Abstract
Establishment and maintenance of differential chromatin structure between transcriptionally competent and repressed genes are critical aspects of transcriptional regulation. The elements and mechanisms that mediate formation and maintenance of these chromatin states in vivo are not well understood. To examine the role of the promoter in maintaining chromatin structure and DNA methylation patterns of the transcriptionally active X-linked HPRT locus, 323 bp of the endogenous human HPRT promoter (from position −222 to +102 relative to the translation start site) was replaced by plasmid sequences by homologous recombination in cultured HT-1080 male fibrosarcoma cells. The targeted cells, which showed no detectable HPRT transcription, were then assayed for effects on DNase I hypersensitivity, general DNase I sensitivity, and DNA methylation patterns across the HPRT locus. In cells carrying the deletion, significantly diminished DNase I hypersensitivity in the 5′ flanking region was observed compared to that in parental HT-1080 cells. However, general DNase I sensitivity and DNA methylation patterns were found to be very similar in the mutated cells and in the parental cells. These findings suggest that the promoter and active transcription play a relatively limited role in maintaining transcriptionally potentiated epigenetic states.
An epigenetic hallmark of transcriptionally competent loci is that they adopt and maintain an open and accessible chromatin conformation, whereas inactive genes form a closed, inaccessible conformation (46). As assayed by sensitivity to digestion by nucleases such as DNase I, transcriptionally competent chromatin is characterized by generalized accessibility and sensitivity to DNase I digestion relative to bulk (as well as transcriptionally repressed) chromatin in vivo. This general DNase I sensitivity occurs along the entire length of an active gene and commonly extends both upstream and downstream of the gene itself (20, 23, 29). The difference in general DNase I sensitivity between transcriptionally competent chromatin and repressed chromatin is thought to reflect a difference in higher-order chromatin structure (43), though the molecular basis of general DNase I sensitivity and the resistance of chromatin are not well understood.
DNase I-hypersensitive regions of chromatin in transcriptionally active loci are commonly associated with cis-acting regulatory elements and are thought to reflect localized disruption of nucleosomal arrays (17). This localized hypersensitivity to DNase I is commonly believed to result from the binding of transcription factors to their cognate recognition sequences, leading to alteration of local nucleosomal organization and/or structure (2, 16). However, several studies also suggest that formation and/or maintenance of DNase I-hypersensitive sites in the 5′ flanking regions of active genes may arise independent of stable transcription factor binding in the promoter (10, 25, 28, 31, 33).
Mechanisms that establish and maintain transcriptionally competent or repressed chromatin states in vivo are not well characterized, although recent studies of the runt gene in Drosophila suggest that establishment and maintenance of transcriptional repression are mechanistically distinct events (47). Establishing an accessible (i.e., nuclease-sensitive and transcriptionally competent) chromatin structure has been postulated to involve the initial binding of transcription factors to the promoter region, leading to the opening of local chromatin structure, initiation of active transcription, and subsequent formation of an open chromatin structure along the length of the locus (12), though formation of the preinitiation complex prior to opening of local chromatin structure has also been reported (42). Active transcription has also been postulated to be involved in maintaining an open chromatin structure along the length of an active locus (5). For so-called housekeeping genes that exhibit expression in all cells at all stages of development, the epigenetic mechanisms and cis-acting elements that confer constitutive transcriptional competence on these genes are not entirely clear.
Mechanisms and cis-acting elements involved in establishing and maintaining other epigenetic properties of transcriptionally active and inactive genes, such as DNA methylation, also are unclear. Analysis of transgenes carrying portions of the mouse and hamster Aprt gene promoters suggests that Sp1 binding sites may be necessary but not sufficient for preventing methylation of CpG islands (3, 32, 35, 41). Furthermore, analysis of a transgene carrying a 62-bp fragment from the human HPRT promoter region containing five potential Sp1 binding sites suggests that cis-acting elements within this fragment alone are also not sufficient to protect CpG sites within the transgene from undergoing methylation (41). The role of cis-acting promoter elements involved in protecting CpG islands of endogenous genes within their normal chromosomal context has not been reported.
We examined the role of cis-acting promoter elements and active gene transcription in maintaining chromatin structure and DNA methylation patterns across the endogenous X-linked human HPRT gene on the active X chromosome in cultured cells. The active HPRT allele is constitutively expressed (44), and the promoter region is associated with a CpG island containing a cluster of five GC boxes (36). The minimal functional human promoter is contained within 219 bp of sequence upstream of the translation initiation site (40) and is associated with multiple transcription initiation sites (24, 36). The promoter on the active X chromosome (Xa) is DNase I hypersensitive (30), bound by multiple transcription factors in vivo (22), and hypomethylated at CpG-dinucleotides (21), whereas the promoter on the inactive X chromosome (Xi) is transcriptionally repressed, resistant to nucleases (30), devoid of detectable transcription factor binding (22), and hypermethylated (21). Here we mutated the endogenous HPRT promoter on the Xa by homologous recombination in cultured human male fibrosarcoma cells (HT-1080) by targeted replacement of 323 bp of the promoter region with ∼100 bp of vector sequences. Removal of the endogenous promoter in these cells abolished the major DNase I-hypersensitive site normally present in the 5′ flanking region of the active allele but did not affect general DNase I sensitivity at multiple sites across the gene. Furthermore, no major change in the methylation status of CpG-dinucleotides in the 5′ region and in intron 3 of the gene was observed. These results indicate that the HPRT promoter is required to maintain a nuclease-hypersensitive nucleosomal array structure in the 5′ region of the active gene. However, once an open chromatin structure and DNA methylation patterns are established in the active HPRT gene during development, neither cis-acting promoter elements nor active gene transcription appears to be required for maintaining general DNase I sensitivity or the DNA methylation pattern associated with the active gene.
MATERIALS AND METHODS
Cell lines and culture.
HT-1080 (ATCC CCL 121) is a male human fibrosarcoma cell line that contains a single transcriptionally active copy of the HPRT gene. The cells were grown in minimum essential medium (MEM) with 10% fetal bovine serum and 1% penicillin-streptomycin (Life Technologies) at 37°C in 6% CO2. 4.12 and 8121 are human-hamster somatic cell hybrids that contain an active human X chromosome and an inactive human X chromosome, respectively, in the same HPRT-deficient hamster cell background (22). These cells were grown in Dulbecco's modified Eagle medium under the same conditions as those described above. HPRT-expressing (HPRT +) cells were selected in medium containing 1× HAT (Life Technologies), and non-HPRT-expressing (HPRT −) cells were selected in medium containing 1× 6-thioguanine (6-TG; Sigma).
Construction of targeting vector.
The backbone of the targeting vector (pfloxSBH) was a modified pflox plasmid (18) (generously provided by Camilynn Brannan), which contained the positive and negative selectable markers neo and hsv-tk, respectively, flanked by two loxP sites (see Fig. 1B). The HPRT plasmid pU12HH contained a 7-kb HindIII fragment from the 5′ region of the HPRT gene (including the gene promoter). A novel SgrAI site was inserted just downstream of the HindIII site in the pflox vector to create the pfloxS vector. A 3.7-kb HindIII-SgrAI fragment from pU12HH located immediately upstream of the HPRT gene promoter was cloned into the pfloxS vector upstream of the neo gene. A 3-kb BamHI-HindIII fragment from pU12HH located immediately downstream of the HPRT promoter was first cloned into pBluescript SK(+) plasmid and was excised with XbaI and SalI to generate compatible ends to insert into the pfloxS vector downstream of the hsv-tk gene to create the targeting vector pfloxSBH.
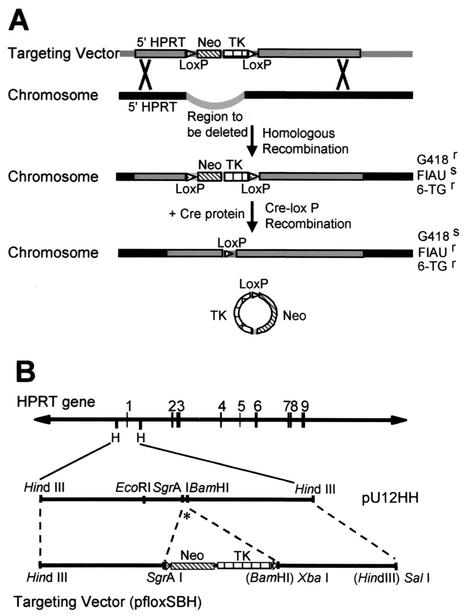
FIG. 1.
