Abstract
We have compared chemokine secretion from human lung A549 cells infected with simian virus 5 (SV5) with other members of the Rubulavirus genus of paramyxoviruses. High levels of the chemokines interleukin-8 (IL-8) and macrophage chemoattractant protein-1 (MCP-1) were secreted from A549 cells infected with Human parainfluenza virus type 2 (HPIV-2) but not from cells infected with wild-type (WT) SV5. The lack of IL-8 secretion from SV5-infected cells was not due to a global block in all signal transduction pathways leading to IL-8 secretion, since SV5-infected A549 cells secreted IL-8 after stimulation with exogenously added tumor necrosis factor alpha or by coinfection with HPIV-2. A previously described, recombinant SV5 containing substitutions in the shared region of the P/V gene (rSV5-P/V-CPI−) induced IL-8 secretion by a mechanism that was dependent on viral gene expression. By contrast, an SV5 variant isolated from persistently infected cells (Wake Forest strain of Canine parainfluenza virus) induced IL-8 secretion by a mechanism that was largely not affected by inhibitors of viral gene expression. Together, these data demonstrate that SV5 is unusual compared to other closely related paramyxoviruses, since SV5 is a very poor inducer of the cytokines IL-8 and MCP-1. The isolation of two recombinant SV5 mutants that are defective in preventing chemokine induction will allow an identification of mechanisms utilized by WT SV5 to avoid activation of host cell innate immune responses to infection.
The synthesis of proinflammatory cytokines is a critical arm of the innate immune response to virus infection of the respiratory tract that can determine the outcome of an infection, virus tropism, and the potency of the adaptive immune response (2, 18). In the respiratory tract, virus-infected epithelial cells are a major source of a large number of cytokines, including the alpha and beta interferons (IFN-α and -β, respectively [type I]), chemokines of the CC family, such as macrophage chemoattractant protein-1 (MCP-1) and RANTES (regulated upon activation, normally T cell expressed and presumably secreted), and chemokines of the CXC family, such as interleukin-8 (IL-8). Virus-induced cytokines can act on infected cells through the activation of antiviral pathways within the infected cell or in neighboring cells. In addition, released cytokines can recruit immune cells that infiltrate the site of infection, thereby aiding in viral clearance as well as in the generation of adaptive immune responses (reviewed in references 2 and 18). The chemokine IL-8 is among the most important of the cytokines activated by infection of airway epithelial cells, since it is a potent chemoattractant and activator of neutrophils, T cells, eosinophils, and basophils (20, 21, 25) and is thought to play a key role in the lung inflammation observed during some viral infections. MCP-1 is a major chemoattractant of monocytes and activated lymphocytes.
Many members of the Paramyxovirus family of nonsegmented negative-strand RNA viruses have been shown to be potent inducers of cytokine synthesis, including Respiratory syncytial virus (RSV), Sendai virus, Human parainfluenza virus type 2 (HPIV-2), HPIV-3, and Newcastle disease virus (9, 19, 27, 29, 30). RSV infection of A549 human airway epithelial cells is one of the best-studied models for paramyxovirus induction of proinflammatory cytokines (3, 10, 13). RSV infection of A549 cells induces the secretion of a large number of interleukins, cytokines, and chemokines (5, 7, 15, 30), mimicking the in vivo activation of cytokines that recruit leukocytes, monocytes, and lymphocytes into the respiratory tract (reviewed in references 13 and 26).
Simian virus 5 (SV5) is a member of the Rubulavirus genus of paramyxoviruses, a group that includes HPIV-2, mumps virus, and SV41 (17). Previous results have shown that SV5 evades the host IFN response by multiple mechanisms. For example, SV5 counteracts the cellular response to type I IFN at least in part by targeting the transcription factor STAT1 for degradation, thus preventing signaling through the IFN pathway (28). The SV5 V protein, which is expressed from the bicistronic viral P/V gene, has been shown to be responsible for targeting STAT1 for degradation (6). Recent work has shown that in addition to blocking IFN signaling pathways, SV5 is also a very poor inducer of the IFN-β gene and that A549 cells infected with SV5 do not secrete detectable levels of type I IFN (27, 28). This result contrasts sharply with that of cells infected by other paramyxoviruses, such as HPIV-2, Sendai virus, or RSV, all of which are potent inducers of IFN synthesis (15, 19, 27). Our finding that SV5-infected lung epithelial cells produce very little IFN raises the hypothesis that SV5 is also a poor inducer of chemokines, such as IL-8 and MCP-1.
