Abstract
Herpes simplex virus (HSV) 1 disaggregates the nuclear domain 10 (ND10) nuclear structures and disperses its organizing promyelocytic leukemia protein (PML). An earlier report showed that ectopic overexpression of PML precludes the disaggregation of ND10 but has no effect on viral replication. PML has been reported to mediate the effects of interferon (IFN) and viral mutants lacking ICP0 (Δα0 mutants). To test the hypothesis that HSV disaggregates ND10 structures and disperses PML to preclude IFN-mediated antiviral effects, we tested the accumulation of viral proteins and virus yields from murine PML+/+ and PML−/− cells mock treated or exposed to IFN-α, IFN-γ, or both and infected with the wild-type or Δα0 mutant virus. We report the following results. (i) The levels of growth of wild-type and mutant viruses and of accumulation of viral proteins were not significantly different in untreated PML+/+ and PML−/− cells. (ii) Major effects of IFN-α and -γ were observed in PML+/+ cells infected with the Δα0 mutant virus, and more minor effects were observed in cells infected with the wild-type virus. The effects of the IFNs on either wild-type or the mutant virus in PML−/− cells were minimal. (iii) The mixture of IFN-α and -γ was more effective than either IFN alone, but again, the effect was more drastic in PML+/+ cells than in PML−/− cells. We concluded that the anti-HSV state induced by exogenous IFN is mediated by PML and that the virus targets the ND10 structures and disseminates PML in order to preclude the establishment of the antiviral state induced by IFNs.
A characteristic feature of cells infected with herpes simplex virus 1 (HSV-1) is the rapid disappearance of the nuclear structures known by any of several names such as nuclear domain 10 (ND10), nuclear bodies, Kremer (Kr) bodies, or promyelocytic leukemia protein (PML) oncogenic domains (13, 20, 24). PML is dispersed from the ND10 structures soon after infection (7, 22, 23). The disaggregation of these structures and the dispersal of PML have been shown to be mediated by infected-cell protein no. 0 (ICP0), a product of the α0 gene (25, 26).
PML is considered to be the organizing protein of the ND10 structures (14, 35). Indeed, in transduced cells forced to overexpress PML, the ND10 structures increase in size and the apparent content of their other constituent proteins increases (19). A central question that is the basis of the studies reported here is why HSV and many other viruses target PML and the ND10 structures. An earlier article from this laboratory showed that in transduced cells forced to overexpress PML the ND10 structures remained intact throughout infection but that overexpression of PML had no effect on viral gene expression or viral yields (19).
PML has been reported to have numerous functions in uninfected cells. It has been implicated in several cellular processes, including cell proliferation, senescence, tumorigenesis, apoptosis, resistance to virus infection, and hormone signaling (5, 11, 16, 18, 21, 27, 30, 31, 32, 34, 36). Perhaps the function attributed to PML that is most relevant to its potential role in HSV-1-infected cells is its mediation of the function of interferons (IFNs). The antiviral functions of PML have been described previously (21, 24, 27). The PML promoter contains IFN response elements (29), and the accumulation of PML, ISG120, SP140, SP100, and proteasome components is increased after IFN treatment (8-10, 12, 17). If the virus targets PML because the latter enables and mediates the repressive effects of IFNs on viral replication, then the overexpression of PML should have a minimal effect on viral replication since the amounts normally resident in the cells should suffice. The prediction, therefore, is that, if the function of IFN is mediated by PML, IFNs should have a greater effect on HSV-1 replication in PML+/+ cells than in PML−/− cells.
IFNs inhibit viral protein synthesis more effectively in PML+/+ cells than in PML−/− cells.
To test the hypothesis that IFNs inhibit viral protein synthesis more effectively in PML+/+ cells that in PML−/− cells, we carried out a series of experiments with mouse fibroblasts derived from embryos of wild-type (PML+/+) and PML−/− mice described elsewhere (31). In these experiments, replicate PML+/+ or PML−/− cells grown in 25-cm2 flasks were either mock treated or treated with 1,000 U of either murine IFN-α or IFN-γ or with 1,000 U of both IFN-α and IFN-γ per ml of medium. Recombinant murine IFN-α (catalog number 12500-1) and IFN-γ (catalog number 12100-1) were purchased from PBL Biomedical Laboratories (New Brunswick, N.J.). After 24 h of exposure, the cells were infected with either 0.1, 1, or 10 PFU of wild-type virus [HSV-1(F)] or the recombinant virus R7910, from which both copies of the α0 gene had been deleted. The derivation of these viruses has been described elsewhere. After 24 h of infection, the cells were harvested and subjected to one of two procedures.