Cre-loxP gene-targeting strategy. (A) A schematic representation of the Cre-loxP recombination strategy. Gray boxes indicate targeting vector sequences. Black boxes indicate endogenous chromosomal sequences. Thick black lines indicate plasmid backbone sequences. (B) A schematic representation of construction of the targeting vector. The asterisk indicates exon 1. See text for details.
Electroporation.
HT-1080 cells were washed with phosphate-buffered saline (PBS), trypsinized, and counted. A total of 107 cells were resuspended in 800 μl of ice-cold PBS and were mixed with 10 to 40 μg of SalI-linearized targeting vector (pfloxSBH) DNA in a prechilled 0.4-cm Gene Pulser (Bio-Rad) cuvette. The cuvette was loaded into a Gene Pulser II (Bio-Rad) electroporation chamber and was exposed to one pulse at 250 μF and 450 V. The cells were immediately plated in 150-mm tissue culture plates in MEM overnight. Transient transfection of the Cre expression plasmid, pBS189 (Life Technologies), was performed similarly, except that supercoiled plasmid DNA was used. All plasmid DNA used for electroporation was purified with a Qiagen Maxiprep Kit.
Selection and isolation of targeted colonies.
To select for homologous recombination of the targeting vector into the HPRT promoter region, electroporated cells were grown in 400 μg of G418 (Geneticin; Life Technologies)-supplemented MEM per ml for 7 to 10 days and were then switched to 1× 6-TG (Sigma)-supplemented media for 7 days. The number of G418-resistant/6-TG-resistant (G418r/6-TGr) colonies was counted, and individual colonies were isolated with cloning rings. The single-cell-derived colonies were placed in 24-well plates and were expanded for genomic DNA isolation. G418r/6-TGr clones were screened by Southern blot analysis with the gene-specific probe HH1, a 1.1-kb HindIII-HpaI fragment from intron 1 that hybridized 3′ and outside the region of HPRT homology in the targeting vector (see Fig. 3). Positive clones were transfected with 6 μg of pBS189 (Cre expression plasmid) by electroporation to transiently express the Cre protein and facilitate Cre-mediated recombination between the two loxP sites. Transfected cells were plated first in nonselective medium overnight and were then grown in 0.2 μM 2′-fluoro 2′-deoxy-5-iodouracil-β-d-arabinofuranoside (FIAU; Moravek Biochemicals, Brea, Calif.) for 10 to 14 days. FIAU-resistant (FIAUr) clones were isolated by using cloning rings and were expanded for genomic DNA isolation. FIAUr clones were screened by Southern blot analysis with HPRT probes HH1 and 5′del, a PCR-amplified fragment (primers 5′del probe 1 and 5′del probe 2; see Table 1 for sequences) located 5′ of the HindIII site ∼2.7 kb upstream of the deleted region (see Fig. 3). All probes were radiolabeled by random priming (Life Technologies) with [α-32P]dCTP (3,000 Ci/mmol).
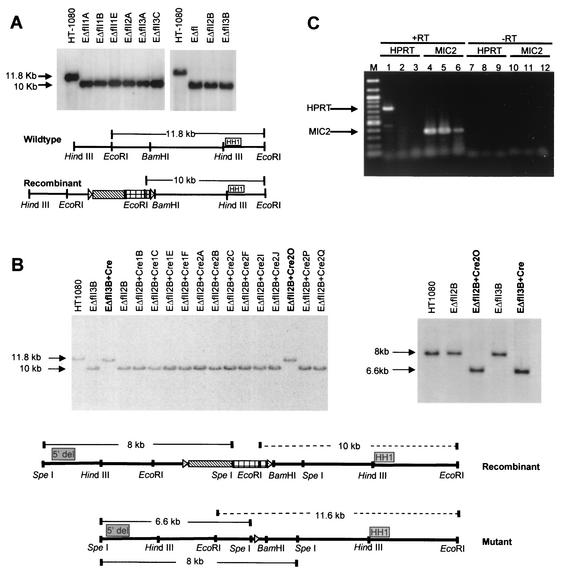
FIG. 3.
Identification of HPRT promoter deletion cell lines. (A) Southern blot verification of homologous recombination. DNA from G418r/6-TGr clones was digested with EcoRI for Southern blot analysis. HH1 indicates the position of an HPRT-specific probe. (B) Southern blot analysis of DNA from FIAUr clones digested with EcoRI and probed with HH1 (left panel). Shown is Southern blot verification of HPRT promoter deletions using DNA digested with SpeI from clones EΔfII3B+Cre (Δ3B) and EΔfII2B+Cre2O (Δ2B) and hybridized with probe 5′del (right panel). The diagrams below are schematic representations of the positions of probes relative to the HPRT promoter region. Dotted lines represent possible EcoRI fragments detected by probe HH1. Solid lines represent possible SpeI fragments detected by probe 5′del. (C) RT-PCR analysis for HPRT mRNA. RT-PCR was performed on total RNA from HT-1080 (lanes 1, 4, 7, and 10), Δ3B (lanes 2, 5, 8, and 11), and Δ2B (lanes 3, 6, 9, and 12) cells. HPRT and MIC2 indicate analysis with HPRT- or MIC2-specific primers, respectively. +RT or −RT indicates that the PCR was performed from template DNA generated in the presence (+) or absence (−) of RT. M indicates the 100-bp marker lane.
TABLE 1.
Sequences of PCR primers
| Primer name | Primer sequence |
|---|---|
| RT-PCR primers | |
| HPRT1 | 5′ TCCTCCTGAGCAGTCAGC 3′ |
| HPRT3 | 5′ GGCGATGTCAATAGGACTC 3′ |
| XMIC2 | 5′ ACCCAGTGCTGGGGATGACTTT 3′ |
| XMIC2R | 5′ CTCTCCATGTCCACCTCCCCT 3′ |
| Probe primers | |
| 5′del probe 1 | 5′ CATACTGCTCTGTGAAGGACTT 3′ |
| 5′del probe 2 | 5′ AAGGCATTCTCCAGCACATT 3′ |
| HPRT2 1 | 5′ GGGCCTGCTTGAATGTTGAGAGAA 3′ |
| HPRT2 2 | 5′ TCCTGAACTCAGAGCGTCACTGTCA 3′ |
| HPRT3 1 | 5′ TGCTCACCTCTCCCACACCCTTT 3′ |
| HPRT3 2 | 5′ TCCGTGCTGAGTGTACCATGGTCA 3′ |
| FSMIC2-1 | 5′ GCCCACTTTCTCCCCAACGCTT 3′ |
| FSMIC2-2 | 5′ TTCCAAATTTAGGCCCGGGACG 3′ |
| Bisulfite primers | |
| Upstream primer 1 | 5′ GTTAGGAAAATGGAAGTTATAGGTAGTG 3′ |
| Upstream primer 2 | 5′ CAACCRCCTAAAACTAAAAAAAAACAA 3′ |
| Downstream primer 1 | 5′ GTGGGGTTTGTTTTTTTTTAGTTTTA 3′ |
| Downstream primer 2 | 5′ CAAAACTCCCCCAAAAAAAAA 3′ |
| Deletion primer 1 | 5′ YGAAGTTATTAGGTTTTTYGATTTGTAGT 3′ |
| Deletion primer 2 | 5′ CTAATTCTAAAAAATCAACTTAAACTACAAAT 3′ |
| Intron 3 primer 1 | 5′ TTATTTATTTATTTTTTTTTTTGAGATAGA 3′ |
| Intron 3 primer 2 | 5′ TATATAAAATATACCCCAATAAAACCTT 3′ |
DNA and RNA isolation and RT-PCR.
Genomic DNA was isolated according to standard methods of proteinase K digestion, phenol extraction, ethanol precipitation, and resuspension in 1× Tris-EDTA (10 mM Tris-HCl, pH 8.0, and 1 mM EDTA). RNA was isolated from cultured cells as described by Chomczynski and Sacchi (8). Reverse transcriptase PCR (RT-PCR) was performed as described by Chen et al. (6). Ten percent of the RT reaction was used to perform PCR in a final volume of 100 μl containing a 0.2 mM concentration of each deoxynucleotide triphosphate, 1.75 mM MgCl2, 10 mM HPRT primers (HPRT1 and HPRT3) or MIC2 primers (XMIC2 and XMIC2R), 1× PCR buffer (Life Technologies), and 1 U of Taq polymerase (Life Technologies). The reaction was initially denatured at 94°C for 2 min and was then cycled 35 times to 94°C for 1 min, 59°C for 30 s, and 70°C for 90 s, followed by a final extension for 4 min at 72°C. Twenty percent of the PCR was size fractionated on a 1.5% agarose gel and was visualized by ethidium bromide staining under UV light.
