Abstract
The human immunodeficiency virus (HIV) Tat protein has a critical role in viral transcription, but this study focuses on its additional role as an extracellular effector of lymphocyte cell death. It is well known that Tat induces tumor necrosis factor-related apoptosis-induced ligand (TRAIL) in peripheral blood mononuclear cells (PBMC), and we show that the majority of TRAIL is produced by the monocyte subset of PBMC. Human monocytes and U937 monoblastoid cells did not take up soluble HIV Tat-86, as T cells did, yet produced more TRAIL than did T cells. TRAIL secretion was induced by Tat and by a cysteine-rich peptide of Tat but not by sulfhydryl-modified Tat toxoid. Although there was only a slight increase in cell surface expression of TRAIL on monocytes, sufficient TRAIL was secreted to be toxic for T cells. The cytotoxicity of Tat-stimulated monocyte medium could be blocked by a TRAIL-neutralizing antibody. T cells treated with Tat did not secrete enough TRAIL to mediate cell death in our assay. Remarkably, uninfected T cells are more susceptible to TRAIL than are HIV-infected T cells. The production of TRAIL by Tat-stimulated monocytes provides a mechanism by which HIV infection can destroy uninfected bystander cells.
The human immunodeficiency virus type 1 (HIV-1) Tat protein is essential for HIV-1 transcription and replication (11, 19, 55) and also modulates the expression of genes responsible for cell survival and proliferation (15, 37, 48, 59). Tat is also a secreted protein that can be detected in sera from HIV-infected individuals (59) and binds to cell surfaces through electrostatic interactions, chemokine receptors, or cell surface integrins (3, 6, 18). Cells treated with Tat showed increased expression of chemokine receptors, overproduction of alpha interferon (IFN-α), and enhanced expression of Fas ligand (FasL) on monocytes/macrophages that could cause cell death in cultured peripheral blood mononuclear cells (PBMC) (7, 10, 27, 53, 62). Tat protein can also be taken up by infected and uninfected PBMC, thus inducing biological effects through an autocrine/paracrine mechanism (18, 63). Here we show that Tat is internalized by T cells but not by monocytes. However, Tat-treated monocytes secrete proapoptotic factors that cause the death of uninfected bystander T cells.
Most circulating monocytes are in a nonactivated, predifferentiated state on their way to becoming macrophages or dendritic cells (1). Activated monocytes mediate host defense mechanisms by releasing inflammatory mediators, activating major histocompatibility complex-restricted T lymphocytes (21, 49), and killing virus-infected cells (12, 29). Activated monocytes also express several soluble mediators of apoptosis, including tumor necrosis factor alpha (TNF-α), CD30L, FasL, 4-1BBL, and TNF-related apoptosis-inducing ligand (TRAIL) (4, 14, 47, 50, 54). HIV infection activates monocyte differentiation either indirectly by inducing IFN-α (62) or directly through the action of extracellular Tat protein (16, 27, 64).
TRAIL, a member of the TNF superfamily, plays a role in HIV-mediated cell death (32, 64). TRAIL is expressed in mouse and human T and B cells, natural killer cells, dendritic cells, and monocytes (26, 31, 41) and is a potent inducer of apoptosis in a variety of cell types (23, 28, 30, 42, 51). At least five receptors for TRAIL have been identified. Two of these, DR4 (TRAIL-R1) and DR5 (TRAIL-R2), contain a cytoplasmic death domain and are capable of transducing an apoptotic signal. TRAIL-R3, TRAIL-R4, and osteoprotegerin lack functional death domains and serve as decoy receptors to block TRAIL-mediated apoptosis (24, 56). In addition to the receptor distribution, intracellular regulation is important for determining the effects of binding soluble TRAIL to cells (23, 34).
This study demonstrates an intracellular and extracellular increase in TRAIL once monocytes are exposed to HIV Tat or to a cysteine-rich peptide of Tat. In contrast to monocytes, T cells secreted relatively little TRAIL upon exposure to Tat. Remarkably, HIV-infected Jurkat cells and primary CD4+ cells were relatively resistant to TRAIL-mediated killing compared with uninfected cells. Cell death by secreted TRAIL is one mechanism for the death of uninfected bystander cells that is observed frequently in vivo after HIV infection (22).
MATERIALS AND METHODS
Reagents.
