Abstract
As the most numerous cells in the brain, astrocytes play a critical role in maintaining central nervous system homeostasis, and therefore, infection of astrocytes by human immunodeficiency virus (HIV) or simian immunodeficiency virus (SIV) in vivo could have important consequences for the development of HIV encephalitis. In this study, we establish that astrocytes are infected in macaques during acute SIV infection (10 days postinoculation) and during terminal infection when there is evidence of SIV-induced encephalitis. Additionally, with primary adult rhesus macaque astrocytes in vitro, we demonstrate that the macrophage-tropic, neurovirulent viruses SIV/17E-Br and SIV/17E-Fr replicate efficiently in astrocytes, while the lymphocyte-tropic, nonneurovirulent virus SIVmac239 open-nef does not establish productive infection. Furthermore, aminoxypentane-RANTES abolishes virus replication, suggesting that these SIV strains utilize the chemokine receptor CCR5 for entry into astrocytes. Importantly, we show that SIV Nef is required for optimal replication in primary rhesus macaque astrocytes and that normalizing input virus by particle number rather than by infectivity reveals a disparity between the ability of a Nef-deficient virus and a virus encoding a nonmyristoylated form of Nef to replicate in these central nervous system cells. Since the myristoylated form of Nef has been implicated in functions such as CD4 and major histocompatibility complex I downregulation, kinase association, and enhancement of virion infectivity, these data suggest that an as yet unidentified function of Nef may exist to facilitate SIV replication in astrocytes that may have important implications for in vivo pathogenesis.
Human immunodeficiency virus (HIV) encephalitis is a debilitating condition occurring in 20 to 30% of untreated HIV-infected individuals, characterized by the recruitment of macrophages into the central nervous system (CNS), elevated chemokine and cytokine levels, and the development of inflammatory lesions, ultimately resulting in dementia (21, 33, 47). The majority of HIV strains isolated from the CNS are macrophage-tropic (13, 25). Although perivascular macrophages and microglia are the predominant cells infected in the CNS (7), more sensitive detection techniques have revealed infection of other cell types, such as astrocytes (3, 45, 48, 61). Astrocytes are the most abundant cells in the brain and provide structural support for the complex network of neuronal synapses necessary for CNS function. Among numerous other functions, these glial cells facilitate the uptake of neurotoxic substances, providing a neuroprotective effect, and they stimulate the formation of tight junctions in endothelial cells to form the blood-brain barrier (6, 46, 51).
Infection of astrocytes by HIV raises significant concerns regarding the ability of infected astrocytes to perform normal functions critical for the maintenance of CNS integrity; the ability of highly active antiretroviral therapies that do not penetrate the CNS to inhibit astrocyte infection; and hence, the potential for infected astrocytes to provide a persistent cellular reservoir for virus replication. Numerous reports have suggested that astrocytes are susceptible to HIV infection in vivo (48, 53, 62). However, infection appears to be restricted to the expression of early gene products, as typically only Nef and Rev proteins are detected by immunohistochemistry (53, 63), and only rarely are structural proteins such as HIV gp160 detected (48).
Studies of HIV cell tropism in vitro have demonstrated that human astrocytoma cell lines are susceptible to infection (24, 36, 43, 57). Likewise, these infections are typically restricted to the production of early gene products, which may involve defective Rev function (42). Other studies have demonstrated that primary fetal astrocytes are also susceptible to infection by HIV, but detection of infectious virus typically requires coculture with susceptible cells or stimulation with cytokines such as tumor necrosis factor alpha or interleukin-1 beta (5, 9, 15, 28-30, 52, 63, 64). It should be noted, however, that the majority of in vitro studies of both primary fetal astrocytes and astrocytoma cell lines examined replication of T-cell-tropic HIV isolates. Because few macrophage-tropic strains or primary blood- or brain-derived isolates have been studied, no consensus has emerged regarding the genotypic or phenotypic nature of viruses that replicate in astrocytes in vivo or in vitro.
Given the inherent difficulties in studying the development and progression of CNS disease in HIV-infected individuals and the limited access to normal human adult primary astrocytes, it is essential to establish cohesive in vitro and parallel in vivo models that closely recapitulate the viral and cellular processes that lead to encephalitis. Therefore, we examined the susceptibility of adult primary macaque astrocytes to productive simian immunodeficiency virus (SIV) replication in vivo and in vitro in the context of a well-characterized SIV-macaque model of HIV neuropathogenesis.
The SIV model of HIV infection provides the best animal model with which to study viral pathogenesis resulting in AIDS and AIDS-related diseases (20, 23, 49). Like HIV, the SIV model results in immune suppression and AIDS and has been shown to induce inflammatory and degenerative changes in the CNS that are very similar to the neuropathology described in HIV-infected patients (34, 58, 59, 68). Similar to HIV, the SIV strains that replicate in the CNS are macrophage-tropic, indicating that certain viral genotypes have a distinct replicative advantage in various tissues and cell types (2, 38, 68). However, unlike studies in humans infected with HIV, the SIV model can be used to perform longitudinal analyses of pathogenesis associated with specific SIV genotypes. To date, no studies have demonstrated directly that astrocytes are infected with SIV in vivo; however, Guillemin et al. (27) were able to demonstrate limited in vitro infection of primary rhesus macaque astrocytes by SIVmac251, a variably neurovirulent virus, which was unable to replicate to high amounts, and particles were detectable only after stimulation with cytokines.
An accelerated, consistent SIV-macaque model has been developed in our laboratory in which macaques dually inoculated with the immunosuppressive swarm SIVDeltaB670 and the previously characterized, neurovirulent molecular clone SIV/17E-Fr progress to AIDS, and greater than 90% develop encephalitis by terminal infection (68), at which time SIV/17E-Fr is a predominant genotype present in the brain (2). With this established model, we demonstrate that astrocytes are infected in vivo during acute and terminal infection, providing the first direct in vivo evidence that astrocytes are targets for SIV infection in macaques. In addition, with primary adult rhesus macaque astrocyte cultures, we demonstrate that these cells support productive replication of the neurovirulent virus swarm SIV/17E-Br as well as the macrophage-tropic, neurovirulent clone SIV/17E-Fr (18, 38), whereas the lymphocyte-tropic, nonneurovirulent molecular clone SIVmac239 was unable to establish productive infection. Furthermore, Nef-deficient molecular clones derived from SIV/17E-Fr exhibited a profound defect in replication, demonstrating a requirement of Nef for optimal replication in astrocytes. Given that Nef is required for in vivo pathogenesis but not in most in vitro systems (22, 32), that Nef is required for optimal SIV replication in primary rhesus macaque astrocytes suggests the existence of a Nef function that may be important for SIV neuropathogenesis.
MATERIALS AND METHODS
Cloning of SIV molecular clones.
SIV/17E-Fr, SIV/17E-FrΔnef, SIV/Fr-2, and SIV/17E-Fr(−myr) were constructed as previously described (18, 19). SIV/17E-Fr(−nef) was constructed by mutating the start codon in SIV/17E-Fr nef and inserting three consecutive stop codons. Briefly, with the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, Calif.), PCR was performed with the mutagenesis primers 5′-CCTACAATACGGGTTGATAAATTTCCATAAGGCGGTC-3′ and 5′-GACCGCCTTATGGAAATTTATCAACCCGTATTGTAGG-3′ and the template pLG-3′SIV/17E-Fr-NheI-BlpI. Following mutagenesis, the plasmid was digested with NheI and BlpI (New England BioLabs, Beverly, Mass.), the insert was ligated into full-length SIV/17E-Fr, and the presence of the mutation was confirmed by DNA sequencing.
Virus stocks.
Virus stocks were obtained by transfecting infectious viral plasmid DNA [SIV/17E-Fr, SIVmac239 open-nef, SIV/17E-FrΔnef, SIV/17E-Fr(−nef), SIV/Fr-2, and SIV/17E-Fr(−myr)] into CEMx174 cells [a gift of James Hoxie, University of Pennsylvania (54)]. Supernatants containing high reverse transcriptase activity (>70,000 cpm/ml; see below) were filtered through 0.45-μm filters (Millipore, Bedford, Mass.) to remove cell debris, and virus was pelleted through 20% sucrose-TNE (40 mM Tris-HCl, 1 mM EDTA, 150 mM NaCl) at 125,000 × g in a Sorvall OTD65B ultracentrifuge with an AH-629 rotor for 2 h at 4°C and resuspended in Dulbecco's modified Eagle's medium supplemented with 2% fetal bovine serum. Stocks of SIV/17E-Br (a virus swarm) were obtained by infecting primary rhesus macaque macrophages and harvesting as described above.
Culture and infection of primary rhesus macaque astrocytes.
Primary rhesus macaque astrocytes (Cambrex, Walkersville, Md.) were grown in astrocyte growth medium (Cambrex) at 37°C in 5% CO2. Twenty-four hours prior to virus infection, the medium was changed to Dulbecco's modified Eagle's medium (Invitrogen, Grand Island, N.Y.) containing 10% fetal bovine serum, l-glutamine (2 mM), sodium pyruvate (2 mM), and gentamicin (50 μg/ml). Immunofluorescence analysis of glial cells demonstrated that all cells in the cultures (passages 1 to 8) were positive for expression of glial fibrillary acidic protein (GFAP); at no time were GFAP-negative cells observed, indicating that the astrocyte cultures were devoid of contaminating cell types.
