Abstract
Calcineurin is a calcium-regulated serine-threonine protein phosphatase that controls developmental and inducible biological responses in diverse cell types, in part through activation of the transcription factor nuclear factor of activated T cells (NFAT). In skeletal muscle, calcineurin has been implicated in the regulation of myoblast differentiation, hypertrophy of mature myofibers, and fiber type switching in response to alterations in intracellular calcium concentration. However, considerable disagreement persists about the functional role of calcineurin signaling in each of these processes. Here we evaluated the molecular phenotypes of skeletal muscle from both calcineurin Aα and calcineurin Aβ gene-targeted mice. Calcineurin Aα was observed to be the predominant catalytic isoform expressed in nearly all skeletal muscles examined. Neither calcineurin Aα or Aβ null mice showed any gross growth-related alterations in skeletal muscle, nor was fiber size or number altered in glycolytic/fast muscle types. In contrast, both calcineurin Aα and Aβ gene-targeted mice demonstrated an alteration in myofiber number in the soleus, an oxidative/slow-type muscle. More significantly, calcineurin Aα and Aβ gene-targeted mice showed a dramatic down-regulation in the oxidative/slow fiber type program in multiple muscles (both slow and fast). Associated with this observation, NFAT-luciferase reporter transgenic mice showed significantly greater activity in slow fiber-containing muscles than in fast. However, only calcineurin Aα null mice showed a defect in NFAT nuclear occupancy or NFAT-luciferase transgene activity in vivo. Collectively, our results suggest that calcineurin signaling plays a critical role in regulating skeletal muscle fiber type switching but not hypertrophy. Our results also suggest that fiber type switching occurs through an NFAT-independent mechanism.
Calcineurin is a calcium-calmodulin-activated serine-threonine phosphatase composed of a catalytic A subunit (59 to 62 kDa) and a calcium-binding regulatory B subunit (19 kDa). Three catalytic genes (A subunit) have been identified in vertebrate species, of which calcineurin Aα and calcineurin Aβ are ubiquitously expressed, while calcineurin Aγ expression is restricted to the testis and brain (11, 23, 38). Receptor stimulation that promotes a sustained elevation in intracellular calcium concentration leads to a direct activation of calcineurin, which in turn facilitates alterations in gene expression through transcriptional effector proteins (11, 23, 38). One such mechanism involves a family of transcriptional regulators referred to as nuclear factor of activated T cells (NFAT), which are normally sequestered in the cytoplasm in a hyperphosphorylated state (36). Activated calcineurin then directly binds NFAT transcription factors in the cytoplasm, resulting in their dephosphorylation and subsequent translocation into the nucleus. Once in the nucleus, NFAT factors function as important coinducers of calcium-activated, inducible gene expression in multiple cell types (36). Five NFAT transcription factors have been identified; among these, NFATc1, NFATc2, NFATc3, and NFATc4 are regulated by calcineurin-mediated dephosphorylation (26, 36). Calcineurin activity and the translocation of NFAT factors can be inhibited by the immunosuppressive agents cyclosporine A (CsA) and FK506 through complexes with cyclophilins and FK506 binding proteins, respectively (11, 23, 38).
Calcineurin-NFAT signaling has been shown to play a critical role in regulating T-cell maturation and cytokine production, synaptic transmission in neurons, vascular patterning during embryonic development, and hypertrophic growth of the heart (reviewed in reference 10). More recently, calcineurin-NFAT signaling has been proposed to regulate skeletal muscle differentiation, hypertrophy, and fiber type specification, although each of these assertions has been disputed (reviewed in reference 32). For example, calcineurin has been implicated in the control of insulin-like growth factor 1 (IGF-1)-dependent myocyte hypertrophy, functional overload hypertrophy in vivo, and the mediation of growth after a period of atrophy (14, 28, 30, 40). However, other investigators have shown no discernible effect of CsA or FK506 on skeletal muscle hypertrophy at baseline, in response to functional overload, after a period of atrophy, or downstream of IGF-1 signaling (5, 16, 17, 33, 37, 41). More significantly, overexpression of an activated calcineurin cDNA in transgenic mice has no growth effect on skeletal muscle at baseline or after functional overload (16, 31).
In addition to hypertrophic growth, skeletal muscle can also undergo adaptive switching in fiber types in response to alterations in workload or frequency of use. Muscle fibers are generally characterized as being oxidative/slow (expressing primarily type I myosin heavy chain [MyHC]), intermediate, or glycolytic/fast (expressing type IId/x/b MyHC). Calcium levels in resting fast fibers are reported to be approximately 50 nM, while prolonged or chronic stimulation of fast fibers increases intracellular calcium concentration, resulting in slow-fiber transformation, implicating calcium as a fundamental regulator of this process (3, 6, 39, 43, 48, 49). Since calcineurin is only activated in response to sustained elevations in calcium levels (13), it is thought to participate in the regulation of fiber type switching in skeletal muscle (32). A significant number of reports have demonstrated that calcineurin-inhibitory agents induce a loss of oxidative/slow fiber number, with a concomitant increase in glycolytic/fast fibers in vivo or in cultured myotubes (9, 12, 14, 27, 33, 41, 46). However, two reports failed to identify a pervasive correlation between calcineurin inhibition and down-regulation of the oxidative/slow program in vivo (4, 44). A similar disparity exists between reports employing a gain-of-function approach. Specifically, overexpression of activated calcineurin in skeletal muscle by using direct injection of recombinant adenovirus or by transgenesis with a skeletal muscle-expressed promoter induced the oxidative/slow program in vivo (12, 31). In contrast, another group has reported no induction of the oxidative/slow program in similarly constructed transgenic mice at baseline or after functional overload (16). Collectively, these reports underscore the ongoing controversy surrounding the functional role of calcineurin-NFAT signaling in skeletal muscle hypertrophy and/or fiber type specification.
To address the areas of controversy discussed above, we analyzed both calcineurin Aα and Aβ gene-targeted mice. Such an approach permitted an evaluation of the phenotypic effects associated with reduced calcineurin activity, apart from potential nonspecific effects associated with CsA and FK506. Expression of the calcineurin Aα isoform was determined to predominate over calcineurin Aβ expression in nearly all muscles analyzed. Consistent with this expression profile, the nuclear content of NFATc1, -c2, -c3, and -c4 was significantly reduced in calcineurin Aα null mice but not in Aβ null mice. Similarly, with an NFAT-luciferase reporter transgenic line, only calcineurin Aα null mice showed a reduction in NFAT activity in skeletal muscle. However, both lines of gene-targeted mice showed a significant and generalized reduction in oxidative/slow fiber content in glycolytic/fast muscles, while only Aβ null mice showed a reduction in the oxidative/slow fiber content in the soleus muscle (primarily oxidative/slow muscle). Analysis of skeletal muscle growth-related indices revealed no significant alterations in the weights of any skeletal muscle analyzed in either gene-targeted line. Moreover, myofiber cross-sectional area and fiber number were unchanged in glycolytic/fast muscles (extensor digitorum longus [EDL] and biceps) from either gene-targeted line. However, the soleus muscle showed a minimal but significant reduction in fiber number in the absence of calcineurin Aα.
MATERIALS AND METHODS
Animals.
calcineurin Aβ−/− mice have been described previously (7, 8). calcineurin Aα−/− mice were generously provided by Jonathan Seidman (Harvard Medical School) and were previously described in detail (52). All animals had free access to food and water, and all experimentation was performed in the Cincinnati Children's Hospital Research Foundation animal care facility in accordance with the guidelines of the National Institutes of Health. Experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee. Mice between 8 and 10 weeks of age were used for analysis.
Western blotting.
Extracts were prepared in cell lysis buffer (20 mM sodium phosphate [pH 7.0], 150 mM NaCl, 2 mM MgCl2, 10 mM NaF, 0.1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 1 mM dithiothreitol, 1% NP-40, 10% glycerol, 10 μg of leupeptin per ml, 10 μg of aprotinin per ml, 10 μg of pepstatin per ml, 10 μg of Nα-tosyl-phenylalanyl chloromethyl ketone [TPCK] per ml, 10 μg of Nα-tosyl-lysyl chloromethyl ketone [TLCK] per ml), and proteins were resolved on a sodium dodecyl sulfate-8% polyacrylamide gel, transferred to a polyvinylidene difluoride membrane, and immunodetected by using an enhanced chemifluorescence kit as specified by the manufacturer (Amersham Biosciences, Piscataway, N.J.). The following antibodies were used: calcineurin pan-A rabbit polyclonal antibody (Chemicon International, Inc., Temecula, Calif.), α-tubulin monoclonal antibody (MAb) (Santa Cruz Biotechnology, Santa Cruz, Calif.), and isoform-specific calcineurin A rabbit polyclonal antibodies. Affinity purified, isoform-specific antibodies were custom synthesized in rabbits by Zymed Laboratories, Inc., by using the calcineurin Aα epitope NH2-CSETNGTDSNGSNSSNIQ-COOH and the calcineurin Aβ epitope NH2-CHTTENHGTGNHTPQ-COOH. Western blot reactivity was quantified on a Storm860 PhosphorImager (Molecular Dynamics, Piscataway, N.J.) using ImageQuant software.