In the work reported here, we have tested this hypothesis by examining the effect of rSV5 infection on the induction of chemokines from A549 human lung epithelial cells. Our data indicate that A549 cells infected with rSV5 do not produce elevated levels of the cytokines IL-8 and MCP-1. By contrast, we have found that two SV5 mutants are potent inducers of IL-8 secretion from A549 cells. A previously described rSV5 mutant containing substitutions in the P/V gene that prevented V-mediated degradation of STAT1 was found to induce IL-8 synthesis by a mechanism that required viral gene expression. By contrast, a naturally occurring SV5 variant isolated from persistently infected cells induced IL-8 secretion by a mechanism that was not sensitive to inhibitors of viral gene expression. Our results demonstrate that WT SV5 is unusual among this family of negative-strand RNA viruses, since it has efficient mechanisms to avoid activation of both type I IFNs and chemokines.
Monolayer cultures of A549 human lung cells were grown in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS). The wild-type (WT) recombinant SV5 (rSV5) and rSV5-green fluorescent protein (GFP) viruses were recovered from cDNA (12) and were grown in MDBK cells. The Greer strain of HPIV-2, HPIV-3, and SV41 (the kind gift of Y. Ito) were grown in CV-1 cells. The rSV5-P/V-CPI− virus, containing six amino acid substitutions in its P/V gene, was grown in Vero cells as described previously (27). The Wake Forest strain of canine parainfluenza virus (WF-PIV) was isolated from Vero cells persistently infected with Canine parainfluenza virus (the kind gift of S. Krakowka; Ohio State University). To measure cytokine induction, cells cultured in 24-well dishes were infected at a multiplicity of infection (MOI) of 30 for rSV5 and rSV5-P/V-CPI− and at an MOI of 10 for WF-PIV, HPIV-2, HPIV-3, and SV41, unless otherwise indicated. At 24 h postinfection (hpi), unless otherwise indicated, cell supernatants were harvested and clarified of cellular debris by microcentrifugation. Enzyme-linked immunosorbent assays (ELISAs) were performed by using the OptEIA Human IL-8 and MCP-1 sets (BD Pharmingen). A parallel sample of uninfected cells was counted at 24 hpi for use in normalizing the concentration of secreted cytokine to 106 cells. Identical results were obtained by using virus stocks that were prepared by centrifugation of virus away from culture media (data not shown).
For inactivation of virus by UV treatment, viruses diluted in DMEM-10% bovine serum albumin (BSA) were held in 60-mm-diameter dishes for 20 min under a handheld germicidal UV lamp at a distance of 6.5 cm. This procedure eliminated all infectivity as determined by plaque assays. To inhibit viral gene expression, cells were incubated at 37°C for 4 h prior to infection in DMEM containing ribavirin (0, 10, 100, or 250 μg/ml). Following the 1-h incubation with virus, monolayers were washed with phosphate-buffered saline and were incubated in ribavirin diluted in DMEM-2% FBS. Media was analyzed by ELISA for IL-8. To analyze viral protein expression, 24-well dishes of infected cells were washed with phosphate-buffered saline and then were lysed in 100 μl of 1% sodium dodecyl sulfate (SDS). Protein concentrations were determined by using the bicinchoninic acid assay (Pierce Chemicals). Equal amounts of protein were analyzed by ECL Western blotting by using rabbit polyclonal serum raised to a synthetic peptide corresponding to the C-terminal end of NP or serum specific for the SV5 P protein as described previously (23). Radiolabeling of infected cells and immunoprecipitation with polyclonal anti-SV5 serum was as described previously (23).
SV5 is a poor inducer of proinflammatory cytokine synthesis compared to other closely related rubulaviruses.
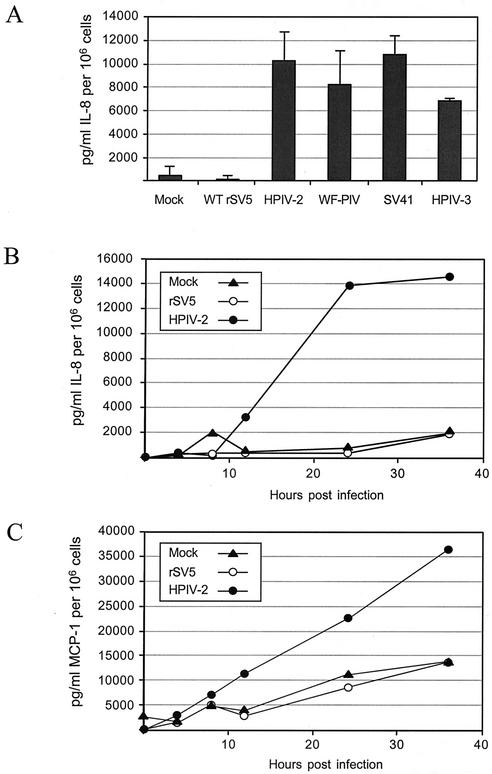
To compare the extent to which rSV5 and other closely related paramyxoviruses induce proinflammatory cytokines, A549 cells were mock infected or were infected with rSV5, HPIV-2, HPIV-3, SV41, or the SV5 variant WF-PIV. The WF-PIV virus was isolated in our laboratory from Vero cells persistently infected with a canine parainfluenza virus and is related to WT SV5 both serologically and by high nucleotide sequence identity (data not shown). Details of the growth properties and nucleotide sequence of WF-PIV will be described elsewhere. At 22 hpi media were harvested from infected cells and were analyzed by ELISA for IL-8. As shown in Fig. 1A, high levels of IL-8 were detected in media from cells infected with HPIV-2, HPIV-3, SV41, and WF-PIV. The levels of IL-8 secretion in these samples (∼8 to 11 ng/ml per 106 cells) were very similar to those reported previously for RSV-infected A549 cells (10). Similar levels of IL-8 were induced by purified WF-PIV and HPIV-2. For example, in one experiment IL-8 was induced to ∼8,146 and ∼8,632 pg/ml/106 cells following infection with purified WF-PIV and HPIV-2, respectively. Most importantly, Fig. 1 shows that WT rSV5 is unusual compared to these closely related paramyxoviruses, since the levels of IL-8 in media from cells infected with WT rSV5 were very similar to that seen with mock-infected cells.