Infections of primary rhesus macaque astrocytes were performed in poly-l-lysine-coated vessels that were prepared as follows: plates were incubated with 0.1 mg of poly-l-lysine (Sigma, St. Louis, Mo.) per ml for 15 min at room temperature, after which the wells were washed three times with Hanks' buffered salt solution (HBSS) (Invitrogen), and the cells were plated. At 60 to 80% confluency, the astrocytes were incubated for 6 h with SIV that had been normalized by particle number (50 ng of p27) or by 50% tissue culture infectious dose (TCID50) (multiplicity of infection = 0.1 titered). The p27-to-TCID50 ratio of the virus stocks used in the Nef mutant comparisons were as follows: SIV/17E-Fr, 90:1; SIV/Fr-2, 495:1; SIV/17E-Fr(−myr), 127:1; SIV/17E-FrΔnef, 790:1; and SIV/17E-Fr(−nef), 1,356:1. The TCID50 values were derived from CEMx174 cells, which do not require Nef for replication. After infection, astrocyte cultures were washed three times with HBSS, and fresh Dulbecco's modified Eagle's medium with 10% fetal bovine serum was added. Cell supernatants were collected approximately every 5 days, the cells were washed once with HBSS, and fresh medium was added.
SIV p27 and reverse transcriptase assays.
Supernatants were assayed for p27 production with the SIV core antigen assay kit (Coulter, Miami, Fla.). Reverse transcriptase activity was assayed as previously described (11).
LuSIV assay.
The LuSIV assay was developed in our laboratory and performed as previously described (50). Briefly, virus (5 ng of p27) derived from supernatants of astrocytes or macrophages infected with SIV/17E-Br was used to infect 1.5 × 105 LuSIV cells, which were then incubated for 72 h and assayed for the presence of luciferase activity with a Labsystems Fluoroscan Ascent FL luminometer.
Blocking experiments.
Prior to infection, astrocytes were incubated for 1 h with 500 ng of aminoxypentane-RANTES (AOP-RANTES; Gryphon Sciences, San Francisco, Calif.) per ml. Astrocytes were incubated with SIV/17E-Br or SIV/17E-Fr normalized by TCID50 (multiplicity of infection = 0.1 titered on CEMx174 cells). Six hours postinfection, the cells were washed three times with HBSS, and medium with or without AOP-RANTES was added. Supernatants were removed every 5 days, and fresh medium with or without 500 ng of AOP-RANTES per ml was added. Supernatants were assayed for the presence of SIV p27 as described above.
Quantitation of viral DNA.
Astrocytes were infected with SIV/17E-Fr, SIV/17E-FrΔnef, SIV/17E-Fr(−nef), or SIVmac239 open-nef virus stocks (multiplicity of infection = 0.1, titered on CEMx174 cells) that had been DNase treated (20 U/ml; Promega, Madison, Wis.) in 10% Dulbecco's modified Eagle's medium containing 10 mM MgCl for 30 min at 37°C to remove exogenous DNA. At 24 h postinfection, the medium was removed, after which the cells were trypsinized, pelleted, washed with phosphate-buffered saline, and cellular DNA was extracted with the DNeasy tissue extraction kit (Qiagen, Valencia, Calif.). Real-time PCR was performed with 250 ng of total cellular DNA as described previously (67) with primers and probes specific for the SIV gag gene.
Quantitation of viral RNA.
Astrocytes were inoculated with SIV/17E-Fr, SIV/17E-FrΔnef, SIV/17E-Fr(−nef), or SIVmac239 open-nef normalized by TCID50 (multiplicity of infection = 0.1 on CEMx174cells) for 24 h and washed three times with HBSS, after which fresh medium was added, and the cells were allowed to incubate for 7 days before total cellular RNA was isolated with RNA-STAT 60 (Tel-Test, Friendswood, Tex.). RNA was treated with DNase I (Promega) for 2 h at 37°C, and RNA was purified with the RNA Clean-Up protocol in the RNeasy mini kit (Qiagen). Real-time reverse transcription (RT)-PCR was performed with 1 μg of total cellular RNA as previously described (67) with primers and probes specific for the SIV gag gene.
Immunofluorescence analysis.
GFAP staining was achieved with clone G-A-5 indocarbocyanine-conjugated monoclonal antiserum (Sigma) diluted 1:250 in phosphate-buffered saline (Invitrogen) in 2% bovine serum albumin. SIVmac p27 monoclonal antibody (55-2f12) was obtained from Niels Pederson through the AIDS Research and Reference Reagent Program (AIDS Program, National Institutes of Health). Cells were grown to 50% confluency on poly-l-lysine-coated coverslips, after which the astrocyte growth medium was changed to 10% Dulbecco's modified Eagle's medium and the cells were incubated for 24 h. For GFAP and p27 dual-labeling studies, cells were inoculated with 50,000 cpm of SIV/17E-Br reverse transcriptase for 6 h, washed as described above, and allowed to incubate for 1 week before immunofluorescence analysis.
For immunofluorescence analysis, cells were fixed for 20 min at −20°C in ice-cold methanol-acetone (1:1), permeabilized for 10 min at room temperature with 0.1% Triton X-100 (Sigma), and washed with phosphate-buffered saline. Samples were blocked for 30 min at room temperature with 2% bovine serum albumin-phosphate-buffered saline, incubated for 1 h at room temperature with appropriate antiserum, washed three times with phosphate-buffered saline (5 min/wash), incubated for 1 h at room temperature with secondary antiserum if appropriate [immunoglobulin G2b-fluorescein isothiocyanate (Roche) for p27 staining] and washed again. For double-labeling experiments, p27 staining was completed first, and cells were washed and processed for GFAP staining. Appropriate controls, including normal mouse serum, secondary only, and isotype controls [immunoglobulin G2b- and G1-indocarbocyanine (Dako, Carpinteria, Calif.)] were used in all experiments.
Transmission electron microscopy.
Astrocytes were infected as above with 100,000 cpm of SIV/17E-Fr reverse transcriptase overnight and washed three times with HBSS, and the cells were allowed to incubate for 14 days. At day 14, both SIV-infected and mock-infected cells were fixed in 2.5% glutaraldehyde in Millonig's sodium phosphate buffer [pH 7.4] for 3 h at 4°C, washed three times with Millonig's buffer, and sent to Electron Microscopy Bioservices (Monrovia, Md.) for ultrathin-section transmission electron microscopy analysis.
Immunohistochemistry.
To demonstrate viral gene expression in astrocytes in vivo, brain sections were obtained from macaques coinoculated with SIV/17E-Fr and SIV delta B670 during the acute (10 days postinoculation) and late (84 days postinoculation) stages (68). To determine whether viral proteins were expressed in astrocytes, brain sections were stained immunohistochemically with polyclonal antiserum to SIV Nef (19) or monoclonal antiserum to gp41 (obtained from Karen Kent and Caroline Powell though the AIDS Research and Reference Reagent Program) and then with polyclonal antiserum to GFAP (Dako). For Nef-gp41 costaining, incubation with Nef antiserum was performed first, followed by incubation with anti-gp41.
RESULTS
Expression of Nef but not gp41 in macaque astrocytes in vivo.
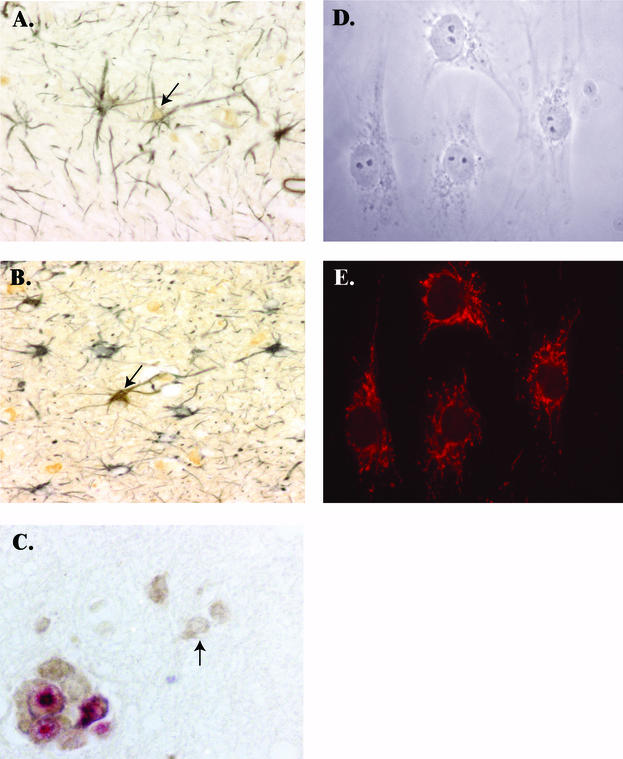
In our accelerated, consistent model of SIV-AIDS and encephalitis, all infected macaques develop AIDS, and over 90% develop encephalitis by 84 days postinoculation (68). Because the vast majority of inoculated animals develop CNS lesions, changes in the brain during acute infection can be considered preencephalitic. We have previously shown that SIV establishes infection in the CNS during acute infection. To determine whether astrocytes are infected at this early time after infection and to examine the extent of SIV infection of astrocytes over the course of infection, the brains from animals euthanatized during acute (10 days postinoculation) and terminal (84 days postinoculation) infection were examined for expression of viral proteins by immunohistochemistry for SIV Nef and gp41 proteins; astrocytes were identified by colabeling with GFAP antibody. During acute infection (10 days postinoculation), both gp41 and Nef were detected in occasional perivascular macrophages. Nef protein expression was clearly detected in astrocytes of six of six macaques by immunohistochemistry (Table 1 and Fig. 1A), while the late gene product gp41 was not detected in astrocytes of any of the six macaques examined. Approximately 10 to 20% of astrocytes expressed Nef in occasional foci throughout the white matter, whereas in other areas there were no Nef-expressing astrocytes.
