Abstract
The capacity of malarial infection to suppress the patient's immune responses both to the parasite and to other antigens has long puzzled researchers. A prime suspect, the parasite-produced pigment hemozoin, has now been clearly shown to mediate immunosuppression by inhibiting dendritic cell activity.
Malaria, caused by protozoan parasites of the genus Plasmodium, is one of the leading causes of illness and death worldwide. Its effects are exacerbated by its ability to modulate immune responses, which not only impairs the patient's ability to fight the malarial infection, but can leave them vulnerable to some secondary infections and reduce the immune response to certain vaccines. Although this partial immunosuppression has been recognized for many years, the underlying mechanisms are not well understood, and the results of studies in both humans and animals have sometimes been contradictory. In recent years, attention has focused on interference of parasites with the myeloid cells of the immune system, in particular the antigen-presenting dendritic cells that are essential for the initiation of almost all adaptive immune responses.
One consequence of malarial infection is the production of the so-called 'malaria pigment' from the breakdown of hemoglobin by the parasite in infected red blood cells. Malaria pigment is a polymerized form of heme also known as hemozoin and has long been suspected to affect the function of myeloid cells. In this issue of Journal of Biology, Millington and colleagues [1] show how ingestion of malaria pigment by dendritic cells alters their function over the course of the infection, with consequences for the adaptive immune response to different asexual blood stages of the parasite.
Immature phagocytic dendritic cells reside in most tissues and constantly sample their environment by phagocytosis and pinocytosis, surveying for invading pathogens [2]. When pathogens enter a tissue or the blood stream, they are usually first recognized by pattern recognition receptors (PRRs) on the surface of dendritic cells, which recognize molecules common to different classes of pathogen. Binding of pathogens or their products to the PRRs triggers the migration of the dendritic cell into lymphoid tissues and its maturation into a powerful antigen-presenting cell [3]. Activated dendritic cells migrate to a draining lymph node or to the spleen, where they initiate an adaptive immune response by presenting pathogen antigens to T lymphocytes (T cells). As they mature, their phagocytic activity decreases, but they increase expression of the cell-surface major histocompatibility complex (MHC) molecules that bind processed pathogen-derived peptides and display them on the dendritic-cell surface. At the same time the maturing dendritic cell starts to increase the expression of cell-surface proteins known as co-stimulatory molecules, which together with MHC-peptide complexes enable dendritic cells to activate any T cells that bind to the antigens displayed on its surface.
To activate or not to activate?
As in many other infectious diseases, dendritic-cell function in Plasmodium infections has been studied extensively in vivo and in vitro, but results have been contradictory, depending on the Plasmodium species, the inoculation dose and the type of dendritic cells [4] under investigation. Mice, in which most work has been done, are susceptible to infection with the species Plasmodium chabaudi chabaudi, P. yoeli, and P. berghei. A number of studies have shown that dendritic cells can mature in response to Plasmodium infection and induce a powerful T-cell response. In one example, the culture of immature bone-marrow-derived dendritic cells with mouse red blood cells infected with P. chabaudi chabaudi resulted in limited maturation of the dendritic cells, which could be enhanced by external maturational stimuli such as bacterial lipopolysaccharide (LPS), tumor necrosis factor-α (TNF-α) or CD40 ligand [5,6]. In a different study covering the first four days of a P. chabaudi chabaudi infection, a subset of splenic dendritic cells found in the marginal zone of the spleen and characterized by the cell-surface marker protein CD11c were observed to migrate from the marginal zone into the T-cell rich periarteriolar sheath, the area in which immune T-cell responses are initiated [7]. These dendritic cells showed enhanced expression of MHC class II molecules and co-stimulatory molecules typical of mature dendritic cells. In yet another study, splenic CD11c+ dendritic cells isolated at the peak of a P. yoeli infection were able to activate antigen-specific T cells and induce secretion by the T cells of the cytokines interleukin 2 (IL-2), interferon-γ (IFN-γ) and TNF-α, a cytokine profile characteristic of the Th1 class of differentiated effector T cells [8].
In contrast, other studies have reported that dendritic cells failed to mature when co-cultured with red blood cells infected with P. chabaudi chabaudi or P. yoeli, even though the dendritic cells were subsequently capable of inducing T-cell responses in vivo when transferred into uninfected mice [9]. In one study, at the peak of blood-stage infection (the stage at which the parasite infects red blood cells) the response of CD8+ cytotoxic T cells (the killer subset of T lymphocytes) to liver-stage parasites was inhibited in vivo, most probably as a result of cytokines secreted by dendritic cells [10]. More recently, Wilson and colleagues [11] showed that inoculation of mice with P. berghei induced a systemic activation of splenic dendritic cells similar to that observed after administration of DNA containing unmethylated CpG, which is a ligand for a type of PRR known as Toll-like receptor 9 (TLR-9). As with activation by CpG, however, if another antigen was administered on day 3 or 4 of a P. berghei infection, the dendritic cells proved unable to cross-present this exogenous anitgen and activate CD8+ T cells. At this stage of infection, the parasite load in the blood (parasitemia) is still increasing. The defect in antigen presentation appeared to be due to reduced dendritic-cell phagocytic activity.
