however, if this is the case, a supplementary lysine takes over this role in the Δ(498-530) Xkid mutant. Thus, we concluded that the mutant ΔN(1-530) is probably not big enough to be degraded.
When C-terminal mutants were analyzed, we observed that the proteolysis of the C-terminal truncated forms ΔC(619-651), ΔC(601-651), and ΔC(581-651) were not affected. However, mutants ΔC(521-651), ΔC(541-651), and ΔC(561-651) were completely stabilized in both CSF+Ca2+ and Cdh1-containing interphase extracts (Fig. 4A). These results indicate that a putative destruction sequence may be present at the C-terminal sequence of Xkid between amino acids 561 and 581.
Given the identification of this region as required for Xkid degradation, we created three internal mutated forms of Xkid lacking amino acids from 561 to 568, from 569 to 576, and from 577 to 581. Mutant Δ(561-568) was clearly stabilized compared to mutants Δ(569-576) and Δ(577-581), which were normally degraded, showing that a destruction signal may be present between amino acids 561 and 568 (sequence 561GLENQPTW568).
To further identify the minimal sequence required for signaling APC/Cdc20 and APC/Cdh1-dependent degradation of Xkid, we constructed four internal mutated forms of Xkid: Δ(561-564) (lacking sequence GLEN), Δ(563-566) (lacking sequence ENQP), and Δ(565-568) (lacking sequence QPTW). We also constructed an internal mutated form of Xkid (Δ556-560), which comprised a contiguous sequence to the 561-to-568 region in order to eliminate a possible participation of this sequence to the destruction signal. As shown in Fig. 4B and C, only the mutated forms lacking sequences GLEN [mutant Δ(561-564)] and ENQP [mutant Δ(563-566)] were importantly stabilized in both CSF+Ca2+ and Cdh1-containing interphase extracts. These results indicate that the destruction signal resides within the sequence GLEN.
Finally, to completely identify this new degradation signal, we constructed a series of point mutated forms of Xkid in which every amino acid of the sequence 561GLEN564 was substituted by an alanine. The results of the degradation analysis showed that destruction of E563A and N564A mutants was considerably delayed in the CSF+Ca2+ extracts and completely blocked in the Cdh1-containing interphase extracts, whereas the substitution of L562 to alanine did not affect the proteolysis of this protein in either extract. Surprisingly, the G561A form was importantly stabilized in the CSF+Ca2+ extracts, but it was normally degraded in Cdh1-containing interphase extracts (Fig. 4D, CSF+Ca2+ and INT+Cdh1, respectively). This would suggest that, despite being within the same domain, the proteolysis signal of Xkid is not the same for APC/Cdc20 and APC/Cdh1.
In vivo degradation of Xkid by APC/Cdc20 and APC/Cdh1 is mediated by the proteolysis sequence GXEN.
In order to further characterize the sequence of Xkid required for APC/Cdc20 and APC/Cdh1 to induce the degradation of this protein, we analyzed in vivo the proteolysis pattern of all of the point mutants described above by both E3 complexes.
To study the in vivo degradation of Xkid by APC/Cdc20, we measured the proteolysis of this chromokinesin at the transition from metaphase I to anaphase I. To this end, the mutated Xkid mRNAs were microinjected into maturing oocytes 2 h after progesterone treatment. Subsequently, at GVBD, total protein synthesis was blocked by the addition of Chx. Two hours later, oocytes were homogenized, and the stability of the ectopically expressed Xkid mutants was analyzed by Western blotting.
To measure in vivo degradation of Xkid by APC/Cdh1, we first microinjected stage VI oocytes, which are devoid of Cdh1, with the mRNA encoding this protein and, 1 h later, with the mRNA for the different mutated forms of Xkid. After 1 h of ectopic Xkid protein expression, Chx was added to the oocyte buffer to block protein synthesis, and the oocytes were homogenized at the indicated times. Protein levels of the Xkid mutants were analyzed by Western blotting.