TABLE 1.
Expression of SIV Nef and gp41 in astrocytes during acute and terminal infection
| Macaque no. | Time postinoculation (days) | Severity of encephalitis | Expression of Nef and gp41 in cells labeled for:
|
|
|---|---|---|---|---|
| GFAP/Nef | GFAP/gp41 | |||
| MF4C | 10 | None | + | − |
| MV326 | 10 | None | + | − |
| MV616 | 10 | None | + | − |
| MV672 | 10 | None | + | − |
| MV674 | 10 | None | + | − |
| MV392 | 10 | None | + | − |
| M17834 | 84 | None | − | − |
| MV713 | 84 | Mild | + | − |
| MV715 | 84 | Mild | − | − |
| MV708 | 84 | Moderate | − | − |
| M18242 | 84 | Moderate | + | − |
| M18031 | 84 | Moderate | − | − |
| MV394 | 84 | Moderate | − | − |
| MV387 | 84 | Moderate | + | − |
| M18292 | 84 | Severe | − | − |
| M18033 | 84 | Severe | + | − |
| MV389 | 84 | Severe | + | − |
FIG. 1.
(A) Immunohistochemical staining of brain from an SIV-infected macaque euthanatized at 10 days postinoculation, demonstrating colocalization of GFAP [black, SG substrate (Vector Laboratories, Burlingame, Calif.)] and SIV Nef [brown, 3,3,-diaminobenzidine; Biogenics, San Ramon, Calif.)] (magnification, 200×). Arrow indicates an astrocyte expressing SIV Nef. (B) Immunohistochemical staining of brain tissue from a macaque with severe encephalitis (3 months postinfection; magnification, 200×). Arrow indicates an SIV Nef-expressing astrocyte. (C) Immunohistochemical staining of brain tissue from a macaque with severe encephalitis (3 months postinfection; magnification, 400×) dually labeled for SIV Nef (3,3,-diaminobenzidine) and SIV gp41 (Texas Red; Vector Labs). Shown is a multinucleated giant cell expressing both SIV Nef and gp41, while the arrow indicates an astrocyte expressing only Nef. (D) Phase contrast of primary rhesus macaque astrocytes plated on glass coverslips and displaying stellate morphology typical of astrocytes (magnification, 400×). (E) GFAP expression in the astrocyte cultures shown in D and stained with a monoclonal antibody to human GFAP (indocarbocyanine-conjugated; Sigma; magnification, 400×).
Astrocytes in the brains of 5 of 11 macaques with SIV encephalitis (84 days postinoculation) were positive for SIV Nef expression by double immunohistochemical staining (Table 1 and Fig. 1B), and Nef expression appeared to be independent of the severity of encephalitis. gp41 was not detected in astrocytes in any of the macaques but was readily detected in macrophages and multinucleated giant cells (Fig. 1C). Astrocytes expressing Nef were more numerous in the brains of macaques with SIV encephalitis than in macaques euthanatized at 10 days postinfection, but these cells still constituted a small percentage of the astrocyte population. Infected astrocytes were most common in the subcortical white matter of the frontal and parietal cortex and in the basal ganglia and thalamus, and expression was less focal than at 10 days postinfection. To our knowledge, these data provide the first direct in vivo evidence of astrocyte infection in SIV-infected macaques. In addition, we provide evidence that astrocytes are infected during acute infection and thus may provide a reservoir for virus in the CNS.
Characterization of primary rhesus macaque astrocytes in vitro.
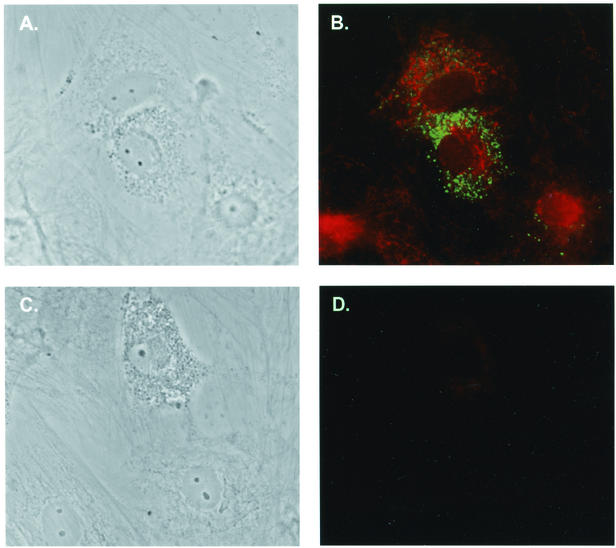
Primary adult rhesus macaque astrocyte cultures were first examined for purity by expression of the intermediate filament-associated GFAP, the classical marker for astrocytes, with immunofluorescence analysis. Astrocyte cultures contained cells with stellate morphology characteristic of in vitro cultures of simian astrocytes (26) (Fig. 1D), and the cultures were uniformly positive for GFAP, which localized characteristically to filaments extending throughout the cytoplasm of the cells (Fig. 1E). Negative isotype controls did not stain astrocyte cultures, indicating that the observed fluorescence was specific for GFAP (see Fig. 3C and 3D). GFAP-negative cells were never detected in the astrocyte cultures at any time during the course of the study.
FIG. 3.
Colabeling of GFAP-positive cells with SIV p27. (A) Phase contrast of infected astrocytes plated on glass coverslips, infected with 50,000 cpm of SIV/17E-Br reverse transcriptase units, washed, and allowed to incubate for 1 week. (B) Immunofluorescence of astrocytes in A stained for viral p27 (fluorescein isothiocyanate; Dako) and GFAP (indocarbocyanine) (magnification, 400×). (C) Phase contrast of infected astrocytes (magnification, 400×). (D) Immunofluorescence of the astrocytes shown in C stained with isotype controls for both p27 and GFAP.
SIV infection of primary astrocytes.
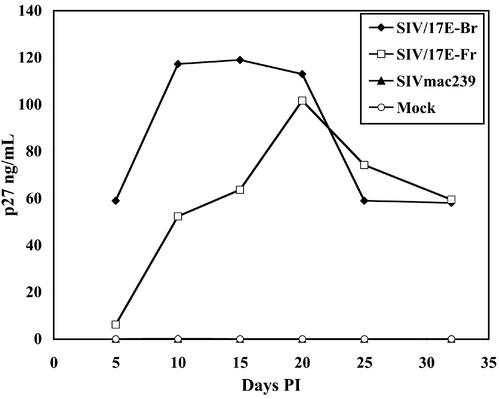
Given our in vivo data indicating restricted replication of SIV in astrocytes (limited to expression of the early gene nef), we next examined whether primary adult rhesus macaque astrocytes were susceptible to SIV infection in vitro and evaluated the extent of productive virus replication in these cells. Astrocytes were inoculated with the neurovirulent viral swarm SIV/17E-Br, the neurovirulent, macrophage-tropic clone SIV/17E-Fr, or the nonneurovirulent, lymphocyte-tropic clone SIVmac239 open-nef. Productive virus replication was monitored by measuring SIV p27 levels in astrocyte cultures over time. The neurovirulent viral swarm SIV/17E-Br and the neurovirulent clone SIV/17E-Fr replicated efficiently in astrocytes, with p27 concentrations readily detectable without the need for coculture with permissive cells or stimulation with cytokines (Fig. 2). In contrast, there was no productive replication of SIVmac239 open-nef at any time in primary astrocytes. Productive SIV infection of astrocytes was predominantly noncytolytic, and only rarely was there evidence of cell-cell fusion in cultures even at 32 days postinoculation. Furthermore, productive replication in these cells occurred over a long period of time [32 days, compared to much shorter infections in macrophages (18)], and examination of viability (by trypan blue dye exclusion) over 32 days of infection did not reveal evidence of cell death (data not shown). As reported previously for other cell types (18, 50), the uncloned, primary isolate SIV/17E-Br routinely replicated to higher levels in astrocyte cultures than the molecular clone SIV/17E-Fr.