These studies used a wide variety of in vitro cell culture systems, functional read outs, Plasmodium species and mouse strains. A general trend emerges, however, suggesting that there is a dual response of dendritic cells to Plasmodium blood-stage infection, with an early phase of activation, in conditions of low parasitemia, and a late phase of functional inhibition, in conditions of high parasitemia.
Nailing the suspect
The study by Millington and colleagues [1] now not only reconciles the disparate results on dendritic-cell function in rodent malaria, but also provides novel insights into the consequences of dendritic-cell modulation in vivo. Their careful studies demonstrated an unambiguous biphasic response of dendritic cells to P. chabaudi chabaudi blood-stage infection in mice in vivo. They began their investigations with a simple question: at which stage during the course of a blood-stage infection does suppression of immune responses occur? To answer this question they immunized mice with the protein antigen ovalbumin (OVA) and bacterial LPS (which provides a nonspecific stimulus for an immune response) at different time points during a P. chabaudi chabaudi infection and monitored the antibody response to OVA. Only during the late stages of the infection, when most parasites had been cleared, did the mice show greatly reduced OVA-specific IgG responses.
Millington et al. [1] then demonstrated, through a series of carefully controlled experiments, that hemozoin acts directly on the dendritic cells and inhibits their maturation in response to maturational stimuli such as LPS or the cell-surface protein CD40 ligand (CD40L). Likewise, CD11c+ dendritic cells isolated from the spleens of infected mice were activated early but not late during infection, and at the later stage were refractory to subsequent stimulation with LPS. Inhibition of dendritic-cell maturation during the late stages of infection had consequences for the initiation of adaptive immune responses: antigen-specific T cells were activated by dendritic cells but failed to proliferate and secrete cytokines. Of particular importance, these T cells did not migrate into B-cell follicles in the spleen to provide the required help to B cells, and so there was also a failure to mount a specific antibody response (Figure 1).
Figure 1.
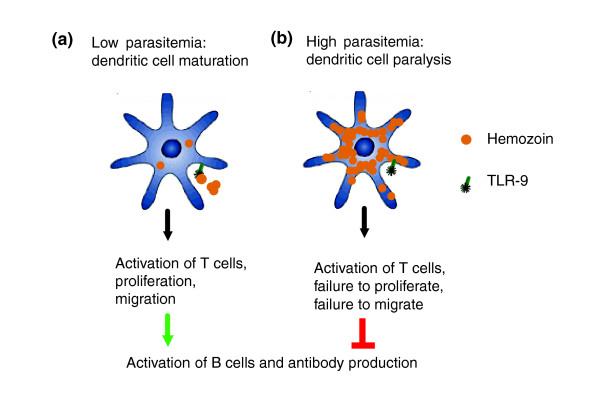
Biphasic response of dendritic cells to Plasmodium blood-stage infection in rodents. (a) Early on during infection, engagement of TLR-9 by hemozoin and interaction with infected red blood cells may result in dendritic-cell maturation. Mature dendritic cells present antigen to T cells and induce their activation. Activated T cells proliferate and migrate into primary B-cell follicles where they provide help for antibody production by B cells. (b) With increasing parasitemia, more and more myeloid dendritic cells in the spleen are paralyzed through ingestion of increasing amounts of hemozoin, with negative effects on downstream T-cell and B-cell responses.
Hemozoin has long been known to be a potent modifier of myeloid cells [12]. Hemozoin is released together with other cell debris when the mature blood-stage forms of the parasite cause red blood cells to rupture, and it is rapidly taken up by monocytes (immature precursors of macrophages and dendritic cells) and dendritic cells. Hemozoin reacts with membrane phospholipids, generating hydroxy-polyunsaturated fatty acids that cause membrane peroxidation [13]. Hydroxy-polyunsaturated fatty acids inhibit monocyte functions such as phagocytosis, activation by inflammatory cytokines, and generation of the oxidative burst [14,15]. Hemozoin also inhibits the differentiation of human monocytes to dendritic cells and their maturation [16]. It has been shown to bind to TLR-9 on the myeloid and plasmacytoid subsets of dendritic cells in rodents, although this observation was not confirmed in another study [17,18]. However, activation of dendritic cells via TLR-9 early during infection, but paralysis of dendritic cells via ingested hemozoin during the late stage of infection would agree with the results of Millington et al. [1]. Thus, hemozoin is directly associated with the alteration of cellular responses observed during acute malaria in mice and humans.