For MyHC Western blotting, extracts were prepared as described previously (24). Briefly, excised muscles were homogenized in Isotris (10 mM Tris-HCl [pH 7.4] and 0.9% NaCl) plus 1 mM phenylmethylsulfonyl fluoride, and 75 μl of 20% sodium dodecyl sulfate was added to the homogenate. The samples were vortexed, boiled, and centrifuged, and the supernatant was transferred to a fresh tube and used for Western blotting. Proteins were resolved and blotted as stated above. The following antibodies were used: NOQ7.5.4D, MyHC I MAb (Sigma, St. Louis, Mo.); BF-F3, MyHC IIb MAb; SC-71, MyHCIIa+IIx MAb (German Collection of Microorganisms and Cell Cultures).
Western blots of proteins from nuclear extracts were resolved on 8% polyacrylamide gels (5% when detecting NFAT isoforms), and the following antibodies were used: pan-myocyte enhancer factor-2 (MEF2), Elk-1, NFATc2, NFATc3, NFATc4, GATA-2, PGC-1α (Santa Cruz Biotechnology, Inc.), and NFATc1 (Pharmingen, San Diego, Calif.). PGC-1α antibody was also generously supplied by Bruce M. Spiegelman.
Immunohistochemistry.
Frozen muscle samples were sectioned into 10-μm-thick sections and placed immediately into phosphate-buffered saline (PBS). Muscle sections were blocked in 10% horse serum-PBS for 1 h at room temperature. The primary antibodies used were NOQ7.5.4D (MyHC I) and My32 (type II MyHCs) (Sigma). Primary antibodies were diluted 1:200 in blocking solution, and incubations were carried out for 2 h at room temperature. Tissue sections were washed three times with PBS for 5 min each, followed by incubation in fluorescein isothiocyanate-conjugated goat anti-mouse secondary antibody. Secondary antibody was diluted 1:400 in blocking solution, and tissue sections were incubated for 1 h at room temperature. Tissue sections were then washed three times with PBS and mounted with VectaShield (Vector Laboratories, Burlingame, Calif.) and coverslips.
Nuclear protein preparation.
Total mouse skeletal muscle was collected from wild-type, calcineurin Aα−/−, and calcineurin Aβ−/− mice, and protein extracts were prepared by using a method modified from one previously described (19). Briefly, crude nuclear extract was spun on a sucrose cushion for 30 min at 4°C at 24,000 rpm using a Beckman SW28 rotor. Nuclei were then collected and lysed in lysis buffer (10 mM HEPES [pH 7.6], 100 mM KCl, 3 mM MgCl2, 0.1 mM EDTA, 10% glycerol, 1 mM dithiothreitol, 0.1 mM phenylmethylsulfonyl fluoride, 5 μg of TLCK/ml, 5 μg of TPCK/ml, and 2 μg each of aprotinin and pepstatin A/ml). Nuclear proteins were precipitated by using one-fifth volume of 2 M ammonium sulfate and spun at 4°C for 25 min at 38,000 rpm using a Beckman 70.1Ti rotor. Pelleted protein was then resuspended in dialysis buffer (25 mM HEPES [pH 7.6], 40 mM KCl, 0.1 mM EDTA, 10% glycerol, 1 mM dithiothreitol, 0.1 mM phenylmethylsulfonyl fluoride, 5 μg each of TLCK and TPCK/ml, and 1 μg of aprotinin/ml,) and dialyzed for 3 h, with one change of dialysis buffer.
Fiber cross-sectional area and average fiber number.
EDL and soleus skeletal muscles from wild-type, calcineurin Aα−/−, and Aβ−/− mice were collected, fixed in 10% formalin containing PBS, and embedded in paraffin. Four sections cut at 5 μm were analyzed from each muscle. Sections were deparaffinized in xylene and rehydrated in decreasing dilutions of ethanol. To visualize fibers, sections were incubated with tetramethyl rhodamine isothiocyanate (TRITC)-labeled wheat germ agglutinin (50 μg/ml in 1× PBS; Sigma) for 1 h, washed three times for 10 min each in PBS, and mounted with VectaShield and coverslips. Sections were viewed and photographed by using an Olympus BX51 microscope in conjunction with an Olympus U-TVI X digital camera. Fiber cross-sectional area and average fiber number were assessed by using MagnaFire Image Capture and ImagePro Plus Imaging software. Average fiber cross-sectional areas from approximately 300 fibers per muscle per mouse were determined. To determine average fiber number, total fibers in an entire muscle were quantified and the total area was divided by the number of fibers. This figure was then used to determine the number of fibers in a 100,000-μm2 area.
NADH-tetrazolium reductase staining.
Frozen tissue sections were incubated for 15 min at room temperature in a solution of 0.2 M Trizma base, 1.5 mM nicotinamide adenine dinucleotide (NADH), and 1.5 mM nitro tetrazolium blue. Sections were then serially incubated with acetone solutions (60, 80, 100, 80, and 60% for 2 min each) and mounted with Aquamount (Lerner Labs, Pittsburgh, Pa.). NADH-tetrazolium staining revealed three distinct fiber profiles: unstained (representing type IIb), moderately stained (representing type IIa+IId/x), and intensely stained (representing type I).
Statistical analysis.
Data are expressed as the means ± standard errors of the means. Statistical significance was determined by Student's t test at the P < 0.05 level.
RESULTS
Calcineurin Aα is the predominant catalytic isoform expressed in skeletal muscle.
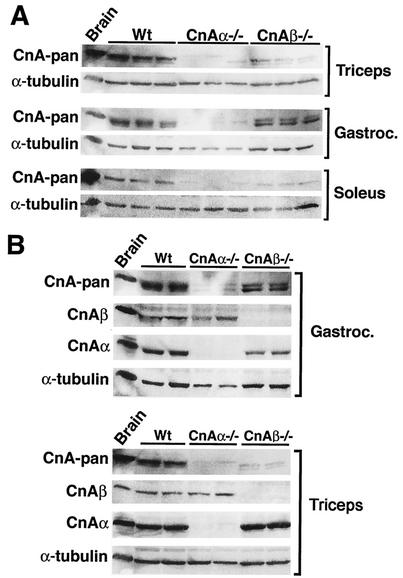
Calcineurin Aα and Aβ catalytic isoforms are encoded by separate genes that are ubiquitously expressed throughout most vertebrate tissues and cell types, although the relative levels of expression vary such that Aα predominates in the brain while Aβ predominates in T and B cells (21). It was of interest to determine which calcineurin catalytic isoform is most highly expressed in skeletal muscle, especially given calcineurin's role in potentially regulating muscle-specific gene expression and fiber-type specification (reviewed in reference 32). The triceps, gastrocnemius, and soleus muscles from wild-type, calcineurin Aα−/−, and calcineurin Aβ−/− mice were each analyzed for calcineurin protein levels by using a cross-reactive catalytic subunit antibody (pan) (Fig. 1A). calcineurin Aα−/− mice displayed a more substantial reduction in total catalytic protein content than did Aβ−/− mice for each of the muscles examined, while α-tubulin levels were largely invariant (Fig. 1A). Quantitation of these data indicated that calcineurin Aα comprises approximately 80% of the total catalytic protein content within the gastrocnemius muscle. However, triceps and soleus showed slightly less dichotomy, so that only ≈60% of the total calcineurin A content was composed of calcineurin Aα (Fig. 1A).
FIG. 1.
Western blots of calcineurin protein expression in mouse skeletal muscles. (A) Western blots of total calcineurin A expression in triceps, gastrocnemius, and soleus from wild-type (Wt), calcineurin Aα−/−, and Aβ−/− mice. α-Tubulin was included as a loading control. (B) Isoform-specific calcineurin A Western blots of protein extracts from gastrocnemius and triceps from wild-type or calcineurin A gene-targeted mice.
Calcineurin Aα and Aβ isoform expression was also quantified in the skeletal muscles of each targeted line to examine potential compensatory alterations in gene expression within this family. Western blotting with pan and isoform-specific antibodies failed to reveal any compensatory alterations in either calcineurin Aα or Aβ protein levels in skeletal muscle from Aβ−/− or Aα−/− mice, respectively (Fig. 1B).
Oxidative/slow fibers have more calcineurin-NFAT activity in vivo.