DNase I sensitivity assays and Southern blot analysis.
DNase I digestion of chromatin was performed in permeabilized cultured cells grown to confluency in eight T-150 tissue culture flasks. Cells were washed with PBS, trypsinized, collected by centrifugation, and washed in solution A (150 mM sucrose, 80 mM KCl, 35 mM HEPES, pH 7.4, 5 mM K2HPO4, 5 mM MgCl2, and 0.5 mM CaCl2). The cells then were resuspended in 12 ml of solution B (150 mM sucrose, 80 mM KCl, 35 mM HEPES, pH 7.4, 5 mM K2HPO4, 5 mM MgCl2, and 2 mM CaCl2) and were divided equally into 1-ml aliquots in 12 tubes and were kept at 37°C. Various amounts of DNase I (Worthington) were added to a total volume of 1 ml of solution B containing 0.4% NP-40 (Sigma) in order to achieve final concentrations of 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, and 100 μg of DNase I/ml. The DNase I-NP-40-solution B mixture was added to the cells and was incubated at 37°C for 2 min. DNA lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 25 mM EDTA, 0.5% sodium dodecyl sulfate, and 300 μg of proteinase K/ml) was added to terminate the reaction. The cells were lysed overnight at room temperature, and genomic DNA was purified by standard phenol extractions and ethanol precipitations. Approximately 7 to 10 μg of each DNase I-treated sample was Southern blotted or slot blotted as described below. A 1.4-kb BamHI-PstI probe (HPRT A) from the 5′ region of the HPRT gene (30) was used to assay for DNase I hypersensitivity in the HPRT promoter region (see Fig. 4). A 770-bp PstI-EcoRI fragment from the 3′ region of the human β-globin gene was used as an internal control probe for DNase I hypersensitivity analysis (generously provided by Jörg Bungert). Probes for analysis of DNase I general sensitivity at the HPRT locus were obtained by PCR amplification with primers from regions of the HPRT locus indicated in Fig. 5. The control probe MIC2 was generated by PCR amplification with primers FSMIC2-1 and FSMIC2-2. The sequences for the primers are shown in Table 1. The control probe XIST was generated by isolating the EcoRI insert from plasmid pXIST-C6 (ATCC 65444). All radiolabeled probes were synthesized by random priming (Life Technologies) with [α-32P]dCTP (3,000 Ci/mmol) and were assayed by Southern blot hybridization with restriction enzyme-digested total human genomic DNA to verify that they were single copy and yielded low backgrounds. For Southern blots, genomic DNA (10 μg) was digested with the appropriate restriction enzyme and was size fractionated on a 0.8% agarose gel in 1× Tris-acetate-EDTA (40 mM Tris-acetate and 2 mM EDTA). Capillary transfer to nylon membranes (Hybond N+; Amersham), hybridization, and washing were performed as described previously (7).
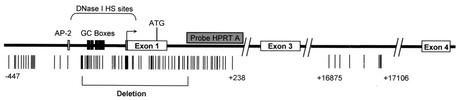
FIG. 4.
Physical map of the human HPRT gene. The thick horizontal line represents the HPRT gene. Open horizontal boxes represent exons, and “ATG” indicates the translation initiation site. The bent arrow represents the major transcription initiation sites, and a putative initiator element is shaded. The gray box indicates the position of probe “HPRT A.” The bracket above the line indicates the position of the 5′ flanking DNase I-hypersensitive site. The positions of CpG-dinucleotides within the gene are shown as vertical lines below the gene map. Numbers indicate the position of CpGs relative to the translation initiation site. The horizontal bracket below the line indicates the region deleted in Δ2B and Δ3B cells.
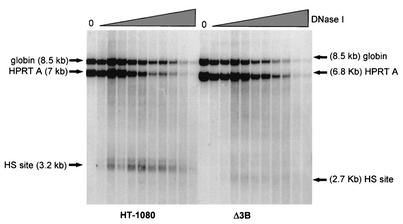
FIG. 5.
Analysis of DNase I hypersensitivity. HT-1080 (male human fibrosarcoma) cells and Δ3B (HT-1080 cells carrying the promoter mutation) cells were treated with 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, and 100 μg of DNase I/ml. Southern blot analysis was performed on DNA digested with EcoRI and was hybridized to the HPRT promoter probe HPRT A. “HS site” indicates the positions of HPRT-hypersensitive sites; “globin” indicates the hybridization band detected by the control human β-globin probe.
Slot blot analysis.
DNase I-treated genomic DNA was either digested with a restriction enzyme or was sheared by vortexing and was quantitated by UV spectrophotometry. In a total volume of 250 μl, 7 μg of each DNase I-treated sample was denatured in 0.2 M NaOH at 37°C for 15 min and was then applied to Hybond N+ nylon membranes in a slot blot apparatus (Convertible Filtration Manifold System; Life Technologies). The blots were vacuum dried at 80°C for 1 to 2 h and were used for DNase I general sensitivity analysis. The intensities of probe hybridization signals within each individual slot on the slot blots were quantitated by PhosphorImager scanning. The data from DNase I-treated samples generated from PhosphorImager were used to calculate the percent hybridization signal remaining (after DNase I treatment) by dividing the quantitated signal intensity from each DNase I-treated sample by that of the mock-treated sample (NP-40 without DNase I) and then multiplying by 100. The percent hybridization signal remaining was plotted as a function of DNase I concentration and was then fitted to a straight line with Microsoft Excel. Quantitation of general DNase I sensitivity was ascertained by calculating the slope of the line generated by the PhosphorImager plots.
High-resolution sodium bisulfite genomic sequencing.
DNA methylation analyses were performed essentially as described in reference 9. Genomic DNA was digested with an appropriate restriction enzyme and was purified by standard phenol extractions and ethanol precipitation. Five micrograms of digested DNA was sheared by vortexing and was denatured in 0.3 M NaOH at 37°C for 30 min. A solution of sodium bisulfite and hydroquinone, pH 5.0, was added to the denatured DNA to a final concentration of 1.55 M sodium bisulfite and 0.5 mM hydroquinone, and the samples were incubated for 18 h at 55°C in the dark. Free bisulfite ion was removed with the Wizard DNA Clean-Up kit (Promega), desulfonated in 0.3 M NaOH for 15 min at 37°C, and ethanol precipitated. Ten percent of the recovered bisulfite-treated sample was subjected to PCR with HotStarTaq Polymerase in the provided buffer (Qiagen) under the following conditions: 94°C for 15 min, followed by 35 cycles of 94°C for 45 s, 52°C for 30 s, 72°C for 90 s, and a final extension at 72°C for 10 min. The sequences for the PCR primers are shown in Table 1. PCR products were gel purified by using Qiagen's QIAquick kit, ligated into the TA cloning vector pCR2.1 (Invitrogen), and transformed into competent bacterial cells. Plasmids from individual colonies were subjected to automated sequencing with the ABI Prism BigDye Terminator Cycle Sequencing Ready Reaction Kit with AmpliTaq DNA Polymerase. A minimum of seven PCR clones was analyzed for each primer set.
RESULTS
Construction of targeted promoter deletion cell lines.
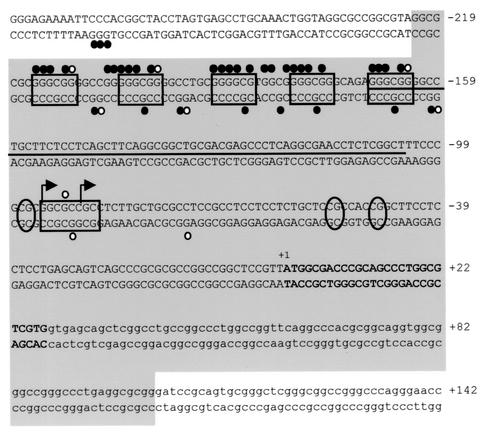
We utilized a Cre-loxP-mediated recombination targeting strategy (summarized in Fig. 1A) in cultured HT-1080 male fibrosarcoma cells to replace the endogenous functional HPRT promoter region with plasmid sequences and a single loxP site. The targeted mutation removed 323 bp of the HPRT gene promoter from the SgrAI site to the BamHI site between positions −222 and +102 (relative to the translation initiation site), including the minimal functional promoter (40), six in vivo footprinted transcription factor binding sites (of which five are potential Sp1 binding sites) (22), the multiple transcription initiation sites (24, 36), three critical methylation sites (6), and the translation initiation site (40) (shown in Fig. 2). In addition, the deleted region included most or all of the 5′ flanking DNase I-hypersensitive site (30).