FIG. 1.
SV5 is a poor inducer of IL-8 and MCP-1 secretion compared to other closely related rubulaviruses. (A) Monolayers of A549 cells were mock infected or were infected with WT rSV5, HPIV-2, WF-PIV, SV41, or HPIV-3. At 22 hpi media were harvested and analyzed by ELISA for IL-8. Values are the mean concentration of triplicate samples of IL-8 in picograms per milliliter, with standard deviations shown by bars, and are normalized to 106 cells. (B and C) Time course of chemokine secretion. A549 cells were mock infected or were infected with rSV5 and HPIV-2 at a high MOI. At the indicated times postinfection media were harvested and assayed by ELISA for IL-8 (B) or MCP-1 (C). Results are representative of three experiments.
In time course experiments (Fig. 1B), significant levels of IL-8 secreted from HPIV-2-infected A549 cells were detected starting at 12 hpi, and then it reached its maximal level by 24 hpi. These kinetics are very similar to that found for RSV-infected A549 cells (10), with the exception that IL-8 levels continued to increase up to 48 hpi for RSV-infected cells. As shown in Fig. 1 above, the levels of IL-8 secreted over time from rSV5-infected cells closely resembled that of mock-infected cells.
To determine if the lack of IL-8 secretion by SV5-infected cells also applied to a different proinflammatory cytokine, media were also analyzed by ELISA for the presence of MCP-1. As shown in Fig. 1C, secretion of MCP-1 from HPIV-2-infected cells increased over time, with kinetics similar to that of IL-8 secretion. By contrast, the levels of MCP-1 secreted from SV5-infected cells closely matched that seen for mock-infected controls. Together, these data indicate that cells infected with rSV5 are not induced to secrete the proinflammatory cytokines IL-8 or MCP-1, a result that contrasts with the potent induction of these cytokines by other rubulaviruses, including the closely related HPIV-2 and WF-PIV.
SV5-infected A549 cells do not have a global block in signaling pathways leading to IL-8 secretion.
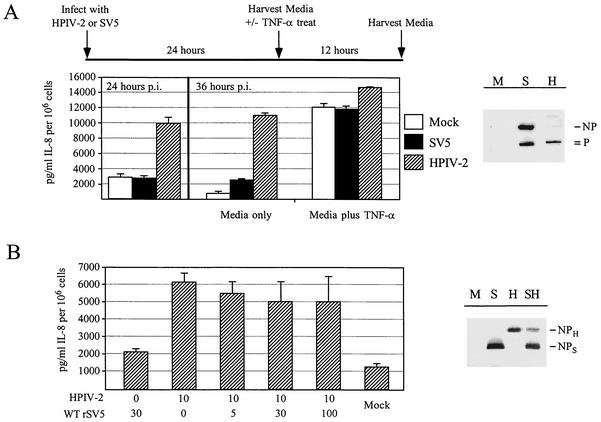
The lack of IL-8 secretion from SV5-infected cells could be explained by an SV5-induced global block in all signaling pathways that lead to activation of the IL-8 promoter. To test this hypothesis, we determined the ability of rSV5-infected cells to produce IL-8 in response to exogenously added tumor necrosis factor alpha (TNF-α). A timeline for the experimental design is shown in Fig. 2A. A549 cells were mock infected or were infected with rSV5 or HPIV-2. At 24 hpi media were analyzed by ELISA for IL-8, and cell lysates were analyzed by Western blotting for the viral NP and P proteins. Parallel sets of infected cells were then treated with media alone or media containing 10 ng of TNF-α/ml for 20 min, followed by incubation for another 12 h to allow IL-8 levels to accumulate. Western blot analysis indicated that all infections were productive, as shown by their accumulation of the viral proteins (Fig. 2A). Consistent with the above results, high levels of IL-8 had been secreted by cells infected with HPIV-2 by 24 hpi, while SV5-infected samples produced only background levels of IL-8 (Fig. 2A). This trend continued for the control samples treated with media only and harvested 12 h later. However, treatment of cells with TNF-α resulted in IL-8 secretion from all samples, including cells infected with rSV5 or HPIV-2 (Fig. 2A). These data indicate that SV5 infection does not result in a block in TNF-α signaling pathways that lead to IL-8 induction.