First procedure.
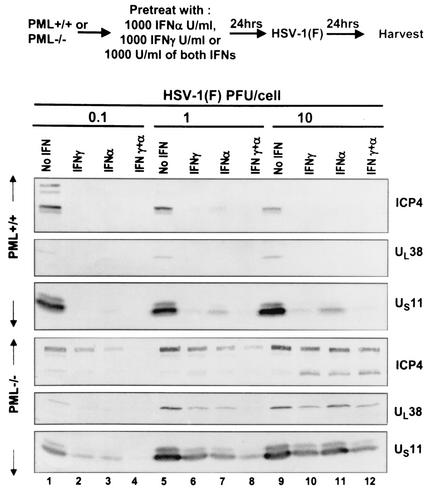
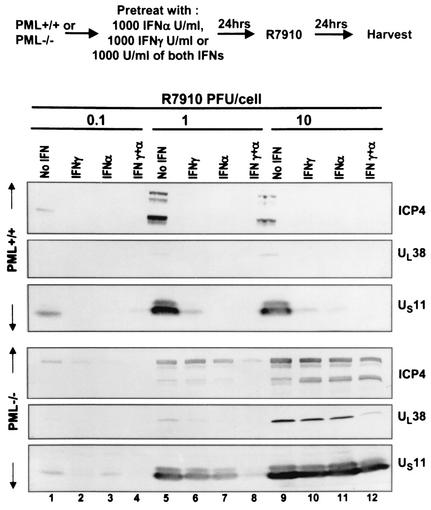
In the first procedure, the infected cells were harvested, rinsed with phosphate-buffered solution, solubilized in a denaturing buffer, subjected to electrophoresis in denaturing polyacrylamide gels, transferred to a nitrocellulose sheet, and probed with antibodies to three viral proteins, ICP4 (the product of the α4 gene) and the products of UL38 and US11 genes. Whereas ICP4 is expressed immediately after infection, the products of the UL38 and US11 genes are made very late in infection and require the synthesis of viral DNA. The results of this series of experiments were as follows (Fig. 1 and 2). (i) As expected, the amounts of viral proteins detected in cells infected with 0.1 PFU/cell were generally significantly lower than those detected in lysates of cells infected at higher ratios of virus per cell. (ii) Only trace amounts of viral proteins were detected in IFN-treated cells compared to the amounts detected in mock-treated, infected cells. (iii) In contrast to the drastic effects of IFN on viral gene expression in PML+/+ cells, IFN had virtually no effect on viral gene expression in PML−/− cells infected with 10 PFU/cell and the mixture of IFN-α and IFN-γ had only a slight effect on the accumulation of viral proteins in cells infected with 1 PFU/cell. We concluded that the suppressive effects of IFN require the presence of PML-infected cells in both the wild type and an ICP0 mutant (Δα0 mutant).
FIG. 1.
Effects of IFN-α and IFN-γ on the accumulation of viral proteins encoded by α4, UL38, and US11 open reading frames. (Top) Experimental design; (bottom) immunoblots of electrophoretically separated lysates of murine PML+/+ or PML−/− infected cells. At The University of Chicago, the PML−/− and PML+/+ cells were initially maintained in culture for approximately 20 serial passages and were then frozen. Prior to the initiation of these studies, the absence of PML from PML−/− cells was verified for both untreated and IFN-treated cells (data not shown). In all experiments, thawed cells were maintained in culture for less than 15 serial passages. HSV-1(F), an isolate that can be passaged a limited number of times, is the prototype HSV-1 strain used in this laboratory (6). The construction and phenotypic properties of the recombinant virus R7910, lacking both copies of the α0 gene, were described elsewhere (15). The cells were harvested 24 h after infection, rinsed with phosphate-buffered saline (PBS), resuspended in PBS* (PBS containing 1% NP-40, 1% deoxycholate, and the Roche complete protease inhibitor cocktail), lysed for 30 min in ice, and centrifuged at 11,000 × g for 15 min to remove the insoluble fraction. Protein concentrations were determined by the Bradford assay (Bio-Rad). Protein lysates (100 μg) were denatured by boiling them for 5 min in disruption buffer (2% sodium dodecyl sulfate, 50 mM Tris [pH 7.0], 2.75% sucrose, 5% β-mercaptoethanol [final concentrations], and bromophenol blue), electrophoretically separated on a denaturing 12% polyacrylamide (acrylamide-DATD) gel, and transferred to nitrocellulose membranes. The nitrocellulose sheets were blocked for 1 h with 5% milk buffer, probed with the appropriate primary antibody for 2 h at room temperature or overnight at 4°C, rinsed, and then reacted with secondary antibodies conjugated with alkaline phosphatase (Bio-Rad) diluted 1:3,000 in PBS containing 0.05% Tween 20 and 1% bovine serum albumin for 1 h. The mouse monoclonal antibodies to ICP4 and US11 were reported elsewhere (1, 28) and were used at dilutions of 1:1,000 and 1:2,000, respectively. The polyclonal antibody to the UL38 protein (33) was used at a 1:500 dilution. Alkaline phosphatase-conjugated antibodies were detected in AP buffer (100 mM Tris [pH 9.5], 100 mM NaCl, and 5 mM MgCl2) containing 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium.