FIG. 2.
DNA sequence of the human HPRT promoter region. Boxes indicate positions of transcription factor binding sites. Circles indicate positions of dimethyl sulfate (DMS) in vivo footprints, closed circles represent DMS protections, and open circles represent sites of enhanced DMS reactivity. The thin horizontal line indicates the region of multiple transcription initiation sites, with bent arrows indicating the major sites of transcription initiation. Ovals indicate positions of methylated CpGs critical for transcriptional repression. +1 indicates the translation initiation site, with exon 1 shown in boldface. The large gray box indicates the region deleted by homologous recombination.
The parental male HT-1080 fibrosarcoma cells were first examined by fluorescence in situ hybridization and Southern blot analysis (relative to normal human diploid male and female fibroblasts) to verify the presence of only a single copy of the HPRT gene (data not shown). The linearized targeting vector (pfloxSBH), which contained the positive selectable marker neo and the negative selectable marker hsv-tk flanked by loxP sites and by sequences homologous to the HPRT gene (Fig. 1B and Materials and Methods), was transfected into HT-1080 cells. By homologous recombination, 323 bp of the endogenous HPRT promoter region was replaced with sequences from the targeting vector, including the two selectable markers, the loxP sites, and plasmid sequences. Insertion of the neo gene conferred G418 resistance, and removal of the functional HPRT promoter conferred 6-TG resistance. Nine colonies from four independent transfections (totaling 4 × 107 cells) survived both G418 and 6-TG selection, and all nine of these single-cell-derived colonies were individually isolated and expanded.
To verify that these colonies contained the desired homologous recombination product, Southern blot analysis was performed on EcoRI-digested DNA from each G418r/6-TGr clone by using an HPRT-specific probe (HH1), which lies 3′ and outside the region of homologous recombination (Fig. 3A). Homologous recombination of the targeting vector into the endogenous HPRT promoter introduced a new EcoRI site from the hsv-tk gene, so that probe HH1 would detect a novel 10-kb EcoRI fragment in correctly targeted cells instead of a normal 11.8-kb EcoRI fragment. All nine G418r/6-TGr clones showed the 10-kb EcoRI fragment, confirming that all contained the desired homologous recombination product.
Two of the nine clones, EΔfII2B and EΔfII3B, were then subjected to transient expression of the Cre protein and subsequent Cre-mediated recombination, resulting in removal of the selectable markers and one loxP site. This recombination event conferred FIAU resistance to the cells and left behind a single loxP site and adjacent plasmid sequences in place of the HPRT promoter. Thirty-one colonies from two independent transfections (totaling 2 × 107 cells) were FIAUr and were individually isolated and expanded for Southern blot screening (Fig. 3B). If Cre-mediated recombination occurred, then EcoRI-digested DNA from the FIAUr clones hybridized with probe HH1 should detect an 11.5-kb EcoRI fragment. Likewise, hybridization of the 5′del probe to SpeI-digested DNA should detect a 6.6-kb fragment if the correct recombination event occurred. Southern blot analysis using both 5′del and HH1 probes verified that, in 2 of the 31 clones, i.e., EΔfII2B+Cre2O (Δ2B) and EΔfII3B+Cre (Δ3B), the selectable markers were removed (Fig. 3B).
Nucleotide sequence analysis of the HPRT 5′ flanking region of the Δ2B and Δ3B cell lines revealed that the desired 323 bp of the HPRT promoter region was deleted and was replaced by the expected ∼100 bp of vector plasmid sequences, including a loxP site (data not shown). The vector sequences replacing the promoter region consisted of the multiple cloning sites from the vector backbone that were inserted into the targeting vector during construction. A search of the transcription factor database, TransFacs (http://www.genomatix.de/cgi-bin/matinspector/matinspector.pl) (37), indicated that the exogenous plasmid sequences (and loxP site) did not introduce any known transcription factor binding sites into the HPRT gene.
To confirm that the two targeted cell lines did not express the HPRT gene, RT-PCR was performed by using total RNA from HT-1080, Δ2B, and Δ3B cells. PCR amplification with primers from the HPRT gene or the control MIC2 gene (an X-linked gene expressed from both the active and inactive X) (14) was performed to assay for HPRT gene expression. Only RNA from the parental HT-1080 cells amplified HPRT cDNA (Fig. 3C), indicating that there was no detectable HPRT transcription in the targeted Δ2B and Δ3B cell lines.
Analysis of DNase I hypersensitivity.
DNase I hypersensitivity is postulated to be associated with regions of transcription factor binding and chromatin lacking canonical nucleosomes. However, several studies suggest that formation of DNase I-hypersensitive sites may not require persistent transcription factor binding (10, 25, 28, 31, 33). Therefore, to determine if cis-acting elements in the HPRT promoter region are required to maintain DNase I hypersensitivity of the region on the transcriptionally active allele in vivo, we assayed deletion cell lines Δ2B and Δ3B for DNase I hypersensitivity in the 5′ flanking region.
Intact HT-1080, Δ2B, and Δ3B cells were permeabilized and treated in vivo with increasing concentrations of DNase I. Genomic DNA was isolated and digested with HindIII for Southern blot analysis and was hybridized with a probe (HPRT A) that detects the promoter region (Fig. 4). As shown in Fig. 5, a normal 7-kb genomic HindIII fragment and a 3.2-kb DNase I-hypersensitive fragment were detected in the parental HT-1080 cells. In the mutant Δ3B cell line, a 6.8-kb genomic HindIII fragment was observed (smaller than the wild type because of the targeted promoter mutation); however, the major 3.2-kb DNase I-hypersensitive fragment was absent and a novel, weak 2.7-kb DNase I-hypersensitive band was detected (Fig. 5). This novel hypersensitive site mapped to the region of the altered gene just downstream of the targeted mutation. A similar result was obtained in Δ2B cells (data not shown).
Analysis of general DNase I sensitivity.
To examine the role of the promoter region and active transcription in maintaining a transcriptionally competent higher-order chromatin structure, general DNase I sensitivity was assayed at various regions across the HPRT gene in cell lines Δ2B and Δ3B and was compared to the general DNase I sensitivity of the intact HPRT gene in parental HT-1080 cells. Intact HT-1080, Δ2B, and Δ3B cell lines were permeabilized and were treated with increasing concentrations of DNase I, and then purified genomic DNA from each sample was immobilized onto nylon membranes in separate wells of a slot blot apparatus. The same membrane was then sequentially hybridized with radiolabeled probes from different regions of the HPRT gene to assay for differences in general DNase I sensitivity between the parental and mutated cells; membranes were stripped of the previous probe prior to rehybridization with a new probe. These probes assayed general DNase I sensitivity at three sites spanning the HPRT gene (Fig. 6A). Probe HPRT A lies just downstream of the promoter in the 5′ region of the gene, probe HPRT 2 hybridizes within intron 3, and probe HPRT 3 hybridizes within intron 6. The hybridization signal detected with each probe at each DNase I concentration (i.e., within each slot) was quantitated by PhosphorImager analysis and was then expressed as a percentage of hybridization signal remaining (after DNase I treatment) relative to the control sample that did not receive treatment with DNase I (Materials and Methods). These percentages were plotted as a function of DNase I concentration to reflect the DNase I sensitivity at each site assayed (Fig. 6B).
FIG. 6.