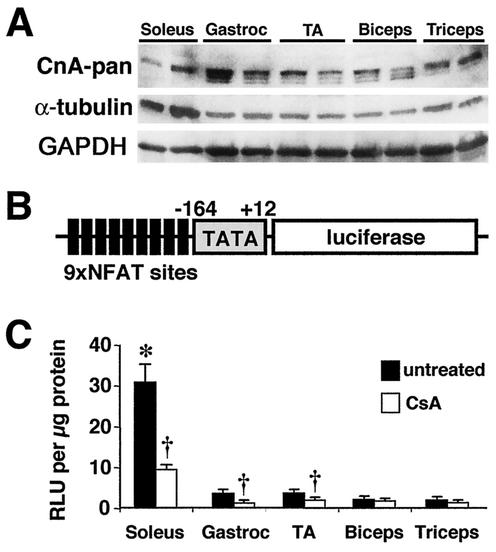
It was also of interest to evaluate calcineurin A protein expression levels and activity between oxidative/slow and glycolytic/fast fiber types. Western blotting with the pan-reactive calcineurin A antibody demonstrated slightly higher calcineurin A protein levels in predominantly glycolytic/fast muscles (gastrocnemius, tibialis anterior, biceps, and triceps) than in oxidative slow muscles (soleus) (Fig. 2A). Glycolytic/fast muscles showed three different calcineurin A isoforms, which might arise due to differential splicing or another posttranslational modification, while the soleus muscle showed only one isoform (Fig. 2A). The calcineurin Western blots were normalized to both α-tubulin and GAPDH, given that each control itself appeared to be differentially expressed in fast versus slow muscles (equal protein amounts were loaded) (Fig. 2A).
FIG. 2.
Calcineurin expression and activity between fast and slow muscles. (A) Western blot of total calcineurin A protein content between oxidative/slow (soleus) and glycolytic/fast (gastrocnemius, tibialis anterior [TA], biceps, and triceps) muscles. α-Tubulin and GAPDH are shown as loading controls. (B) Schematic of the transgene construct that was used to make NFAT-dependent reporter mice. (C) Luciferase activity normalized as relative light units (RLU) per microgram of protein from fast and slow muscles taken from untreated transgenic mice (n = 5 mice) or CsA-treated mice (n = 8 mice) (asterisk indicates P < 0.05 versus any of the four untreated fast muscles, and daggers indicate P < 0.05 versus untreated muscles).
While expression profiling suggested greater calcineurin protein content in glycolytic/fast muscles, it was uncertain how these protein levels relate to actual enzymatic activity in vivo. Traditional methods for assessing calcineurin activity utilize an in vitro enzymatic assay in the presence of saturating calmodulin and a phosphorylated RII peptide substrate (38). This assay does not accurately reflect the specific activity of calcineurin in vivo, so evaluation of NFAT nuclear localization and transcriptional activity is often used as a surrogate for evaluation of the activity of calcineurin in vivo (38). Given this, we generated a series of NFAT reporter transgenic mice containing a DNA construct consisting of nine copies of an NFAT-only DNA binding site upstream of a minimal α-MyHC promoter fused to luciferase (Fig. 2B). After screening a large number of founders, two transgenic lines were identified that demonstrated robust calcineurin transgene-induced expression in the heart that could be inhibited with CsA (B. J. Wilkins and J. D. Molkentin, unpublished data). These transgenic lines also demonstrated expression in skeletal muscle and were subsequently evaluated for differential activity between slow and fast fiber type muscles. To verify the specificity of the calcineurin-sensitive NFAT reporter, these transgenic mice were treated with the calcineurin inhibitor CsA for 3 days at 20 mg/kg of body weight/day (eight mice). Untreated NFAT-luciferase transgenic mice showed significantly greater NFAT activity in the oxidative/slow soleus than in the largely glycolytic/fast muscles (five mice; two muscles per mouse; P < 0.05) (Fig. 2C). Importantly, CsA treatment reduced reporter activity, verifying the specificity of this approach (P < 0.05) (Fig. 2C). Background luciferase activity from nontransgenic muscles was assayed, and a mean value of 0.68 relative light units/μg of protein was found. The biceps and triceps failed to show a significant effect with CsA, most likely due to the low starting signal. Collectively, these results indicate that oxidative/slow fibers have significantly more calcineurin enzymatic activity than glycolytic/fast fibers in vivo.
Calcineurin gene-targeted mice have a reduced oxidative/slow fiber type profile.
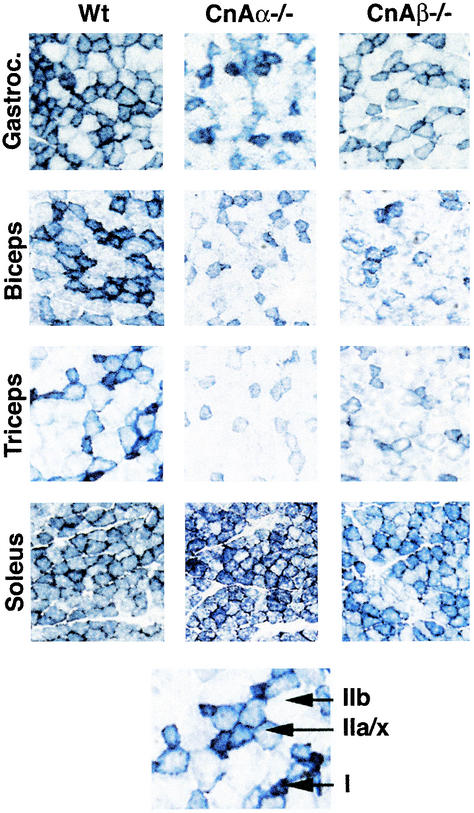
Overexpression of activated calcineurin in the gastrocnemius of adenovirally infected rat pups or in transgenic mice has been reported to ectopically induce the slow/oxidative fiber type program (12, 31), although this conclusion was disputed in another study that employed the same general activated calcineurin construct in transgenic mice (16). To address this area of controversy, a loss-of-function genetic approach was employed. Skeletal muscle from calcineurin Aα−/− and Aβ−/− mice at 8 to 10 weeks of age was analyzed for oxidative capacity by using NADH-tetrazolium reductase staining for mitochondrial oxidative capacity. The data demonstrate a noticeable reduction in the most oxidative fibers (type I associated, dark blue) and the intermediate oxidative fibers (type IIa associated, light blue) in each of the skeletal muscles examined from calcineurin Aβ−/− mice (Fig. 3). By comparison, calcineurin Aα−/− mice also showed a reduction in oxidative capacity in gastrocnemius, biceps, and triceps but not in soleus (Fig. 3). That the soleus muscle was unaffected in calcineurin Aα null mice is intriguing but likely reflects a distinct regulatory program between fast and slow fiber types (soleus is typically the most oxidative/slow muscle type in the body [see Discussion]). Similar results were observed in three independent experiments. Despite the exception observed in the soleus, overall our results indicate a down-regulation in the oxidative program of skeletal muscle in association with a reduction in calcineurin protein. Moreover, these genetic data are consistent with the ability of CsA, a calcineurin inhibitor, to reduce the oxidative/slow muscle program in some experimental systems (9, 12, 14, 27, 33, 41, 46).
FIG. 3.
NADH-tetrazolium staining of various skeletal muscles from wild-type, calcineurin Aα−/−, and Aβ−/− mice. The darkest blue fibers are the most oxidative (type I), the lighter blue fibers are intermediate oxidative/glycolytic (type IIa/x), and the unstained fibers are glycolytic (type IIb).
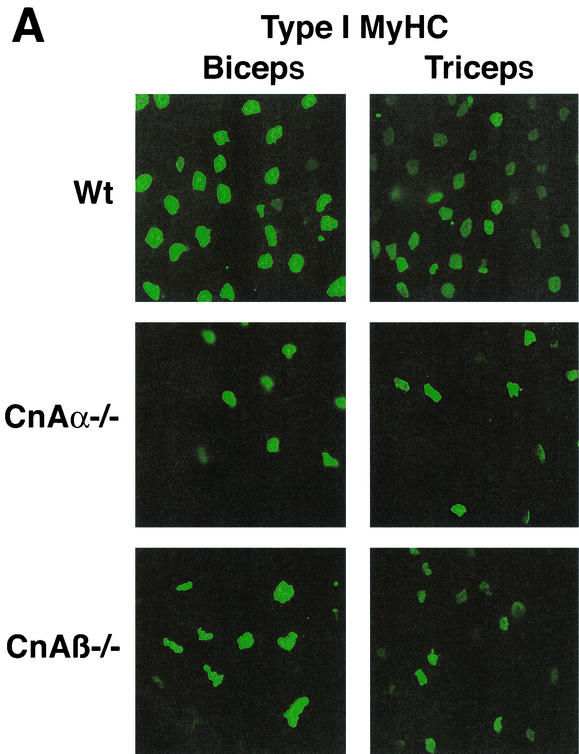
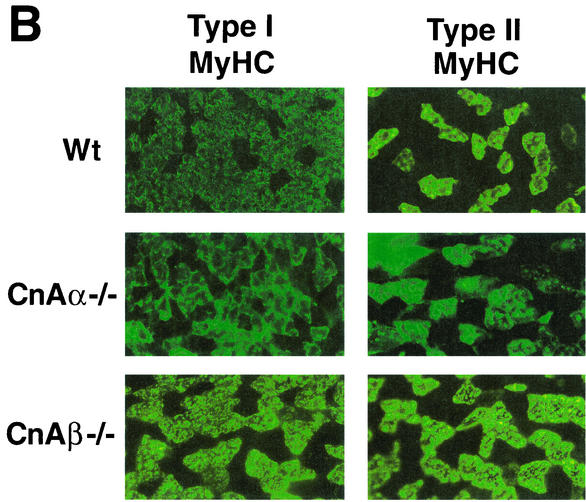
Immunohistochemistry was performed with type I MyHC MAb to more thoroughly examine the phenotypic alterations observed in skeletal muscle from calcineurin Aα−/− and Aβ−/− mice. calcineurin Aα−/− and Aβ−/− mice show a noticeable down-regulation in type I (slow program)-expressing fibers in the biceps and triceps (Fig. 4A) and in the gastrocnemius and extensor carpi radialis largus muscles (data not shown). Similar results were observed in three independent experiments. With respect to the soleus, immunohistochemistry against type I MyHC demonstrated a significant reduction in expression in calcineurin Aβ−/− mice and a corresponding up-regulation of fast type II MyHC expression (Fig. 4B). However, soleus muscle from calcineurin Aα−/− mice consistently failed to show any alteration in type I or type II MyHC protein expression (Fig. 4B), despite the fact that calcineurin Aα expression is slightly greater than that of calcineurin Aβ in the soleus (see Discussion).