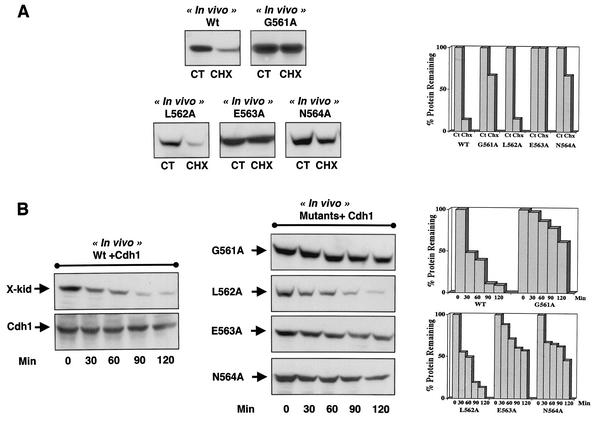
As observed in vitro, Xkid wild-type protein was clearly degraded in vivo by both APC/Cdc20 and APC/Cdh1 (Fig. 5A, Wt, and 5B, Wt+Cdh1). However, unlike the in vitro results, both in vivo APC/Cdc20- and APC/Cdh1-dependent degradation of Xkid was strongly delayed in G561A, E563A, and N564A mutants, whereas substitution of the leucine 562 to alanine did not affect Xkid proteolysis (Fig. 4A and B, respectively). To date, we cannot explain the reason why the in vitro and in vivo APC/Cdh1-dependent degradation patterns of the G561A mutant are different. A possible explanation could involve an incorrect in vitro folding of the ectopically translated protein in interphase egg extracts. However, the in vivo results clearly demonstrate that the glycine residue of the GLEN sequence is part of the degradation signal. Thus, all of these results show the presence of a new destruction motif in the Xkid protein, with the sequence GXEN, recognized by both the APC/Cdc20 and APC/Cdh1 complexes to induce degradation of this chromokinesin.
FIG. 5.
In vivo requirement of the GXEN proteolysis sequence for Xkid degradation. (A) Stage VI oocytes were stimulated to undergo oocyte maturation with progesterone. Two hours after progesterone addition, oocytes were microinjected with the wild type (Wt) or the punctual Xkid mutants G561A, L562A, E563A, and N564A mRNAs. Oocytes were then examined every 30 min, and once they underwent GVBD they were separated and incubated with Chx for two more hours. Oocytes were then homogenized, and ectopic Xkid protein levels were measured by Western blotting. Control Xkid levels were obtained by homogenization of wild-type Xkid-microinjected, nontreated, maturing oocytes 2 h post-GVBD in the same batch of oocytes (CT). (B) Stage VI oocytes were first microinjected with Cdh1 mRNA and then 1 h later with the mRNA of either the wild-type protein (X-kid) or the Xkid point mutants G561A, L562A, E563A, and N564A. After 1 h of incubation, Chx (100 μg/ml) was added and the oocytes were homogenized at the indicated times. Xkid protein levels, as well as Cdh1 expression, were analyzed by Western blotting. Quantification of the Xkid remaining at each degradation assay is depicted on the right-hand side.
The GXEN sequence acts as a transposable degradation signal.
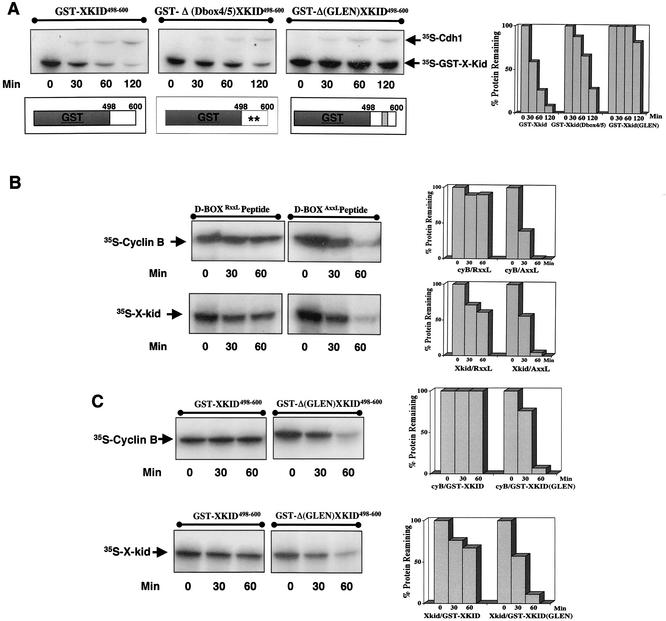
An essential feature of the three previously described D-box, A-box, and KEN-box degradation sequences is that these signals are transposable. Fusion of these sequences to a nondegradable protein results in the proteolysis of the fusion proteins by either APC/Cdc20 or the APC/Cdh1 (15, 19, 22, 26). To test whether the GXEN sequence acts as a transposable signal, we merged GST protein with the C-terminal domain of wild-type Xkid containing amino acids from 498 to 600. Since D-box(4) and D-box(5) were contained in this C-terminal domain, we developed another fusion protein in which the GST was merged to the 498-to-600 C-terminal domain of the Xkid ΔD-box(1-5) mutant. Finally, we constructed a third fusion protein by merging the same C-terminal fragment of the wild-type Xkid in which amino acids 561GLEN564 were deleted. We then assayed the degradation pattern of these constructs in interphase Xenopus egg extracts supplemented with Cdh1. As shown in Fig. 6A, GST-KID498-600 fusion protein was degraded with a pattern similar to that of wild-type Xkid protein. According to the small delay of proteolysis observed in the ΔD-box(1-5) Xkid mutant, the destruction of GST-ΔD-box(4/5)Xkid498-600 was only slightly slowed down. Nevertheless, the GST-Δ(GLEN)XID498-600 fusion mutated form was completely stable in these extracts, indicating that, similar to the other proteolysis signals, the GXEN sequence acts as a transposable degradation signal.