FIG. 2.
SV5-infected A549 cells do not have a global block in signaling pathways leading to IL-8 secretion. (A) Induction of IL-8 secretion by TNF-α in SV5- and HPIV-2-infected A549 cells. A549 cells were mock infected or were infected at high MOI with SV5 and HPIV-2. At 24 hpi media were harvested from one set of cells. At that time, parallel sets of infected cells were further incubated alone or with 10 ng of TNF-α/ml for 20 min. Media from these cells were harvested at 36 hpi and were analyzed by ELISA for IL-8. To confirm that cells were productively infected, cell monolayers were lysed in 1% SDS at 24 hpi and were analyzed by Western blot for the viral proteins NP and P. M, mock infected; S, SV5 infected; H, HPIV-2 infected. Note that the serum raised against the SV5 P protein recognizes both SV5 and HPIV-2 P proteins, but the antipeptide serum raised against the SV5 NP only recognizes SV5 NP protein. (B) IL-8 secretion from cells coinfected with SV5 and HPIV-2. A549 cells were mock infected, infected with SV5 alone, infected with HPIV-2 alone, or coinfected with the indicated amounts of HPIV-2 and SV5. At 24 hpi cell supernatants were harvested and assayed by ELISA for IL-8. To confirm that HPIV-2 did not prevent SV5 gene expression, A549 cells that were mock infected (M lane) or were infected with SV5 alone (S), HPIV-2 alone (H), or coinfected with SV5 and HPIV-2 (SH) were radiolabeled for 30 min with 35S-labeled amino acids at 18 hpi as described previously (23). Cell lysates were immunoprecipitated with anti-SV5 antibodies and were analyzed by SDS-polyacrylamide gel electrophoresis. The position of the SV5 and HPIV-2 NP proteins are indicated.
To determine if rSV5 infection could block IL-8 secretion induced by a heterologous virus, A549 cells were infected with rSV5 alone or HPIV-2 alone or were coinfected with HPIV-2 at a constant MOI and SV5 at an increasing MOI. At 24 hpi cell supernatants were harvested and assayed for IL-8 by ELISA. As shown in Fig. 2B, infection with HPIV-2 alone or coinfection with HPIV-2 and rSV5 resulted in high levels of IL-8 secretion, even in infected cells where the SV5 MOI was 10 times higher than that of HPIV-2. Radiolabeling experiments showed that A549 cells coinfected with both viruses synthesized the SV5 and HPIV-2 NP proteins (Fig. 2B). In addition, microscopy experiments demonstrated that cells coinfected with HPIV-2 and rSV5-GFP did not show decreased expression of GFP relative to cells infected with rSV5-GFP alone (data not shown). These data indicate that the inability of SV5 to block HPIV-2 induction of IL-8 secretion was not simply due to HPIV-2 interference with SV5 gene expression. Thus, IL-8 induction by HPIV-2 is dominant during a coinfection with rSV5, consistent with the idea that rSV5 infection does not have active mechanisms to block all pathways leading to IL-8 synthesis.
Induction of IL-8 secretion by infection with an rSV5 containing substitutions in the P/V gene.
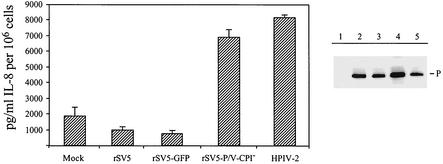
We have previously shown that the IFN-β promoter is activated by infection with an rSV5 containing naturally occurring mutations in its P/V gene (rSV5-P/V-CPI−) but not by WT rSV5 (27). To determine if the rSV5-P/V-CPI− mutant also activates the synthesis of proinflammatory cytokines, A549 cells were mock infected or were infected with rSV5, an rSV5-expressing GFP (the proper control for the P/V mutant which contains GFP), rSV5-P/V-CPI−, or HPIV-2. At 24 hpi, media were harvested and assayed for IL-8 by ELISA, and cell lysates were analyzed by Western blotting for P gene expression. As shown in Fig. 3, cells infected with the rSV5-P/V-CPI− mutant secreted IL-8 to levels similar to that produced by HPIV-2-infected cells, while only low background levels of IL-8 secretion were seen from cells infected with WT rSV5 and rSV5-GFP. These results indicate that the rSV5-P/V-CPI− mutant containing naturally occurring substitutions in the P/V gene differs from WT rSV5 by its ability to induce the synthesis of both type I IFNs as well as the proinflammatory cytokine IL-8.
FIG. 3.