FIG. 2.
The effects of IFN-α and IFN-γ on the accumulation of viral proteins encoded by α4, UL38, and US11 open reading frames. (Top) Experimental design; (bottom) immunoblots of electrophoretically separated lysates of murine PML+/+ or PML−/− infected cells. The procedures were as described in the legend to Fig. 1.
Second procedure.
In the second procedure, the cells harvested 24 h after infection were disrupted by sonication and the titers either in Vero cells (wild-type virus) or in U2-OS cells were determined. The results, shown in Table 1, were as follows. (i) HSV-1(F) replicated in PML−/− cells at least as well as (0.1 PFU/cell) or better than (1 PFU/cell) in PML+/+ cells. IFN-α and IFN-γ were approximately equally effective in reducing viral yields in PML+/+ cells infected with 0.1 PFU/cell. The effects of IFN-α and IFN-γ were significantly reduced (less than 10-fold) in PML−/− cells infected with 1.0 PFU/cell. In PML−/− cells infected with 0.1 PFU/cell, IFN-α reduced the yield by approximately 20-fold—significantly less than the reduction in virus yields obtained with IFNα-treated PML+/+ cells (100-fold). The mixture of IFN-α and IFN-γ was more effective than the individual IFNs in reducing viral yields. In this instance as well, the effects of the mixture of IFNs were greater in PML+/+ than in PML−/− cells at the lower multiplicity of infection. (ii) The differential effects of IFNs in PML−/− and PML+/+ cells were more pronounced in cells infected with the recombinant virus R7910, from which both copies of the α0 gene had been deleted. Although the yields of R7910 were reduced relative to those of the wild-type parent, there was no significant difference between yields obtained from untreated PML−/− and those obtained from PML+/+ cells. In contrast, both IFN-α and IFN-γ had a significantly more drastic effect on viral replication in PML+/+ than in PML−/− cells. While the reduction in virus yields from PML−/− cells treated with IFN-α or IFN-γ and exposed to 0.1 PFU/cell was approximately 30- to 40-fold, the corresponding reduction in PML+/+ cells was approximately 10,000-fold. While viral yields from PML−/− cells infected with 1 PFU/cell and treated with IFN-α or IFN-γ did not differ significantly from those of untreated cells, there was a 100-fold reduction in viral yields from infected PML+/+ cells. Again, as in the case of cells infected with wild-type virus, the combination of IFN-α and IFN-γ was more effective in reducing virus yields in PML+/+ than in PML−/− cells, especially in cells infected at a multiplicity of infection of 0.1 PFU/cell.
TABLE 1.
Viral yields from PML+/+ or PML−/− cells mock treated or treated with IFN-α or IFN-γ
| No. of PFU/cell | IFN treatment | Viral yield from indicated type of cells infected with:
|
|||
|---|---|---|---|---|---|
| HSV-1 (F)
|
R7910 (Δα0)
|
||||
| PML+/+ | PML−/− | PML+/+ | PML−/− | ||
| 0.1 | None | 2.9 × 107 | 4.9 × 107 | 8.0 × 104 | 2.5 × 105 |
| IFN-α | 1.7 × 104 | 1.7 × 106 | 5 | 6.2 × 103 | |
| IFN-γ | 1.6 × 104 | 9.1 × 106 | 10 | 7.3 × 104 | |
| IFN-α + IFN-γ | 35 | 4.1 × 104 | 7 | 80 | |
| 1 | None | 3.5 × 106 | 5.7 × 107 | 4.8 × 105 | 1.5 × 105 |
| IFN-α | 1.3 × 105 | 8.0 × 106 | 260 | 8.3 × 104 | |
| IFN-γ | 6.0 × 104 | 2.0 × 107 | 2.5 × 103 | 7.3 × 104 | |
| IFN-α + IFN-γ | 3.2 × 104 | 3.5 × 105 | 180 | 1.6 × 103 | |
PML mediates an antiviral response in the presence of IFNs.