FIG. 4.
Immunostaining for MyHC expression in muscles from wild-type, calcineurin Aα−/−, and Aβ−/− mice. (A) Type I (slow) MyHC expression is noticeably reduced in biceps and triceps from both calcineurin Aα−/− and Aβ−/− mice (green stain is fluorescein isothiocyanate-conjugated secondary antibody to detect type I MyHC). (B) Type I (slow) and type II (fast) MyHC expression in soleus muscle from calcineurin Aα−/− and Aβ−/− mice.
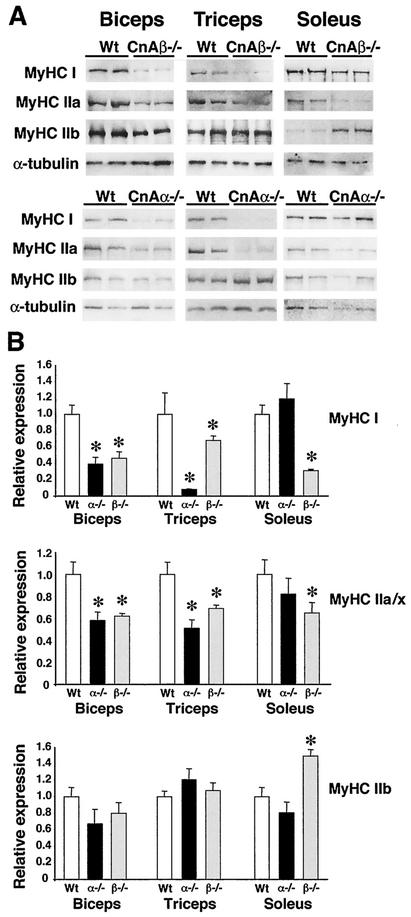
Quantitative Western blotting was also performed to more accurately assess the oxidative/slow program of skeletal muscles of calcineurin Aα−/− and Aβ−/− mice. A significant reduction in both the slowest MyHC (type I) and the intermediate slow/fast MyHC (type IIa) was observed in biceps, triceps, and soleus of calcineurin Aβ−/− mice (Fig. 5). Since both biceps and triceps in the mouse are largely glycolytic/fast, a corresponding up-regulation of fast MyHC (type IIb) was not noticeable (Fig. 5). However, the largely oxidative/slow soleus muscle readily showed an up-regulation in fast MyHC in calcineurin Aβ−/− mice (Fig. 5).
FIG. 5.
Western analysis of MyHC protein expression. (A) Biceps, triceps, and soleus from wild-type, calcineurin Aα−/−, and Aβ−/− mice were subjected to Western blotting to detect MyHC I, IIa, and IIb isoform expression. (B) Quantitation of MyHC Western blots (from four individual mice). Asterisks indicate P < 0.05 versus wild-type mice.
calcineurin Aα−/− mice also demonstrated a significant down-regulation in type I and type IIa MyHC expression in biceps and triceps but not soleus (Fig. 5). That fiber type specification was not altered in the soleus of calcineurin Aα−/− mice is also supported by the lack of alteration in type IIb (fast) MyHC, consistent with the NADH-tetrazolium reductase staining and immunohistochemistry data discussed above (Fig. 5). These data were quantified from four separate mice of each genotype. We also performed another independent series of similar Western blot experiments with approximately 8-month-old calcineurin Aβ−/− mice and observed a similar pattern of down-regulated type I and type IIa MyHC in muscle (data not shown). Collectively, these results show a generalized down-regulation of the oxidative/slow program in association with reduced calcineurin protein content, despite the minor exception of soleus in calcineurin Aα−/− mice.
Loss of calcineurin and muscle fiber size.
Enhanced calcineurin activity has been associated with both skeletal muscle differentiation (1, 12, 18, 30) and IGF-1-mediated hypertrophy (30, 40). However, overexpression of activated calcineurin in skeletal muscle by transgenesis in the mouse did not induce hypertrophy, and the assertion that calcineurin mediates IGF-1-regulated skeletal muscle hypertrophy has been disputed (5, 16, 31, 37). Given these discordant results, the phenotypes of skeletal muscle in both calcineurin Aα−/− and Aβ−/− mice were assessed for growth alterations. Analysis of individual muscle weights normalized to body weight for soleus, triceps, gastrocnemius, tibialis anterior, and quadriceps failed to identify any differences between wild-type, calcineurin Aα−/−, and Aβ−/− mice (at least six muscles were weighed for each group) (Table 1). These five muscles were chosen based on their ability to be unambiguously dissected from surrounding muscle tissue. These results suggest the lack of a significant growth defect in skeletal muscle in the absence of either calcineurin catalytic isoform. However, both lines of calcineurin gene-targeted mice showed a significant reduction in body weight for unknown reasons, although muscle weights are appropriately matched to bone lengths and body weights, negating the possibility that reduced muscle weights cause the reduction in body weight.
TABLE 1.
Physical measurements in wild-type, calcineurin Aα−/−, and Aβ−/− mice
| Mouse | Body weighta (g) | Tibialis anterior/body (mg/g) | Gastrocnemius/body (mg/g) | Soleus/body (mg/g) | Triceps/body (mg/g) | Quadriceps/body (mg/g) |
|---|---|---|---|---|---|---|
| Wild type | 30.3 ± 0.7 (10) | 1.61 ± 0.04 (16) | 4.57 ± 0.15 (16) | 0.28 ± 0.01 (16) | 3.75 ± 0.10 (16) | 6.05 ± 0.15 (16) |
| Aα−/− | 25.7 ± 1.6b (9) | 1.54 ± 0.04 (22) | 4.29 ± 0.09 (17) | 0.28 ± 0.01 (18) | 3.76 ± 0.11 (18) | 6.19 ± 0.14 (18) |
| Aβ−/− | 27.0 ± 1.0b (14) | 1.49 ± 0.07 (7) | 4.33 ± 0.18 (6) | 0.29 ± 0.02 (7) | 3.40 ± 0.19 (7) | 6.33 ± 0.32 (7) |
All values are means ± SEM. Numbers in parentheses indicate number of mice (for measurement of body weight) or number of muscles (for all other measurements).
P < 0.05 versus wild type.
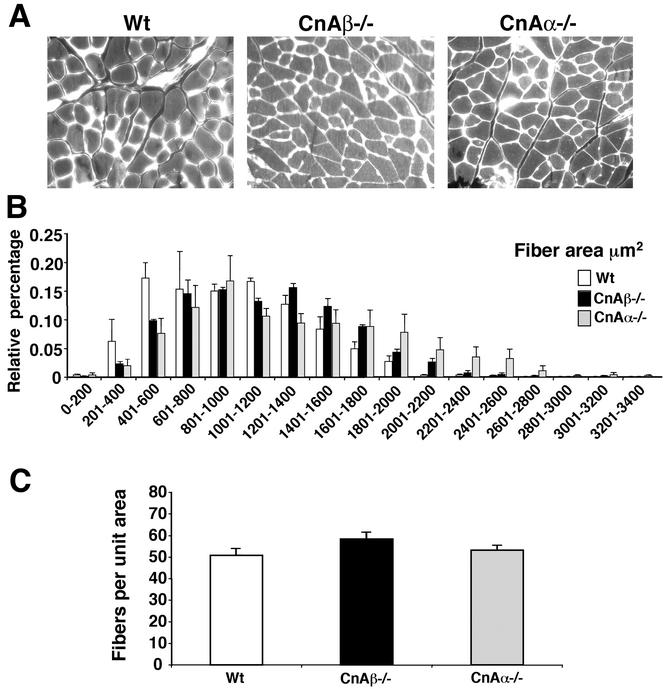
Microscopically, sections from the EDL muscle were stained with wheat germ agglutinin-TRITC conjugate to permit a quantitative assessment of fiber cross-sectional area (from four separate mice of each genotype) (Fig. 6A). The data demonstrate no significant difference in cross-sectional fiber areas of any size range in either calcineurin Aα−/− or Aβ−/− mice compared with littermate control wild-type mice (Fig. 6B). Similar negative results were also observed in biceps (data not shown). Finally, the number of fibers per unit of area was also assessed to quantify any potential differences in total fiber number. Since muscle weights did not significantly vary and fiber cross-sectional areas were similar, no differences in fiber number should be observed. Indeed, no differences were observed in EDL or biceps fiber number from either calcineurin Aα−/− or Aβ−/− mice (Fig. 6C and data not shown). These data indicate that loss of calcineurin Aα or Aβ does not influence the growth or specification of muscle fibers in glycolytic/fast muscles.