HIV Tat protein, Tat-86, has a C-terminal truncation of 15 amino acids and was obtained from the National Institutes of Health AIDS Research and Reference Reagent Program (ARRRP). Antibodies included anti-Tat (ARRRP no. 705), mouse anti-human immunoglobulin G1 (IgG1; Sigma, St. Louis, Mo.), polyclonal rabbit anti-TRAIL (H-257; Santa Cruz Biotechnology, Inc., San Diego, Calif.), and a neutralizing monoclonal anti-TRAIL (N2B2; eBioscience Co., San Diego, Calif.). Tat was chemically inactivated by carboxymethylation to make Tat toxoid, a molecule with an average of two modified cysteines, as described previously (35). 7-Amino actinomycin D (7-AAD; Sigma), actinomycin D (Sigma), propidium iodide (Sigma), annexin V-fluorescein isothiocyanate (FITC), FITC-anti-human CD4+, and phycoerythrin (PE)-anti-human CD8+ (Pharmingen, San Diego, Calif.) were used for flow cytometry. The Tat peptides are as follows, with the included amino acid residues indicated in subscript: Tat1-20 (peptide 1), MEPVDPRLEPWKHPGSQPKT; Tat16-35 (peptide 8), SQPKTACTNCYCKKCCFHCQ; Tat31-50 (peptide 13), CFHCQVCFMTKALGISYGRK; Tat46-65 (peptide 19), SYGRKKRRQRRRAHQDSQTH; Tat61-80 (peptide 25), DSQTHQASLSKQPTSQSRGD; and Tat76-95 (peptide 31), QSRGDPTGPKESKKKVERET.
Cells and cell culture.
The human leukemia Jurkat T-cell line (clone E6.1; American Type Culture Collection) and promonocytic U937 cell line (no. CRL2367; American Type Culture Collection) were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), 2 mM l-glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. Human monocytes were isolated from the PBMC of healthy donors by adherence to plastic (i.e., they were incubated for 2 h in T125 flasks in RPMI 1640 medium containing 10% heat-inactivated AB serum). Fluorescence-activated cell sorter analysis demonstrated that more than 98% of the cells were CD14+, indicating monocytes of high purity. CD4+ lymphocytes were purified by using negative-selection columns (R&D Systems, Minneapolis, Minn.). The purity of recovered cells was more than 90%, with no detectable cross-contamination by CD8+ cells. CD4+ cells were incubated in RPMI 1640 medium supplemented with 10% heat-inactivated FCS, 2 mM l-glutamine, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 50 IU of interleukin-2/ml (RO 23-6019; Hoffmann-La Roche, Nutley, N.J.).
HIV infection of primary CD4+ cells and Jurkat cells.
Before infection, cells were stimulated for 3 days with phytohemagglutinin (PHA; 5 μg/ml) and interleukin-2 (50 IU/ml) in RPMI 1640 medium containing 10% heat-inactivated FCS, 2 mM glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. In preparation for infection, both CD4+ cells and Jurkat cells were treated with 20 μg of DEAE-dextran/ml for 30 min at 37°C. HIV IIIB was added to 107 cells at 150 ng of p24gag (approximately 30 to 40 pg of p24 per 50% tissue culture infective dose). Cells were incubated at 37°C with occasional mixing for 2 h and then centrifuged and decanted. Cells were cultured for 5 days at 37°C in an atmosphere of 5% CO2 to establish the infection and were then used for cell death studies by exposure to Tat-stimulated monocyte (TSM) medium.
Tat uptake into cells.
CD4+ cells, Jurkat cells, U937 cells, or fresh human monocytes at 3 × 105 cells/well were incubated with 100 ng of Tat/ml for 0, 30, 60, and 180 min and washed with phosphate-buffered saline (PBS) three times, and cell lysates were harvested according to the Ne-Per protocol (catalog no. 78833; Pierce Chemicals, Rockford, Ill.). This protocol includes detergent washes that strip proteins off the cell surface, resulting in some loss of cytoplasmic content, so most of the observed Tat is nuclear (57). For immunoblotting (see below), anti-Tat monoclonal antibody (ARRRP no. 705) was used.
Immunoblotting assays.
U937 cells or monocytes were stimulated with HIV Tat (0 to 1,000 ng/ml) for 20 h, and then cell lysates and media were collected. The stimulation took place in 96-well plates at 37°C with 3 × 105 cells/well in a volume of 100 μl of RPMI 1640 medium plus 10% FCS. At the end of these incubations, media were collected and stored at −70°C for later use as TSM media. The cells were lysed at 4°C in a buffer containing 1% NP-40, 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 10 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 0.1% aprotinin, and 1 mM pepstatin A as described previously (13). The lysate was sonicated for 30 s on maximum setting (Ultrasonic Cleaner; Branson, Danbury, Conn.) and was centrifuged at 1,000 × g for 10 min at 4°C to pellet debris. TSM media, Tat-stimulated T-cell media, and cell lysates were analyzed by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-10% PAGE) using 20 μl of undiluted medium per well or 6 × 104 cell equivalents of lysate. The separated proteins were transferred to nitrocellulose membranes. Membranes were sequentially incubated with anti-TRAIL and then with a corresponding secondary antibody conjugated with horseradish peroxidase and were detected with an electrochemiluminescent system (ECL; Amersham Pharmacia Biotech, Piscataway, N.J.).