FIG. 2.
SIV infection of primary rhesus astrocytes. Astrocyte cultures were inoculated with SIV/17E-Br, SIV/17E-Fr, and SIVmac239 normalized by TCID50 (multiplicity of infection = 0.1) and allowed to incubate for 6 h before the cells were washed extensively, and replication was monitored over time postinfection (PI) for the presence of SIV p27. Results shown are representative of several independent experiments.
Although GFAP staining of astrocyte cultures did not reveal any contaminating cell types, it was important to demonstrate that SIV replication occurred in GFAP-positive astrocytes. Astrocyte cultures infected with SIV/17E-Br for 7 days were double-labeled with antisera specific for GFAP and SIV p27 and examined by fluorescence microscopy. Single-cell analysis illustrated costaining of GFAP and p27, with p27 exhibiting punctate staining as described for other cell types (31) (Fig. 3A and 3B). Experiments with isotype control antiserum yielded no staining (Fig. 3C and 3D). Examination of the cultures revealed distinct foci of infected cells representing approximately 10% of all cultured cells. This is consistent with the delayed appearance of significant levels of virus shown in Fig. 2.
One interpretation of the focal nature of SIV replication is that simian astrocyte populations are heterogeneous in nature with respect to expression of various receptors and ability to support virus replication. This has been suggested for astrocytes from other species (35, 65), and accordingly, specific astrocytes within the culture may not be readily susceptible to infection by SIV. This is consistent with our in vivo observations, in which random foci of infected astrocytes were seen. Variable susceptibility of the astrocytes in vitro may affect both initial SIV infection and spread of virus throughout the culture.
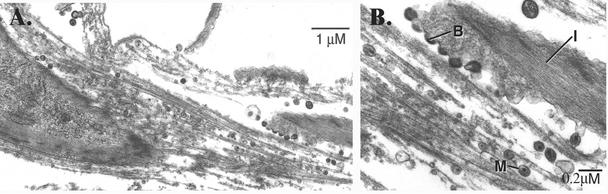
To further characterize SIV infection of macaque astrocytes, we examined cultures of SIV/17E-Fr-infected cells by transmission electron microscopy. After 14 days of infection, electron micrographs revealed SIV particles actively budding from the surface of astrocytes (containing prominent intermediate filaments characteristic of GFAP; Fig. 4A and 4B). Mature particles exhibiting classical lentivirus morphology were both closely associated with the cell surface and in a small number of intracellular vacuoles, indicating that SIV budding from astrocytes has the characteristics of virus budding from other cell types as previously described (54).
FIG. 4.
Transmission electron microscopy of SIV-infected primary macaque astrocytes. (A) Low magnification of astrocyte cultures infected with SIV/17E-Fr and allowed to incubate for 14 days before samples were prepared as described in Materials and Methods and sent to Electron Microscopy Bioservices for analysis. Bar, 1 μm. (B) Higher magnification of the cell shown in panel A, showing SIV budding from the plasma membrane. Bar, 0.2 μm. B, budding virions; M, mature particle; I, intermediate filaments.
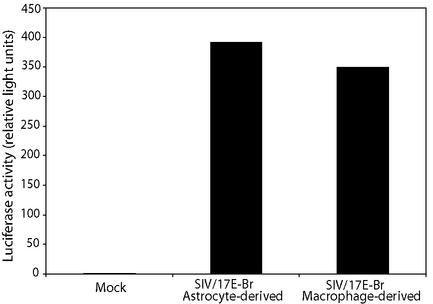
It is important to establish that virions produced by astrocytes are infectious in other cell types, since it might provide an important contribution to virus in the central nervous system. Therefore, we investigated the infectivity of virions produced by astrocytes infected with SIV/17E-Br. The LuSIV infectivity assay, previously developed in our laboratory (50), is based on a CEMx174 cell line stably transfected with a plasmid encoding luciferase under control of the SIV long terminal repeat promoter. SIV/17E-Br virions produced by astrocytes were infectious, as demonstrated by the presence of substantial luciferase activity when astrocyte-derived virus was inoculated onto LuSIV cells (Fig. 5). In addition, comparison of the virus derived from astrocytes with the same virus produced in macrophages demonstrated that the viruses were equally infectious. These data indicate that virus produced by astrocytes is infectious and capable of infecting nonastrocytic cells (in this case, CEMx174 cells) and that virus derived from astrocytes does not lose infectivity despite slow replication kinetics.
FIG. 5.
Infectivity of astrocyte-derived SIV. Supernatants derived from astrocytes and macrophages infected with SIV/17E-Br were normalized for p27, and the infectivities were quantitated by the LuSIV assay as described in Materials and Methods.
Characterization of receptor usage for SIV infection.
SIV entry is known to be mediated by CD4 and the chemokine receptor CCR5; only rarely does SIV utilize other chemokine receptors such as CCR1, CCR2b, or CCR3 for entry (16). However, CD4-independent, CCR5-dependent entry has been described for SIV/17E-Br and SIV/17E-Fr as well as other neurovirulent, macrophage-tropic SIVs (17, 39). Flow cytometric analysis of astrocytes failed to detect surface expression of either CCR5 or CD4 (data not shown). However, RT-PCR was able to detect the presence of both CD4 and CCR5 mRNA (data not shown), indicating that the surface expression of these two molecules could be below the limit of detection of flow cytometry, as previously described (26, 66).
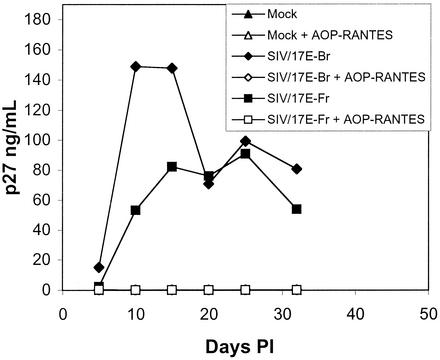
Because rhesus astrocytes were positive for expression of CCR5 mRNA, the major coreceptor for SIV, we attempted to block entry and replication of virus with AOP-RANTES, a nonhydrolyzable ligand that reduces cell surface expression of CCR5 (37). Astrocytes incubated with 500 ng of AOP-RANTES per ml for 1 h were infected with SIV/17E-Br or SIV/17E-Fr. Treatment with AOP-RANTES for 1 h followed by continued incubation with this ligand completely blocked replication (as assessed by p27 production) of both viruses (Fig. 6). Infected astrocytes in the absence of AOP-RANTES produced readily detectable SIV p27. Treatment with as little as 125 ng of AOP-RANTES per ml was also able to completely block SIV infection of astrocytes (data not shown), consistent with low-level surface expression of CCR5. Thus, it appears that although the levels of CCR5 expressed on the surface of astrocytes are undetectable by flow cytometry, sufficient levels exist and are likely utilized by SIV for entry and replication in these primary CNS cells.
FIG. 6.
AOP-RANTES blocks SIV infection of primary rhesus astrocytes. Astrocytes were incubated with or without 500 ng of AOP-RANTES per ml for 1 h prior to infection with SIV/17E-Br or SIV/17E-Fr [input virus normalized by TCID50 (multiplicity of infection = 0.1)], and supernatants were sampled at various times postinfection (PI) and assayed for the presence of SIV p27. Data are representative of several independent experiments.
Requirement of Nef for optimal SIV replication in primary macaque astrocytes.
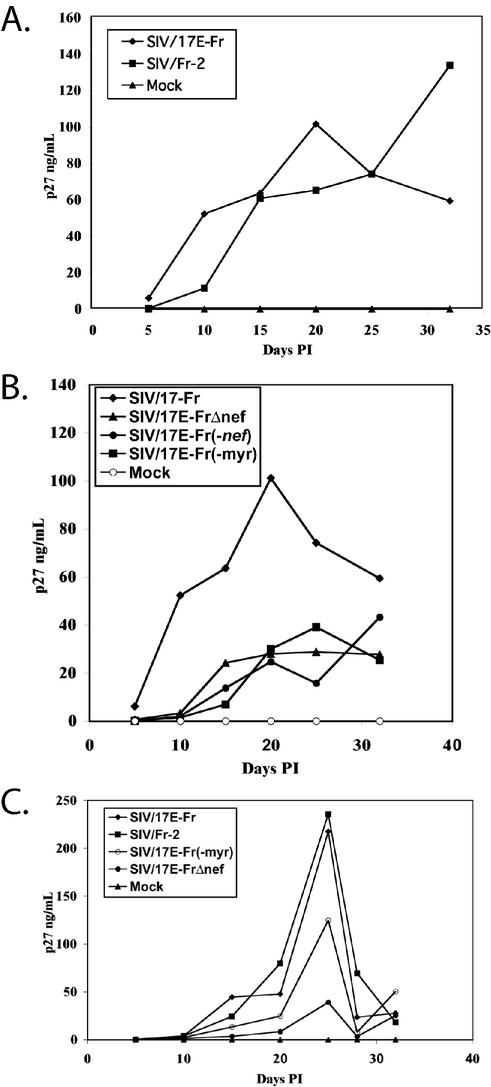
It has been shown previously that env and nef sequences in SIV/17E-Fr confer neurovirulence and the ability to replicate in brain-derived microvessel endothelial cells in vitro (18, 38). Therefore, we examined the importance of specific nef sequences to virus replication in primary macaque astrocytes. First we examined the replicative ability of SIV/Fr-2, a clone that is isogenic to SIV/17E-Fr except that it contains the open-nef gene from SIVmac239. We have previously reported that the sequence of the Nef protein derived from SIV/17E-Fr differs from that of SIVmac239 Nef at five amino acid residues (19) and that these two Nef proteins associate with distinct cellular kinases (4). SIVmac239 Nef associates with p21-associated kinase and an unidentified serine/threonine kinase, while SIV/17E-Fr Nef associates only with a distinct unidentified serine/threonine kinase (4). When input virus was normalized by TCID50, both SIV/17E-Fr and SIV/Fr-2 replicated to similar levels in astrocytes (Fig. 7A), suggesting that no one of the three kinases is specifically required to optimize replication in astrocytes. However, we cannot rule out the possibility that some Nef-associated kinase activity is important for efficient replication, as neither virus is devoid of Nef-associated kinase activity. More importantly, that SIV/Fr-2 replicates while SIVmac239 open-nef does not indicates that the molecular determinant underlying the inability of SIVmac239 open-nef to replicate is independent of nef and likely involves the env gene.