FIG. 6.
Histological analysis of EDL from wild-type, calcineurin Aα−/−, and Aβ−/− mice. (A) Representative sections of wheat germ agglutinin-TRITC-labeled EDL muscle. (B) Fiber areas in four mice each were measured from wild-type, calcineurin Aα−/−, and Aβ−/− mice. Approximately 300 fibers were measured per muscle section and grouped by size in 200-μm2 increments. (C) Average number of fibers per unit of area (100,000 μm2). No values were significantly different.
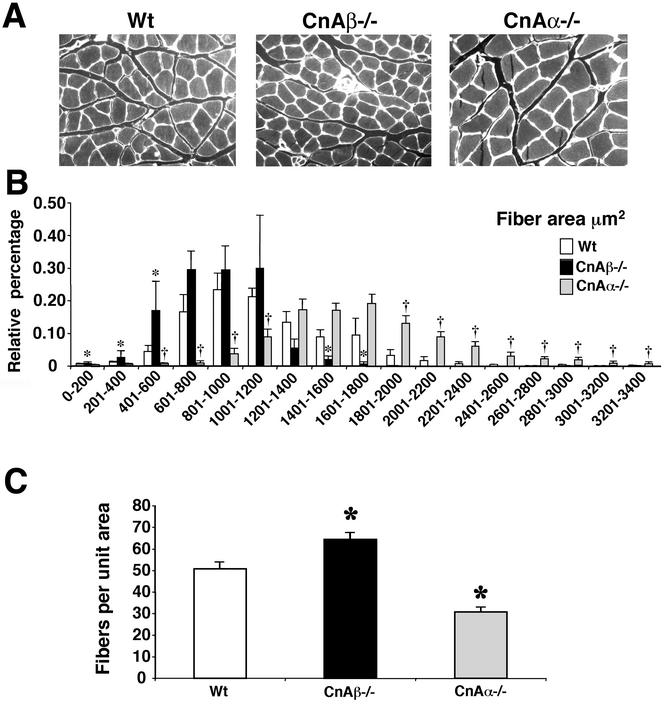
While no alterations were observed in predominantly glycolytic/fast muscles, oxidative/slow muscles have a different profile of calcineurin expression and subsequent transcriptional responses, suggesting that calcineurin might have distinct functions in slow versus fast muscles (4, 44). Indeed, we observed that the soleus muscle from calcineurin Aα−/− mice showed significantly greater cross-sectional fiber areas and fewer fibers than did wild-type mice (P < 0.05) (Fig. 7). In contrast, the soleus muscle from calcineurin Aβ−/− mice showed a trend towards smaller cross-sectional areas and greater fiber numbers that reached significance in a few of the fiber diameter ranges (P < 0.05) (Fig. 7). These data were interpreted to indicate that loss of calcineurin Aα reduces the number of fibers in the predominantly oxidative/slow soleus muscle, which likely causes a secondary increase in fiber diameter in an attempt to maintain overall muscle size. In contrast, loss of calcineurin Aβ was associated with a trend towards the opposite phenotype, likely due to a greater specification of muscle fibers in the soleus (see Discussion). These data are also partially consistent with a reduction in total fiber number observed in NFATc3 null mice (22).
FIG. 7.
Histological analysis of soleus from wild-type, calcineurin Aα−/−, and Aβ−/− mice. (A) Representative sections of wheat germ agglutinin-TRITC-labeled soleus muscle. (B) Fiber areas in four mice each were measured from wild-type, calcineurin Aα−/−, and Aβ−/− mice. Approximately 300 fibers were measured per muscle section and grouped by size in 200-μm2 increments. Asterisks indicate P < 0.05 for wild-type versus calcineurin Aβ−/− mice, and daggers indicate P < 0.05 for wild-type versus calcineurin Aα−/− mice. (C) Average number of fibers per unit of area (100,000 μm2). Asterisks indicate P < 0.05 versus wild type.
Calcineurin-deficient mice show alterations in NFAT nuclear content and activity.
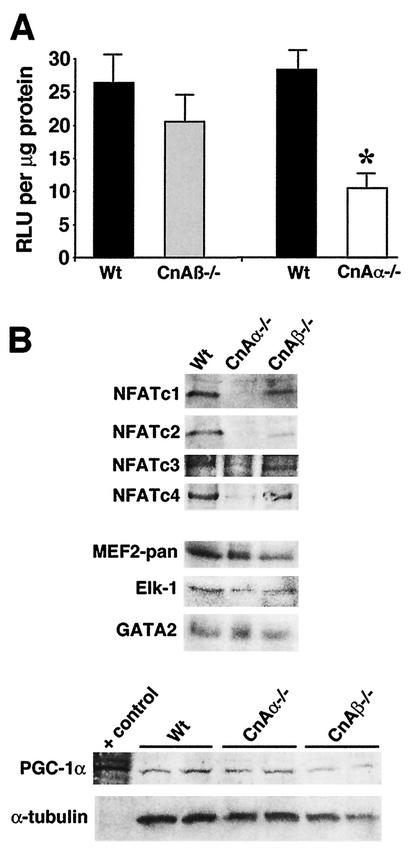
NFAT is a critical downstream effector of calcineurin that can influence skeletal muscle development (20, 22) but probably not fiber type specification (12). The activity and nuclear localization of NFATc1, -c2, -c3, and -c4 was analyzed in muscle from wild-type, calcineurin Aα−/−, and Aβ−/− mice by two distinct assays. First, mice containing the NFAT-luciferase transgene described above were crossed into the calcineurin Aα−/− and Aβ−/− backgrounds to assay for a potential reduction in activity in the muscle with the most robust activity (soleus). Interestingly, loss of calcineurin Aβ had no significant effect on NFAT-luciferase expression compared with what was seen with wild-type littermates with the transgene (Fig. 8A). In contrast, loss of calcineurin Aα resulted in a dramatic down-regulation in NFAT-luciferase activity in the soleus (Fig. 8A), similar to the reduction observed with CsA (Fig. 2C). Using a separate approach, three independent experiments were performed with purified nuclear extracts obtained from total body muscle (three mice each), followed by three series of Western blots for NFATc1, -c2, -c3, and -c4 nuclear content. A representative panel of these Western blots from wild-type, calcineurin Aα−/−, and Aβ−/− mice is shown (Fig. 8B), demonstrating a remarkable reduction in all NFATs in the nucleus of Aα−/− muscle, without a change in Aβ−/− muscle. By comparison, no difference in MEF2, Elk-1, PGC-1α, or GATA2 nuclear content was observed in the absence of either calcineurin isoform (Fig. 8B). These data suggest that calcineurin Aα, which is the predominant isoform expressed in skeletal muscle, plays an important role in regulating NFAT activity and suggest a potential mechanism underlying the reduction in fiber number observed in the soleus of Aα−/− mice. However, these data also suggest that NFAT activation does not directly influence fiber type specification since calcineurin Aβ−/− mice have normal NFAT nuclear occupancy and activity despite a consistent reduction in oxidative/slow fibers.
FIG. 8.
(A) Relative luciferase activity (in relative light units [RLU] per microgram of protein) from soleus muscle from mice containing the NFAT-luciferase reporter crossed into either calcineurin Aα−/− (n = 4) or Aβ−/− (n = 5) mice compared with that of littermate wild-type controls (n = 6). Asterisks indicate P < 0.05 versus wild type. (B) Western blots of nuclear proteins from skeletal muscle of wild-type, calcineurin Aα−/−, and Aβ−/− mice (n = 3 mice per nuclear protein preparation per experiment). Western blotting for NFATc1, -c2, -c3, and -c4 revealed a significant reduction in nuclear content in the absence of calcineurin Aα, but not Aβ. No change in total MEF2, Elk-1, or GATA2 was detected from any of the nuclear extracts or in PGC-1α from soleus whole-cell protein extracts.
DISCUSSION
Calcineurin isoform-specific regulatory roles.