Reverse transcription-PCR.
Total RNA was extracted from 2 × 106 cells by using Trizol reagent (Invitrogen, Carlsbad, Calif.), followed by treatment with RNase-free DNase (Promega, Madison, Wis.). cDNA was synthesized from 1 μg of RNA by using AMV reverse transcriptase (Promega) as described previously (60). PCRs were performed using the following primers: for TRAIL, 5′-GACCCCAATGACGAAGAGAGTATG-3′ (forward) and 5′-GTTGCTCAGGAATGAATGCCC-3′ (reverse), and for β-actin, 5′-GGGTCAGAAGGATTCCTATG-3′ (forward) and 5′-GGTCTCAAACATGATCTGGG-3′ (reverse). The TRAIL PCR cycle conditions were 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min for a total of 35 cycles. The β-actin cycle conditions were 94°C for 45 s, 72°C for 1 min, and 55°C for 45 s for a total of 25 cycles. Samples were resolved on a 1% agarose gel and visualized with ethidium bromide.
Chromium release assay.
Chromium release assays were performed as described previously (60, 61). Briefly, monocytes were treated with HIV Tat for 24 h and supernatants and cells were collected for use as effectors. The chromium-labeled target cells were either uninfected or HIV-infected Jurkat cells or primary human CD4+ cells. Target cells were distributed to 96-well U-bottom plates (Costar, Cambridge, Mass.) at 103 cells/well, and effector monocytes were added at effector-to-target cell ratios of 6.25:1, 12.5:1, 25:1, and 50:1. Chromium release assay mixtures were incubated in triplicate for 20 h. The percentage of specific lysis was determined as follows: 100 × [(experimental release − spontaneous release)/(maximum release − spontaneous release)]. Maximum release was determined by the lysis of targets in 1% Triton X-100, and spontaneous release was determined by lysis of targets in media without effectors and was found to be consistently less than 20%. Target cells were not lysed by cell-free Tat in this assay.
Cell viability and flow cytometry assay.
To detect cell surface TRAIL, U937 cells, primary human monocytes, Jurkat cells, or primary human CD4+ cells (2 ×106 cells) were cultured with or without HIV Tat at 37°C for 24 h and then incubated with PE-labeled anti-TRAIL (RIK-2; Pharmingen) before a final wash and fixation in 1% paraformaldehyde-PBS for flow cytometry.
To assay the cytotoxicity of Tat-stimulated monocytes, TSM media were collected from human monocyte cultures that had been exposed to 100 ng of Tat/ml for 20 h. Uninfected or HIV-infected target cells were incubated with 100 μl of TSM medium for 16 to 20 h at 37°C. Cell viability was routinely checked by trypan blue exclusion. For controls, TSM medium was preincubated with anti-human IgG1 at a concentration of 20 μg/ml or with anti-TRAIL (N2B2) at a concentration of 100 ng/ml for 1 h. For flow cytometry for detection of apoptosis, aliquots of 105 cells were washed and incubated in the dark at 4°C for 20 min in PBS containing either 5 μl of annexin V-FITC (to detect early apoptosis) or 20 μg of 7-AAD/ml (to detect late apoptosis) (58). Cells were processed according to the manufacturer's protocol and fixed in a PBS-bovine serum albumin-NaN3 buffer containing 1% paraformaldehyde. Samples were analyzed 20 min later by FACScan flow cytometry (36).
RESULTS
HIV Tat is taken up by T cells but not by monocytes.
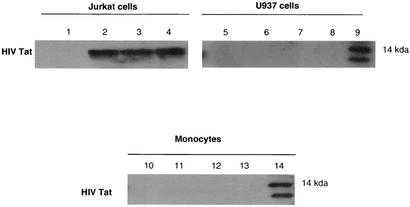
To demonstrate how HIV Tat influences the activity of monocytes, we exposed cells to HIV Tat protein and visualized its uptake into monocytes and T cells. Immunoblotting results indicated that HIV Tat was not internalized in U937 cells or monocytes, while it appears in Jurkat cells as early as 30 min after exposure (57) and can still be detected 3 h after the initial exposure to Tat (Fig. 1).
FIG. 1.
HIV Tat enters Jurkat cells but not U937 cells or monocytes. Jurkat cells, U937 cells, and monocytes were exposed to HIV Tat (100 ng/ml) for 0 min (lanes 1, 5, and 10), 30 min (lanes 2, 6, and 11), 1 h (lanes 3, 7, and 12), or 3 h (lanes 4, 8, and 13). After the indicated times, cells were collected, cell lysates were separated by SDS-10% PAGE, and cell proteins were transferred to nitrocellulose membranes and immunoblotted with the TR1 murine monoclonal antibody to Tat (57). Lanes 9 and 14 contain recombinant Tat protein.