FIG. 7.
Nef is required for optimal SIV replication in rhesus astrocytes. (A) Astrocytes were infected with SIV/17E-Fr or SIV/Fr-2 [input normalized by TCID50 (multiplicity of infection = 0.1)]. (B) Astrocytes were infected with SIV/17E-Fr, SIV/17E-Fr(−myr), SIV/17E-Fr(−nef), or SIV/17E-FrΔnef [input normalized by TCID50 (multiplicity of infection = 0.1)]. (C) Astrocytes were infected with SIV/17E-Fr, SIV/Fr-2, SIV/17E-Fr(−myr), or SIV/17E-FrΔnef [input normalized by particle number (50 ng of p27)]. Supernatants in all experiments were sampled at various times postinfection (PI) and assayed for viral p27. Data are representative of several independent experiments.
As Nef likely has important roles in astrocytes in vivo and in vitro (5, 48), we next determined whether nef was required for replication in astrocytes. We compared the replicative abilities of SIV/17E-Fr and SIV/17EFrΔnef, a clone that contains a point mutation in the nef start codon as well as a 180-bp deletion in the nef open reading frame (18). When input virus was normalized for TCID50, SIV/17E-FrΔnef replication was substantially delayed and reduced in rhesus macaque astrocytes compared to SIV/17E-Fr in all experiments (Fig. 7B), indicating that Nef is vital for efficient and optimal replication of SIV in these CNS cells. Typically, SIV/17E-Fr p27 was detectable 5 to 10 days earlier than SIV/17E-FrΔnef p27 in parallel astrocyte cultures, and SIV/17E-Fr replicated to much higher levels than SIV/17E-FrΔnef.
In addition, we constructed another Nef-deficient molecular clone, SIV/17E-Fr(−nef), containing a point mutation in the nef start codon as well as three subsequent stop codons to determine if the 180-bp deletion in SIV/17E-FrΔnef affects SIV replication in astrocytes because of altered nef RNA structure (Fig. 7B). We found no difference in the replicative ability of SIV/17E-Fr(−nef) and SIV/17E-FrΔnef, indicating that the diminished capacity of these viruses to replicate occurs at the level of Nef protein, not RNA structure. Thus, it is clear that SIV replication requires Nef protein for efficient and optimal replication in primary adult rhesus macaque astrocytes.
We next examined the importance of Nef myristoylation for virus replication, since myristoylation of the Nef protein is required for many of the in vitro functions attributed to Nef such as downregulation of CD4 (1, 55) and major histocompatibility complex I (56), p21-associated kinase association (44), virion incorporation of Nef (8), and enhancement of virion infectivity (10). Therefore, we compared the replicative abilities of SIV/17E-Fr and SIV/17E-Fr(−myr), a clone containing a mutation in the N-terminal glycine residue required for myristoylation (19). When input virus was normalized for TCID50, SIV/17E-Fr(−myr) also displayed substantially delayed and reduced replication kinetics in astrocytes compared to SIV/17E-Fr, similar to those noted for the Nef-deficient molecular clones (Fig. 7B). Because Nef is required in the producer cell as well as the virion for optimal infection of the target cell (18, 41), these data indicate that virion incorporation of Nef and enhancement of virion infectivity are important for infection of primary astrocytes.
Standardizing virus input by TCID50 normalizes SIV/17E-FrΔnef and SIV/17E-Fr(−myr) for their common defect in virus infectivity attributed to lack of virion-incorporated Nef. However, normalizing input virus by particle number unmasks this defect and separates Nef's function in the virion from the role of newly synthesized, cellular Nef in the infected cell. Thus, we examined the replication kinetics of these clones by normalizing input virus for p27 (number of particles). When input SIV/17E-Fr, SIV/Fr-2, SIV/17E-FrΔnef, and SIV/17E-Fr(−myr) were normalized for particle number, SIV/17E-Fr(−myr) was able to replicate to higher levels than SIV/17E-FrΔnef (Fig. 7C). Consistent results were obtained from several experiments with independently prepared virus stocks, confirming the reproducibility of the nonmyristoylated phenotype. Thus, de novo-synthesized, nonmyristoylated Nef enhances virus replication in astrocytes, and this function is only detectable when input virus is normalized by particle number. Since newly synthesized Nef is not required for replication in CEMx174 cells, which were used to titer the virus stocks, normalizing infection of astrocytes by TCID50 masks the function of intracellular, nonmyristoylated Nef that functions in astrocytes to optimize replication. Collectively, these results illustrate a novel function of cellular Nef that, in addition to the enhancement of infectivity by virion-incorporated Nef, optimizes replication in astrocytes.
DNA expression of SIV molecular clones in primary astrocytes.
Viral growth curves demonstrated that SIVmac239 open-nef, a lymphocyte-tropic molecular clone, was unable to establish productive infection in primary astrocytes, while two Nef-deficient molecular clones displayed substantially reduced replication. To determine whether replication of these viruses was blocked or delayed at entry or at a later stage, we used real-time PCR to determine SIV DNA copy number (Table 2). All SIV-infected astrocyte cultures were positive for the presence of viral DNA 24 h after infection. Surprisingly, SIVmac239 open-nef, the clone that was unable to establish productive infection, had significant levels of viral DNA. SIV/17E-Fr had far more DNA copies detectable at 24 h than the other viruses tested, indicating that this virus may have undergone one round of replication and spread to uninfected cells. Interestingly, SIVmac239 open-nef, SIV/17E-Fr(−nef), and SIV/17E-FrΔnef all had similar levels of SIV DNA present, suggesting that SIVmac239 open-nef is able to enter astrocytes and undergo reverse transcription to levels sufficient for replication and that Nef is important for initial entry and reverse transcription in astrocytes. However, it is reasonable to assume that since the Nef-deficient clones are able to replicate eventually to detectable levels, entry and reverse transcription are not the only functions of Nef required for efficient replication in primary astrocytes, which is further supported by the viral growth curves described above.
TABLE 2.
Expression of SIV DNA and RNA in primary astrocytes infected with different molecular clones
| Molecular clone | SIV DNA (copy equivalents/250 ng of total cellular DNA) | SIV RNA (copy equivalents/μg of total cellular RNA) |
|---|---|---|
| None | 0a | 0a |
| SIV/17E-Fr | 1,041,772 ± 21,392 | 348,424 ± 71,243 |
| SIV/17E-FrΔnef | 48,640 ± 3,257 | 8,249 ± 226 |
| SIV/17E-Fr(−nef) | 46,793 ± 545 | 30,088 ± 3,034 |
| SIVmac239 open-nef | 59,429 ± 5,154 | 6,705 ± 1,041 |
The threshold of detection was set as less than 200 copies.
Production of viral mRNA in infected primary astrocytes.
Given that SIVmac239 open-nef, SIV/17E-Fr(−nef), and SIV/17E-FrΔnef were able to enter and undergo reverse transcription to similar levels, we next examined production of viral mRNA in astrocytes infected for 7 days with SIV/17E-Fr, SIV/17E-FrΔnef, SIV/17E-Fr(−nef), and SIVmac239 open-nef. Real-time PCR analysis for SIV gag was performed to determine the amount of SIV RNA produced by infection with each virus. As shown in Table 2, all strains of SIV had detectable amounts of RNA, although SIV/17E-Fr had the highest levels of RNA compared with the Nef-deficient clones or SIVmac239 open-nef.
It is interesting that SIVmac239 open-nef transcribed viral RNA and yet progeny virions were not detectable at any time during the course of infection. This could be a result of posttranscriptional deficiencies in protein assembly or budding or possibly inefficient spread from astrocyte to astrocyte within the culture, perhaps implicating Env in the replication block. Astrocytes infected with SIV/17E-FrΔnef and SIV/17E-Fr(−nef) had detectable amounts of SIV RNA but to a lesser extent than SIV/17E-Fr, further indicating that Nef is required for optimal and efficient replication in primary astrocytes.