While vertebrates contain three distinct calcineurin A catalytic genes (α, β, and γ), only the Aα and Aβ isoforms are expressed in skeletal muscle. Using muscle from calcineurin Aα and Aβ null mice, the Aα isoform was shown to constitute the majority of catalytic isoform content in each skeletal muscle analyzed. This conclusion is based on Western blotting with a pan-calcineurin A antibody that recognizes the more conserved catalytic region of the protein (greater than 95% identity between Aα and Aβ in this region). Use of a single antibody with tissue from gene-deleted mice permits direct assessment of total protein content for each isoform, assuming similar antibody affinities. In contrast, using separate isoform-specific antibodies, Dunn et al. reported that calcineurin Aβ was the most abundant isoform expressed in skeletal muscle (16).
Expression of calcineurin Aα and Aβ are differentially regulated such that Aα protein is most abundant in brain while Aβ is most abundant in immune cells. Each isoform is also regionally expressed in the kidney, brain, and thymus (21, 47). More significantly, calcineurin Aα protein was reported to be enriched in the nuclei of neurons while Aβ protein was largely cytoplasmic (47). In the heart, each isoform comprises approximately 50% of the total catalytic isoform expression (8), although calcineurin Aβ protein levels increase during hypertrophy while Aα levels remain unchanged (45). At the biochemical level, calcineurin Aα and Aβ differ slightly in their enzymatic properties such that Aα has a lower Km and a higher Vmax toward the phosphorylated RII peptide than does Aβ (35). While calcineurin Aα and Aβ each show identical calmodulin dissociation rates and similar inhibition curves to the autoinhibitory peptide, the Aα isoform is more sensitive to FK506 inhibition than is Aβ (35). Collectively, these previous reports indicate that calcineurin Aα and Aβ are subject to different levels of regulation in multiple tissues and that each has slightly different biochemical properties. Such differences in relative expression levels, subcellular localization, association with cofactors, or biochemical properties might underlie the subtle differences in skeletal muscle phenotypes observed in calcineurin Aα and Aβ gene-targeted mice (see below).
Role of calcineurin in regulating the oxidative/slow fiber type program.
The longstanding conclusion that motoneuron activity directly regulates skeletal muscle fiber type specificity suggests a linkage between usage and gene expression (3, 6, 39, 43, 48, 49). Neuromuscular stimulation associated with oxidative/slow fibers shows a more continuous oscillation of calcium between 100 and 300 nM, while glycolytic/fast fibers show discontinuous action potentials and resting calcium levels of 50 nM (reviewed in reference 32). These fundamental differences in calcium handling suggested the hypothesis that calcium itself directly regulates the fiber type gene program through an unknown signal transduction pathway. Chin et al. initially proposed that calcineurin might function as the molecular sensor of motoneuron activity through calcium (9), especially given the observation that calcineurin is only activated by sustained elevations in intracellular calcium concentrations that typify oxidative/slow fibers (13). Indeed, Chin et al. observed that inhibition of calcineurin with cyclosporine in the rat promoted a dramatic down-regulation in oxidative/slow fibers in vivo (9). Since their study, a number of groups have reported that calcineurin-inhibitory agents similarly induce a loss of oxidative/slow fiber number with a concomitant increase in glycolytic/fast fibers in vivo or in cultured myotubes (9, 12, 14, 27, 33, 41, 46).
Despite the large number of supportive studies discussed above, others have disputed calcineurin's ability to regulate the fiber type program in skeletal muscle (4, 16, 44). For example, overexpression of activated calcineurin by using the muscle creatine kinase promoter in transgenic mice dramatically enhanced the oxidative/slow fiber type program in vivo (31). In support of this conclusion, direct injection of an activated calcineurin-encoding adenovirus into the gastrocnemius induced slow MyHC (type I) protein expression that was coincident with infected fibers, while control adenoviral infection had no effect (12). In contrast, Dunn et al. recently described transgenic mice expressing activated calcineurin under control of the muscle creatine kinase or myosin light chain promoter regions, neither of which showed any alterations in fiber type composition at baseline or after functional overload (16). While the transgenic lines employed by Naya et al. and Dunn et al. were different (although the same promoter was used), the ultimate reason for the discordant results might reflect differing expression levels of the activated calcineurin protein, nonuniform transgene expression so that most fibers do not express the transgene, or developmental windows whereby the activated calcineurin transgene can have its influence.
The data presented in this report support the conclusions of Naya et al. and help solidify the hypothesis that calcineurin directly participates in programming the oxidative/slow program of skeletal muscle. Both calcineurin Aα and Aβ gene-targeted mice demonstrated a significant reduction in oxidative/slow fiber content within highly glycolytic muscles (gastrocnemius, biceps, and triceps) according to three separate criteria (NADH-TR staining, immunohistochemistry, and Western blotting). Moreover, calcineurin Aβ−/− mice also showed a significant down-regulation in the oxidative/slow program in the soleus muscle and a corresponding up-regulation in glycolytic/fast fibers. Intriguingly, calcineurin Aα null mice failed to show a defect in the oxidative/slow program in the soleus, despite the fact that the Aα isoform is the most abundantly expressed catalytic isoform in this muscle. However, the soleus muscle is one of the very few predominantly oxidative/slow muscle types in the mouse, suggesting that it may have different regulatory features. More importantly, the soleus muscle has already been shown to have alterations in calcineurin signaling constituents compared with more highly glycolytic muscles. For example, CsA more severely affected the growth of the plantaris (fast) muscle than that of the soleus muscle (28). In addition, calcineurin A expression levels were reported to be significantly higher in fast-type muscles than in soleus (28, 42, 44), a finding which we have also confirmed by Western blotting (Fig. 2A). In contrast, soleus muscle has higher NFAT expression than EDL (fast) or gastrocnemius (fast) (15, 44), while the fast plantaris muscle expresses significantly higher levels of the regulatory calcineurin B subunit than does soleus (28). In this report, we show that the oxidative/slow soleus muscle has greater calcineurin enzymatic activity in vivo than do glycolytic/fast muscles. These results underscore the regulatory differences that likely occur between fast and slow muscle types with respect to calcineurin signaling.
Another interesting aspect of our present study was the observed discordance between NFAT nuclear content and fiber type switching. For example, NFAT nuclear content was not significantly altered in skeletal muscle from calcineurin Aβ gene-targeted mice compared with that from wild-type mice, yet the oxidative/slow program was uniformly down-regulated. By comparison, calcineurin Aα−/− mice showed a significant reduction in NFAT nuclear protein levels, suggesting less-efficient calcineurin-directed translocation. The simplest interpretation of these data is that NFAT is not the determinative factor for mediating fiber type specification downstream of calcineurin. In support of this hypothesis, Delling et al. previously reported that NFAT overexpression in cultured cells did not enhance the slow program, in contrast to calcineurin overexpression (12).
While NFAT factors are unlikely to directly participate in the fiber type gene program, another calcineurin effector protein, MEF2, represents a more attractive candidate (32). Calcineurin directly activates MEF2 transcriptional activity in the heart and skeletal muscle without changing protein levels or DNA binding capacity (34, 50, 51). This calcineurin-dependent alteration in MEF2 activity has also been proposed to directly mediate fiber type switching in skeletal muscle (50, 51). More recently, this concept was extended to show that peroxisome-proliferator-activated receptor gamma coactivator-1 (PGC-1α) directly mediates fiber type switching to the oxidative/slow program through a calcineurin-MEF2-dependent signaling pathway (25). However, calcineurin Aα and Aβ null mice showed no alteration in PGC-1α protein content in the soleus. While NFAT has been shown to augment expression of the MyHC type I and type IIa promoters in transfected cultured cells (2, 41), the evidence contained in this report and the other data discussed above collectively suggest that NFAT factors are not direct physiologic activators of the oxidative/slow program. It remains to be determined whether MEF2 transcriptional activity is altered in skeletal muscle of calcineurin Aα or Aβ null mice.
Role of calcineurin in regulating skeletal muscle differentiation.
The results discussed above suggest that NFAT factors do not directly participate in regulating the fiber type gene program of skeletal muscle. Such a conclusion calls into question the function or importance of NFAT factors as transducers of calcineurin signaling in skeletal muscle. Previous studies have indicated that NFAT factors are involved in regulating skeletal muscle development and/or myotube differentiation. For example, overexpression of calcineurin or NFAT has been shown to dramatically enhance myotube differentiation in cultured cells (12). Loss-of-function studies have also supported a role for NFAT factors in skeletal muscle development. NFATc3−/− mice show embryonic defects in the formation of primary myofibers (22), while NFATc2−/− mice show defects in postnatal maturation of skeletal muscle (20). Here, calcineurin Aα−/− mice demonstrated a defect in NFAT nuclear occupancy that was associated with a reduction in total myofiber number within the soleus. This result is consistent with the reduction in myofiber number reported in NFATc3−/− mice (22). However, each of the glycolytic/fast muscles that were examined in calcineurin Aα or Aβ null mice failed to show a defect in fiber number or maturation, suggesting no alteration in differentiation (Fig. 6 and 7 and data not shown). That skeletal muscle differentiation is largely normal in both calcineurin Aα and Aβ null mice could indicate either no role for calcineurin in this process or that one gene compensates for the other in ultimately permitting differentiation. Indeed, calcineurin Aα and Aβ double-null mice are embryonic lethal, indicating that each gene can partially compensate for the other (data not shown). Future studies to conditionally delete all calcineurin activity from skeletal muscle should ultimately define its importance, if any, in the regulation of myocyte differentiation.