FIG. 6.
The GXEN sequence acts as a transposable degradation signal and competes with the D-box degradation signal to induce cyclin B and Xkid degradation. (A) Interphase egg extract was first supplemented with Cdh1 mRNA and then 1 h later with 1 μl of either 35S-labeled GST-KID498-600, the same fusion protein in which D-box(4) and D-box(5) were mutated (GST-Δ (Dbox4/5)XKID498-600, or the GST-KID498-600 form, in which the GLEN sequence was deleted [GST-Δ(GLEN)KID498-600]. Sample (2 μl) of the mixtures were obtained at different times for analysis of the levels of the three GST-Xkid fusion proteins by autoradiography. The presence in the autoradiography of a band corresponding to 35S-labeled Cdh1 due to the free [35S]methionine of the reticulocyte lysates was variable; however, it confirmed the correct translation of this protein. Constructs of the two fusion proteins are schematically depicted. (B) Interphase egg extract previously supplemented with Cdh1 mRNA was mixed to a final concentration of 0.5 μg/μl with either a D-box-containing peptide (amino acid sequence RRTALGDVTNKVSE) or the D-box mutated form of this peptide (amino acid sequence RATALGDVTNKVSE) (underlining and boldface indicate the wild-type and mutated sequences, respectively). Subsequently, 1 μl of 35S-labeled Xkid or the same volume of 35S-labeled cyclin B was added, and 2-μl samples were obtained at different times for analysis of the levels of both proteins by autoradiography. (C) Interphase extract was first supplemented with either the GST-Kid498-600 or the GST-Δ(GLEN)Kid498-600 mRNAs for 1 h and subsequently with the Cdh1 mRNA. After an incubation of a supplementary hour, 1 μl of 35S-labeled Xkid and the same volume of 35S-labeled cyclin B were added, and the levels of these two proteins were analyzed by autoradiography. Quantification of the remaining protein levels of Xkid or cyclin B is depicted on the right-hand side of each degradation assay.
D-box- and GXEN-containing peptides completely block degradation of both cyclin B and Xkid by APC/Cdh1.
It has been previously described that the KEN-box-dependent degradation of Cdc20 by APC/Cdh1 is blocked by a D-box-containing peptide (26). In order to investigate the mechanism by which the GXEN degradation signal targets substrates to be degraded by APC/Cdh1, we investigated whether a D-box peptide could also compete with the GXEN sequence and block, in a similar fashion, proteolysis of the GXEN-containing substrate Xkid. To this end, we measured the degradation profile of Xkid in Cdh1-containing interphase egg extract previously supplemented with either a D-box-containing peptide or the corresponding D-box mutated form of this peptide. As shown in Fig. 6B, Xkid proteolysis was clearly blocked by the D-box-containing peptide (D-boxRXXL peptide), whereas the addition to the extracts of the D-box mutated form (D-boxAXXL peptide) did not induce any effect on the degradation pattern of this protein. As expected, the addition of the D-box-containing peptide also prevented cyclin B degradation by APC/Cdh1, whereas the D-box mutated form did not. Thus, from these results we can conclude that the D-box-containing peptide competes for the degradation of the substrate targeted by the GXEN sequence.