IL-8 secretion from cells infected with an rSV5 containing naturally occurring mutations in the P/V gene. A549 cells were mock infected (lane 1) or were infected at high MOI with SV5, rSV5-GFP, the rSV5-P/V-CPI− mutant containing substitutions in the P/V gene, or HPIV-2 (lanes 2 to 5, respectively). At 24 hpi media were harvested and assayed by ELISA for IL-8. Monolayers were then lysed in 1% SDS, and equivalent amounts of protein were analyzed by Western blotting for the viral protein P.
Inhibition of viral gene expression prevents IL-8 induction by HPIV-2 and rSV5-P/V-CPI− but not by WF-PIV.
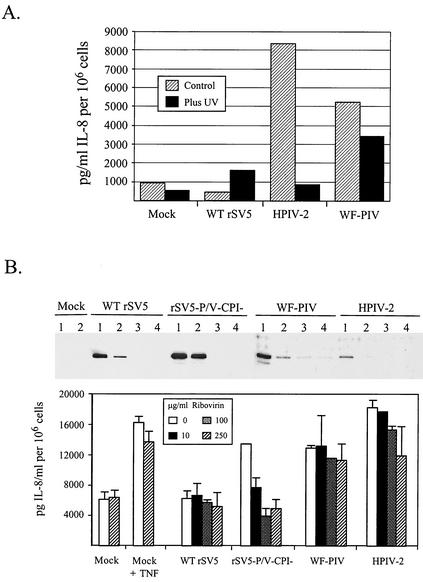
Viral gene expression was inhibited by UV inactivation to determine the mechanism by which infection with the SV5 variants WF-PIV and rSV5-P/V-CPI− induce IL-8 secretion. A549 cells were mock infected or were infected with WT rSV5, HPIV-2, or WF-PIV. In parallel, cells were infected with virus that had been inactivated by treatment with UV light. A representative result of IL-8 induced by these viruses is shown in Fig. 4A. UV treatment of HPIV-2 prior to infection greatly reduced the ability of the virus to induce IL-8 secretion from A549 cells. Similarly, IL-8 was not induced by infection with UV-treated rSV5-P/V-CPI− (data not shown). In contrast, cells infected with UV-treated WF-PIV showed only a small but reproducible decrease in IL-8 secretion; however, the levels of IL-8 secreted were still much higher than that of mock-infected or WT rSV5-infected cells (Fig. 4A).
FIG. 4.
Effect of UV light treatment and ribavirin on the induction of IL-8 secretion by mutant and WT SV5. (A) UV light treatment eliminates IL-8 induction by HPIV-2 but not by WF-PIV. A549 cells were mock infected or were infected at high MOI with WT rSV5, HPIV-2, or WF-PIV that had or had not been previously treated with UV light as described in Materials and Methods. At 24 hpi media were harvested and analyzed for IL-8 by ELISA as described in the legend to Fig. 1. Results are representative of two experiments. (B) Ribavirin inhibits IL-8 induction by rSV5-P/V-CPI− but not by WF-PIV. A549 cells were incubated in various amounts (0, 10, 100, or 250 μg/ml; lanes 1 to 4, respectively) of ribavirin for 4 h prior to being mock infected or infected at high MOI with SV5, rSV5-P/V-CPI−, WF-PIV, or HPIV-2. Following infection, ribavirin was added to the media at the same concentration. At 24 hpi media were harvested and analyzed for IL-8 by ELISA. Cell monolayers were lysed in 1% SDS and were analyzed by Western blotting for the viral P protein. At 20 hpi a set of mock-infected cells was incubated with 10 ng of TNF-α/ml for 20 min to serve as a positive control.
Results of the UV inactivation experiment were confirmed by using ribavirin, a known inhibitor of paramyxovirus gene expression and growth (8). Titration experiments showed that viral protein synthesis in A549 cells infected with WT rSV5, rSV5-P/V-CPI−, WF-PIV, and HPIV-2 was severely reduced by treatment with ribavirin at 100 μg/ml or higher (see blots in Fig. 4B). To determine the effect of ribavirin on virus-induced IL-8 secretion, A549 cells were treated with various concentrations of the drug (0, 10, 100, 250 μg/ml) for 4 h prior to mock infection or infection with WT rSV5, rSV5-P/V-CPI−, WF-PIV, or HPIV-2. Following attachment of the virus for 1 h without drug, cells were incubated for 24 h in the presence of the drug. As a control, a parallel set of mock-infected cells were induced with TNF-α with or without ribavirin. As shown in Fig. 4B, increasing ribavirin concentrations inhibited the amount of IL-8 secreted from cells infected with rSV5-P/V-CPI− but only slightly reduced IL-8 secretion from cells infected with WF-PIV and HPIV-2. Likewise, the background levels of IL-8 secreted from mock- and SV5-infected cells was not affected by ribavirin treatment, nor was the induction of IL-8 by TNF-α affected by the presence of ribavirin. Taken together, these data indicate that IL-8 induction by WF-PIV occurs by a mechanism that is relatively insensitive to UV light and to ribavirin, two treatments that reduce viral gene expression. By contrast, rSV5-P/V-CPI− induces IL-8 secretion by a mechanism that is sensitive to inhibitors of gene expression.
Previous work has shown that the IL-8 promoter can be induced in A549 cells by at least two pathways that involve activation of distinct transcription factors: a pathway that can be activated by extracellular TNF-α and an intracellular pathway that is activated by viral replication (4). Mutational analysis of the IL-8 promoter has shown that TNF-α-induced IL-8 secretion requires only an intact NF-κB-binding site, whereas RSV-induced IL-8 secretion required promoter binding sites for AP-1, NF-kB/NF-IL-6, and a novel binding site termed the RSV-responsive element (RSVRE) which is similar in sequence to a consensus IFN-stimulated response element (ISRE) (4, 14). Our data show that A549 cells infected with HPIV-2 show a time- and MOI-dependent induction of high levels of IL-8 and MCP-1 secretion. The mechanism of IL-8 induction by HPIV-2 infection involves a pathway that is sensitive to inhibitors of gene expression, consistent with activation of IL-8 secretion by a mechanism similar to that shown for replicating RSV (5). Our finding that HPIV-2 is a strong inducer of proinflammatory cytokine synthesis was not unexpected, since a number of other paramyxoviruses have been shown to have this property (for examples see reference 2). The major result reported here is that human lung epithelial cells infected with WT rSV5 do not secrete large amounts of the proinflammatory cytokines IL-8 or MCP-1. These data extend a previous finding that WT SV5 is a poor inducer of the IFN-β promoter (27) and support the general proposal that SV5 has efficient mechanisms to avoid activation of important innate immune responses in human cells.
NF-κB is a key transcription factor that is required for activation of the IL-8 promoter through the intracellular and TNF-α pathways (3, 10). Our finding that TNF-α induction of IL-8 secretion is not altered in rSV5-infected A549 cells suggests that the lack of cytokine secretion from rSV5-infected cells is not due to an inhibition of NF-κB activity. IL-8 secretion is also induced from cells coinfected with HPIV-2 and SV5. Thus, the lack of IL-8 secretion from SV5-infected cells is due either to a failure to produce a viral component that activates the intracellular pathway or blocking a step in activation that is distinct from any steps shared in the pathways activated by HPIV-2 and TNF-α.
While WT rSV5 is a very poor inducer of IL-8 and MCP-1 synthesis, we have found two SV5 variants that are potent inducers of both of these cytokines. Remarkably, the engineered mutant rSV5-P/V-CPI− and the naturally occurring variant WF-PIV appear to activate IL-8 secretion by different mechanisms, which can be distinguished by their sensitivity to inhibitors of viral gene expression. We have previously shown that infection of A549 cells with the rSV5-P/V-CPI− mutant activates the IFN-β promoter, and the IFN signaling pathway is functional due to the inability of this mutant to target STAT1 degradation (27). It is possible that this mutant activates IL-8 secretion by a mechanism involving synthesis of IFN-β and signaling through the IFN receptor to activate transcription through the ISRE-like binding site in the IL-8 promoter (4). This mechanism is supported by coinfection experiments showing that infection with WT rSV5 prevents IL-8 secretion induced by rSV5-P/V-CPI−, and STAT1 is degraded in these coinfected cells (data not shown).
The SV5 variant WF-PIV induces IL-8 synthesis by a mechanism that is distinct from that of HPIV-2 or rSV5-P/V-CPI−. The finding that IL-8 secretion is induced by UV-inactivated WF-PIV or in ribavirin-treated cells suggests that a very early event, such as binding to the host cell, induces activation of the IL-8 promoter. Recently, the RSV fusion (F) protein has been shown to induce the synthesis of IL-8 and other proinflammatory cytokines through its interaction with Toll-like receptor 4 (TLR-4) (16). Similar findings have been reported for the activation of TLRs by other viral glycoproteins, including the mouse mammary tumor virus env protein with TLR-4 (24) and the WTF measles virus H protein with TLR-2 (1). Thus, in addition to TNF-α and an intracellular signal, the interaction of microbial pathogens with cell surface receptors is a third mechanism for IL-8 induction. While our present data cannot distinguish between IL-8 activation through WF-PIV attachment or through an early postattachment step, we propose that binding of WF-PIV to a cell surface pattern recognition receptor is the mechanism for induction of IL-8 by WF-PIV. Chimeric rSV5 viruses containing exchanges of WT rSV5 and WF-PIV genes will be useful tools for mapping the genes (e.g., HN and/or F) and the mechanisms responsible for IL-8 induction by WF-PIV.