Role of calcineurin in regulating skeletal muscle hypertrophy.
The results presented here suggest that calcineurin does not play a critical role in regulating skeletal muscle fiber growth. calcineurin Aα and Aβ gene-targeted mice showed normal fiber cross-sectional areas in glycolytic/fast muscles, although the soleus was subtly affected. However, we interpret this alteration in soleus myofiber cross-sectional area to be a secondary consequence of altered differentiation due to a corresponding change in myofiber number. This overall conclusion is supported by a number of pharmacological inhibitory studies in rodent models. For example, use of CsA or FK506 failed to show a reduction in skeletal muscle fiber size under resting conditions or in response to functional overload (16, 17, 33, 41). Calcineurin signaling has also been reported not to directly influence IGF-1-mediated hypertrophy (5, 37). In contrast, a nearly equal number of credible studies have identified a defect in myofiber growth using calcineurin-inhibitory agents at baseline in resting animals, in the growth response following a period of atrophy after hind limb suspension, or downstream of IGF-1 signaling (14, 28, 30, 40). The interpretation of the results discussed above might also be influenced by potential nonspecific effects associated with CsA and FK506. Given this consideration, a case can be made that a genetic gain-of-function approach might be more definitive in addressing causality between calcineurin signaling and skeletal muscle growth. Indeed, overexpression of an activated calcineurin cDNA in the heart induces massive hypertrophy, suggesting a causal role in the cardiac growth response (29). However, transgenic overexpression of the same activated calcineurin cDNA in skeletal muscle failed to induce hypertrophy at baseline or after a period of functional overload (16, 31). This latter result strongly suggests that calcineurin does not directly regulate skeletal muscle hypertrophy. Consistent with this interpretation, analysis of calcineurin Aα and Aβ gene-targeted mice failed to identify a significant growth defect. Indeed, calcineurin Aα null mice even showed an increase in fiber areas in the soleus compared with what was seen with wild-type or calcineurin Aβ mice, although this effect is likely secondary to a reduction in primary fiber numbers. In any event, the results presented here indicate that calcineurin is potentially only important in regulating fiber type switching and not hypertrophy or differentiation. However, before such a conclusion is fully embraced it may be necessary to inactivate all calcineurin in skeletal muscle by using a conditional gene-targeting approach.
Acknowledgments
This work was supported by National Institutes of Health grants and the Pew Charitable Trust Foundation (J.D.M), by National Institutes of Health training grant no. 5T32 HL07382 (S.P.), by postdoctoral fellowship F32HL10336 from the National Institutes of Health (O.F.B.), and by an M.D./Ph.D. scholar award (University of Cincinnati Physician Scientist Training Program) and Illick Fellowship (Albert J. Ryan Foundation) (B.J.W.).
We thank Jonathan Seidman for supplying calcineurin Aα gene-targeted mice.
REFERENCES
- 1.Abbott, K. L., B. B. Friday, D. Thaloor, T. J. Murphy, and G. K. Pavlath. 1998. Activation and cellular localization of the cyclosporine A-sensitive transcription factor NF-AT in skeletal muscle cells. Mol. Biol. Cell 9:2905-2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, D. L., and L. A. Leinwand. 2002. Intracellular calcium and myosin isoform transitions. Calcineurin and CAM kinase pathways regulate preferential activation of the IIa myosin heavy chain promoter. J. Biol. Chem. 277:45323-45330. [DOI] [PubMed] [Google Scholar]
- 3.Barton-Davis, E. R., W. A. LaFramboise, and M. J. Kushmerick. 1996. Activity-dependent induction of slow myosin gene expression in isolated fast-twitch mouse muscle. Am. J. Physiol. 271:C1409-C1414. [DOI] [PubMed] [Google Scholar]
- 4.Bigard, X., H. Sanchez, J. Zoll, P. Mateo, V. Rousseau, V. Veksler, and R. Ventura-Clapier. 2000. Calcineurin co-regulates contractile and metabolic components of slow muscle phenotype. J. Biol. Chem. 275:19653-19660. [DOI] [PubMed] [Google Scholar]
- 5.Bodine, S. C., T. N. Stitt, M. Gonzalez, W. O. Kline, G. L. Stover, R. Bauerlein, E. Zlotchenko, A. Scrimgeour, J. C. Lawrence, D. J. Glass, and G. D. Yancopoulos. 2001. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 3:1014-1019. [DOI] [PubMed] [Google Scholar]
- 6.Brunetti, O., A. M. Barazzoni, G. Dell Torre, P. Clavenzani, V. E. Pettorossi, and R. Bortolami. 1997. Partial transformation from fast to slow muscle fibers induced by deafferentation of capsaicin-sensitive muscle afferents. Muscle Nerve 20:1404-1413. [DOI] [PubMed] [Google Scholar]
- 7.Bueno, O. F., E. B. Brandt, M. E. Rothenberg, and J. D. Molkentin. 2002. Defective T cell development and function in calcineurin Aβ-deficient mice. Proc. Natl. Acad. Sci. USA 99:9398-9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bueno, O. F., B. J. Wilkins, K. M. Tymitz, B. J. Glascock, T. F. Kimball, J. N. Lorenz, and J. D. Molkentin. 2002. Impaired cardiac hypertrophic response in calcineurin Aβ-deficient mice. Proc. Natl. Acad. Sci. USA 99:4586-4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chin, E. R., E. N. Olson, J. A. Richardson, Q. Yang, C. Humphries, J. M. Shelton, H. Wu, W. Zhu, R. Bassel-Duby, and R. S. Williams. 1998. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev. 12:2499-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crabtree, G. R., and E. N. Olson. 2002. NFAT signaling: choreographing the social lives of cells. Cell 109:S67-S79. [DOI] [PubMed] [Google Scholar]
- 11.Crabtree, G. R. 1999. Generic signals and specific outcomes: signaling through Ca2+, calcineurin, and NF-AT. Cell 96:611-614. [DOI] [PubMed] [Google Scholar]
- 12.Delling, U., J. Tureckova, H. W. Lim, L. J. De Windt, P. Rotwein, and J. D. Molkentin. 2000. A calcineurin-NFATc3-dependent pathway regulates skeletal muscle differentiation and slow myosin heavy-chain expression. Mol. Cell. Biol. 20:6600-6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolmetsch, R. E., R. S. Lewis, C. C. Goodnow, and J. I. Healy. 1997. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 386:855-858. [DOI] [PubMed] [Google Scholar]
- 14.Dunn, S. E., J. L. Burns, and R. N. Michel. 1999. Calcineurin is required for skeletal muscle hypertrophy. J. Biol. Chem. 274:21908-21912. [DOI] [PubMed] [Google Scholar]
- 15.Dunn, S. E., A. R. Simard, R. Bassel-Duby, R. S. Williams, and R. N. Michel. 2001. Nerve activity-dependent modulation of calcineurin signaling in adult fast and slow skeletal muscle fibers. J. Biol. Chem. 276:45243-45254. [DOI] [PubMed] [Google Scholar]
- 16.Dunn, S. E., E. R. Chin, and R. N. Michel. 2000. Matching of calcineurin activity to upstream effectors is critical for skeletal muscle fiber growth. J. Cell Biol. 151:663-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dupont-Versteegden, E. E., M. Knox, C. M. Gurley, J. D. Houle, and C. A. Peterson. 2002. Maintenance of muscle mass is not dependent on the calcineurin-NFAT pathway. Am. J. Physiol. Cell Physiol. 282:C1387-C1395. [DOI] [PubMed] [Google Scholar]
- 18.Friday, B. B., V. Horsley, and G. K. Pavlath. 2000. Calcineurin activity is required for the initiation of skeletal muscle differentiation. J. Cell Biol. 149:657-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorski, K., M. Carneiro, and U. Schibler. 1986. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell 47:767-776. [DOI] [PubMed] [Google Scholar]
- 20.Horsley, V., B. B. Friday, S. Matteson, K. M. Kegley, J. Gephart, and G. K. Pavlath. 2001. Regulation of the growth of multinucleated muscle cells by an NFATC2-dependent pathway. J. Cell Biol. 153:329-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang, H., F. Xiong, S. Kong, T. Ogawa, M. Kobayashi, and J. O. Liu. 1997. Distinct tissue and cellular distribution of two major isoforms of calcineurin. Mol. Immunol. 34:663-669. [DOI] [PubMed] [Google Scholar]