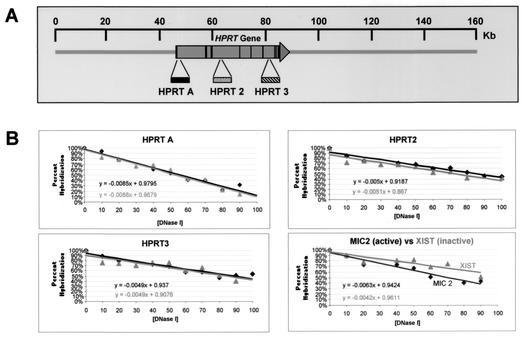
Analysis of DNase I general sensitivity of the HPRT locus. (A) Approximate positions of slot blot hybridization probes HPRT A, HPRT2, and HPRT3, relative to the HPRT gene (large horizontal arrow); vertical lines within the arrow indicate exons. The scale at the top is relative to the human X chromosome BAC clone bWXD187 (accession no. AC004383). (B) Quantitation of general DNase I sensitivity assayed by each hybridization probe. Each panel represents analysis of general DNase I sensitivity at a given site in the HPRT gene. Quantitation of probe hybridization to slot blots was performed by PhosphorImager analysis (Materials and Methods). Linear plots were fitted to the data points, and the equation representing each line is shown. Dark lines represent DNase I digestion of HT-1080 chromatin; gray lines represent DNase I digestion of chromatin in cell line Δ3B containing the HPRT promoter mutation. The panel in the lower right corner shows a comparison of general DNase I sensitivities of chromatin from the transcriptionally active MIC2 locus (darker line) and of chromatin from the transcriptionally silenced XIST locus (lighter line) in HT-1080 cells.
The linear plot generated from this analysis of Δ3B cells was compared to that from parental HT-1080 cells for each hybridization probe (Fig. 6B). General DNase I sensitivity was quantitated by determining the slope of each line. Comparing the slopes of lines for HT-1080 and Δ3B cells for each hybridization probe revealed, at most, a 4% difference in general DNase I sensitivity at all sites assayed in the HPRT gene. If the promoter region were required to maintain a DNase I-sensitive conformation across the HPRT gene, we would have expected a significantly greater difference in sensitivity between HT-1080 and Δ3B cells, because previous analysis of general DNase I sensitivity demonstrated that chromatin from the active HPRT allele was approximately twofold more sensitive to digestion by DNase I than was the inactive HPRT allele (30). Thus, replacing the promoter region with a loxP site and vector sequences had, at most, only a very minor effect on general DNase I sensitivity at all sites assayed across the HPRT gene. This was true even for the 5′ flanking region, which, by Southern blot analysis, appeared to be more sensitive to DNase I in the parental cell line than in the mutated cell line (compare the genomic HindIII band in Fig. 5 between HT-1080 and Δ3B cells). This apparent discrepancy in analyzing general DNase I sensitivity with probe HPRT A by Southern blotting (Fig. 5) versus slot blot analysis (Fig. 6) occurs because the region examined by probe HPRT A contains a DNase I-hypersensitive site. Therefore, the Southern blot in Fig. 5 separated the hybridization signals from the upper parental band and the lower hypersensitive band, while the hybridization signal in the slot blot analysis was the sum of the signals from both the parental and hypersensitive bands. By combining the hybridization signals from the parental and hypersensitive bands, the results of the slot blot method provide a measure of the overall general sensitivity of the region, even in the presence of a DNase I-hypersensitive site.
It is interesting that the slopes generated by two of the three probes (HPRT2 and HPRT3) were almost identical, suggesting no significant difference in the general DNase I sensitivity between these regions. However, the nuclease sensitivity of the promoter region (probe HPRT A) generated a slope that was at least 1.7-fold greater than for other sites in the gene. It is possible that the steeper slope detected by this probe is indicative of an intrinsically higher accessibility of the 5′flanking region to DNase I (or preferential DNase I cleavage of the underlying DNA sequence).
As a control, we also compared the general DNase I sensitivity between the transcriptionally expressed MIC2 gene (14) and the transcriptionally repressed XIST gene (4) on the active X chromosome. Probes specific to MIC2 and XIST were rehybridized to the same blots that were used for assaying general DNase I sensitivity of the HPRT gene above, and the percent hybridization at each DNase I concentration was plotted as a function of DNase I concentration as before. As shown in the lower right panel of Fig. 6B, the slopes of the lines representing DNase I digestion of chromatin from the MIC2 locus and the XIST locus revealed a 1.5-fold difference in general DNase I sensitivity between the expressed MIC2 gene and the repressed XIST gene. This difference in general DNase I sensitivity between these two genes in HT-1080 cells is similar to that seen previously at other active and inactive loci, including the HPRT gene (20, 23, 29, 30). Thus, analysis of the MIC2 and XIST genes demonstrates that our assay system for quantitating relative general DNase I sensitivity was capable of detecting small differences in general DNase I sensitivity of the HPRT gene in the parental and mutant cells.
Results from our analysis of general DNase I sensitivity revealed that, at each site examined in the HPRT gene, little difference was detected in general DNase I sensitivity between the parental HT-1080 cells and the targeted cells containing a promoter-less HPRT gene.
DNA methylation analysis.
A characteristic of X-linked housekeeping genes subject to X chromosome inactivation is the differential methylation of CpG islands in promoter regions where the active promoter is hypomethylated and where the inactive promoter is hypermethylated (15). For example, the HPRT promoter region contains five potential Sp1 binding sites and is hypermethylated on the inactive allele and virtually unmethylated on the active allele (21). Additionally, a region within intron 3 of the HPRT gene is reported to exhibit the reverse methylation pattern, where the active allele is methylated and where the inactive allele is unmethylated (48). The mechanisms that establish these DNA methylation patterns of individual genes during development and the mechanisms that recruit (or exclude) de novo DNA methylases to specific sites at specific stages of development remain unclear. Studies of the hamster Aprt gene suggested that Sp1 binding elements are involved in protecting CpG islands from de novo methylation (3). However, similar analysis of a 62-bp fragment of the human HPRT promoter region containing the five Sp1 binding sites showed that this fragment failed to protect CpG sites in a transgene from methylation (41). Therefore, we examined the effect of removing 323 bp of the promoter (including the 62 bp containing the Sp1 binding sites) from the endogenous active HPRT gene on maintaining DNA methylation patterns in the 5′ flanking region and within the body of the gene. If cis-acting elements within the 323-bp interval are required for preventing methylation of the 5′ CpG island in the endogenous HPRT promoter (or for methylation of the CpGs in intron 3), we would have expected hypermethylation of the remaining CpGs in the 5′ CpG island (or demethylation in intron 3) after removal of the 323-bp region.
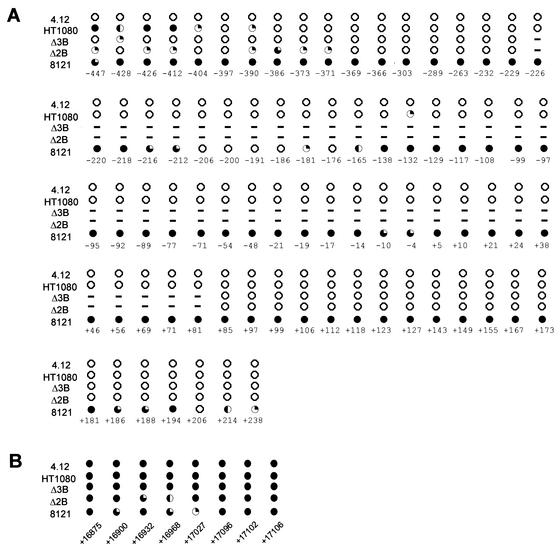
We performed high-resolution bisulfite genomic sequencing to assay the methylation status of 87 individual CpG-dinucleotides in the endogenous HPRT gene. Figure 4 shows the relative positions of 79 CpG-dinucleotides in the promoter region and 8 CpGs in intron 3 of the HPRT gene that were analyzed in cell lines HT-1080, 4.12 (somatic cell hybrid containing a human Xa), 8121 (somatic cell hybrid containing a human Xi), Δ2B, and Δ3B. Methylation analysis of these cells allowed us to determine whether or not cis-acting elements within the deleted 323-bp region of the endogenous promoter (including the five Sp1 binding sites) are necessary to maintain the DNA methylation patterns within the 5′ CpG island and intron 3 of the active HPRT allele. Figure 7 summarizes the DNA methylation status of each CpG-dinucleotide from analysis of all five cell lines. Analysis of the mutated Δ2B and Δ3B cells in both the promoter region and intron 3 revealed that, with the exception of the far upstream region examined, the DNA methylation pattern was the same as that seen for the active HPRT allele in both HT-1080 and 4.12 cells (Fig. 7A). The five CpG sites farthest upstream (at positions −447, −428, −426, −412, and −404) did show some degree of hypomethylation in the mutant Δ2B and Δ3B cells relative to HT-1080 cells, while three other upstream sites (at positions −386, −373, and −371) showed a slight degree of hypermethylation only in the Δ2B cells relative to that in HT-1080 cells. However, neither the Δ2B nor the Δ3B cells exhibited the same degree of hypermethylation seen for the inactive HPRT allele in the 8121 cells. Surprisingly, our analysis also revealed that the CpGs in intron 3 previously reported as a region of differential methylation by Southern blot analysis that used methyl-sensitive enzymes (48) were fully methylated on the active allele and were methylated at all but one site on the inactive allele (Fig. 7B).