The results presented in this report lead to several conclusions. (i) We did not observe major differences between PML−/− and PML+/+ cells with respect to the accumulation of viral proteins or viral yields in the absence of IFNs. (ii) PML mediates to a large extent the ability of exogenous IFN-α or IFN-γ to induce a host antiviral response in infected cells. This mediation is evident from analyses of both viral-protein accumulation in and viral yields from mock-infected and IFN-treated cells. (iii) The effects of IFN were less drastic at lower than at higher multiplicities of infection and more drastic in PML+/+ than in PML−/− cells. (iv) The effects of exogenously added IFNs, albeit very much reduced in PML−/− cells, suggest either that PML is not the sole mediator or that its role is indirect with respect to the induction of an antiviral state in HSV-1-infected cells.
The significance of the report presented here stems from several considerations. Several viruses, including HSV-1, induce the disaggregation of ND10 structures and the dissemination of PML (2, 3, 4). In HSV-1-infected cells, these functions are mediated by ICP0. As noted earlier in the text, a large number of seemingly unrelated functions have been attributed to PML or, possibly, to the ND10 structures to which PML is localized. While the evidence that HSV-1 targets ND10 structures is persuasive, the reason why the virus would target these structures is not. Since ectopically induced overexpression of PML had no effect on viral replication, the data suggested either that the destruction of ND10 was a consequence of a virus-induced activity against another cellular target or that the function of PML did not require excessive amounts of the protein. The test of the latter hypothesis, as described in this report, was based on the reports that, among other functions, PML mediates the effects of IFN and that cells lacking the α0 genes and hence that are unable to synthesize ICP0 are more sensitive to IFN than wild-type cells are.
The scenario that is emerging from these studies is that HSV-1 targets ND10 and PML concurrently to preclude the ability of the cell to respond to the presence of IFN, which would lead to the development of an antiviral response. This possibility is consistent with the report cited earlier that IFN induces the synthesis of mRNA and controls ND10 protein levels as well as the number and size of ND10 structures (8-10, 12, 17). In essence, the model that emerges from these studies is that the destruction of ND10 and the dispersal of PML are not critical in the absence of IFN. In the absence of IFN, the Δα0 mutant multiplied in PML−/− cells as well or as poorly (depending on cell type) as in PML+/+ cells. In the presence of IFN, the failure to disperse PML and disaggregate the ND10 structures results in a drastic effect on viral replication. Our studies also indicated that overexpression of PML had no effect because the cells already contained the full complement of PML required to effect an antiviral response if IFN were present.
In the studies presented here, we have noted that the mixture of IFN-α and IFN-γ has a more profound effect than either of the IFNs alone, even in PML−/− cells. This observation suggests at least two possibilities. The first is that the anti-HSV state is induced by different pathways by IFN-α and IFN-γ and that both pathways must be induced to overcome the virus. In this scenario, each pathway is mediated by PML and differentially affected by the expression of ICP0. The alternative, nonexclusive hypothesis is that PML acts to facilitate the induction by IFN of the antiviral state but is not the effector of the IFN signal transduction pathways and that, therefore, the low-level activity of exogenous IFNs may be additive.
Acknowledgments
These studies were aided by grants from the National Cancer Institute of the U.S. Public Health Service to The University of Chicago (grants CA87661, CA83939, CA71933, CA78766, and CA88860) and to Sloan-Kettering Cancer Center (grant CA71692).
REFERENCES
- 1.Ackermann, M., D. K. Braun, L. Pereira, and B. Roizman. 1984. Characterization of herpes simplex virus 1 α proteins 0, 4, and 27 with monoclonal antibodies. J. Virol. 52:108-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn, J. H., E. J. Brignole III, and G. S. Hayward. 1998. Disruption of PML subnuclear domains by the acidic IE1 protein of human cytomegalovirus is mediated through interaction with PML and may modulate a RING finger-dependent cryptic transactivator function of PML. Mol. Cell. Biol. 18:4899-4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chelbi-Alix, M. K., F. Quignon, L. Pelicano, M. H. M. Koken, and H. de Thé. 1998. Resistance to virus infection conferred by the interferon-induced promyelocytic leukemia protein. J. Virol. 72:1043-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doucas, V., A. M. Ishov, A. Romo, H. Juguilon, M. D. Weitzman, R. M. Evans, and G. G. Maul. 1996. Adenovirus replication is coupled with the dynamic properties of the PML nuclear structure. Genes Dev. 10:196-207. [DOI] [PubMed] [Google Scholar]
- 5.Doucas, V., M. Tini, D. A. Egan, and R. M. Evans. 1999. Modulation of CREB binding protein function by the promyelocytic (PML) oncoprotein suggests a role for nuclear bodies in hormone signaling. Proc. Natl. Acad. Sci. USA 96:2627-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ejercito, P. M., E. D. Kieff, and B. Roizman. 1968. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J. Gen. Virol. 2:357-364. [DOI] [PubMed] [Google Scholar]
- 7.Everett, R. D., and G. G. Maul. 1994. HSV-1 IE protein Vmw110 causes redistribution of PML. EMBO J. 13:5062-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gongora, C., G. David, L. Pintard, C. Tissot, T. D. Hua, A. Dejean, and N. Mechti. 1997. Molecular cloning of a new interferon-induced PML nuclear body-associated protein. J. Biol. Chem. 272:19457-19463. [DOI] [PubMed] [Google Scholar]
- 9.Grotzinger, T., K. Jensen, and H. Will. 1996. The interferon (IFN)-stimulated gene Sp100 promoter contains an IFN-gamma activation site and an imperfect IFN-stimulated response element which mediate type I IFN inducibility. J. Biol. Chem. 271:25253-25260. [DOI] [PubMed] [Google Scholar]
- 10.Grotzinger, T., T. Sternsdorf, K. Jensen, and H. Will. 1996. Interferon-modulated expression of genes encoding the nuclear-dot-associated proteins Sp100 and promyelocytic leukemia protein (PML). Eur. J. Biochem. 238:554-560. [DOI] [PubMed] [Google Scholar]
- 11.Guiochon-Mantel, A., J. F. Savouret, F. Quignon, K. Delabre, E. Milgrom, and H. De The. 1995. Effect of PML and PML-RAR on the transactivation properties and subcellular distribution of steroid hormone receptors. Mol. Endocrinol. 9:1791-1803. [DOI] [PubMed] [Google Scholar]
- 12.Guldner, H. H., C. Szostecki, T. Grotzinger, and H. Will. 1992. IFN enhance expression of Sp100, an autoantigen in primary biliary cirrhosis. J. Immunol. 149:4067-4073. [PubMed] [Google Scholar]
- 13.Hodges, M., C. Tissot, K. Howe, D. Grimwade, and P. S. Freemont. 1998. Structure, organization, and dynamics of promyelocytic leukemia protein nuclear bodies. Am. J. Hum. Genet. 63:297-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishov, A. M., A. G. Sotnikov, D. Negorev, O. V. Vladimirova, N. Neff, T. Kamitani, E. T. Yeh, J. F. Strauss III, and G. G. Maul. 1999. PML is critical for ND10 formation and recruits the PML-interacting protein daxx to this nuclear structure when modified by SUMO-1. J. Cell Biol. 147:221-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawaguchi, Y., C. Van Sant, and B. Roizman. 1997. Herpes simplex virus 1 α regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J. Virol. 71:7328-7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LaMorte, V. J., J. A. Dyck, R. L. Ochs, and R. M. Evans. 1998. Localization of nascent RNA and CREB binding protein with the PML-containing nuclear body. Proc. Natl. Acad. Sci. USA 95:4991-4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavau, C., A. Marchio, M. Fagioli, J. Jansen, B. Falini, P. Lebon, F. Grosveld, P. P. Pandolfi, P. G. Pelicci, and A. Dejean. 1995. The acute promyelocytic leukaemia-associated PML gene is induced by interferon. Oncogene 11:871-876. [PubMed] [Google Scholar]