HIV Tat increases the expression of TRAIL in U937 cells and human monocytes.
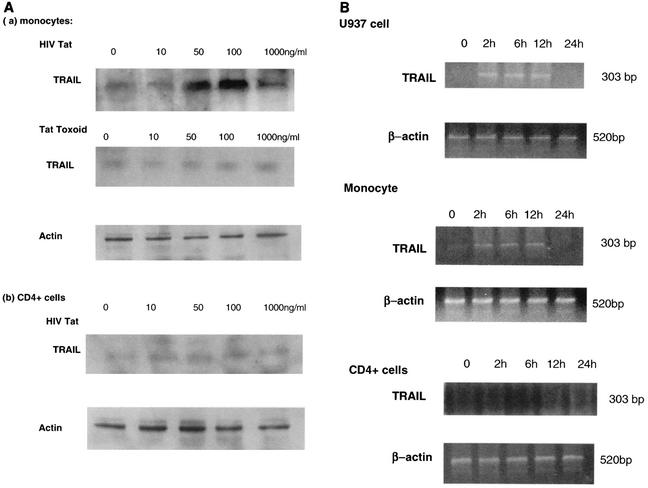
Fresh human monocytes or the promonocytic cell line U937 were stimulated with HIV Tat protein (0 to 1,000 ng/ml) at 37°C, and lysates were collected after 20 h. Immunoblotting results showed that HIV Tat increases the expression of TRAIL in a dose-dependent manner, with 100 ng/ml being the optimum concentration of HIV Tat (Fig. 2A, part a). At a higher concentration of Tat (1,000 ng/ml), the expression of TRAIL was decreased. The same results were obtained with U937 cells; however, no Tat stimulation of TRAIL could be detected in fresh human T cells or in Jurkat cells (Fig. 2A, part b). Chemically modified Tat toxoid, with an average of two cysteines inactivated per molecule, was incapable of inducing TRAIL in monocytic cells (Fig. 2A, part a).
FIG. 2.
HIV Tat increases TRAIL expression in U937 cells and human monocytes, but Tat toxoid does not. (A) TRAIL expression increases with increasing doses of Tat protein. Monocytes (a) and CD4+ cells (b) were incubated with or without HIV Tat or Tat toxoid (10 to 1,000 ng/ml) at 37°C for 20 h, and 10 μl of cell lysate (6 × 104 cell equivalents) was separated by SDS-10% PAGE. Cell proteins were transferred to nitrocellulose membranes and immunoblotted with polyclonal anti-TRAIL (H-257; 1:1,000). Lanes (from left): 1, no HIV Tat protein; 2 to 5, cell extracts exposed to 10, 50, 100, and 1,000 ng of Tat/ml, respectively. Actin (antibody A-2668; Sigma) was an internal control. Similar results were obtained with fresh human monocytes and with the U937 cell line. TRAIL production in fresh human T cells or in Jurkat cells was not stimulated by exposure to Tat. (B) HIV Tat induces the transcription of TRAIL mRNA in U937 cells and monocytes. Total RNA from U937 cells, monocytes, or CD4+ cells was isolated and subjected to reverse transcription-PCR. β-Actin mRNA was amplified over 25 cycles and TRAIL mRNA was amplified over 35 cycles of PCR. Transcription of control β-actin or TRAIL/APO2L mRNA in U937 cells is shown after 0, 2, 6, 12, or 24 h of incubation in the presence of HIV Tat (100 ng/ml).
HIV Tat increases TRAIL mRNA transcription in U937 cells and human monocytes.
The transcription of TRAIL mRNA was rapidly induced within 2 h after the addition of Tat, and expression was sustained throughout overnight culture (Fig. 2B) in both U937 cells and in freshly isolated human monocytes. Since Tat is easily oxidized, mRNA expression dropped off after 12 h. In parallel experiments with T cells, TRAIL mRNA was not detectable.
HIV cysteine-rich peptide is responsible for TRAIL induction.
To identify the portion of Tat responsible for inducing TRAIL, we synthesized six overlapping peptides covering different portions of Tat. Tat16-35, which contains six of the seven cysteines of Tat, induced TRAIL expression in monocytes, while other peptides did not (Fig. 3).
FIG. 3.
The cysteine-rich region of Tat is responsible for inducing monocytes to express TRAIL. Peptides Tat1-20 (peptide 1), Tat16-35 (peptide 8), Tat31-50 (peptide 13), Tat46-65 (peptide 19), Tat61-80 (peptide 25), and Tat76-95 (peptide 31) at concentrations of 0 to 1,000 ng/ml were incubated with monocytes at 37°C for 20 h, and then 10 μl of cell lysate was separated by SDS-10% PAGE. