Abstract
Mouse CD33/Siglec-3 (mCD33) is the apparent ortholog of human CD33/Siglec-3 (hCD33), a member of the Siglec (sialic acid-binding Ig superfamily lectin) family of sialic acid-recognizing cell-surface lectins. We examined the binding specificity and expression pattern of mCD33 and explored its functions by generating mice deficient in this molecule. Like hCD33, mCD33 is expressed on myeloid precursors in the bone marrow, albeit mostly in the more mature stages of the granulocytic lineage. Moreover, unlike hCD33, mCD33 in peripheral blood is primarily expressed on granulocytes. Also, unlike hCD33, mCD33 did not bind to α2-3- or α2-6-linked sialic acids on lactosamine units. Instead, it showed distinctive sialic acid-dependent binding only to the short O-linked glycans of certain mucins and weak binding to the sialyl-Tn epitope. Binding was enhanced by removal of 9-O-acetyl groups and attenuated by truncation of the glycerol-like side chain of sialic acids. Mice deficient in CD33 were viable and fertile in a controlled-access specific-pathogen-free vivarium, showed no major morphological or histological abnormalities, had no changes in bone marrow or peripheral leukocyte subpopulations, and had very minor differences in biochemical and erythrocyte parameters. Cellular responses to intraperitoneally injected proinflammatory stimulants, as well as subsequent interleukin-6 secretion, were also apparently unaffected. These results indicate substantial species differences in CD33 expression patterns and ligand recognition and suggest functional degeneracy between mCD33 and the other CD33-related Siglec proteins expressed on cells of the myeloid lineage.
Human CD33 (hCD33) is an immunoglobulin (Ig) superfamily protein expressed on myeloid progenitor cells in the bone marrow and on peripheral blood monocytes. It is known to recognize α2-3- and α2-6-linked sialic acids (7, 18), which are expressed mostly at the nonreducing termini (outermost positions) of glycan chains (5, 38). Human CD33 (also known as hSiglec-3) is a member of the Siglec (sialic acid-binding Ig superfamily lectin) family of molecules defined by their mutual sequence similarity in the first two Ig-like domains and by their ability to recognize sialic acids (1, 9, 10; P. R. Crocker, E. A. Clark, M. Filbin, S. Gordon, Y. Jones, J. H. Kehrl, S. Kelm, D. N. Le, L. Powell, J. Roder, R. L. Schnaar, D. C. Sgroi, K. Stamenkovic, R. Schauer, M. Schachner, T. K. Van den Berg, P. A. Van der Merwe, S. M. Watt, and A. Varki, Letter, Glycobiology 8:v, 1998). A cDNA for the putative mouse ortholog of hCD33 was cloned many years ago (33). Similarities within the extracellular domain between hCD33 and mouse CD33 (mCD33; 62% identity in amino acid sequence) (33) and similar gene structure and chromosomal position relative to adjacent genes (2) warrant its designation as the mouse ortholog of hCD33. However, the lack of sequence similarity in the cytosolic domains and difficulties in resolving phylogenetic relationships among the related Siglec molecules in humans and mice have raised questions about its functional equivalence to hCD33 (2, 4).
A subset of Siglec proteins showing relatively high sequence similarity to CD33 are called “CD33/Siglec-3-related Siglecs” (1, 9, 10). These include CD33/Siglec-3, Siglec-5, Siglec-6/OB-BP1, Siglec-7/AIRM1, Siglec-8, Siglec-9, Siglec-10, and Siglec-11, and a Siglec-like molecule (Siglec-L1) in humans, as well as five confirmed or putative Siglec proteins in mice (CD33, Siglec-E, Siglec-F, Siglec-G, and Siglec-H) (2). Many CD33-related Siglecs are expressed on cells involved in innate immunity. For example, hSiglec-7/AIRM-1 is expressed on natural killer cells and monocytes (14, 26); hSiglec-8 is expressed on eosinophils (17), and mSiglec-F is expressed on immature cells of myeloid lineage (2). The cytosolic tails of most CD33-related Siglecs have two conserved tyrosine-based putative signaling motifs, one of which conforms to the immunoreceptor tyrosine-based inhibitory motif (ITIM), while the other is a putative motif of unknown function (1, 9, 10). It has been shown in some CD33-related Siglecs that the ITIM is the preferred docking site for the protein tyrosine phosphatase SHP-1 (27, 32, 36, 41), and cross-linking of these Siglecs elicits negative signaling in the cells expressing these molecules (15, 27, 36, 39, 40). Although the expression pattern of CD33-related Siglecs and their function as signaling molecules suggest their involvement in the regulation of innate immunity, their in vivo function in a model organism has not been studied so far.
Although mCD33 was cloned nearly a decade ago (33), its protein expression pattern and ligand-binding properties have not yet been reported. In this study, we produced recombinant mCD33 and analyzed its binding properties. We also raised a monospecific antibody against mCD33 and studied its expression pattern in the intact mouse. Finally, we generated mice deficient in CD33, and analyzed CD33's possible involvement in innate immunity. We report the initial phenotypic characterization of the CD33-deficient mice and discuss possible species differences and functional degeneracy between CD33 and other Siglecs.
MATERIALS AND METHODS
Materials.
All antibodies were obtained from BD Biosciences Pharmingen, San Diego, Calif., unless otherwise stated. Ovine and porcine submaxillary mucins (OSM and PSM, respectively) were purchased from Accurate Chemical and Scientific (Westbury, N.Y.). Bovine submaxillary mucin (BSM) was prepared as described previously (24).
Production of recombinant mCD33-Fc fusion protein.
The coding region for the extracellular domain of mCD33 was amplified by PCR and cloned into an expression vector, which upon mammalian cell transfection produces mCD33-Fc, a recombinant fusion protein of the mCD33 extracellular domain and human IgG Fc fragment, with an enterokinase recognition sequence/FLAG tag (DYKDDDDK) in between (3, 4). CHO cells stably transfected with this vector were grown in Opti-MEM (Invitrogen) supplemented with 1.5% low-IgG fetal bovine serum (HyClone, Logan, Utah) for 5 to 7 days. The culture supernatant was collected, and the fusion protein was purified on protein A-Sepharose as described previously (3, 4). The recombinant mCD33-Fc protein was treated with Arthrobacter ureafaciens sialidase (AUS) while bound to protein A-Sepharose (7) to eliminate possible masking of the binding site by sialylated glycans attached to the same molecule.
Analysis of the sialic acid-binding properties of mCD33 with various cells.
The full-length coding region of mCD33 (33) was amplified by reverse transcription-PCR (RT-PCR) with mouse bone marrow RNA as a template and cloned into pcDNA3.1(+). This construct was transiently transfected into COS-7 cells with Lipofectamine reagent (Invitrogen), and an erythrocyte rosetting assay with human erythrocytes was performed with these transfected cells as described previously (3, 4).
Mouse peripheral blood lymphocytes, bone marrow cells, and splenocytes were incubated with mCD33-Fc (5 μg/ml; AUS treated) that had been precomplexed (for 15 min at 4°C) with phycoerythrin-conjugated goat F(ab′)2 anti-human IgG (1:100 diluted; Caltag). Binding was analyzed by flow cytometry with FACScan (BD Biosciences Immunocytometry Systems). The same analysis was performed in parallel with AUS-treated hCD33-Fc.
Analysis of the sialic acid-binding properties of mCD33 by ELISA.
The sialic acid-binding properties of the mCD33-Fc were analyzed by enzyme-linked immunosorbent assay (ELISA) as described earlier (7). In brief, a 96-well plate (Nunc) was coated with protein A (500 ng per well), and after blocking with ELISA buffer (20 mM HEPES, 1% bovine serum albumin [BSA], 125 mM NaCl [pH 7.45]), the wells were incubated with mCD33-Fc (500 ng per well). Subsequently, biotin-conjugated polyacrylamide probes substituted with Neu5Acα2-6Galβ1-4Glc, Neu5Acα2-3Galβ1-4Glc, Neu5Acα2-6GalNAcα(sialyl-Tn), or GalNAcα (Glycotech; 500 ng/well) or biotin-conjugated mucins (OSM, PSM, or BSM; 500 to 1,000 ng per well) were added, followed by incubation with alkaline-phosphatase-conjugated streptavidin (Invitrogen; 1:500 diluted, 100 μl per well). For development, p-nitrophenyl phosphate was used, and plates were read at 405 nm.
Mild periodate treatment of the sialylated probes.
To specifically truncate the glycerol side chain of sialic acid, essential for sialic acid binding by most Siglecs (1), probes were treated mildly with periodate as described previously (7). Briefly, they were treated with 2 mM NaIO4 in phosphate-buffered saline (PBS) for 30 min on ice in the dark, and the aldehydes were reduced with 10 mM NaBH4 in PBS for 30 min on ice in the dark. Reaction mixtures were then diluted 1:5 with ELISA buffer and used directly in assays. As a control, 2 mM NaIO4 and 10 mM NaBH4 were incubated for an hour on ice and then diluted with ELISA buffer, and the probes were added to this mixture just before use in assays.
Biotinylation of the mucins.
OSM, PSM, and BSM were biotinylated as described previously (7). Briefly, 90 μg of EZ-Link Sulfo-NHS-biotin (Pierce) was added to 1 mg of mucin in 0.1 M NaHCO3 (pH 8.3), and this mixture was incubated for 2 h at room temperature. This mixture was extensively dialyzed against PBS at 4°C.
Removal of O-acetyl esters from BSM.
BSM was incubated with 0.1 M NaOH for 30 min at room temperature to remove base-labile O-acetyl esters (11). Subsequently this mixture was neutralized with HCl and extensively dialyzed against PBS at 4°C. Influenza C virus hemagglutinin-esterase fused with the Fc part of human-IgG (CHE-Fc) was produced and purified as described previously (22). This 9-O-acetyl sialic acid-specific esterase was also used to remove 9-O-acetyl esters from the sialic acids of BSM. Ten micrograms of BSM was incubated with CHE-Fc (11.1 μg) in 300 μl of PBS for 1 h at 37°C. To remove the CHE-Fc, 35 μl of protein A-Sepharose was added to the mixture, which was then incubated at room temperature for 1 h. The protein A-Sepharose was removed, and 700 μl of ELISA buffer was added to the mixture, of which 100 μl per well was used in the subsequent ELISA. As a control, the CHE-Fc was boiled for 15 min and then incubated with BSM as described above. To confirm the effects of CHE-Fc treatment, binding was analyzed in the same ELISA using CHE-FcD (CHE-FcD is CHE-Fc treated with diisopropylfluorophosphate that inactivates the esterase activity, resulting in a probe that specifically binds to 9-O-acetylated sialic acids) and human CD22-Fc (binding is blocked by 9-O-acetylation of sialic acids) (20-22, 30).
Animal care.
All animals used were maintained in an access-controlled barrier facility under specific-pathogen-free conditions. Studies were performed in accordance with Public Health Service guidelines and approved by the Animal Subjects Committee of the University of California, San Diego.
Production of mCD33-specific polyclonal antibody.
Mice deficient in CD33, generated as described below, were immunized with 50 μg of mCD33 extracellular domain (prepared by enterokinase digestion of mCD33-Fc recombinant fusion protein and removal of the Fc portion by adsorption to protein A-Sepharose) in 400 μl of a 1:1 emulsion of PBS and Titermax Gold (Titermax USA, Norcross, Ga.) by intraperitoneal (i.p.) injection, followed by booster adminisration of 50 μg of mCD33 extracellular domain in 400 μl of 1:1 PBS-Titermax Gold emulsion 14 days later. After another 14 days, mice were given further boosts by i.p. injections of 10 to 20 μg of mCD33 extracellular domain in sterile PBS (200 μl) every week. Blood (∼200 μl per animal) was collected from the tail vein every other week, and serum was prepared by a standard protocol. Monospecific polyclonal antibody was prepared from the collected serum by affinity purification. In brief, hSiglec-9-Fc (0.5 mg) and mCD33-Fc (0.5 mg) were separately coupled to 0.5 ml of AffiGel-10 (Bio-Rad) according to the manufacturer's instructions. Serum was first applied to a column of hSiglec-9-conjugated resin (0.25 ml) to eliminate antibodies that reacted with the enterokinase/FLAG tag or the human IgG Fc portion of the recombinant protein. The flowthrough from this column was applied to a column of mCD33-conjugated resin (0.25 ml), which was washed well, and the adsorbed antibodies were eluted with 0.1 M glycine-HCl buffer (pH 3.0) immediately followed by neutralization with 1/10 volume of 1 M Tris-HCl (pH 8.0). The affinity-purified antibody was concentrated, and the buffer was exchanged to PBS with an ultrafiltration device (UltraFree 15; molecular weight cutoff, 30,000; Millipore). A portion of this affinity-purified antibody was labeled with biotin by using EZ-Link Sulfo-NHS-biotin (Pierce) at an approximate antibody/biotin molar ratio of 1:100 according to the manufacturer's protocol. The specificity of the antibody preparation was confirmed by an ELISA in which mCD33-Fc, mSiglec-F-Fc, and mSiglec-E-Fc (a kind gift from P. Crocker, University of Dundee) were used as test samples (data not shown).
Analysis of mCD33 expression on mouse hematopoietic cells by flow cytometry.
Single-cell suspensions were prepared from thymus, spleen, bone marrow (from femurs), lymph nodes, and blood collected from anesthetized mice. Cells (106 cells per sample) were first incubated with 0.5 μg of mouse Fc-Block (anti-CD16/CD32 monoclonal antibody) and then with 1 μg of biotinylated anti-mCD33 antibody in 100 μl of 1% BSA-PBS at 4°C for 30 min, washed, and then incubated with phycoerythrin-conjugated streptavidin (1:100 dilution; Jackson ImmunoResearch) at 4°C for 15 min. The cells were washed again and suspended in 1 ml of 1% BSA-PBS, and analyzed with a FACSCalibur (BD Biosciences Immunocytometry Systems).
Sorting and morphological analysis of CD33-positive cells.
Single-cell suspensions from peripheral blood and bone marrow were stained for CD33 antigen as described above and sorted with FACSVantage (BD Biosciences Immunocytometry Systems). The CD33-positive cell population and unsorted population were immobilized onto glass slides with a Cytospin unit (Thermo Shandon, Pittsburgh, Pa.). The cells were stained with Giemsa reagent and analyzed for their morphology under oil-immersion microscopy.
Generation of mice deficient in CD33.
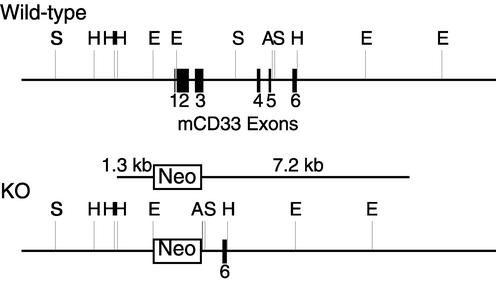
A genomic DNA fragment containing the mCD33 gene was obtained by screening a phage library of 129/SvJ mouse genomic DNA (Invitrogen) with the cDNA for the first Ig-like domain used as a probe. A gene-targeting construct was prepared by replacing a 3.8-kb DNA segment containing exons 1 to 5 of the CD33 gene with a pPGK-neo-bpA cassette (see Fig. 3), with flanking 1.3- and 7.2-kb arms to mediate homologous recombination. The targeting construct was transfected to R1 mouse embryonic stem cells, and G418-resistant clones were screened for homologous recombinants by PCR and Southern blotting. One homologous recombinant clone was used in blastocyst injection, and the resulting chimeric mice were bred to C57BL/6 mice. All mice used for the phenotype analysis were intercross offspring of the heterozygotes backcrossed for at least six generations into a C57BL/6 background.
FIG. 3.
Targeted deletion of the mCD33 gene. A CD33 gene-targeting construct was prepared by replacing the 3.8-kb DNA fragment containing exons 1 to 5 of the CD33 gene with the pPGK-neo-bpA cassette, with flanking 1.3- and 7.2-kb arms to mediate homologous recombination. A, ApaI; E, EcoRI; H, HindIII; S, SacI.
Hematology.
Blood from the tail vein of methoxyflurane-anesthetized mice (6 to 8 weeks old) was collected into EDTA-containing polypropylene microtubes (Becton Dickinson). Analyses of erythrocytes, leukocyte count, and platelet cell numbers and morphology were carried out with a Cell-Dyn 3500 automated analyzer (Abbott Diagnostics) or Hemavet 850 FS (CDC Technologies, Oxford, Conn.), both programmed for mouse blood parameters. Differential analysis was performed manually on smears of EDTA-anticoagulated blood stained with Wright's stain.
Serum chemistry.
Blood was collected in the absence of anticoagulants by tail bleed and allowed to clot for several hours at room temperature in plastic microtubes, and the serum was collected by centrifugation. Serum chemistry analyses were performed with a Beckman CX-7 automated chemistry analyzer with a general coefficient of variation of <5%.
Analysis of hematopoietic cell populations in blood and lymphoid organs.
Single-cell suspensions from thymus, spleen, lymph nodes, bone marrow, and blood were prepared from CD33-deficient mice and wild-type littermates (8 to 10 weeks old), erythrocytes were removed by lysis with ammonium chloride, and surviving cells were analyzed by flow cytometry for subpopulation distribution, using antibodies against various cell-surface markers appropriately labeled with fluorescent chromophore. The antibodies used were as follows: CD4, CD5, CD8, CD11b (Mac-1), CD21, CD22, CD23, CD24, CD25, CD28, CD40, CD43, CD44, CD62L (l-selectin), CD69, CD79b, B220, B7.2, I-Ab (major histocompatibility complex class II), surface IgM, surface IgD, Ly6G (Gr-1), NK1.1, and TER119. Appropriate combinations were employed for multicolor analysis.
Quantification of Igs.
Serum isotype-specific antibody titers were determined by sandwich ELISA with plates coated with anti-mouse isotype-specific antibodies. A standard curve was generated with purified mouse IgM, IgA (Sigma), IgG1, IgG2a, IgG2b, and IgG3 to convert optical density (OD) values to antibody concentration. Sera were diluted to 1/200 and analyzed with antimouse isotype-specific antibodies conjugated to alkaline phosphatase (IgM and IgA from Sigma; others from Pharmingen). OD at 405 nm (OD405) values were obtained with a microplate reader.
Casein-induced peritonitis.
Casein (0.2% wt/vol in PBS, 1 ml per mouse; Sigma) was i.p. injected into CD33-deficient mice and wild-type littermates (8 to 12 weeks old), and the cells accumulating in the peritoneal cavity were collected by lavage with 10 ml of cold PBS containing 1% BSA and 0.5 mM EDTA at 0, 2, 4, 6, or 24 h postinjection. Peritoneal cells were stained with antibodies against Ly6G (Gr-1) and F4/80 and analyzed by flow cytometry. In some experiments, peripheral blood was collected from the retroorbital plexus of anesthetized mice prior to euthanasia and peritoneal lavage, anticoagulated with EDTA, and subjected to differential cell counting, as described above. The interleukin-6 (IL-6) concentration in EDTA-anticoagulated plasma (1:100 diluted) was measured by sandwich ELISA with monoclonal antibodies MP5-20F3 (capture antibody) and biotinylated MP5-32C11 (detection antibody), according to the manufacturer's instructions (BD Biosciences Pharmingen). IL-6 was chosen because it is considered a more reliable marker of inflammation than tumor necrosis factor alpha or IL-1β (12) and is secreted at much higher level, enabling its accurate measurement in biological fluids available only in small amounts.
LPS-induced systemic inflammation.
Lipopolysaccharide (LPS; 2 μg/g of body weight, from Escherichia coli O55:B5 strain; Sigma) in 1 ml of PBS was i.p. injected into CD33-deficient mice and wild-type littermates (8 to 12 weeks old), and peripheral blood (∼100 μl) was collected from the tail vein or retroorbital plexus at 0, 3, 6, and 24 h postinjection. Differential cell counts were performed as described above. The IL-6 concentration in EDTA-anticoagulated plasma (1:100 diluted) was measured as described above. Bone marrow cells were collected from the right femur at 26 h postinjection and analyzed for the ratio of TER119+ (erythroid lineage) and CD11b+ cells (myeloid lineage).
RESULTS
Sialic acid-dependent binding by mCD33.
mCD33-Fc did not show binding to any of the mouse hematopoietic cells (peripheral blood lymphocytes, bone marrow cells, and splenocytes) tested. In contrast, considerable sialic acid-dependent binding was found for hCD33-Fc, and binding was sensitive to mild periodate treatment of the cells, which truncates sialic acid side chains (data not shown). Furthermore, in contrast with hCD33, no rosettes were found to form between human erythrocytes and COS-7 cells transfected with full-length mCD33 (data not shown). These results indicate that the ligand for mCD33 is different from that for hCD33.
The sialic acid-binding abilities of mCD33-Fc were studied by ELISA with various sialylated probes. Of the biotinylated polyacrylamide probes tested, sialic acid-dependent binding was only found for Neu5Acα2-6GalNAcα (sialyl-Tn). While the signal for this interaction was very low, it was sensitive to mild periodate treatment (data not shown). However, no sialic acid-dependent binding was found to OSM, a mucin containing sialyl-Tn with sialic acid exclusively in the N-acetyl form (Neu5Ac) (19, 23), and very weak sialic acid-dependent binding to PSM was found (data not shown). PSM is a mucin that contains sialyl-Tn and the structures Galβ1-3[Siaα2-6]GalNAc and Fucα1-2Galβ1-3[Siaα2-6]GalNAc (23, 37), with the sialic acid almost exclusively in the N-glycolylneuraminic acid (Neu5Gc) form. mCD33-Fc did bind to BSM in a partly sialic acid-dependent manner (Fig. 1). In addition to sialyl-Tn, BSM contains Galβ1-3[Neu5Ac/Gcα2-6]GalNAc and GlcNAcβ1-3[Neu5Ac/Gcα2-6]GalNAc structures (23, 28, 35). Furthermore, BSM contains ∼40% 9-O-acetylated sialic acids as determined by a chemical analysis (7). It is known that 9-O-acetylation of sialic acid reduces the binding to many Siglecs, probably by blocking the glycerol side chain of sialic acid (20, 21, 30, 31). This turns out to be the case for mCD33 as well, since binding was considerably increased after removal of 9-O-acetyl esters by either base treatment or CHE-Fc treatment of BSM (Fig. 1). As a control for the removal of 9-O-acetyl esters from BSM by CHE-Fc, binding to CD22/Siglec-2-Fc and CHE-FcD was analyzed in the same assay. This showed the expected considerable increase and decrease in binding, respectively (data not shown). The results with the polyacrylamide probes and mucins show that the sialylated ligand for mCD33 may be similar, but not identical, to the sialyl-Tn epitope, with a possible preference for the Neu5Gc form of sialic acid. In addition, the results with the mucins indicate that it is likely that the presentation of the sialylated ligand on its protein backbone or the adjacent or underlying sugars is important for recognition by mCD33.
FIG. 1.
Binding of mCD33-Fc to BSM: effect of 9-O-acetylation and side chain modification of sialic acid. Biotinylated BSM was treated with base or with CHE-Fc to remove 9-O-acetyl esters from its sialic acids. ELISA was used to assay the interaction between mCD33-Fc and BSM as described in Materials and Methods. In some instances, the BSM was also treated with mild periodate to truncate sialic acid side chains. The mean A405 values of triplicate wells are plotted for each sample. The error bars indicate standard deviations.
Mild periodate truncation of the side chains of sialic acids on BSM reduced but did not eliminate binding of the mCD33-Fc (Fig. 1, left panel). In this respect, mCD33 is again different from hCD33, which shows an absolute requirement for an intact sialic acid side chain, for binding to BSM (7).
Generation of a polyclonal mono-specific antibody against mCD33 protein.
Despite three attempts in collaboration with BD Biosciences Pharmingen to generate rat monoclonal antibodies against mCD33, antibodies of the desired property and stability were not obtained. We therefore took advantage of our CD33-deficient mice (described below) and immunized them with recombinant mCD33 protein. While a strong serum response against the immunogen was seen, the group at BD Biosciences Pharmingen was again unable to isolate stable specific monoclonal antibodies by using the spleens from these mice. The reason for this difficulty is unclear, since the same group has had much success in generating other such monoclonal antibodies. Whatever the reason, this may explain why no other group has yet reported a monoclonal antibody against mCD33. Regardless, we were able to use the serum from the CD33-deficient mouse to affinity purify a monospecific polyclonal antibody against mCD33 and used this antibody to analyze CD33 protein expression.
Expression of mCD33 protein on myeloid precursors and on granulocytes.
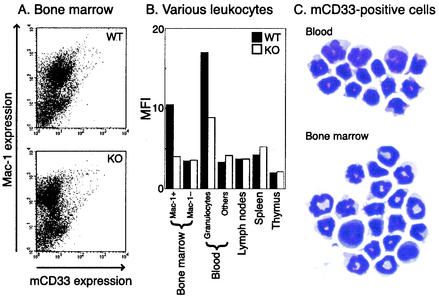
Among many hematopoietic cell-types, CD33 expression was observed only on certain bone marrow and blood cells (Fig. 2). The CD33-positive cells in bone marrow were also CD11b (Mac-1) positive, indicating that mCD33 is expressed on the cells of the myeloid lineage. When sorted and examined directly after Geimsa staining, most of the CD33-positive cells in the bone marrow showed a mature granulocyte morphology under the microscope, with occasional cells showing an immature myeloid cell morphology (Fig. 2C). In peripheral blood, the CD33-positive cells were initially identified as mainly granulocytes, based on their distinctive light-scatter profile. This conclusion was supported by microscopic analysis of Geimsa-stained fluorescence-activated cell sorter (FACS)-sorted CD33-positive cells, which mostly had the morphology of mature granulocytes (Fig. 2C). The relatively low-level expression was not due to a low titer of the antibody preparation, as evident from the strong staining of 293 cells transiently transfected with mCD33 full-length expression construct (data not shown). Peripheral blood granulocytes from the CD33-deficient mice did not show any staining, confirming the specificity of the expression seen in the wild-type mice. Due to the lack of a definitive mouse monocyte marker, the paucity of monocytes in mouse blood, difficulties in morphological distinction between mouse granulocytes and monocytes, and the weak signals obtained with anti-CD33 antibody staining, we could not definitively prove or disprove mCD33 expression on circulating mouse monocytes. However, the lack of monocyte enrichment in CD33-positive sorted cells (Fig. 2) strongly suggests that mouse monocytes are negative for mCD33.
FIG. 2.
CD33 expression on mouse hematopoietic cells. (A) Hematopoietic cells in bone marrow from wild-type (top panel) and CD33-deficient (bottom panel) mice were stained with the anti-CD33 antibody and analyzed by flow cytometry. The majority of Mac-1-positive cells stained weakly. (B) CD33 expression on leukocytes from various tissues. Solid bars, wild-type cells; open bars, CD33-deficient cells. MFI, median fluorescence intensity. (C) Examples of the light microscopic appearance of Giemsa-stained CD33-positive cells obtained by cell sorting from peripheral blood or bone marrow. The dominant morphology in both compartments is that of mature granulocytes.
Frozen sections of pellets of transiently transfected cells gave a positive signal with the antibody, showing that it was suitable for immunohistochemical analyses (data not shown). Thus, we also attempted to analyze mCD33 expression in both frozen and formaldehyde-fixed paraffin-embedded sections of various organs (e.g., brain, liver, kidney, lung, gastrointestinal tract, etc.) by various immunohistochemical staining methods. However, we did not observe any signal above the background level. Thus, if cells in solid tissues (e.g., tissue macrophages) do express this molecule, they must do so at very low levels. Taken together, our data indicate that mCD33 is expressed at low levels on granulocytes and on some developing cells of myeloid lineage. This is in contrast to hCD33, which is easily detectable on immature cells of the myeloid lineage and circulating monocytes, but very weakly, if at all, on circulating granulocytes.
Generation of CD33-deficient mice.
To investigate physiological functions of CD33 in vivo, we generated mice deficient in CD33 through conventional homologous targeting in embryonic stem cells (Fig. 3). Loss of CD33 expression in the CD33-deficient mice was confirmed by flow cytometric analysis of hematopoietic cells, as described above. In the limited-access barrier facility under specific-pathogen-free conditions, the CD33-deficient mice showed no gross morphological or histological abnormalities in many organs studied, and their growth was equivalent to that of wild-type littermates (data not shown). Also, CD33-deficient mice were fertile. Although many mice with the CD33 Δ/Δ genotype (even the offspring of C57BL/6 F10 × F10 intercross) had red eyes and a white-grayish coat, in contrast to the black eyes and black coat of wild-type and heterozygous littermates, this apparent phenotype was not strictly correlated with the CD33 genotype, in that some breeding pairs produced black pups with CD33 Δ/Δ genotype. This observation is likely to be explained by a background mutation of the gene pink-eyed dilution (gene symbol P; Mouse Genome Informatics accession identification no. MGI:97454), which is only ∼5 centimorgans apart from Cd33. The R1 ES cell line is heterozygous at the P locus because it is a hybrid between 129X1/SvJ and 129S1/Sv-p+Tyr+KitlSl-J/+ (16, 29, 34), and the p allele (on 129X1-derived chromosome 7) probably coincided with the targeted CD33 allele on the same chromosome copy. We have also observed mild transmission distortion of the null allele in female F6 × F6 offspring (W/W:W/Δ:Δ/Δ = 34:64:23), but this distortion was not statistically significant (P = 0.30 for χ2 test).
Minor effect of CD33 deficiency on hematological and biochemical parameters.
Differential cell counting and analysis of blood chemistries of CD33-deficient mice and wild-type littermates were performed as described in Materials and Methods and summarized in Table 1. The only statistically significant differences between wild-type and CD33-deficient mice were a slight reduction in mean erythrocyte count and hematocrit and an increase in the mean concentration of serum glutamic-oxaloacetic transaminase (aspartate aminotransferase) in blood. Although these parameters were still within the normal range for wild-type mice, these differences may be biologically significant. Thus, the absence of CD33 has only minor hematological and biochemical consequences in mice.
TABLE 1.
Peripheral blood analyses of CD33-deficient mice and wild-type littermates
| Parameter observed | Observed valuea
|
|
|---|---|---|
| CD33+/+ | CD33−/− | |
| Differential cell countsb | ||
| Total leukocytes (103/μl) | 6.05 ± 0.80 | 5.55 ± 0.60 |
| Neutrophils (%) | 16.0 ± 2.6 | 21.4 ± 3.2 |
| Lymphocytes (%) | 79.5 ± 2.7 | 73.8 ± 3.6 |
| Monocytes (%) | 2.0 ± 0.5 | 2.8 ± 0.7 |
| Eosinophils (%) | 1.9 ± 0.5 | 1.8 ± 0.5 |
| Basophils (%) | 0.3 ± 0.2 | 0.2 ± 0.2 |
| Erythrocytes (106/μl) | 9.0 ± 0.1 | 8.6 ± 0.1c |
| Platelets (103/μl) | 894 ± 41 | 765 ± 63 |
| Blood chemistryd | ||
| Glucose (mg/dl) | 184.4 ± 16.8 | 165.1 ± 11.9 |
| Urea nitrogen (mg/dl) | 20.3 ± 2.0 | 21.7 ± 1.2 |
| Bicarbonate (meq/liter) | 17.3 ± 0.8 | 16.3 ± 0.5 |
| Potassium (meq/liter) | 4.2 ± 0.1 | 4.3 ± 0.3 |
| SGOT (IU/liter) | 49.9 ± 4.4 | 63.3 ± 2.7e |
| SGPT (IU/liter) | 34.1 ± 4.2 | 27.7 ± 2.5 |
| Alkaline phosphatase (IU/liter) | 117.0 ± 9.8 | 121.6 ± 14.9 |
Data are means ± standard deviations.
n = 12 for CD33+/+ mice, and n = 13 for CD33−/− mice.
Statistically significant reduction in CD33-deficient mice (P = 0.007).
n = 9 for CD33+/+ mice, and n = 9 for CD33−/− mice. SGOT, serum glutamic-oxaloacetic transaminase; SGPT, serum glutamic-pyruvic transaminase.
Statistically significant increase in CD33-deficient mice (P = 0.019).
No obvious effect of CD33 deficiency on hematopoietic cell development.
Detailed analysis of the distribution and number of hematopoietic cell subpopulations in primary and secondary lymphoid organs by flow cytometry (n = 3 for each genotype) revealed no significant difference between CD33-deficient and wild-type mice (data not shown), suggesting no major nonredundant role of CD33 in the development and distribution of hematopoietic cells. Quantification of various Igs by ELISA in the serum of these mice also showed no statistically significant differences between CD33-deficient and wild-type mice (Table 2). These results suggest no major nonredundant contribution of CD33 in normal development of the humoral immune system.
TABLE 2.
Serum Ig levels in CD33-deficient mice and wild-type littermates
| Ig type | Serum Ig level (μg/ml)a
|
|
|---|---|---|
| CD33+/+ (n = 3) | CD33−/− (n = 3) | |
| IgM | 96.7 ± 24.8 | 66.0 ± 5.9 |
| IgA | 2.1 ± 0.1 | 2.4 ± 0.4 |
| IgG1 | 67.4 ± 2.9 | 67.8 ± 1.0 |
| IgG2a | 6.3 ± 3.3 | 10.0 ± 4.2 |
| IgG2b | 83.7 ± 23.3 | 100.7 ± 42.5 |
| IgG3 | 18.7 ± 12.5 | 33.9 ± 19.2 |
Data are means ± standard deviations.
Minor effect of CD33 deficiency in casein-induced peritonitis and LPS-induced systemic inflammation.
Since the expression of CD33 was restricted to granulocytes and myeloid lineage cells, we analyzed whether CD33 is involved in the regulation of innate immunological responses. A pilot casein-induced peritonitis experiment using a limited number of mice suggested that CD33-deficient mice might be hyporesponsive to this stimulus, showing reduced granulocyte (Gr-1+) infiltration at earlier stage of inflammation (up to 6 h postinjection), while the number of macrophages (F4/80+) in the peritoneum was not influenced by the mCD33 genotype (data not shown). We reexamined this finding by using more mice (10 wild-type and 9 mCD33-deficient mice), while limiting the time point to only 4 h postinjection. In this study, we found no significant difference in the ratio of Gr-1+ to :F4/80+ cells (granulocytes and macrophages) between wild-type and CD33-deficient mice (data not shown). The levels of IL-6 in blood induced by peritonitis (wild-type mice, 892 ± 452 pg/ml; knockout mice, 780 ± 679 pg/ml) were also not significantly different (Student's t test, P = 0.68). There was no significant difference between wild-type and mCD33-deficient mice in peripheral blood cell counts either, with the possible exception of the platelet count (Table 3). However, platelets are not positive for mCD33 expression (data not shown).
TABLE 3.
Differential blood cell count in casein-induced peritonitis (4 h post-casein injection)
| Blood cell type | Blood cell count (cells/μl)a
|
|
|---|---|---|
| CD33+/+ (n = 10) | CD33−/− (n = 9) | |
| Leukocytes (103) | 7.23 ± 2.05 | 6.23 ± 2.17 |
| Lymphocytes (103) | 3.89 ± 1.30 | 3.57 ± 1.20 |
| Neutrophils (103) | 3.01 ± 0.91 | 2.40 ± 0.97 |
| Monocytes (103) | 0.20 ± 0.10 | 0.18 ± 0.10 |
| Erythrocytes (106) | 10.7 ± 0.93 | 10.2 ± 0.55 |
| Platelets (103) | 1,070 ± 181 | 902 ± 149b |
Data are means ± standard deviations.
Statistically significant reduction in CD33-deficient mice (P = 0.042).
We then examined the effect of mCD33 deficiency on LPS-induced systemic inflammation. The IL-6 response at the peak (3 h postinjection) was somewhat weaker in CD33-deficient mice (wild-type mice, 120 ± 42 ng/ml; knockout mice, 78.4 ± 54 ng/ml), but the statistical significance of this difference was marginal (Student's t test, P = 0.11). Again, there was no significant difference in peripheral blood cell counts at any time points up to 24 h after LPS injection (Table 4). Along with the finding that there was no substantial difference in the ratio of Mac-1+ (myeloid cells) to TER119+ (erythroid cells) in wild-type and CD33-deficient mouse bone marrow (data not shown), these data suggest that mCD33 may not have any nonredundant functions in leukocyte trafficking nor in activity regulation of cells via common pathways involved in innate immunity.
TABLE 4.
Differential blood cell count in LPS-induced systemic inflammation in mice
| Blood cell type | Blood cell count (103/μl)a
|
|
|---|---|---|
| CD33+/+ (n = 8) | CD33−/− (n = 8) | |
| Total leukocytes | ||
| 0 h | 5.41 ± 1.86 | 6.17 ± 1.90 |
| 3 h | 2.44 ± 1.34 | 3.84 ± 2.19 |
| 6 h | 4.14 ± 1.91 | 4.73 ± 0.99 |
| 24 h | 3.70 ± 1.57 | 3.45 ± 2.09 |
| Lymphocytes | ||
| 0 h | 3.82 ± 1.46 | 4.57 ± 1.75 |
| 3 h | 1.64 ± 0.79 | 2.14 ± 0.52 |
| 6 h | 1.91 ± 0.86 | 2.14 ± 0.77 |
| 24 h | 1.38 ± 0.54 | 1.37 ± 0.93 |
| Neutrophils | ||
| 0 h | 1.45 ± 0.51 | 1.46 ± 0.44 |
| 3 h | 0.64 ± 0.51 | 1.59 ± 1.73 |
| 6 h | 2.03 ± 1.13 | 2.28 ± 0.38 |
| 24 h | 2.10 ± 1.15 | 1.94 ± 1.42 |
| Monocytes | ||
| 0 h | 0.12 ± 0.07 | 0.12 ± 0.05 |
| 3 h | 0.10 ± 0.05 | 0.08 ± 0.04 |
| 6 h | 0.16 ± 0.07 | 0.21 ± 0.07 |
| 24 h | 0.17 ± 0.11 | 0.10 ± 0.09 |
| Platelets | ||
| 0 h | 1,210 ± 250 | 1,160 ± 158 |
| 3 h | 677 ± 327 | 800 ± 57 |
| 6 h | 712 ± 197 | 812 ± 201 |
| 24 h | 610 ± 134 | 673 ± 153 |
Data are means ± standard deviations.
DISCUSSION
In this report, we have described the sialic acid-binding properties of mCD33 and its expression on bone marrow and blood granulocytes. We also generated and characterized mice deficient in CD33 and analyzed its possible involvement in innate immunity. mCD33 showed distinct binding to BSM in a sialic acid-dependent manner, and this binding was affected by 9-O-acetylation or by truncation of the glycerol-like side chain of sialic acids, confirming the designation of mCD33 as a member of the Siglec family. Although the exact glycan structure preferred by mCD33 was not identified, it is likely to be related to sialyl-Tn (Neu5Acylα2-6GalNAc) structure, as suggested by preferential binding of mCD33 to a polymeric probe with substitution of multiple copies of sialyl-Tn disaccharides. However, its preferred ligand structure may not be sialyl-Tn itself, since neither OSM nor PSM (both rich in sialyl-Tn structure) showed strong binding to mCD33. A class of glycan structures found abundantly in BSM but not in OSM or PSM is likely to explain the preferential binding of BSM. An alternative explanation is that the preferred structure is present in all mucin probes tested, but the spacing between individual glycans is not favorable in the poorer ligands.
The above results are in striking contrast to the sialic acid-binding specificity of hCD33, which showed robust binding to α2-3- and α2-6-linked sialic acids on lactosamine units (7). The sequence identity between mouse and hCD33 is higher in the second Ig-like domain (72% identity over 93 amino acids) than in the first Ig-like domain (54% identity over 127 amino acids), which is the primary site of sialic acid recognition in Siglecs (9, 10). The striking difference in the sialic acid-binding properties between hCD33 and mCD33 is likely to be explained by these extensive sequence differences in their first Ig-like domains. Of course, it is possible that both mCD33 and hCD33 have an alternative ligand interacting with their second Ig-like domain, which has prevented the sequence divergence of the second Ig-like domain of CD33. However, curiously, this trend (lower sequence identity in the first Ig-like domain) is also observed in some other human-mouse orthologous pairs of CD33-related Siglecs (i.e., mSiglec-E/hSiglec-9 and mSiglec-G/hSiglec-10). It is tempting to interpret this trend as an indication that the first Ig-like domain of CD33-related Siglecs has been under selective pressure to undergo rapid evolution, rather than as evidence of passive decay due to functional insignificance. Such facilitated sequence evolution may be explained by its interaction with rapidly evolving pathogens, which could potentially have influenced the evolution of the first Ig-like domain of CD33-related Siglecs. Pathogens carrying sialic acids (or sialic acid-like sugars, such as legionaminic and pseudaminic acids) (5) are possible agents that could have facilitated such rapid evolution of CD33-related Siglecs via interaction with the cells expressing them.
Our study has shown that the pattern of expression of mCD33 is also not identical to that of hCD33, in that the former is expressed mostly on mature granulocytes rather than on early myeloid lineage cells in bone marrow. In contrast, hCD33 is more prominently expressed on monocytes in peripheral blood and only very weakly expressed on granulocytes, if at all. Differences in expression patterns have also been observed for another pair of putative Siglec orthologs (i.e., mSiglec-F and hSiglec-5) (2). The former is expressed on immature cells of the myeloid lineage in bone marrow (2), while the latter is prominently expressed on mature neutrophils and monocytes (8). This finding, along with the lack of a canonical ITIM in the cytosolic tail of mCD33 and difference in the sialic acid-binding properties between mCD33 and hCD33, raises the possibility that mCD33 and hCD33 are functionally not equivalent. If this is the case, it somewhat reduces the utility of CD33-deficient mice as a model to understand the function of CD33 per se in humans. Nevertheless, this is the first reported animal model in which a CD33-related Siglec was genetically deleted and can still serve as a model to study generic functions of CD33-related Siglecs.
Mice deficient in CD33 were viable and fertile, without any obvious deficiency in overall organ development and growth. Moreover, they showed no distinct difference from wild-type mice in the development and distribution of mature hematopoietic cells. These observations are not surprising, given the restricted expression pattern of mCD33. However, it was somewhat unexpected that they showed no overt phenotypical difference from wild-type mice in the models of acute inflammation, such as casein-induced peritonitis and LPS-induced systemic response. One interpretation is that CD33 does not substantially contribute to generic acute-phase inflammation. Another is that it has functional redundancy to other Siglecs known to be expressed on the same cells. A different mode of stimulus (e.g., a more sustained stimulation of the innate immune system or some specific stimulus engaging CD33) may be necessary to identify differences between wild-type and CD33-deficient mice in immune responses. In this respect, recent studies using DNA microarrays (6, 25) have shown that different bacteria induced both “stereotyped” (common to all bacteria) and “specific” (bacterial species specific) responses in human cells involved in innate immunity, suggesting involvement of both common (leading to stereotyped response) and different (leading to specific response) cellular receptors to bacterial components or products in the host recognition of bacteria. mCD33 is possibly one of these receptors involved in the specific responses. It would be interesting to know if wild-type and CD33-deficient cells respond differently to various types of bacteria (or bacterial products) by using DNA microarray analysis. We should also emphasize that the mice used in this study were maintained under specific-pathogen-free conditions, potentially masking a deficiency in innate immunity that may manifest under less controlled conditions. Transfer of these mice to a conventional vivarium and deliberate challenge with dead or live pathogens are under consideration.
Edelman and Gally (13) have noted that in many cases (up to 30%), mouse models deficient in selected gene products fail to manifest phenotypic consequences, which at least in part, is likely to be explained by the functional “degeneracy” of different gene products. The lack of a distinct phenotype in CD33-deficient mice may be also explained by the presence of another Siglec (e.g., Siglec-F) (2) in similar cell subsets that express CD33. Although the ligand-binding specificities of Siglec-F and CD33 are quite different (the former preferentially recognizes the Neu5Acα2-3Galβ1-4GlcNAc structure), some glycoproteins might express glycan structures recognized by both Siglec-F and CD33, and one of these glycoproteins may be the functional ligand for Siglec-F and CD33. In order to examine this possibility, we are currently working to generate mice deficient for Siglec-F as well as those with a combined deficiency in mCD33 and mSiglec-F. Also, there are some other mouse Siglecs (Siglec-E, -G, and -H) whose expression patterns have yet to be reported, leaving open the possibility that one (or more) of these Siglecs is coexpressed with CD33. Antibody development for these Siglecs is currently under way (P. Crocker, personal communication), and a report on the expression analysis of these Siglecs is awaited.
Acknowledgments
This work was supported by Public Health Service grant P01-HL57345 (to A.V.) from the National Institutes of Health.
We thank David Ditto, Lisa Wiggleton, and Mark Boton for excellent technical assistance and Jamey Marth, Dzung Le, and Nissi Varki for expert advice.
The first three authors contributed equally to this work.
REFERENCES
- 1.Angata, T., and E. Brinkman-Van der Linden. 2002. I-type lectins. Biochim. Biophys. Acta 1572:294-316. [DOI] [PubMed] [Google Scholar]
- 2.Angata, T., R. Hingorani, N. M. Varki, and A. Varki. 2001. Cloning and characterization of a novel mouse Siglec, mSiglec-F: differential evolution of the mouse and human (CD33) Siglec-3-related gene clusters. J. Biol. Chem. 276:45128-45136. [DOI] [PubMed] [Google Scholar]
- 3.Angata, T., and A. Varki. 2000. Siglec-7: a sialic acid-binding lectin of the immunoglobulin superfamily. Glycobiology 10:431-438. [DOI] [PubMed] [Google Scholar]
- 4.Angata, T., and A. Varki. 2000. Cloning, characterization, and phylogenetic analysis of Siglec-9, a new member of the CD33-related group of Siglecs—evidence for co-evolution with sialic acid synthesis pathways. J. Biol. Chem. 275:22127-22135. [DOI] [PubMed] [Google Scholar]
- 5.Angata, T., and A. Varki. 2002. Chemical diversity in the sialic acids and related alpha-keto acids: an evolutionary perspective. Chem. Rev. 102:439-470. [DOI] [PubMed] [Google Scholar]
- 6.Boldrick, J. C., A. A. Alizadeh, M. Diehn, S. Dudoit, C. L. Liu, C. E. Belcher, D. Botstein, L. M. Staudt, P. O. Brown, and D. A. Relman. 2002. Stereotyped and specific gene expression programs in human innate immune responses to bacteria. Proc. Natl. Acad. Sci. USA 99:972-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brinkman-Van der Linden, E. C. M., and A. Varki. 2000. New aspects of siglec binding specificities, including the significance of fucosylation and of the sialyl-Tn epitope. J. Biol. Chem. 275:8625-8632. [DOI] [PubMed] [Google Scholar]
- 8.Cornish, A. L., S. Freeman, G. Forbes, J. Ni, M. Zhang, M. Cepeda, R. Gentz, M. Augustus, K. C. Carter, and P. R. Crocker. 1998. Characterization of siglec-5, a novel glycoprotein expressed on myeloid cells related to CD33. Blood 92:2123-2132. [PubMed] [Google Scholar]
- 9.Crocker, P. R., and A. Varki. 2001. Siglecs, sialic acids and innate immunity. Trends Immunol. 22:337-342. [DOI] [PubMed] [Google Scholar]
- 10.Crocker, P. R., and A. Varki. 2001. Siglecs in the immune system. Immunology 103:137-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz, S., H. H. Higa, B. K. Hayes, and A. Varki. 1989. O-acetylation and de-O-acetylation of sialic acids. 7- and 9-O-acetylation of alpha2,6-linked sialic acids on endogenous N-linked glycans in rat liver Golgi vesicles. J. Biol. Chem. 264:19416-19426. [PubMed] [Google Scholar]
- 12.Dinarello, C. A. 1996. Cytokines as mediators in the pathogenesis of septic shock. Curr. Top. Microbiol. Immunol. 216:133-165. [DOI] [PubMed] [Google Scholar]
- 13.Edelman, G. M., and J. A. Gally. 2001. Degeneracy and complexity in biological systems. Proc. Natl. Acad. Sci. USA 98:13763-13768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falco, M., R. Biassoni, C. Bottino, M. Vitale, S. Sivori, R. Augugliaro, L. Moretta, and A. Moretta. 1999. Identification and molecular cloning of p75/AIRM1, a novel member of the sialoadhesin family that functions as an inhibitory receptor in human natural killer cells. J. Exp. Med. 190:793-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferlazzo, G., G. M. Spaggiari, C. Semino, G. Melioli, and L. Moretta. 2000. Engagement of CD33 surface molecules prevents the generation of dendritic cells from both monocytes and CD34+ myeloid precursors. Eur. J. Immunol. 30:827-833. [DOI] [PubMed] [Google Scholar]