In humans, modulation of dendritic-cell function might be caused by adhesion of infected erythrocytes to the adhesive cell-surface protein CD36 expressed on dendritic cells. Adhesion of P. falciparum-infected erythrocytes to CD36 is mediated by the P. falciparum erythrocyte membrane protein 1 (PfEMP-1). Does the work of Millington et al. [1] rule out a role for CD36 in the modulation of dendritic-cell function? PfEMP-1 is not produced by the Plasmodium species that infect mice [19], and although rodent Plasmodium species can adhere to CD36 to a certain extent, the ligand (or ligands) mediating CD36 adhesion in rodents are not known, and expression of adhesion ligand occurs much later, during the asexual blood-stage phase [20,21]. In addition, the expression pattern of CD36 on human and mouse dendritic cells is fundamentally different: only CD8α+ dendritic cells in the periarteriolar lymphatic sheath express CD36 [22,23]. Nevertheless, work by Arese and colleagues [16] has shown that monocyte function, differentiation to dendritic cells, and dendritic-cell maturation were impaired in vitro after ingestion of hemozoin by human monocytes. Unlike PfEMP-1, hemozoin is present in both human and rodent Plasmodium species, yet both mechanisms can contribute to the modulation of dendritic-cell function in humans.
The seminal in vivo studies presented here in Journal of Biology by Millington et al. [1] convincingly show that hemozoin impairs dendritic-cell function in vivo in a rodent model of malaria, and that it significantly contributes to immune suppression during acute blood-stage malaria. The deleterious effects of dendritic-cell paralysis on humans infected with P. falciparum in endemic malaria areas is easy to envisage. Many parasite antigens are either clonally variant or polymorphic. If immune suppression is induced, adaptive immune responses to variant antigens expressed during later stages of an infection or to super-infecting parasites may not be induced efficiently.
Acknowledgments
Acknowledgements
B.C.U. is supported by a Wellcome Trust Career Development Award.
References
- Millington OR, Di Lorenzo C, Phillips RS, Garside P, Brewer JM. Suppression of adaptive immunity to heterologous antigens during Plasmodium infection through hemozoin-induced failure of dendritic-cell function. J Biol. 2006;5:5. doi: 10.1186/jbiol34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu Y-J, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Edwards AD, Manickasingham SP, Sporri R, Diebold SS, Schulz O, Sher A, Kaisho T, Akira S, Reis e Sousa C. Microbial recognition via Toll-like receptor-dependent and independent pathways determines the cytokine response of murine dendritic cell subsets to CD40 triggering. J Immunol. 2002;169:3652–3660. doi: 10.4049/jimmunol.169.7.3652. [DOI] [PubMed] [Google Scholar]
- Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- Seixas E, Cross C, Quin S, Langhorne J. Direct activation of dendritic cells by the malaria parasite, Plasmodium chabaudi chabaudi. Eur J Immunol. 2001;31:2970–2978. doi: 10.1002/1521-4141(2001010)31:10<2970::AID-IMMU2970>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Ing R, Segura M, Thawani N, Tam M, Stevenson MM. Interaction of mouse dendritic cells and malaria-infected erythrocytes: uptake, maturation, and antigen presentation. J Immunol. 2006;176:441–450. doi: 10.4049/jimmunol.176.1.441. [DOI] [PubMed] [Google Scholar]
- Leisewitz AL, Rockett KA, Gumede B, Jones M, Urban B, Kwiatkowski DP. Response of the splenic dendritic cell population to malaria infection. Infect Immun. 2004;72:4233–4239. doi: 10.1128/IAI.72.7.4233-4239.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JA, Rush A, Wilson RJ, Olver CS, Avery AC. Dendritic cells from malaria-infected mice are fully functional APC. J Immunol. 2004;172:475–482. doi: 10.4049/jimmunol.172.1.475. [DOI] [PubMed] [Google Scholar]