FIG. 7.
High-resolution DNA methylation analysis of CpG-dinucleotides in the human HPRT promoter and intron 3. The methylation status of individual CpG-dinucleotides on the upper strand was determined by sodium bisulfite genomic sequencing. Each CpG-dinucleotide was sequenced a minimum of seven times after chemical conversion with bisulfite. (A) Methylation pattern of a 698-bp region in the HPRT promoter region. (B) Methylation pattern of eight CpGs within intron 3 of the HPRT gene. All position numbers are relative to the translation initiation site. Closed circles indicate CpG sites that are >80% methylated, three-quarter-filled circles indicate 60 to 80% methylation, half-filled circles indicate 40 to 60% methylation, one-quarter-filled circles indicate 20 to 40% methylation, and open circles indicate <20% methylation. Dashed lines indicate CpGs deleted in Δ2B and Δ3B.
To determine if the observed lack of de novo methylation in the 5′ CpG island of the mutated Δ2B and Δ3B cells could be due simply to the absence of the de novo DNA methyltransferases (DNMTs) in these cells, we performed RT-PCR analysis on total RNA from both wild-type HT-1080 and mutant Δ3B cells by using primers specific for amplification of the human DNMT3A and DNMT3B mRNAs. Our results showed that transcripts from both methyltransferases were readily detectable in both the HT-1080 and Δ3B cells (data not shown), which is consistent with the presence of a low level of de novo methylation activity in our targeted cells, suggested by the weak hypermethylation observed in the Δ2B cells at positions −386, −373, and −371 relative to the parental HT-1080 cells (Fig. 7).
Thus, our results indicated that cis-acting elements within the 323-bp region deleted in the mutant cells are not required to maintain the hypomethylated state of what remains of the 5′ CpG island, nor is the hypermethylated state of CpG sites in intron 3 of the endogenous gene. These results are in contrast to those reported for the Aprt gene, where cis-acting elements within the immediate promoter region could prevent methylation of CpGs within and flanking a transgene (41).
DISCUSSION
Chromatin structure.
Two independent cell lines were constructed in which 323 bp of the active endogenous HPRT promoter region in cultured male fibrosarcoma cells was replaced with plasmid sequences. The promoter mutation deleted sequences that colocalize with the major DNase I-hypersensitive site in the 5′ region of the HPRT gene (7, 30). In mutated cells, the major hypersensitive site was abolished; however, a new weak hypersensitive site was detected just downstream of the targeted promoter mutation. It is unclear whether this weak hypersensitive site in targeted cells represents a novel site present in only the mutant cell lines or was present in wild-type HT-1080 cells but was masked by the major hypersensitive site. Nevertheless, these results indicate that cis-acting sequences within the deleted 323 bp of the promoter region are required to maintain the DNase I-hypersensitive nucleosomal array structure associated with the 5′ flanking region of the transcriptionally active HPRT allele (7). However, it is unclear if it is the transcription factor binding sites or other DNA sequences within the deleted region that are responsible for maintaining DNase I hypersensitivity of the promoter region. Several studies suggest that DNase I hypersensitivity of promoter regions can arise or be retained in the absence of persistent transcription factor binding to the promoter (10, 25, 28, 31, 33). For example, mutation of transcription factor binding sites in the yeast hsp82 promoter in vivo did not abolish DNase I hypersensitivity in the 5′flanking region (28). Likewise, DNase I hypersensitivity was retained at the human HSP70 promoter region in chromatin from mitotic chromosomes, though transcription factor binding was not detectable (33). Our results suggest that cis-acting elements within the 323-bp interval deleted in Δ2B and Δ3B cells are nonetheless required for maintaining a hypersensitive promoter region, perhaps by transient interaction of factors (or complexes) with these elements. This would be consistent with a study demonstrating that, in mitotic chromosomes, where transcription is globally repressed and where double-stranded DNA-binding proteins (i.e., transcription factors) are released, chromatin distortion was still detectable on promoters that were to be reactivated after mitosis but not on those that would remain inactive (34). Furthermore, studies by Kontaraki et al. suggest that formation of an active chromatin pattern in the chicken lysozyme 5′ regulatory region during development was detectable prior to stable binding of end stage transactivators and active transcription (25). Thus, both establishment and maintenance of a DNase I-hypersensitive chromatin structure in the 5′ flanking region of active genes may involve cis-acting sequences that are not stably bound by transcriptional activators.
Analysis of general DNase I sensitivity of several loci, including the lysozyme (23), HPRT (30), apolipoprotein B (29), and β-globin (20) genes, has typically shown an approximately two- to threefold-greater sensitivity of an active locus than for the corresponding inactive locus or to bulk chromatin. Formation of this more open and accessible higher-order chromatin structure of active loci has been postulated to involve progression of the transcription elongation complex through active genes, leading to disruption of higher-order chromatin structure and increased general DNase I sensitivity (5, 27). However, our analysis of general DNase I sensitivity in the targeted Δ2B and Δ3B cells demonstrated that, even in the absence of detectable transcription in the HPRT locus (Fig. 3C), the gene remained in a DNase I-sensitive and open chromatin configuration. These results suggest that maintenance of a general DNase I-sensitive chromatin structure along the entire HPRT gene does not require either active gene transcription or cis-acting elements in the minimal promoter region (i.e., within the 323-bp region deleted in Δ2B and Δ3B cells). These results are consistent with a report published on the HPRT gene (31) and a study performed on a testis-specific chromatin domain by Kramer et al. (26) showing that chromatin remodeling to an open conformation occurs prior to gene transcription.
Moreover, our data suggest that it is unlikely that chromatin-remodeling complexes or histone modification enzymes (e.g., histone acetylases) are recruited by promoter-bound transcription factors to maintain an active chromatin conformation of a locus in vivo. However, because our targeted mutation replaced the promoter region with plasmid sequences, we cannot formally eliminate the possibility that these exogenous sequences may somehow contribute to the general DNase I sensitivity observed in the mutated cells, although this is unlikely.
If cis-acting elements are involved in maintaining general DNase I sensitivity of the HPRT gene on the active X chromosome, these elements must be located in a regulatory region(s) outside the immediate promoter (i.e., the 323-bp region deleted in Δ2B and Δ3B cells). However, analysis of DNase I hypersensitivity of the active HPRT locus has revealed no hypersensitive sites (suggestive of regulatory regions) other than the one associated with the promoter region (30). Thus, since the immediate promoter region and/or active transcription of the HPRT locus is not necessary to maintain general DNase I sensitivity, then the nature of the elements and mechanisms that perform this function remains unclear.
These results are similar to those from functional studies investigating the role of the β-globin locus control region (LCR) in establishing and maintaining an open chromatin structure across the active β-globin locus in erythroid cells (1, 11, 38). The role of the LCR in opening chromatin of the human β-globin locus in erythroid cells was suggested by analysis of a Hispanic patient carrying a large deletion that included hypersensitive sites 2 thru 5 of the LCR (and ∼20 kb of additional upstream sequences); this patient exhibited general DNase I resistance throughout the mutated β-globin locus in erythroid cells (13). However, targeted deletion of the endogenous mouse β-globin LCR in embryonic stem cells resulted in retention of general DNase I sensitivity across the β-globin locus in erythroid cells from mice carrying the LCR deletion (11, 38). Furthermore, deletions of the LCR from the human β-globin locus in a mouse erythroid cell background resulted in loss of human β-globin gene transcription (i.e., no signal was detected from either strand by using radiolabeled RT-PCR) but also resulted in preservation of general DNase I sensitivity at the human β-globin locus (1). Taken together, these functional studies of the mouse and human β-globin LCR indicate that the LCR alone is unlikely to establish and/or maintain an open chromatin structure across the locus. Thus, it remains unclear what, if any, cis-acting elements function to establish and maintain an open chromatin conformation (i.e., general DNase I sensitivity) in active loci such as the β-globin locus in erythroid cells and the HPRT locus on the active X chromosome. Recent studies of the β-globin locus suggest the involvement of low-level intergenic transcription as well as transcription upstream and within the LCR in the opening of chromatin structure across the locus (28a). However, the lack of detectable transcription from either strand of the HPRT gene by RT-PCR in our promoter-less mutants (Fig. 3C) indicates that this is unlikely to be a mechanism for maintaining general nuclease sensitivity of the HPRT gene in the absence of a functional promoter.