- 18.Li, H., C. Leo, J. Zhu, X. Wu, J. O'Neil, E. J. Park, and J. D. Chen. 2000. Sequestration and inhibition of Daxx-mediated transcriptional repression by PML. Mol. Cell. Biol. 20:1784-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez, P., R. J. Jacob, and B. Roizman. 2002. Overexpression of promyelocytic leukemia protein precludes the dispersal of ND10 structures and has no effect on accumulation of infectious herpes simplex virus 1 or its proteins. J. Virol. 76:9355-9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matera, A. G. 1999. Nuclear bodies: multifaceted subdomains of the interchromatin space. Trends Cell Biol. 9:302-309. [DOI] [PubMed] [Google Scholar]
- 21.Maul, G. G. 1998. Nuclear domain 10, the site of DNA virus transcription and replication. Bioessays 20:660-667. [DOI] [PubMed] [Google Scholar]
- 22.Maul, G. G., and R. D. Everett. 1994. The nuclear location of PML, a cellular member of the C3HC4 zinc-binding domain protein family, is rearranged during herpes simplex virus infection by the C3HC4 viral protein ICP0. J. Gen. Virol. 75:1223-1233. [DOI] [PubMed] [Google Scholar]
- 23.Maul, G. G., H. H. Guldner, and J. G. Spivack. 1993. Modification of discrete nuclear domains induced by herpes simplex virus type 1 immediate early gene 1 product (ICP0). J. Gen. Virol. 74:2679-2690. [DOI] [PubMed] [Google Scholar]
- 24.Maul, G. G., D. Negorev, P. Bell, and A. M. Ishov. 2000. Properties and assembly mechanisms of ND10, PML bodies, or PODs. J. Struct. Biol. 129:278-287. [DOI] [PubMed] [Google Scholar]
- 25.Muller, S., M. J. Matunis, and A. Dejean. 1998. Conjugation with the ubiquitin-related modifier SUMO-1 regulates the partitioning of PML within the nucleus. EMBO J. 17:61-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parkinson, J., and R. D. Everett. 2000. Alphaherpesvirus proteins related to herpes simplex virus type 1 ICP0 affect cellular structures and proteins. J. Virol. 74:10006-10017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Regad, T., and M. K. Chelbi-Alix. 2001. Role and fate of PML nuclear bodies in response to interferon and viral infections. Oncogene 20:7274-7286. [DOI] [PubMed] [Google Scholar]
- 28.Roller, R. J., and B. Roizman. 1992. The herpes simplex virus 1 RNA binding protein US11 is a virion component and associates with ribosomal 60S subunits. J. Virol. 66:3624-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stadler, M., M. K. Chelbi-Alix, M. H. Koken, L. Venturini, C. Lee, A. Saib, F. Quignon, L. Pelicano, M. C. Guillemin, C. Schindler, et al. 1995. Transcriptional induction of the PML growth suppressor gene by interferons is mediated through an ISRE and a GAS element. Oncogene 11:2565-2573. [PubMed] [Google Scholar]
- 30.Vallian, S., K. V. Chin, and K. S. Chang. 1998. The promyelocytic leukemia protein interacts with Sp1 and inhibits its transactivation of the epidermal growth factor receptor promoter. Mol. Cell. Biol. 18:7147-7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, Z. G., L. Delva, M. Gaboli, R. Rivi, M. Giorgio, C. Cordon-Cardo, F. Grosveld, and P. P. Pandolfi. 1998. Role of PML in cell growth and the retinoic acid pathway. Science 279:1547-1551. [DOI] [PubMed] [Google Scholar]
- 32.Wang, Z. G., D. Ruggero, S. Ronchetti, S. Zhong, M. Gaboli, R. Rivi, and P. P. Pandolfi. 1998. PML is essential for multiple apoptotic pathways. Nat. Genet. 20:266-272. [DOI] [PubMed] [Google Scholar]
- 33.Ward, P. L., W. O. Ogle, and B. Roizman. 1996. Assemblons: nuclear structures defined by aggregation of immature capsids and some tegument proteins of herpes simplex virus 1. J. Virol. 70:4623-4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhong, S., L. Delva, C. Rachez, C. Cenciarelli, D. Gandini, H. Zhang, S. Kalantry, L. P. Freedman, and P. P. Pandolfi. 1999. A RA-dependent, tumour-growth suppressive transcription complex is the target of the PML-RARalpha and T18 oncoproteins. Nat. Genet. 23:287-295. [DOI] [PubMed] [Google Scholar]
- 35.Zhong, S., S. Muller, S. Ronchetti, P. S. Freemont, A. Dejean, and P. P. Pandolfi. 2000. Role of SUMO-1-modified PML in nuclear body formation. Blood 95:2748-2752. [PubMed] [Google Scholar]
- 36.Zhong, S., P. Salomoni, and P. P. Pandolfi. 2000. The transcriptional role of PML and the nuclear body. Nat. Cell Biol. 2:E85-E90. [DOI] [PubMed] [Google Scholar]