The results reported here have implications for a recent proposal to develop rSV5 as a vaccine vector (22). A mouse model system has been developed to analyze the adaptive immune response to rSV5 infection of the respiratory tract and to show that intranasal infection with rSV5 vectors can elicit a cytotoxic T-lymphocyte response to a foreign protein that is comparable to that seen with analogous recombinant vaccinia vectors (11, 22). Since IL-8 is a potent chemoattractant and activator of neutrophils and T cells, recruitment of lymphocytes to sites of SV5 infection would be predicted to be greatly reduced compared to that with infection with HPIV-2, a possibility that could be tested by using our mouse model system (11, 22). Our finding here that WT rSV5 is not a potent inducer of proinflammatory cytokines in human cells is important, since it is widely accepted that innate immune responses can be a critical determinant of the subsequent adaptive immune response to infection (for examples see reference 2), and this could contribute to the ability of SV5 to establish noncytopathic persistent infections. An exciting possibility is that the WF-PIV genes that are responsible for activating cytokine synthesis could be incorporated into the WT rSV5 genome to allow an evaluation of the role of innate responses in adaptive immunity to rubulavirus infection.
In summary, our results have shown that WT rSV5 is unusual among the related rubulaviruses that we have examined, since SV5-infected cells do not secrete large amounts of the proinflammatory cytokines IL-8 and MCP-1. We have isolated two variants of rSV5 that activate IL-8 secretion by two apparently different mechanisms. Work is in progress to directly test the ability of our rSV5 variants to activate individual transcription factors needed for IL-8 synthesis and to identify the step that is blocked in cells infected with WT rSV5.
Acknowledgments
We thank Liz Wansley and Doug Lyles for helpful comments on the manuscript.
This work was supported by NIH grant AI42023. Virginia Young is a Howard Hughes Medical Institute Predoctoral Fellow.
REFERENCES
- 1.Bieback, K., E. Lien, I. M. Klagge, E. Avota, J. Schneider-Schaulies, W. P. Duprex, H. Wagner, C. J. Kirschning, V. ter Meulen, and S. Schneider-Schaulies. 2002. Hemagglutinin protein of wild-type measles virus activates Toll-like receptor 2 signaling. J. Virol. 76:8729-8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biron, C. A., and G. C. Sen. 2001. Interferons and other cytokines, p. 321-349. In B. Fields, D. Knipe, and P. Howley (ed.), Fields virology, 4th ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 3.Brasier, A. R., M. Jamaluddin, A. Casola, W. Duan, Q. Shen, and R. P. Garofalo. 1998. A promoter recruitment mechanism for tumor necrosis factor-α-induced interleukin-8 transcription in type II pulmonary epithelial cells. J. Biol. Chem. 273:3551-3561. [DOI] [PubMed] [Google Scholar]
- 4.Casola, A., R. P. Garofalo, M. Jamaluddin, S. Vlahopoulos, and A. R. Brasier. 2000. Requirement of a novel upstream response element in respiratory syncytial virus-induced IL-8 gene expression. J. Immunol. 164:5944-5951. [DOI] [PubMed] [Google Scholar]
- 5.Casola, A., R. P. Garofalo, H. Haeberle, T. F. Elliott, R. Lin, M. Jamaluddin, and A. R. Brasier. 2001. Multiple cis regulatory elements control RANTES promoter activity in alveolar epithelial cells infected with respiratory syncytial virus. J. Virol. 75:6428-6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Didcock, L., D. F. Young, S. Goodbourn, and R. E. Randall. 1999. The V protein of SV5 inhibits interferon signaling by targeting STAT1 for proteasome-mediated degradation. J. Virol. 73:9928-9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Domachowske, J. B., C. A. Bonville, and H. F. Rosenberg. 2001. Gene expression in epithelial cells in response to pneumonvirus infection. Respir. Res. 2:225-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiedler, M. A., K. Wernke-Dollries, and J. M. Stark. 1996. Inhibition of viral replication reverses respiratory syncytial virus-induced NF-κB activation and interleukin-8 gene expression in A549 cells. J. Virol. 70:9079-9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao, J., B. P. De, Y. Han, S. Choudhary, R. Ransohoff, and A. K. Banerjee. 2001. Human parainfluenza virus type 3 inhibits gamma interferon-induced major histocompatibility complex class II expression directly and by inducing alpha/beta interferon. J. Virol. 75:1124-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garofalo, R., M. Sabry, M. Jamaluddin, R. K. Yu, A. Casola, P. L. Ogra, and A. R. Brasier. 1996. Transcriptional activation of the interleukin-8 gene by respiratory syncytial virus infection in alveolar epithelial cells: nuclear translocation of the RelA transcription factor as a mechanism producing airway mucosal inflammation. J. Virol. 70:8773-8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray, P. M., G. D. Parks, and M. A. Alexander-Miller. 2001. A novel CD8-independent high-avidity cytotoxic T-lymphocyte response directed against an epitope in the phosphoprotein of the paramyxovirus simian virus 5. J. Virol. 75:10065-10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He, B., R. G. Paterson, C. D. Ward, and R. A. Lamb. 1997. Recovery of infectious SV5 from cloned DNA and expression of a foreign gene. Virology 237:249-260. [DOI] [PubMed] [Google Scholar]