Accumulating evidence suggests a possible scenario for maintaining a general DNase I-sensitive chromatin structure in the absence of a promoter or active transcription. After an open and potentiated chromatin structure of a locus is established during development, it is maintained throughout subsequent cell divisions by heritable epigenetic mechanisms that do not require specific cis-acting elements or transcription within the locus. This notion is consistent with recent reports that suggest mechanisms by which active and inactive states of chromatin structure may be perpetuated and inherited (reviewed in references 39 and 45). In particular, Hassan et al. have shown that specific bromodomains of the chromatin-modifying SAGA and SWI/SNF complexes can anchor these complexes to acetylated nucleosomes, even in the absence of DNA-bound transcriptional activators, thereby creating a potential mechanism for maintaining a transcriptionally active epigenetic state (19). In addition, recent studies suggest a chromatin-based mechanism for maintaining epigenetic states that involves temporal compartmentalization of transcriptionally active and inactive genes during DNA replication (i.e., early and late replication of genes) and the subsequent reassembly of chromatin structures that reflect replication timing (49). These mechanisms for perpetuating epigenetics states could also confer constitutive transcriptional competence on housekeeping genes.
DNA methylation.
Studies of the Aprt gene suggest that Sp1 binding sites are necessary but are not sufficient to protect CpG sites in transgenes from methylation (3, 41). A 120-bp fragment from the Aprt promoter region, including two Sp1 binding sites, appeared to protect CpGs in a transgene from methylation (41) in mouse tissues. However, in similar studies, a 62-bp fragment from the human HPRT promoter containing five Sp1 binding sites was not sufficient to prevent methylation of CpGs in a transgene in mice, suggesting that sequences other than Sp1 sites within the 120-bp fragment of the Arpt promoter region are also involved in preventing DNA methylation of the transgene (41). Similarly, it is conceivable that other sequences in the HPRT promoter region outside this 62-bp fragment may play a role in protecting CpGs from DNA methylation.
Our removal of a 323-bp fragment from the endogenous human HPRT promoter (which included the five Sp1 binding sites) in cultured somatic cells showed little effect on hypomethylation of the 5′ CpG island on the active allele. This might be most simply explained by the lack of significant de novo methylase activity in the HT-1080 cells so that CpG islands no longer require Sp1 binding sites to protect from de novo methylation and maintain a methylation-free region. However, RT-PCR analysis of our mutant cell lines detected transcripts from genes encoding the de novo methylases DNMT3A and DNMT3B (data not shown). Furthermore, the presence of a low level of de novo methylation in our targeted cells is suggested by the weak hypermethylation observed in the Δ2B cells at positions −386, −373, and −371 relative to that in the parental HT-1080 cells (Fig. 7). Therefore, even in the probable presence of de novo methylase activity, the promoter-less mutant Δ2B and Δ3B cells maintained the methylation-free state of the 5′ CpG island that was established during embryogenesis. This might suggest that other as-yet-unidentified cis-acting elements outside the 323-bp promoter fragment may be involved in maintaining a methylation-free CpG island in the HPRT locus. Our studies also demonstrated that promoter elements also are not required to maintain a hypermethylated state in a region within the body of the HPRT gene (i.e., within intron 3) that is not a CpG island.
Thus, the 323-bp fragment removed from the HPRT promoter region does not appear to be involved in maintaining DNA methylation patterns on the active HPRT allele in somatic cells. However, because our deletion of the promoter fragment occurred in somatic HT-1080 cells after the DNA methylation pattern on the active allele had already been established in early development, it is still possible that cis-acting elements within the 323-bp promoter fragment may be required for establishing DNA methylation patterns in the endogenous HPRT gene (e.g., protecting the 5′ CpG island from methylation) during embryogenesis.
In summary, our functional analysis of the HPRT gene on the active X chromosome in HT-1080 fibrosarcoma cells suggests that, once a transcriptionally potentiated epigenetic state of the gene is established during development, the promoter region (and active transcription) plays a relatively limited role in maintaining this epigenotype.
Acknowledgments
We thank Camilynn Brannan and Jörg Bungert for helpful advice and plasmids, and Fai Siu for probes.
This work was supported by NIH grant RO1 GM44286 to T.P.Y.
REFERENCES
- 1.Bender, M. A., M. Bulger, J. Close, and M. Groudine. 2000. Beta-globin gene switching and DNase I sensitivity of the endogenous beta-globin locus in mice do not require the locus control region. Mol. Cell 5:387-393. [DOI] [PubMed] [Google Scholar]
- 2.Boyes, J., and G. Felsenfeld. 1996. Tissue-specific factors additively increase the probability of the all-or-none formation of a hypersensitive site. EMBO J. 15:2496-2507. [PMC free article] [PubMed] [Google Scholar]
- 3.Brandeis, M., D. Frank, I. Keshet, Z. Siegfried, M. Mendelsohn, A. Nemes, V. Temper, A. Razin, and H. Cedar. 1994. Sp1 elements protect a CpG island from de novo methylation. Nature 371:435-438. [DOI] [PubMed] [Google Scholar]
- 4.Brown, C. J., A. Ballabio, J. L. Rupert, R. G. Lafreniere, M. Grompe, R. Tonlorenzi, and H. F. Willard. 1991. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature 349:38-44. [DOI] [PubMed] [Google Scholar]
- 5.Brown, S. A., and R. E. Kingston. 1997. Disruption of downstream chromatin directed by a transcriptional activator. Genes Dev. 11:3116-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, C., M. C. Yang, and T. P. Yang. 2001. Evidence that silencing of the HPRT promoter by DNA methylation is mediated by critical CpG sites. J. Biol. Chem. 276:320-328. [DOI] [PubMed] [Google Scholar]
- 7.Chen, C., and T. P. Yang. 2001. Nucleosomes are translationally positioned on the active allele and rotationally positioned on the inactive allele of the HPRT promoter. Mol. Cell. Biol. 21:7682-7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 9.Clark, S. J., J. Harrison, C. L. Paul, and M. Frommer. 1994. High sensitivity mapping of methylated cytosines. Nucleic Acids Res. 22:2990-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costlow, N. A., J. A. Simon, and J. T. Lis. 1985. A hypersensitive site in hsp70 chromatin requires adjacent not internal DNA sequence. Nature 313:147-149. [DOI] [PubMed] [Google Scholar]
- 11.Epner, E., A. Reik, D. Cimbora, A. Telling, M. A. Bender, S. Fiering, T. Enver, D. I. Martin, M. Kennedy, G. Keller, and M. Groudine. 1998. The beta-globin LCR is not necessary for an open chromatin structure or developmentally regulated transcription of the native mouse beta-globin locus. Mol. Cell 2:447-455. [DOI] [PubMed] [Google Scholar]
- 12.Felsenfeld, G. 1992. Chromatin as an essential part of the transcriptional mechanism. Nature 355:219-224. [DOI] [PubMed] [Google Scholar]
- 13.Forrester, W. C., E. Epner, M. C. Driscoll, T. Enver, M. Brice, T. Papayannopoulou, and M. Groudine. 1990. A deletion of the human beta-globin locus activation region causes a major alteration in chromatin structure and replication across the entire beta-globin locus. Genes Dev. 4:1637-1649. [DOI] [PubMed] [Google Scholar]
- 14.Goodfellow, P., B. Pym, T. Mohandas, and L. J. Shapiro. 1984. The cell surface antigen locus, MIC2X, escapes X-inactivation. Am. J. Hum. Genet. 36:777-782. [PMC free article] [PubMed] [Google Scholar]