- 22.Kegley, K. M., J. Gephart, G. L. Warren, and G. K. Pavlath. 2001. Altered primary myogenesis in NFATC3 −/−mice leads to decreased muscle size in the adult. Dev. Biol. 232:115-126. [DOI] [PubMed] [Google Scholar]
- 23.Klee, C. B., H. Ren, and X. Wang. 1998. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J. Biol. Chem. 273:13367-13370. [DOI] [PubMed] [Google Scholar]
- 24.Kumar, A., K. Crawford, L. Close, M. Madison, J. Lorenz, T. Doetschman, S. Pawlowski, J. Duffy, J. Neumann, J. Robbins, G. P. Boivin, B. A. O'Toole, and J. L. Lessard. 1997. Rescue of cardiac alpha-actin-deficient mice by enteric smooth muscle gamma-actin. Proc. Natl. Acad. Sci. USA 94:4406-4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin, J., H. Wu, P. T. Tarr, C. Y. Zhang, Z. Wu, O. Boss, L. F. Michael, P. Puigserver, E. Isotani, E. N. Olson, B. B. Lowell, R. Bassel-Duby, and B. M. Spiegelman. 2002. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418:797-801. [DOI] [PubMed] [Google Scholar]
- 26.Lopéz-Rodríguez, C., J. Aramburu, A. S. Rakeman, and A. Rao. 1999. NFAT5, a constitutively nuclear NFAT protein that does not cooperate with Fos and Jun. Proc. Natl. Acad. Sci. USA 96:7214-7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meissner, J. D., G. Gros, R. J. Scheibe, M. Scholz, and H. P. Kubis. 2001. Calcineurin regulates slow myosin, but not fast myosin or metabolic enzymes, during fast-to-slow transformation in rabbit skeletal muscle cell culture. J. Physiol. 533:215-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell, P. O., S. T. Mills, and G. K. Pavlath. 2002. Calcineurin differentially regulates maintenance and growth of phenotypically distinct muscles. Am. J. Physiol. Cell Physiol. 282:C984-C992. [DOI] [PubMed] [Google Scholar]
- 29.Molkentin, J. D., J. R. Lu, C. L. Antos, B. Markham, J. Richardson, J. Robbins, S. R. Grant, and E. N. Olson. 1998. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93:215-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musaró, A., K. J. A. McCullagh, F. J. Naya, E. N. Olson, and N. Rosenthal. 1999. IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature 400:581-585. [DOI] [PubMed] [Google Scholar]
- 31.Naya, F. J., B. Mercer, J. Shelton, J. A. Richardson, R. S. Williams, and E. N. Olson. 2000. Stimulation of slow skeletal muscle fiber gene expression by calcineurin in vivo. J. Biol. Chem. 275:4545-4548. [DOI] [PubMed] [Google Scholar]
- 32.Olson, E. N., and R. S. Williams. 2000. Remodeling muscles with calcineurin. Bioessays 22:510-519. [DOI] [PubMed] [Google Scholar]
- 33.Pallafacchina, G., E. Calabria, A. L. Serrano, J. M. Kalhovde, and S. Schiaffino. 2002. A protein kinase B-dependent and rapamycin-sensitive pathway controls skeletal muscle growth but not fiber type specification. Proc. Natl. Acad. Sci. USA 99:9213-9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Passier, R., H. Zeng, N. Frey, F. J. Naya, R. L. Nicol, T. A. McKinsey, P. Overbeek, J. A. Richardson, S. R. Grant, and E. N. Olson. 2000. CaM kinase signaling induces cardiac hypertrophy and activates the MEF2 transcription factor in vivo. J. Clin. Investig. 105:1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perrino, B. A., A. J. Wilson, P. Ellison, and L. H. Clapp. 2002. Substrate selectivity and sensitivity to inhibition by FK506 and cyclosporin A of calcineurin heterodimers composed of the alpha or beta catalytic subunit. Eur. J. Biochem. 269:3540-3548. [DOI] [PubMed] [Google Scholar]
- 36.Rao, A., C. Luo, and P. G. Hogan. 1997. Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 15:707-747. [DOI] [PubMed] [Google Scholar]
- 37.Rommel, C., S. C. Bodine, B. A. Clarke, R. Rossman, L. Nunez, T. N. Stitt, G. D. Yancopoulos, and D. J. Glass. 2001. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat. Cell Biol. 3:1009-1013. [DOI] [PubMed] [Google Scholar]
- 38.Rusnak, F., and P. Mertz. 2000. Calcineurin: form and function. Physiol. Rev. 80:1483-1521. [DOI] [PubMed] [Google Scholar]
- 39.Schuler, M., and D. Pette. 1996. Fiber transformation and replacement in low-frequency stimulated rabbit fast-twitch muscles. Cell. Tissue Res. 285:297-303. [DOI] [PubMed] [Google Scholar]
- 40.Semsarian, C., M.-J. Wu, Y.-K. Ju, T. Marciniec, T. Yeoh, D. G. Allen, R. P. Harvey, and R. M. Graham. 1999. Skeletal muscle hypertrophy is mediated by a Ca2+-dependent calcineurin signaling pathway. Nature 400:576-580. [DOI] [PubMed] [Google Scholar]
- 41.Serrano, A. L., M. Murgia, G. Pallafacchina, E. Calabria, P. Coniglio, T. Lomo, and S. Schiaffino. 2001. Calcineurin controls nerve activity-dependent specification of slow skeletal muscle fibers but not muscle growth. Proc. Natl. Acad. Sci. USA 98:13108-13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spangenburg, E. E., J. H. Williams, R. R. Roy, and R. J. Talmadge. 2001. Skeletal muscle calcineurin: influence of phenotype adaptation and atrophy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280:R1256-R1260. [DOI] [PubMed] [Google Scholar]
- 43.Sreter, F. A., J. R. Lopez, L. Alamo, K. Mabuchi, and J. Gergely. 1987. Changes in intracellular ionized calcium concentration associated with muscle fiber type transformation. Am. J. Physiol. 253:C296-C300. [DOI] [PubMed] [Google Scholar]
- 44.Swoap, S. J., R. B. Hunter, E. J. Stevenson, H. M. Felton, N. V. Kansagra, J. M. Lang, K. A. Esser, and S. C. Kandarian. 2000. The calcineurin-NFAT pathway and muscle fiber-type gene expression. Am. J. Physiol. Cell Physiol. 279:C915-C924. [DOI] [PubMed] [Google Scholar]
- 45.Taigen, T., L. J. De Windt, H. W. Lim, and J. D. Molkentin. 2000. Targeted inhibition of calcineurin prevents agonist-induced cardiomyocyte hypertrophy. Proc. Natl. Acad. Sci. USA 97:1196-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torgan, C. E., and M. P. Daniels. 2001. Regulation of myosin heavy chain expression during rat skeletal muscle development in vitro. Mol. Biol. Cell 12:1499-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Usuda, N., H. Arai, H. Sasaki, T. Hanai, T. Nagata, T. Muramatsu, R. L. Kincaid, and S. Higuchi. 1996. Differential subcellular localization of neural isoforms of the catalytic subunit of calmodulin-dependent protein phosphatase (calcineurin) in central nervous system neurons: immunohistochemistry on formalin-fixed paraffin sections employing antigen retrieval by microwave irradiation. J. Histochem. Cytochem. 44:13-18. [DOI] [PubMed] [Google Scholar]
- 48.Westerbald, H., and D. G. Allen. 1991. Changes in myoplasmic calcium concentration during fatigue in single mouse muscle fibers. J. Gen. Physiol. 98:615-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams, R. S., S. Salmons, E. A. Newsholme, R. E. Kaufman, and J. Mellor. 1986. Regulation of nuclear and mitochondrial gene expression by contractile activity in skeletal muscle. J. Biol. Chem. 261:376-380. [PubMed] [Google Scholar]
- 50.Wu, H., B. Rothermel, S. Kanatous, P. Rosenberg, F. J. Naya, J. M. Shelton, K. A. Hutcheson, J. M. DiMaio, E. N. Olson, R. Bassel-Duby, and R. S. Williams. 2001. Activation of MEF2 by muscle activity is mediated through a calcineurin-dependent pathway. EMBO J. 20:6414-6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu, H., F. J. Naya, T. A. McKinsey, B. Mercer, J. M. Shelton, E. R. Chin, A. R. Simard, R. N. Michel, R. Bassel-Duby, E. N. Olson, and R. S. Williams. 2000. MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fiber type. EMBO J. 19:1963-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang, B. W., G. Zimmer, J. Chen, D. Ladd, E. Li, F. W. Alt, G. Wiederrecht, J. Cryan, E. A. O'Neill, C. E. Seidman, A. K. Abbas, and J. G. Seidman. 1996. T cell responses in calcineurin A alpha-deficient mice. J. Exp. Med. 183:413-420. [DOI] [PMC free article] [PubMed] [Google Scholar]