DISCUSSION
The results of this study clearly demonstrate SIV replication in macaque astrocytes both in vivo and in vitro. To our knowledge, this is the first in vivo study to examine and characterize infection of astrocytes directly and longitudinally in the brain of SIV-infected macaques. Furthermore, the data presented here demonstrate for the first time that astrocytes were infected in six of six macaques examined as early as 10 days postinfection, providing further proof that SIV enters the CNS during acute infection. While astrocytes may not produce detectable amounts of virus during early infection, they do express early viral proteins, which may alter astrocyte function and impact the CNS response to virus infection.
Early establishment of infection in astrocytes and other CNS cells may alter cytokine and chemokine levels in the brain (67), causing infiltration of immune cells that may contribute to central nervous system dysfunction (12). We have previously shown an increase in the expression of T-cell restricted intracellular antigen-1 (TIA-1) in the brains of macaques prior to the development of encephalitis (12). TIA-1 is expressed exclusively in cytotoxic lymphocytes, including cytotoxic T lymphocytes and natural killer cells, which may target infected cells such as astrocytes in the brain. Individual differences in immune responses leading to variable targeting and loss of infected astrocytes could contribute to our observation that Nef protein is detectable in astrocytes in only half of the macaque brains at terminal infection, independent of the severity of encephalitis.
In contrast to astrocyte infection in vivo, in which we found that virus replication is restricted, neurovirulent viruses were able to establish productive infection in astrocyte cultures in vitro. While it is difficult to identify the exact mechanism underlying the restricted replication phenotype in vivo, our in vitro data nonetheless demonstrate that pure cultures of astrocytes are capable of supporting efficient viral replication. Unlike the consistent and well-defined environment in tissue culture, the dynamic environment of the infected brain with its associated immunological responses is extremely complex and largely unknown. Thus, one possibility is that the restricted replication of HIV or SIV in astrocytes in vivo is maintained by innate and cellular immune responses in the CNS that occur throughout the course of infection.
In vitro the macrophage-tropic viruses SIV/17E-Br and SIV/17E-Fr replicated productively in primary macaque astrocytes and did not require cytokine stimulation or coculture with permissive cells. Comparing levels of SIV/17E-Fr replication in astrocytes to those previously reported for replication of SIVmac251 [macrophage-tropic SIV with a variable neurovirulent phenotype (27)] revealed that SIV/17E-Fr replicated to 100-fold-higher levels than SIVmac251, which was barely detectable by enzyme-linked immunosorbent assay even from cells stimulated with cytokines. The question arises why SIV/17E-Fr is able to replicate efficiently in astrocytes without the need for coculture or cytokine stimulation. SIV/17E-Fr is a macrophage-tropic, neurovirulent molecular clone with env and nef sequences from the SIV/17E-Br viral swarm, which was isolated from an animal with CNS disease (18), and SIV/17E-Fr is able to reproducibly cause CNS disease in vivo (38), while SIVmac251 is not reproducibly neurovirulent. Thus, it is likely that SIV/17E-Fr replicates more efficiently in astrocytes in vitro because of unique sequences associated with its neurovirulent phenotype in vivo. We have previously shown that viral genotypes that gain access to the CNS and replicate differ genotypically from those in the periphery (2), and thus, the most relevant in vitro model of SIV infection of simian astrocytes in vivo may be one that uses a reproducibly neurovirulent virus such as SIV/17E-Fr.
The ability of AOP-RANTES to completely abolish productive replication in astrocytes strongly suggests that SIV utilizes CCR5 as its predominant coreceptor in these cells. It has been shown previously that SIV predominantly uses CD4 with CCR5 and rarely other chemokine receptors such as CCR1, CCR2b, or CCR3 for fusion (16). All of the SIV strains tested in this study were able to enter and undergo reverse transcription in macaque astrocytes, demonstrating that SIV Env can efficiently utilize receptors on the surface for entry and further suggesting that restricted replication of viruses with differing tropisms may occur by distinct mechanisms. The low surface expression of both CCR5 and CD4 (undetectable by flow cytometry) coupled with the fact that SIV/17E-Br and SIV/17E-Fr can use CCR5 independent of CD4 while SIVmac239 open-nef cannot suggests that CD4-independent viruses may have an increased capacity to establish productive replication in macaque astrocytes, a finding similar to that found for SIV infection of primary brain capillary endothelial cells (17). Because both SIV/17E-Br and SIV/17E-Fr are neurovirulent, these data further support the findings of Gorry et al. (25), who suggested that increased CCR5 affinity and reduced CD4 dependence may represent a phenotype contributing to the neurodegenerative pathology associated with AIDS.
Perhaps the most striking result of our study is the requirement of Nef for optimal SIV replication in adult macaque astrocytes in vitro. Because Nef is required for the development of AIDS but is dispensable for most in vitro systems, using cell culture systems to elucidate the precise Nef function that is required for in vivo pathogenesis has proven difficult. The few in vitro culture systems that require Nef include primary cell cultures of lymphocytes (60), immature dendritic cells (40), and now primary adult macaque astrocytes. The exact function of Nef in astrocytes that optimizes replication is still unclear. However, it is clear that the presence of the Nef protein, albeit nonmyristoylated, enhances virus replication compared to astrocytes infected with a Nef-deficient virus. This suggests a novel function of de novo-synthesized Nef in primary astrocytes that facilitates optimal replication.
The myristoylation-dependent functions of Nef characterized in vitro, such as downregulation of cell surface CD4 and major histocompatibility complex I, optimization of virion infectivity, and association with cellular signaling molecules, may play an important role in pathogenesis. However, it is equally likely that Nef's versatility allows it to perform different functions in vivo (in different cell types) that have yet to be identified and also play a significant role in pathogenesis. For example, the inability of the SIV Δnef viruses to replicate to significant levels in adult macaques in vivo is manifested early (within 2 weeks) postinoculation (14), suggesting that Nef is required to establish a threshold level of replication in the infected individual. This lack of an early establishment of replication of Δnef viruses may be a direct reflection in vivo of the slow replication kinetics of the virus in specific cell types such as astrocytes, lymphocytes, and immature dendritic cells that has been demonstrated in vitro. Thus, the inability of SIV/17E-Fr(−nef) and SIV/17E-FrΔnef to replicate optimally in astrocytes from adult macaques may provide an important in vitro system to identify novel functions of Nef in cells that play a direct role in pathogenesis of HIV and/or SIV.
Acknowledgments
We thank Ming Li, Brandon Bullock, and Laurie Queen for invaluable technical assistance and the rest of the Retrovirus Laboratory for contributing to useful discussions regarding the research presented herein.
This work was supported by National Institutes of Health grants T32 NS07392, NS35751, and NS38008 to J.E.C. and MH61189 and NS36911 to M.C.Z.
Under a licensing agreement between Bayer AG and the Johns Hopkins University, J. E. Clements is entitled to a share of a payment received by the university on sales of products embodying the technology described in this article. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict-of-interest policies.
REFERENCES
- 1.Aiken, C., J. Konner, N. R. Landau, M. E. Lenburg, and D. Trono. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76:853-864. [DOI] [PubMed] [Google Scholar]
- 2.Babas, T., D. Munoz, J. L. Mankowski, P. M. Tarwater, J. E. Clements, and M. C. Zink. 2003. Role of microglial cells in selective replication of simian immunodeficiency virus genotypes in the brain. J. Virol. 77:208-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagasra, O., E. Lavi, L. Bobroski, K. Khalili, J. P. Pestaner, R. Tawadros, and R. J. Pomerantz. 1996. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS 10:573-585. [DOI] [PubMed] [Google Scholar]
- 4.Barber, S. A., M. T. Flaherty, S. M. Plafker, and J. E. Clements. 1998. A novel kinase activity associated with Nef derived from neurovirulent simian immunodeficiency virus. Virology 251:165-175. [DOI] [PubMed] [Google Scholar]
- 5.Bencheikh, M., G. Bentsman, N. Sarkissian, M. Canki, and D. J. Volsky. 1999. Replication of different clones of human immunodeficiency virus type 1 in primary fetal human astrocytes: enhancement of viral gene expression by Nef. J. Neurovirol. 5:115-124. [DOI] [PubMed] [Google Scholar]
- 6.Brack-Werner, R. 1999. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS 13:1-22. [DOI] [PubMed] [Google Scholar]
- 7.Budka, H. 1991. Neuropathology of human immunodeficiency virus infection. Brain Pathol. 1:163-175. [DOI] [PubMed] [Google Scholar]
- 8.Bukovsky, A. A., T. Dorfman, A. Weimann, and H. G. Göttlinger. 1997. Nef association with human immunodeficiency virus type 1 virions and cleavage by the viral protease. J. Virol. 71:1013-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canki, M., M. J. Potash, G. Bentsman, W. Chao, T. Flynn, M. Heinemann, H. Gelbard, and D. J. Volsky. 1997. Isolation and long-term culture of primary ocular human immunodeficiency virus type 1 isolates in primary astrocytes. J. Neurovirol. 3:10-15. [DOI] [PubMed] [Google Scholar]
- 10.Chowers, M. Y., C. A. Spina, T. J. Kwoh, N. J. S. Fitch, D. D. Richman, and J. C. Guatelli. 1994. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J. Virol. 68:2906-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clabough, D. L., D. Gebhard, M. T. Flaherty, L. E. Whetter, S. T. Perry, L. Coggins, and F. J. Fuller. 1991. Immune-mediated thrombocytopenia in horses infected with equine infectious anemia virus. J. Virol. 65:6242-6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clements, J. E., T. Babas, J. L. Mankowski, K. Suryanarayana, M. Piatak, Jr., P. M. Tarwater, J. D. Lifson, and M. C. Zink. 2002. The central nervous system as a reservoir for simian immunodeficiency virus (SIV): steady-state levels of SIV DNA in brain from acute through asymptomatic infection. J. Infect. Dis. 186:905-913. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham, A. L., H. Naif, N. Saksena, G. Lynch, J. Chang, S. Li, R. Jozwiak, M. Alali, B. Wang, W. Fear, A. Sloane, L. Pemberton, and B. Brew. 1997. HIV infection of macrophages and pathogenesis of AIDS dementia complex: interaction of the host cell and viral genotype. J. Leukoc. Biol. 62:117-125. [DOI] [PubMed] [Google Scholar]
- 14.Daniel, M. D., F. Kirchhoff, C. Czajak, P. K. Sehgal, and R. C. Desrosiers. 1992. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science 258:1938-1941. [DOI] [PubMed] [Google Scholar]
- 15.Di Rienzo, A. M., F. Aloisi, A. C. Santarcangelo, C. Palladino, E. Olivetta, D. Genovese, P. Verani, and G. Levi. 1998. Virological and molecular parameters of HIV-1 infection of human embryonic astrocytes. Arch. Virol. 143:1599-1615. [DOI] [PubMed] [Google Scholar]
- 16.Edinger, A. L., A. Amedee, K. Miller, B. J. Doranz, M. Endres, M. Sharron, M. Samson, Z. Lu, J. E. Clements, M. Murphey-Corb, S. C. Peiper, M. Parmentier, C. C. Broder, and R. W. Doms. 1997. Differential utilization of CCR5 by macrophage and T cell tropic simian immunodeficiency virus strains. Proc. Natl. Acad. Sci. USA 94:4005-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edinger, A. L., J. L. Mankowski, B. J. Doranz, B. J. Margulies, B. Lee, J. Rucker, M. Sharron, T. L. Hoffman, J. F. Berson, M. C. Zink, V. M. Hirsch, J. E. Clements, and R. W. Doms. 1997. CD4-independent, CCR5-dependent infection of brain capillary endothelial cells by a neurovirulent simian immunodeficiency virus strain. Proc. Natl. Acad. Sci. USA 94:14742-14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flaherty, M., D. A. Hauer, J. L. Mankowski, M. C. Zink, and J. E. Clements. 1997. Molecular and biological characterization of a neurovirulent molecular clone of SIV. J. Virol. 71:5790-5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flaherty, M. T., S. A. Barber, and J. E. Clements. 1998. Neurovirulent simian immunodeficiency virus incorporates a Nef-associated kinase into virions. AIDS Res. Hum. Retroviruses 14:163-170. [DOI] [PubMed] [Google Scholar]
- 20.Fox, H. S., L. H. Gold, S. J. Henriksen, and F. E. Bloom. 1997. Simian immunodeficiency virus: a model for neuro-AIDS. Neurobiol. Dis. 4:265-274. [DOI] [PubMed] [Google Scholar]
- 21.Gendelman, H. E., S. A. Lipton, M. Tardieu, M. I. Bukrinsky, and H. S. Nottet. 1994. The neuropathogenesis of HIV-1. J. Leukoc. Biol. 56:387-388. [DOI] [PubMed] [Google Scholar]
- 22.Gibbs, J. S., D. A. Regier, and R. C. Desrosiers. 1994. Construction and in vitro properties of HIV-1 mutants with deletions in “nonessential” genes. AIDS Res. Hum. Retroviruses 10:343-350. [DOI] [PubMed] [Google Scholar]
- 23.Gold, L. H., H. S. Fox, S. J. Henriksen, M. J. Buchmeier, M. R. Weed, M. A. Taffe, S. Huitron-Resendiz, T. F. Horn, and F. E. Bloom. 1998. Longitudinal analysis of behavioral, neurophysiological, viral and immunological effects of SIV infection in rhesus monkeys. J. Med. Primatol. 27:104-112. [DOI] [PubMed] [Google Scholar]
- 24.Gorry, P., D. Purcell, J. Howard, and D. McPhee. 1998. Restricted HIV-1 infection of human astrocytes: potential role of nef in the regulation of virus replication. J. Neurovirol. 4:377-386. [DOI] [PubMed] [Google Scholar]
- 25.Gorry, P. R., G. Bristol, J. A. Zack, K. Ritola, R. Swanstrom, C. J. Birch, J. E. Bell, N. Bannert, K. Crawford, H. Wang, D. Schols, E. De Clercq, K. Kunstman, S. M. Wolinsky, and D. Gabuzda. 2001. Macrophage tropism of human immunodeficiency virus type 1 isolates from brain and lymphoid tissues predicts neurotropism independent of coreceptor specificity. J. Virol. 75:10073-10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guillemin, G., Boussin, F. D., Croitoru, J., Franck-Duchenne, M., Le Grand, R., Lazarini, F., Dormant, D. 1997. Obtention and Characterization of primary astrocyte and microglial cultures from adult monkey brains. J. Neurol. Res. 49:576-591. [DOI] [PubMed] [Google Scholar]
- 27.Guillemin, G., J. Croitoru, R. L. Le Grand, M. Franck-Duchenne, D. Dormont, and F. D. Boussin. 2000. Simian immunodeficiency virus mac251 infection of astrocytes. J. Neurovirol. 6:173-186. [DOI] [PubMed] [Google Scholar]
- 28.Hao, H. N., F. C. Chiu, L. Losev, K. M. Weidenheim, W. K. Rashbaum, and W. D. Lyman. 1997. HIV infection of human fetal neural cells is mediated by gp120 binding to a cell membrane-associated molecule that is not CD4 nor galactocerebroside. Brain Res. 764:149-157. [DOI] [PubMed] [Google Scholar]
- 29.Hao, H. N., and W. D. Lyman. 1999. HIV infection of fetal human astrocytes: the potential role of a receptor-mediated endocytic pathway. Brain Res. 823:24-32. [DOI] [PubMed] [Google Scholar]
- 30.Harouse, J., C. Kunch, H. Hartle, M. Laughlin, J. Hoxie, B. Wigdahl, and F. Gonzalez-Scarano. 1989. CD4-independent infection of human neural cells by human immunodeficiency virus type I. J. Virol. 63:2527-2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hermida-Matsumoto, L. 2000. Localization of human immunodeficiency virus type 1 Gag and Env at the plasma membrane by confocal imaging. J. Virol. 74:8670-8679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jamieson, B. D., G. M. Aldrovandi, V. Planelles, J. B. M. Jowett, L. Gao, L. M. Bloch, I. S. Y. Chen, and J. A. Zack. 1994. Requirement of human immunodeficiency virus type 1 nef for in vivo replication and pathogenicity. J. Virol. 68:3478-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kato, T., A. Hirano, J. F. Llena, and H. M. Dembitzer. 1987. Neuropathology of the acquired immune deficiency syndrome (AIDS) in 53 autopsy cases with particular emphasis on microglial nodules and multinucleated giant cells. Acta Neuropathol. 73:287-294. [DOI] [PubMed] [Google Scholar]
- 34.Kestler, H., T. Kodama, D. Ringler, M. Marthas, N. Pedersen, A. Lackner, D. Regier, P. Sehgal, M. Daniel, N. King, and R. Desrosiers. 1990. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science 248:1109-1112. [DOI] [PubMed] [Google Scholar]
- 35.Linskey, M. G. M. 1995. Glial differentiation: a review with implications for new directions in neuro-oncology. Neurosurgery 36:1-22. [DOI] [PubMed] [Google Scholar]