- 16.Festing, M. F., E. M. Simpson, M. T. Davisson, and L. E. Mobraaten. 1999. Revised nomenclature for strain 129 mice. Mamm. Genome 10:836.. [DOI] [PubMed] [Google Scholar]
- 17.Floyd, H., J. Ni, A. L. Cornish, Z. Z. Zeng, D. Liu, K. C. Carter, J. Steel, and P. R. Crocker. 2000. Siglec-8—a novel eosinophil-specific member of the immunoglobulin superfamily. J. Biol. Chem. 275:861-866. [DOI] [PubMed] [Google Scholar]
- 18.Freeman, S. D., S. Kelm, E. K. Barber, and P. R. Crocker. 1995. Characterization of CD33 as a new member of the sialoadhesin family of cellular interaction molecules. Blood 85:2005-2012. [PubMed] [Google Scholar]
- 19.Hill, H. D. J., J. A. Reynolds, and R. L. Hill. 1977. Purification, composition, molecular weight, and subunit structure of ovine submaxillary mucin. J. Biol. Chem. 252:3791-3798. [PubMed] [Google Scholar]
- 20.Kelm, S., R. Brossmer, R. Isecke, H. J. Gross, K. Strenge, and R. Schauer. 1998. Functional groups of sialic acids involved in binding to siglecs (sialoadhesins) deduced from interactions with synthetic analogues. Eur. J. Biochem. 255:663-672. [DOI] [PubMed] [Google Scholar]
- 21.Kelm, S., R. Schauer, J.-C. Manuguerra, H.-J. Gross, and P. R. Crocker. 1994. Modifications of cell surface sialic acids modulate cell adhesion mediated by sialoadhesin and CD22. Glycoconj. J. 11:576-585. [DOI] [PubMed] [Google Scholar]
- 22.Klein, A., M. Krishna, N. M. Varki, and A. Varki. 1994. 9-O-acetylated sialic acids have widespread but selective expression: analysis using a chimeric dual-function probe derived from influenza C hemagglutinin-esterase. Proc. Natl. Acad. Sci. USA 91:7782-7786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lloyd, K. O., and A. Savage. 1991. High performance anion exchange chromatography of reduced oligosaccharides from sialomucins. Glycoconj. J. 8:493-498. [DOI] [PubMed] [Google Scholar]
- 24.Manzi, A. E., A. Dell, P. Azadi, and A. Varki. 1990. Studies of naturally occurring modifications of sialic acids by fast-atom bombardment-mass spectrometry. Analysis of positional isomers by periodate cleavage. J. Biol. Chem. 265:8094-8107. [PubMed] [Google Scholar]
- 25.Nau, G. J., J. F. Richmond, A. Schlesinger, E. G. Jennings, E. S. Lander, and R. A. Young. 2002. Human macrophage activation programs induced by bacterial pathogens. Proc. Natl. Acad. Sci. USA 99:1503-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicoll, G., J. Ni, D. Liu, P. Klenerman, J. Munday, S. Dubock, M. G. Mattei, and P. R. Crocker. 1999. Identification and characterization of a novel siglec, siglec-7, expressed by human natural killer cells and monocytes. J. Biol. Chem. 274:34089-34095. [DOI] [PubMed] [Google Scholar]
- 27.Paul, S. P., L. S. Taylor, E. K. Stansbury, and D. W. McVicar. 2000. Myeloid specific human CD33 is an inhibitory receptor with differential ITIM function in recruiting the phosphatases SHP-1 and SHP-2. Blood 96:483-490. [PubMed] [Google Scholar]
- 28.Savage, A. V., C. M. Donoghue, S. M. D'Arcy, C. A. M. Koeleman, and D. H. Van den Eijnden. 1990. Structure determination of five sialylated trisaccharides with core types 1, 3 or 5 isolated from bovine submaxillary mucin. Eur. J. Biochem. 192:427-432. [DOI] [PubMed] [Google Scholar]
- 29.Simpson, E. M., C. C. Linder, E. E. Sargent, M. T. Davisson, L. E. Mobraaten, and J. J. Sharp. 1997. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nat. Genet. 16:19-27. [DOI] [PubMed] [Google Scholar]
- 30.Sjoberg, E. R., L. D. Powell, A. Klein, and A. Varki. 1994. Natural ligands of the B cell adhesion molecule CD22beta can be masked by 9-O-acetylation of sialic acids. J. Cell Biol. 126:549-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strenge, K., R. Schauer, N. Bovin, A. Hasegawa, H. Ishida, M. Kiso, and S. Kelm. 1998. Glycan specificity of myelin-associated glycoprotein and sialoadhesin deduced from interactions with synthetic oligosaccharides. Eur. J. Biochem. 258:677-685. [DOI] [PubMed] [Google Scholar]
- 32.Taylor, V. C., C. D. Buckley, M. Douglas, A. J. Cody, D. L. Simmons, and S. D. Freeman. 1999. The myeloid-specific sialic acid-binding receptor, CD33, associates with the protein-tyrosine phosphatases, SHP-1 and SHP-2. J. Biol. Chem. 274:11505-11512. [DOI] [PubMed] [Google Scholar]
- 33.Tchilian, E. Z., P. C. Beverley, B. D. Young, and S. M. Watt. 1994. Molecular cloning of two isoforms of the murine homolog of the myeloid CD33 antigen. Blood 83:3188-3198. [PubMed] [Google Scholar]
- 34.Threadgill, D. W., D. Yee, A. Matin, J. H. Nadeau, and T. Magnuson. 1997. Genealogy of the 129 inbred strains: 129/SvJ is a contaminated inbred strain. Mamm. Genome 8:390-393. [DOI] [PubMed] [Google Scholar]
- 35.Tsuji, T., and T. Osawa. 1986. Carbohydrate structures of bovine submaxillary mucin. Carbohydr. Res. 151:391-402. [DOI] [PubMed] [Google Scholar]
- 36.Ulyanova, T., J. Blasioli, T. A. Woodford-Thomas, and M. L. Thomas. 1999. The sialoadhesin CD33 is a myeloid-specific inhibitory receptor. Eur. J. Immunol. 29:3440-3449. [DOI] [PubMed] [Google Scholar]
- 37.Van Halbeek, H., L. Dorland, J. Haverkamp, G. A. Veldink, J. F. Vliegenthart, B. Fournet, G. Ricart, J. Montreuil, W. D. Gathmann, and D. Aminoff. 1981. Structure determination of oligosaccharides isolated from A+, H+ and A−, H− hog-submaxillary-gland mucin glycoproteins, by 360-MHz 1H-NMR spectroscopy, permethylation analysis and mass spectrometry. Eur. J. Biochem. 118:487-495. [DOI] [PubMed] [Google Scholar]
- 38.Varki, A. 1992. Diversity in the sialic acids. Glycobiology 2:25-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vitale, C., C. Romagnani, M. Falco, M. Ponte, M. Vitale, A. Moretta, A. Bacigalupo, L. Moretta, and M. C. Mingari. 1999. Engagement of p75/AIRM1 or CD33 inhibits the proliferation of normal or leukemic myeloid cells. Proc. Natl. Acad. Sci. USA 96:15091-15096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vitale, C., C. Romagnani, A. Puccetti, D. Olive, R. Costello, L. Chiossone, A. Pitto, A. Bacigalupo, L. Moretta, and M. C. Mingari. 2001. Surface expression and function of p75/AIRM-1 or CD33 in acute myeloid leukemias: engagement of CD33 induces apoptosis of leukemic cells. Proc. Natl. Acad. Sci. USA 98:5764-5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitney, G., S. L. Wang, H. Chang, K. Y. Cheng, P. Lu, X. D. Zhou, W. P. Yang, M. McKinnon, and M. Longphre. 2001. A new siglec family member, siglec-10, is expressed in cells of the immune system and has signaling properties similar to CD33. Eur. J. Biochem. 268:6083-6096. [DOI] [PubMed] [Google Scholar]