- 13.Hedges, S. R., R. W. Agace, and C. Svanborg. 1995. Epithelial cytokine responses and mucosal cytokine networks. Trends Microbiol. 3:266-270. [DOI] [PubMed] [Google Scholar]
- 14.Jamaluddin, M., R. Garofalo, P. L. Ogra, and A. R. Brasier. 1996. Inducible translational regulation of the NF-IL6 transcription factor by respiratory syncytial virus infection in pulmonary epithelial cells. J. Virol. 70:1554-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jamaluddin, M., S. Wang, R. Garofalo, T. Elliot, A. Casola, S. Baron, and A. R. Brasier. 2001. IFN-β mediates coordinate expression of antigen-processing genes in RSV-infected pulmonary epithelial cells. Am. J. Physiol. 280:248-257. [DOI] [PubMed] [Google Scholar]
- 16.Kurt-Jones, E. A., L. Popova, L. Kwinn, L. M. Haynes, L. P. Jones, R. A. Tripp, E. W. Walsh, M. W. Freeman, D. T. Golenbock, L. J. Anderson, and R. W. Finberg. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1:398-401. [DOI] [PubMed] [Google Scholar]
- 17.Lamb, R. A., and D. Kolakofsky. 2001. Paramyxoviridae: the viruses and their replication, p. 1305-1340. In B. Fields, D. Knipe, and P. Howley (ed.), Fields virology, 4th ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 18.Luster, A. D. 2002. The role of chemokines in linking innate and adaptive immunity. Curr. Opin. Immunol. 14:129-135. [DOI] [PubMed] [Google Scholar]
- 19.Megyeri, K., W. C. Au, I. Rosztoczy, N. B. Raj, R. L. Miller, M. A. Tomai, and P. M. Pitha. 1995. Stimulation of interferon and cytokine gene expression by imiquimod and stimulation by Sendai virus utilize similar signal transduction pathways. Mol. Cell. Biol. 15:2207-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukaida, N. 2003. Pathophysiological roles of interleukin-8/CXCL8 in pulmonary diseases. Am. J. Physiol. Lung Cell Mol. Physiol. 284:L566-L577. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura, H., K. Yoshimura, H. A. Jaffe, and R. G. Crystal. 1991. Interleukin-8 gene expression in human bronchial epithelial cells. J. Biol. Chem. 266:19611-19617. [PubMed] [Google Scholar]
- 22.Parks, G. D., and M. A. Alexander-Miller. 2002. High avidity CTL to a foreign antigen are efficiently activated following immunization with a recombinant paramyxovirus simian virus 5. J. Gen. Virol. 83:1167.. [DOI] [PubMed] [Google Scholar]
- 23.Parks, G. D., K. R. Ward, and J. C. Rassa. 2001. Increased readthrough transcription across the simian virus 5 M-F gene junction leads to growth defects and a global inhibition of viral mRNA synthesis. J. Virol. 75:2213-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rassa, J. C., J. L. Meyers, Y. Zhahng, R. Kudaravalli, and S. R. Ross. 2002. Murine retroviruses activate B cells via interaction with Toll-like receptor 4. Proc. Natl. Acad. Sci. USA 99:2281-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staniford, T. J., S. L. Kunkel, M. A. Basha, S. W. Chensue, J. P. I. Lynch, G. B. Toews, J. Westwick, and R. M. Strieter. 1990. Interleukin-8 gene expression by a pulmonary epithelial cell line: a model for cytokine networks in the lung. J. Clin. Investig. 86:1945-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strieter, R. M., J. A. Belperio, and M. P. Keane. 2002. Cytokines in innate host defense in the lung. J. Clin. Investig. 109:699-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wansley, E. K., and G. D. Parks. 2002. Naturally occurring substitutions in the P/V gene convert the noncytopathic paramyxovirus SV5 into virus that induces type I interferon synthesis and cell death. J. Virol. 76:10109-10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young, D. F., L. Didcock, S. Goodbourn, and R. E. Randall. 2000. Paramyxoviruses use distinct virus-specific mechanisms to circumvent the interferon response. Virology 269:383-390. [DOI] [PubMed] [Google Scholar]
- 29.Yoneyama, M., W. Suhara, Y. Fukuhara, M. Fukuda, E. Nishida, and T. Fujita. 1998. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 17:1087-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang, Y., B. A. Luxon, A. Casola, R. P. Garofalo, M. Jamaluddin, and A. R. Brasier. 2001. Expression of respiratory syncytial virus-induced chemokine gene networks in lower airway epithelial cells revealed by cDNA microarrays. J. Virol. 75:9044-9058. [DOI] [PMC free article] [PubMed] [Google Scholar]