- 36.Ludwig, E., F. C. Silberstein, J. van Empel, V. Erfle, M. Neumann, and R. Brack-Werner. 1999. Diminished rev-mediated stimulation of human immunodeficiency virus type 1 protein synthesis is a hallmark of human astrocytes. J. Virol. 73:8279-8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mack, M., B. Luckow, P. J. Nelson, J. Cihak, G. Simmons, P. R. Clapham, N. Signoret, M. Marsh, M. Stangassinger, F. Borlat, T. N. Wells, D. Schlondorff, and A. E. Proudfoot. 1998. Aminooxypentane-RANTES induces CCR5 internalization but inhibits recycling: a novel inhibitory mechanism of HIV infectivity. J. Exp. Med. 187:1215-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mankowski, J. L., M. T. Flaherty, J. P. Spelman, D. A. Hauer, P. J. Didier, A. M. Amedee, M. Murphey-Corb, L. M. Kirstein, A. Muñoz, J. E. Clements, and M. C. Zink. 1997. Pathogenesis of simian immunodeficiency virus encephalitis: viral determinants of neurovirulence. J. Virol. 71:6055-6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Means, R. E., T. Matthews, J. A. Hoxie, M. H. Malim, T. Kodama, and R. C. Desrosiers. 2001. Ability of the V3 loop of simian immunodeficiency virus to serve as a target for antibody-mediated neutralization: correlation of neutralization sensitivity, growth in macrophages, and decreased dependence on CD4. J. Virol. 75:3903-3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Messmer, D., R. Ignatius, C. Santisteban, R. M. Steinman, and M. Pope. 2000. The decreased replicative capacity of simian immunodeficiency virus SIVmac239Delta(nef) is manifest in cultures of immature dendritic cells and T cells. J. Virol. 74:2406-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller, M. D., M. T. Warmerdam, K. A. Page, M. B. Feinberg, and W. C. Greene. 1995. Expression of the human immunodeficiency virus type 1 (HIV-1) nef gene during HIV-1 production increases progeny particle infectivity independently of gp160 or viral entry. J. Virol. 69:579-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neumann, M., E. Afonina, Ceccherini- F. Silberstein, S. Schlicht, V. Erfle, G. Pavlakis, and B. R. Werner. 2001. Nucleocytoplasmic transport in human astrocytes: decreased nuclear uptake of the HIV Rev shuttle protein. J. Cell Sci. 114:1717-1729. [DOI] [PubMed] [Google Scholar]
- 43.Neumann, M., B. K. Felber, A. Kleinschmidt, B. Froese, V. Erfle, G. N. Pavlakis, and R. Brack-Werner. 1995. Restriction of human immunodeficiency virus type 1 production in a human astrocytoma cell line is associated with a cellular block in Rev function. J. Virol. 69:2159-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nunn, M. F., and J. W. Marsh. 1996. Human immunodeficiency virus type 1 Nef associates with a member of the p21-activated kinase family. J. Virol. 70:6157-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nuovo, G. J., F. Gallery, P. MacConnell, and A. Braun. 1994. In situ detection of polymerase chain reaction-amplified HIV-1 nucleic acids and tumor necrosis factor-alpha RNA in the central nervous system. Am. J. Pathol. 144:659-666. [PMC free article] [PubMed] [Google Scholar]
- 46.Prat, A., K. Biernacki, K. Wosik, and J. P. Antel. 2001. Glial cell influence on the human blood-brain barrier. Glia 36:145-155. [DOI] [PubMed] [Google Scholar]
- 47.Price, R. W., B. Brew, J. Sidtis, M. Rosenblum, A. C. Scheck, and P. Cleary. 1988. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science 239:586-592. [DOI] [PubMed] [Google Scholar]
- 48.Ranki, A., M. Nyberg, V. Ovod, M. Haltia, I. Elovaara, R. Raininko, H. Haapasalo, and K. Krohn. 1995. Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. AIDS 9:1001-1008. [DOI] [PubMed] [Google Scholar]
- 49.Rausch, D. 1999. The SIV-infected rhesus monkey model for HIV-associated dementia and implications for neurological diseases. J. Leukoc. Biol. 65:466-474. [DOI] [PubMed] [Google Scholar]
- 50.Roos, J. W., M. F. Maughan, Z. Liao, J. E. Hildreth, and J. E. Clements. 2000. LuSIV cells: a reporter cell line for the detection and quantitation of a single cycle of HIV and SIV replication. Virology 273:307-315. [DOI] [PubMed] [Google Scholar]
- 51.Rothstein, J. D., M. Dykes-Hoberg, C. A. Pardo, L. A. Bristol, L. Jin, R. W. Kuncl, Y. Kanai, M. A. Hediger, Y. Wang, J. P. Schielke, and D. F. Welty. 1996. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16:675-686. [DOI] [PubMed] [Google Scholar]
- 52.Sabri, F., E. Tresoldi, M. Di Stefano, S. Polo, M. C. Monaco, A. Verani, J. R. Fiore, P. Lusso, E. Major, F. Chiodi, and G. Scarlatti. 1999. Nonproductive human immunodeficiency virus type 1 infection of human fetal astrocytes: independence from CD4 and major chemokine receptors. Virology 264:370-384. [DOI] [PubMed] [Google Scholar]
- 53.Saito, Y., L. R. Sharer, L. G. Epstein, J. Michaels, M. Mintz, M. Louder, K. Golding, T. A. Cvetkovich, and B. M. Blumberg. 1994. Overexpression of nef as a marker for restricted HIV-1 infection of astrocytes in postmortem pediatric central nervous tissues. Neurology 44:474-481. [DOI] [PubMed] [Google Scholar]
- 54.Salter, R. D., D. N. Howell, and P. Cresswell. 1985. Genes regulating HLA class I antigen expression in T-L lymphoblast hybrids. Immunogenetics 21:235-246. [DOI] [PubMed] [Google Scholar]
- 55.Sanfridson, A., B. R. Cullen, and C. Doyle. 1994. The simian immunodeficiency virus Nef protein promotes degradation of CD4 in human T cells. J. Biol. Chem. 269:3917-3920. [PubMed] [Google Scholar]
- 56.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J. M. Heard. 1996. Endocytosis of major histocompatibility complex I molecules is induced by HIV-1 Nef protein. Nat. Med. 2:338-342. [DOI] [PubMed] [Google Scholar]
- 57.Shahabuddin, M., Bentsman, G., Volsky, B., Rodriguez, I., Volsky, D. 1996. A mechanism of restricted human immunodeficiency virus type I expression in human glial cells. J. Virol. 70:7992-8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharer, L. R., G. B. Baskin, E. S. Cho, M. Murphey-Corb, B. B. Blumberg, and L. G. Epstein. 1988. Comparison of Simian immunodeficiency virus and human immunodeficiency virus encephalitis in the immature host. Ann. Neurol. 23:S108-S112. [DOI] [PubMed] [Google Scholar]
- 59.Sharma, D. P., M. C. Zink, M. G. Anderson, R. Adams, J. E. Clements, S. V. Joag, and O. Narayan. 1992. Derivation of neurotropic SIV from exclusively lymphocyte-tropic parental virus: pathogenesis of infection in macaques. J. Virol. 66:3550-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spina, C. A., T. Kwoh, M. Y. Chowers, J. C. Guatelli, and D. D. Richman. 1994. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J. Exp. Med. 179:115-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takahashi, K., S. L. Wesselingh, D. E. Griffin, J. C. McArthur, R. T. Johnson, and J. D. Glass. 1996. Localization of HIV-1 human brain with polymerase chain reaction/in situ hybridization and immunocytochemistry. Ann. Neurol. 39:705-711. [DOI] [PubMed] [Google Scholar]
- 62.Tornatore, C., R. Chandra, J. R. Berger, and E. O. Major. 1994. HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology 44:481-487. [DOI] [PubMed] [Google Scholar]
- 63.Tornatore, C., K. Meyers, W. Atwood, K. Conant, and E. Major. 1994. Temporal patterns of human immunodeficiency virus type 1 transcripts in human fetal astrocytes. J. Virol. 68:93-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tornatore, C., A. Nath, K. Amemiya, and E. O. Major. 1991. Persistent human immunodeficiency virus type 1 infection in human fetal glial cells reactivated by T-cell factor(s) or by the cytokines tumor necrosis factor alpha and interleukin-1 beta. J. Virol. 65:6094-6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walz, W. 2000. Controversy surrounding the existence of discrete functional classes of astrocytes in adult gray matter. Glia 31:95-103. [DOI] [PubMed] [Google Scholar]
- 66.Westmoreland, S. V., J. B. Rottman, K. C. Williams, A. A. Lackner, and V. G. Sasseville. 1998. Chemokine receptor expression on resident and inflammatory cells in the brain of macaques with simian immunodeficiency virus encephalitis. Am. J. Pathol. 152:659-665. [PMC free article] [PubMed] [Google Scholar]
- 67.Zink, M. C., G. D. Coleman, J. L. Mankowski, R. J. Adams, P. M. Tarwater, K. Fox, and J. E. Clements. 2001. Increased macrophage chemoattractant protein-1 in cerebrospinal fluid precedes and predicts simian immunodeficiency virus encephalitis. J. Infect. Dis. 184:1015−1021. [DOI] [PubMed]
- 68.Zink, M. C., K. Suryanarayana, J. L. Mankowski, A. Shen, M. Piatak, Jr., J. P. Spelman, D. L. Carter, R. J. Adams, J. D. Lifson, and J. E. Clements. 1999. High viral load in the cerebrospinal fluid and brain correlates with severity of simian immunodeficiency virus encephalitis. J. Virol. 73:10480-10488. [DOI] [PMC free article] [PubMed] [Google Scholar]