We next sought to determine whether, similar to the D-box-containing peptide, a GXEN-containing peptide could compete and block the D-box-dependent degradation of cyclin B. With this aim we tested the ability of a GXEN-containing peptide to inhibit D-box-dependent degradation of cyclin B by APC/Cdh1 in interphase Xenopus egg extracts. We used the GSTKID498-600 and the GST-Δ(GLEN)XID498-600 fusion proteins as a source of GXEN and control peptides, respectively. As described above (Fig. 6A) GST-KID498-600 fusion protein is completely degraded in Cdh1-containing interphase egg extracts; however, when overexpressed, the APC capacity to induce its degradation is exceeded, and it will compete with other substrates to bind APC. Thus, we measured D-box-dependent degradation of cyclin B in Cdh1-containing egg extracts in which ectopic mRNA translation of GST-KID498-600 and GST-Δ(GLEN)XID498-600 induced a large overexpression of these two fusion proteins (data not shown). As shown in Fig. 6C, although cyclin B was clearly stabilized when GXEN-containing protein (GST-KID498-600) was overexpressed, it was degraded when the GXEN-mutated form of this protein was present into the extracts [GST-Δ(GLEN)KID498-600]. As expected, Xkid proteolysis was only blocked by the GXEN-containing protein. These results indicate that the GXEN sequence clearly competes not only with the GXEN-containing substrates but also with the D-box-containing substrates to be degraded by APC/Cdh1.
GXEN-dependent degradation of Xkid is not regulated by the binding of this chromokinesin to DNA.
It has been recently shown that the ubiquitin-proteasome-dependent degradation of the Cdk inhibitor, Xic1, is regulated by the presence of DNA in a nuclear-like environment (43). Since Xkid protein sequence have two helix-hairpin-helix DNA-binding motifs at its C terminus, near the GXEN motif, we sought to determine whether APC-dependent proteolysis of Xkid could be regulated by the binding of this chromokinesin to the DNA. To answer this question, we added demembranated sperm chromatin (2,000/μl) in Cdh1-containing interphase egg extracts. One hour later, 35S-radiolabeled Xkid was added, and the levels of this protein were analyzed by autoradiography at different times. As shown in Fig. 7, DNA addition did not modify the proteolysis pattern of Xkid, indicating that the APC-dependent degradation of this protein might not be regulated by its binding to the DNA.
FIG. 7.
GXEN-dependent degradation of Xkid is not regulated by the binding of this chromokinesin to DNA. Cdh1-containing interphase extract (20 μl) was preincubated for 1 h (+DNA) or not (−DNA) with demembranated sperm chromatin (2,000 sperm cells/μl). Subsequently, radiolabeled wild-type Xkid protein (1 μl) was added, and 2-μl samples were taken at different times to measure Xkid levels by autoradiography.
DISCUSSION
Previous works in Xenopus egg extracts have demonstrated that Xkid mediates chromosome alignment on the metaphase spindle and that its degradation is required to induce chromosome movements toward the poles during anaphase (2, 13). This prompted us to investigate the mechanisms that regulate Xkid proteolysis. The results presented here show that Xkid is destroyed by both the APC/Cdc20 and the APC/Cdh1 complexes and that its degradation depends upon a proteolysis signal, with sequence GXEN, present within its C terminus.
A great number of proteins are considered to be degraded by the APC, e.g., cyclin A and B (36), Cdc20 (40), securin (46, 47), Nek2A (17), or Aurora A (6, 22). Despite the fact that all of these proteins are targeted for degradation by this ubiquitin ligase, their individual proteolysis is sequentially programmed during the cell cycle. This regulation is mainly due to the association of the APC with two different modulators, Cdc20 and Cdh1. Moreover, APC substrates carry conserved degradation motifs that are required for their proteolysis. The current model proposes the presence of three different motifs, the D-box (RXXL), the A-box, and the KEN-box. The D-box sequence acts as a degradation signal that targets both APC/Cdc20- and APC/Cdh1-dependent proteolysis. The A-box is a regulatory rather than a proteolysis sequence that modulates D-box-dependent degradation of Aurora A. Finally, the KEN-box has been considered a signal that exclusively targets APC/Cdh1-dependent degradation. These degradation signals directly interact with the modulators, allowing APC-substrate interaction (3, 18, 27, 33).