- Pouniotis DS, Proudfoot O, Bogdanoska V, Apostolopoulos V, Fifis T, Plebanski M. Dendritic cells induce immunity and long-lasting protection against blood-stage malaria despite an in vitro parasite-induced maturation defect. Infect Immun. 2004;72:5331–5339. doi: 10.1128/IAI.72.9.5331-5339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocana-Morgner C, Mota MM, Rodriguez A. Malaria blood stage suppression of liver stage immunity by dendritic cells. J Exp Med. 2003;197:143–151. doi: 10.1084/jem.20021072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NS, Behrens GMN, Lundie RJ, Smith CM, Waithman J, Young L, Forehan SP, Mount A, Steptoe RJ, Shortman KD, et al. Systemic activation of dendritic cells by Toll-like receptor ligands or malaria infection impairs cross-presentation and antiviral immunity. Nat Immunol. 2006;7:165–172. doi: 10.1038/ni1300. [DOI] [PubMed] [Google Scholar]
- Loose LD, di Luzio NR. A temporal relationship between reticuloendothelial system phagocytic alterations and antibody responses in mice infected with Plasmodium berghei (NYU-2 strain) Am J Trop Med Hyg. 1976;25:221–228. doi: 10.4269/ajtmh.1976.25.221. [DOI] [PubMed] [Google Scholar]
- Schwarzer E, Ludwig P, Valente E, Arese P. 15(S)-hydroxyeicosatetraenoic acid (15-HETE), a product of arachidonic acid peroxidation, is an active component of hemozoin toxicity to monocytes. Parassitologia. 1999;41:199–202. [PubMed] [Google Scholar]
- Schwarzer E, Turrini F, Giribaldi G, Cappadoro M, Arese P. Phagocytosis of P. falciparum malarial pigment hemozoin by human monocytes inactivates monocyte protein kinase C. Biochim Biophys Acta. 1993;1181:51–54. doi: 10.1016/0925-4439(93)90089-j. [DOI] [PubMed] [Google Scholar]
- Schwarzer E, Alessio M, Ulliers D, Arese P. Phagocytosis of the malarial pigment, hemozoin, impairs expression of major histocompatibility complex class II antigen, CD54, and CD11c in human monocytes. Infect Immun. 1998;66:1601–1606. doi: 10.1128/iai.66.4.1601-1606.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorokhod OA, Alessio M, Mordmuller B, Arese P, Schwarzer E. Hemozoin (malarial pigment) inhibits differentiation and maturation of human monocyte-derived dendritic cells: a peroxisome proliferator-activated receptor-gamma-mediated effect. J Immunol. 2004;173:4066–4074. doi: 10.4049/jimmunol.173.6.4066. [DOI] [PubMed] [Google Scholar]
- Pichyangkul S, Yongvanitchit K, Kum-arb U, Hemmi H, Akira S, Krieg AM, Heppner DG, Stewart VA, Hasegawa H, Looareesuwan S, et al. Malaria blood stage parasites activate human plasmacytoid dendritic cells and murine dendritic cells through a toll-like receptor 9-dependent pathway. J Immunol. 2004;172:4926–4933. doi: 10.4049/jimmunol.172.8.4926. [DOI] [PubMed] [Google Scholar]
- Coban C, Ishii KJ, Kawai T, Hemmi H, Sato S, Uematsu S, Yamamoto M, Takeuchi O, Itagaki S, Kumar N, et al. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J Exp Med. 2005;201:19–25. doi: 10.1084/jem.20041836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyes S, Horrocks P, Newbold C. Antigenic variation at the infected red cell surface in malaria. Annu Rev Microbiol. 2001;55:673–707. doi: 10.1146/annurev.micro.55.1.673. [DOI] [PubMed] [Google Scholar]
- Mota MM, Jarra W, Hirst E, Patnaik PK, Holder AA. Plasmodium chabaudi-infected erythrocytes adhere to CD36 and bind to microvascular endothelial cells in an organ-specific way. Infect Immun. 2000;68:4135–4144. doi: 10.1128/IAI.68.7.4135-4144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke-Fayard B, Janse CJ, Cunha-Rodrigues M, Ramesar J, Buscher P, Que I, Lowik C, Voshol PJ, den Boer MAM, van Duinen SG, et al. Murine malaria parasite sequestration: CD36 is the major receptor, but cerebral pathology is unlinked to sequestration. Proc Natl Acad Sci USA. 2005;102:11468–11473. doi: 10.1073/pnas.0503386102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belz GT, Vremec D, Febbraio M, Corcoran L, Shortman K, Carbone FR, Heath WR. CD36 is differentially expressed by CD8+ splenic dendritic cells but is not required for cross-presentation in vivo. J Immunol. 2002;168:6066–6070. doi: 10.4049/jimmunol.168.12.6066. [DOI] [PubMed] [Google Scholar]
- Schulz O, Pennington DJ, Hodivala-Dilke K, Febbraio M, Reis e Sousa C. CD36 or αvβ3 and αvβ5 integrins are not essential for MHC class I cross-presentation of cell-associated antigen by CD8α+ murine dendritic cells. J Immunol. 2002;168:6057–6065. doi: 10.4049/jimmunol.168.12.6057. [DOI] [PubMed] [Google Scholar]