- 15.Goto, T., and M. Monk. 1998. Regulation of X-chromosome inactivation in development in mice and humans. Microbiol. Mol. Biol. Rev. 62:362-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gross, D. S., C. C. Adams, S. Lee, and B. Stentz. 1993. A critical role for heat shock transcription factor in establishing a nucleosome-free region over the TATA-initiation site of the yeast HSP82 heat shock gene. EMBO J. 12:3931-3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gross, D. S., and W. T. Garrard. 1988. Nuclease hypersensitive sites in chromatin. Annu. Rev. Biochem. 57:159-197. [DOI] [PubMed] [Google Scholar]
- 18.Gu, H., J. D. Marth, P. C. Orban, H. Mossmann, and K. Rajewsky. 1994. Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting. Science 265:103-106. [DOI] [PubMed] [Google Scholar]
- 19.Hassan, A. H., P. Prochasson, K. E. Neely, S. C. Galasinski, M. Chandy, M. J. Carrozza, and J. L. Workman. 2002. Function and selectivity of bromodomains in anchoring chromatin-modifying complexes to promoter nucleosomes. Cell 111:369-379. [DOI] [PubMed] [Google Scholar]
- 20.Hebbes, T. R., A. L. Clayton, A. W. Thorne, and C. Crane-Robinson. 1994. Core histone hyperacetylation co-maps with generalized DNase I sensitivity in the chicken beta-globin chromosomal domain. EMBO J. 13:1823-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hornstra, I. K., and T. P. Yang. 1994. High-resolution methylation analysis of the human hypoxanthine phosphoribosyltransferase gene 5′ region on the active and inactive X chromosomes: correlation with binding sites for transcription factors. Mol. Cell. Biol. 14:1419-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hornstra, I. K., and T. P. Yang. 1992. Multiple in vivo footprints are specific to the active allele of the X-linked human hypoxanthine phosphoribosyltransferase gene 5′ region: implications for X chromosome inactivation. Mol. Cell. Biol. 12:5345-5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jantzen, K., H. P. Fritton, and T. Igo-Kemenes. 1986. The DNase I sensitive domain of the chicken lysozyme gene spans 24 kb. Nucleic Acids Res. 14:6085-6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim, S. H., J. C. Moores, D. David, J. G. Respess, D. J. Jolly, and T. Friedmann. 1986. The organization of the human HPRT gene. Nucleic Acids Res. 14:3103-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kontaraki, J., H. H. Chen, A. Riggs, and C. Bonifer. 2000. Chromatin fine structure profiles for a developmentally regulated gene: reorganization of the lysozyme locus before trans-activator binding and gene expression. Genes Dev. 14:2106-2122. [PMC free article] [PubMed] [Google Scholar]
- 26.Kramer, J. A., J. R. McCarrey, D. Djakiew, and S. A. Krawetz. 1998. Differentiation: the selective potentiation of chromatin domains. Development 125:4749-4755. [DOI] [PubMed] [Google Scholar]
- 27.Lee, M. S., and W. T. Garrard. 1991. Positive DNA supercoiling generates a chromatin conformation characteristic of highly active genes. Proc. Natl. Acad. Sci. USA 88:9675-9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee, M. S., and W. T. Garrard. 1992. Uncoupling gene activity from chromatin structure: promoter mutations can inactivate transcription of the yeast HSP82 gene without eliminating nucleosome-free regions. Proc. Natl. Acad. Sci. USA 89:9166-9170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28a.Levings, P. P., and J. Bungert. 2002. The human beta-globin locus control region. Eur. J. Biochem. 269:1589-1599. [DOI] [PubMed] [Google Scholar]
- 29.Levy-Wilson, B., and C. Fortier. 1989. The limits of the DNase I-sensitive domain of the human apolipoprotein B gene coincide with the locations of chromosomal anchorage loops and define the 5′ and 3′ boundaries of the gene. J. Biol. Chem. 264:21196-21204. [PubMed] [Google Scholar]
- 30.Lin, D., and A. C. Chinault. 1988. Comparative study of DNase I sensitivity at the X-linked human HPRT locus. Somat. Cell Mol. Genet. 14:261-272. [DOI] [PubMed] [Google Scholar]
- 31.Litt, M. D., R. S. Hansen, I. K. Hornstra, S. M. Gartler, and T. P. Yang. 1997. 5-Azadeoxycytidine-induced chromatin remodeling of the inactive X-linked HPRT gene promoter occurs prior to transcription factor binding and gene reactivation. J. Biol. Chem. 272:14921-14926. [DOI] [PubMed] [Google Scholar]
- 32.Macleod, D., J. Charlton, J. Mullins, and A. P. Bird. 1994. Sp1 sites in the mouse aprt gene promoter are required to prevent methylation of the CpG island. Genes Dev. 8:2282-2292. [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Balbas, M. A., A. Dey, S. K. Rabindran, K. Ozato, and C. Wu. 1995. Displacement of sequence-specific transcription factors from mitotic chromatin. Cell 83:29-38. [DOI] [PubMed] [Google Scholar]
- 34.Michelotti, E. F., S. Sanford, and D. Levens. 1997. Marking of active genes on mitotic chromosomes. Nature 388:895-899. [DOI] [PubMed] [Google Scholar]
- 35.Mummaneni, P., P. Yates, J. Simpson, J. Rose, and M. S. Turker. 1998. The primary function of a redundant Sp1 binding site in the mouse aprt gene promoter is to block epigenetic gene inactivation. Nucleic Acids Res. 26:5163-5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel, P. I., P. E. Framson, C. T. Caskey, and A. C. Chinault. 1986. Fine structure of the human hypoxanthine phosphoribosyltransferase gene. Mol. Cell. Biol. 6:393-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quandt, K., K. Frech, H. Karas, E. Wingender, and T. Werner. 1995. MatInd and MatInspector: new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res. 23:4878-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reik, A., A. Telling, G. Zitnik, D. Cimbora, E. Epner, and M. Groudine. 1998. The locus control region is necessary for gene expression in the human β-globin locus but not the maintenance of an open chromatin structure in erythroid cells. Mol. Cell. Biol. 18:5992-6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richards, E. J., and S. C. Elgin. 2002. Epigenetic codes for heterochromatin formation and silencing: rounding up the usual suspects. Cell 108:489-500. [DOI] [PubMed] [Google Scholar]
- 40.Rincon Limas, D. E., D. A. Krueger, and P. I. Patel. 1991. Functional characterization of the human hypoxanthine phosphoribosyltransferase gene promoter: evidence for a negative regulatory element. Mol. Cell. Biol. 11:4157-4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siegfried, Z., S. Eden, M. Mendelsohn, X. Feng, B. Z. Tsuberi, and H. Cedar. 1999. DNA methylation represses transcription in vivo. Nat. Genet. 22:203-206. [DOI] [PubMed] [Google Scholar]
- 42.Soutoglou, E., and I. Talianidis. 2002. Coordination of PIC assembly and chromatin remodeling during differentiation-induced gene activation. Science 295:1901-1904. [DOI] [PubMed] [Google Scholar]
- 43.Stalder, J., A. Larsen, J. D. Engel, M. Dolan, M. Groudine, and H. Weintraub. 1980. Tissue-specific DNA cleavages in the globin chromatin domain introduced by DNAase I. Cell 20:451-460. [DOI] [PubMed] [Google Scholar]
- 44.Stout, J. T., and C. T. Caskey. 1985. HPRT: gene structure, expression, and mutation. Annu. Rev. Genet. 19:127-148. [DOI] [PubMed] [Google Scholar]
- 45.Turner, B. M. 2002. Cellular memory and the histone code. Cell 111:285-291. [DOI] [PubMed] [Google Scholar]
- 46.Weintraub, H., and M. Groudine. 1976. Chromosomal subunits in active genes have an altered conformation. Science 193:848-856. [DOI] [PubMed] [Google Scholar]
- 47.Wheeler, J. C., C. VanderZwan, X. Xu, D. Swantek, W. D. Tracey, and J. P. Gergen. 2002. Distinct in vivo requirements for establishment versus maintenance of transcriptional repression. Nat. Genet. 32:206-210. [DOI] [PubMed] [Google Scholar]
- 48.Wolf, S. F., D. J. Jolly, K. D. Lunnen, T. Friedmann, and B. R. Migeon. 1984. Methylation of the hypoxanthine phosphoribosyltransferase locus on the human X chromosome: implications for X-chromosome inactivation. Proc. Natl. Acad. Sci. USA 81:2806-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang, J., F. Xu, T. Hashimshony, I. Keshet, and H. Cedar. 2002. Establishment of transcriptional competence in early and late S phase. Nature 420:198-202. [DOI] [PubMed] [Google Scholar]