Our results demonstrate that Xkid degradation is mediated by both Cdc20 and Cdh1 in an APC-dependent manner, since immunodepletion of the APC subunit, CDC27, or the addition of the APC inhibitor EMI1 completely blocked proteolysis of this chromokinesin. Moreover, although Xkid presents five putative D-box motifs, its proteolysis is D-box independent. We have identified a domain of Xkid required for APC/Cdc20- and APC/Cdh1-dependent degradation of this protein. This domain comprises the sequence GXEN that shares a certain sequence similarity to the KEN-box. However, despite this similarity, the GXEN-box clearly differs from the KEN-box by the fact that, contrary to the latter domain, it is not only recognized by APC/Cdh1 but also by APC/Cdc20. We conclude that the GXEN-box could correspond to a new different proteolysis signal.
One of the distinctives that defines the degradation signals is their capacity to confer proteolysis of a nondegradable protein when a small motif containing this degradation signal is fused. Our results show that fusion of GST to a C-terminal domain of Xkid containing the GXEN sequence results in the degradation of the merged protein, confirming that this domain shares this property with the A-box, D-box, and KEN-box degradation sequences.
Another characteristic of a proteolysis signal is to confer a correct timing of protein degradation during the cell cycle. Xenopus Kid is proteolyzed at the metaphase-anaphase transition and during late mitosis and overexpression of a nondegradable form of this protein during this transition impairs chromosome separation (2, 13). The APC/Cdc20 and the APC/Cdh1 complexes are sequentially activated from metaphase to mitosis exit and G1 (10, 20, 48). APC/Cdc20 activity first appears at metaphase and is later replaced in anaphase by APC/Cdh1. Current thinking is that Cdh1 has to be dephosphorylated before it can bind and activate the APC, and this happens late in metaphase when cyclin B/cdk1 activity disappears (21). A switch from APC/Cdc20 to APC/Cdh1 happens at anaphase (16). Thus, Xkid degradation must be performed by both APC/Cdc20 and APC/Cdh1 in order to assure continuous and complete disappearance of this protein at the metaphase-anaphase transition and late mitosis. We showed here that both APC/Cdc20- and APC/Cdh1-dependent degradation of Xkid are mediated by the GXEN sequence, indicating that this proteolysis motif is also capable to confer the appropriate proteolysis timing of this protein during the cell cycle.
A third feature of a proteolysis signal is to mediate the recognition of the substrate by the APC complex and to induce their association. Accordingly, our results show that both D-box and GXEN-box peptides compete for the APC/Cdh1-dependent degradation of Xkid and cyclin B. These findings indicate that, similar to the D-box and KEN-box sequences, the GXEN-box might mediate the recognition and the interaction of the substrate with the APC.
Finally, a last property of a proteolysis signal is that this motif is conserved in different species. Regarding the GXEN sequence, we have found only limited homology in the region corresponding to the GXEN motif (561GLEN564 in Xenopus spp. versus 568PEEK571 in humans and 563AVEK566 in mice). Nothing is known about Kid proteolysis in the mouse; however, degradation of human Kid does not seem to be regulated in the same fashion as Xenopus Kid proteolysis. In this regard, human Kid is still present in anaphase chromosomes (14, 37), whereas Xkid is completely degraded at the metaphase-anaphase transition (2, 13). Interestingly, the fact that the mechanism of Kid proteolysis could not be conserved in other species suggests the presence of other degradation pathways that would result in a distinct timing of kid proteolysis and underlines the possibility of different roles of this protein in different species. Indeed, a recent report has described a distinct mechanism for human Kid degradation that involves the SIAH-1 ubiquitin ligase (14). This pathway seems to be specific for human Kid protein, since SIAH-1 is not required for Xkid degradation (our unpublished results). It is possible that different species have evolved two different strategies to induce Kid proteolysis: the first one mediated by APC and the second one mediated by the SIAH-1 ubiquitin ligase.
In conclusion, we have described a new target signal that, despite being only partially conserved in other species, fulfills all of the main characteristic features of a degradation motif and that is essential for the Xkid protein to be correctly degraded during the cell cycle. Due to the fact that Xkid amino acid sequence contains five potential D-box sequences and that it binds Cdh1 and Cdc20 in vitro, it was assumed that its degradation was mediated by a D-box (27); however, surprisingly, we clearly demonstrate here that this is not the case. On the basis of these results, a detailed study will be required to establish the degradation signal that targets the proteolysis of every new APC substrate. It is likely that the number of new degradation signals will be enlarged by future studies and will reveal the presence of a complex association network of APC/Cdc20 and APC/Cdh1 with their different substrates.
Acknowledgments
We thank Ian Robbins for helpful comments on the manuscript and Didier Fesquet for technical assistance.
This work was supported by the Ligue Nationale Contre le Cancer (Equipe Labelisée). A.C. is a postdoctoral fellow supported by the Ligue Nationale Contre le Cancer.
REFERENCES
- 1.Afshar, K., N. R. Barton, R. S. Hawley, and L. S. Goldstein. 1995. DNA binding and meiotic chromosomal localization of the Drosophila nod kinesin-like protein. Cell 81:129-138. [DOI] [PubMed] [Google Scholar]
- 2.Antonio, C., I. Ferby, H. Wilhelm, M. Jones, E. Karsenti, A. R. Nebreda, and I. Vernos. 2000. Xkid, a chromokinesin required for chromosome alignment on the metaphase plate. Cell 102:425-435. [DOI] [PubMed] [Google Scholar]
- 3.Burton, J. L., and M. J. Solomon. 2001. D box and KEN box motifs in budding yeast Hsl1p are required for APC-mediated degradation and direct binding to Cdc20p and Cdh1p. Genes Dev. 15:2381-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton, J. L., and M. J. Solomon. 2000. Hsl1p, a Swe1p inhibitor, is degraded via the anaphase-promoting complex. Mol. Cell. Biol. 20:4614-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carpenter, A. T. 1991. Distributive segregation: motors in the polar wind? Cell 64:885-890. [DOI] [PubMed] [Google Scholar]
- 6.Castro, A., Y. Arlot-Bonnemains, S. Vigneron, J. C. Labbe, C. Prigent, and T. Lorca. 2002. APC/Fizzy-Related targets Aurora-A kinase for proteolysis. EMBO Rep. 3:457-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castro, A., M. Peter, L. Magnaghi-Jaulin, S. Vigneron, S. Galas, T. Lorca, and J. C. Labbe. 2001. Cyclin B/cdc2 induces c-Mos stability by direct phosphorylation in Xenopus oocytes. Mol. Biol. Cell 12:2660-2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castro, A., S. Vigneron, C. Bernis, J. C. Labbe, C. Prigent, and T. Lorca. 2002. D-Box-activating domain (DAD) is a new proteolysis signal that stimulates the silent D-Box sequence of Aurora-A. EMBO Rep. 3:1209-1214. [DOI] [PMC free article] [PubMed]
- 9.Cohen-Fix, O., J. M. Peters, M. W. Kirschner, and D. Koshland. 1996. Anaphase initiation in Saccharomyces cerevisiae is controlled by the APC-dependent degradation of the anaphase inhibitor Pds1p. Genes Dev. 10:3081-3093. [DOI] [PubMed] [Google Scholar]
- 10.Fang, G., H. Yu, and M. W. Kirschner. 1998. Direct binding of CDC20 protein family members activates the anaphase-promoting complex in mitosis and G1. Mol. Cell 2:163-171. [DOI] [PubMed] [Google Scholar]
- 11.Fesquet, D., N. Morin, M. Doree, and A. Devault. 1997. Is Cdk7/cyclin H/MAT1 the genuine cdk activating kinase in cycling Xenopus egg extracts? Oncogene 15:1303-1307. [DOI] [PubMed] [Google Scholar]
- 12.Fuller, M. T. 1995. Riding the polar winds: chromosomes motor down east. Cell 81:5-8. [DOI] [PubMed] [Google Scholar]
- 13.Funabiki, H., and A. W. Murray. 2000. The Xenopus chromokinesin Xkid is essential for metaphase chromosome alignment and must be degraded to allow anaphase chromosome movement. Cell 102:411-424. [DOI] [PubMed] [Google Scholar]
- 14.Germani, A., H. Bruzzoni-Giovanelli, A. Fellous, S. Gisselbrecht, N. Varin-Blank, and F. Calvo. 2000. SIAH-1 interacts with alpha-tubulin and degrades the kinesin Kid by the proteasome pathway during mitosis. Oncogene 19:5997-6006. [DOI] [PubMed] [Google Scholar]
- 15.Glotzer, M., A. W. Murray, and M. W. Kirschner. 1991. Cyclin is degraded by the ubiquitin pathway. Nature 349:132-138. [DOI] [PubMed] [Google Scholar]
- 16.Hagting, A., N. Den Elzen, H. C. Vodermaier, I. C. Waizenegger, J. M. Peters, and J. Pines. 2002. Human securin proteolysis is controlled by the spindle checkpoint and reveals when the APC/C switches from activation by Cdc20 to Cdh1. J. Cell Biol. 157:1125-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hames, R. S., S. L. Wattam, H. Yamano, R. Bacchieri, and A. M. Fry. 2001. APC/C-mediated destruction of the centrosomal kinase Nek2A occurs in early mitosis and depends upon a cyclin A-type D-Box. EMBO J. 20:7117-7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hilioti, Z., Y. S. Chung, Y. Mochizuki, C. F. Hardy, and O. Cohen-Fix. 2001. The anaphase inhibitor Pds1 binds to the APC/C-associated protein Cdc20 in a destruction box-dependent manner. Curr. Biol. 11:1347-1352. [DOI] [PubMed] [Google Scholar]
- 19.King, R. W., M. Glotzer, and M. W. Kirschner. 1996. Mutagenic analysis of the destruction signal of mitotic cyclins and structural characterization of ubiquitinated intermediates. Mol. Biol. Cell 7:1343-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kramer, E. R., C. Gieffers, G. Holzl, M. Hengstschlager, and J. M. Peters. 1998. Activation of the human anaphase-promoting complex by proteins of the CDC20/Fizzy family. Curr. Biol. 8:1207-1210. [DOI] [PubMed] [Google Scholar]
- 21.Kramer, E. R., N. Scheuringer, A. V. Podtelejnikov, M. Mann, and J. M. Peters. 2000. Mitotic regulation of the APC activator proteins CDC20 and CDH1. Mol. Biol. Cell 11:1555-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Littlepage, L. E., and J. V. Ruderman. 2002. Identification of a new APC/C recognition domain, the A box, which is required for the Cdh1-dependent destruction of the kinase Aurora-A during mitotic exit. Genes Dev. 16:2274-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorca, T., A. Castro, A. M. Martinez, S. Vigneron, N. Morin, S. Sigrist, C. Lehner, M. Doree, and J. C. Labbe. 1998. Fizzy is required for activation of the APC/cyclosome in Xenopus egg extracts. EMBO J. 17:3565-3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maney, T., A. W. Hunter, M. Wagenbach, and L. Wordeman. 1998. Mitotic centromere-associated kinesin is important for anaphase chromosome segregation. J. Cell Biol. 142:787-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez, L. H., C. Antonio, S. Flament, I. Vernos, and A. R. Nebreda. 2002. Xkid chromokinesin is required for the meiosis I to meiosis II transition in Xenopus laevis oocytes. Nat. Cell Biol. 4:737-742. [DOI] [PubMed] [Google Scholar]
- 26.Pfleger, C. M., and M. W. Kirschner. 2000. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 14:655-665. [PMC free article] [PubMed] [Google Scholar]
- 27.Pfleger, C. M., E. Lee, and M. W. Kirschner. 2001. Substrate recognition by the Cdc20 and Cdh1 components of the anaphase-promoting complex. Genes Dev. 15:2396-2407. [DOI] [PMC free article] [PubMed]
- 28.Reimann, J. D., E. Freed, J. Y. Hsu, E. R. Kramer, J. M. Peters, and P. K. Jackson. 2001. Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell 105:645-655. [DOI] [PubMed] [Google Scholar]
- 29.Reimann, J. D., B. E. Gardner, F. Margottin-Goguet, and P. K. Jackson. 2001. Emi1 regulates the anaphase-promoting complex by a different mechanism than Mad2 proteins. Genes Dev. 15:3278-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rieder, C. L., E. A. Davison, L. C. Jensen, L. Cassimeris, and E. D. Salmon. 1986. Oscillatory movements of monooriented chromosomes and their position relative to the spindle pole result from the ejection properties of the aster and half-spindle. J. Cell Biol. 103:581-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savoian, M. S., M. L. Goldberg, and C. L. Rieder. 2000. The rate of poleward chromosome motion is attenuated in Drosophila zw10 and rod mutants. Nat. Cell Biol. 2:948-952. [DOI] [PubMed] [Google Scholar]
- 32.Schwab, M., A. S. Lutum, and W. Seufert. 1997. Yeast Hct1 is a regulator of Clb2 cyclin proteolysis. Cell 90:683-693. [DOI] [PubMed] [Google Scholar]
- 33.Schwab, M., M. Neutzner, D. Mocker, and W. Seufert. 2001. Yeast Hct1 recognizes the mitotic cyclin Clb2 and other substrates of the ubiquitin ligase APC. EMBO J. 20:5165-5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharp, D. J., G. C. Rogers, and J. M. Scholey. 2000. Cytoplasmic dynein is required for poleward chromosome movement during mitosis in Drosophila embryos. Nat. Cell Biol. 2:922-930. [DOI] [PubMed] [Google Scholar]
- 35.Sigrist, S. J., and C. F. Lehner. 1997. Drosophila fizzy-related downregulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell 90:671-681. [DOI] [PubMed] [Google Scholar]
- 36.Sudakin, V., D. Ganoth, A. Dahan, H. Heller, J. Hershko, F. C. Luca, J. V. Ruderman, and A. Hershko. 1995. The cyclosome, a large complex containing cyclin-selective ubiquitin ligase activity, targets cyclins for destruction at the end of mitosis. Mol. Biol. Cell 6:185-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tokai, N., A. Fujimoto-Nishiyama, Y. Toyoshima, S. Yonemura, S. Tsukita, J. Inoue, and T. Yamamota. 1996. Kid, a novel kinesin-like DNA binding protein, is localized to chromosomes and the mitotic spindle. EMBO J. 15:457-467. [PMC free article] [PubMed] [Google Scholar]
- 38.Visintin, R., S. Prinz, and A. Amon. 1997. CDC20 and CDH1: a family of substrate-specific activators of APC-dependent proteolysis. Science 278:460-463. [DOI] [PubMed] [Google Scholar]
- 39.Walczak, C. E., T. J. Mitchison, and A. Desai. 1996. XKCM1: a Xenopus kinesin-related protein that regulates microtubule dynamics during mitotic spindle assembly. Cell 84:37-47. [DOI] [PubMed] [Google Scholar]
- 40.Weinstein, J. 1997. Cell cycle-regulated expression, phosphorylation, and degradation of p55Cdc: a mammalian homolog of CDC20/Fizzy/slp1. J. Biol. Chem. 272:28501-28511. [DOI] [PubMed] [Google Scholar]
- 41.Wood, K. W., R. Sakowicz, L. S. Goldstein, and D. W. Cleveland. 1997. CENP-E is a plus end-directed kinetochore motor required for metaphase chromosome alignment. Cell 91:357-366. [DOI] [PubMed] [Google Scholar]
- 42.Yeong, F. M., H. H. Lim, C. G. Padmashree, and U. Surana. 2000. Exit from mitosis in budding yeast: biphasic inactivation of the Cdc28-Clb2 mitotic kinase and the role of Cdc20. Mol. Cell 5:501-511. [DOI] [PubMed] [Google Scholar]
- 43.You, Z., Harvey, K., Kong, L., and J. Newport. 2002. Xic1 degradation in Xenopus egg extracts is coupled to initiation of DNA replication Genes Dev. 16:1182-1194. [DOI] [PMC free article] [PubMed]
- 44.Zachariae, W., and K. Nasmyth. 1999. Whose end is destruction: cell division and the anaphase-promoting complex. Genes Dev. 13:2039-2058. [DOI] [PubMed] [Google Scholar]
- 45.Zhang, P., B. A. Knowles, L. S. Goldstein, and R. S. Hawley. 1990. A kinesin-like protein required for distributive chromosome segregation in Drosophila. Cell 62:1053-1062. [DOI] [PubMed] [Google Scholar]
- 46.Zou, H., T. J. McGarry, T. Bernal, and M. W. Kirschner. 1999. Identification of a vertebrate sister-chromatid separation inhibitor involved in transformation and tumorigenesis. Science 285:418-422. [DOI] [PubMed] [Google Scholar]
- 47.Zur, A., and M. Brandeis. 2001. Securin degradation is mediated by fzy and fzr, and is required for complete chromatid separation but not for cytokinesis. EMBO J. 20:792-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zur, A., and M. Brandeis. 2002. Timing of APC/C substrate degradation is determined by fzy/fzr specificity of destruction boxes. EMBO J. 21:4500-4510. [DOI] [PMC free article] [PubMed] [Google Scholar]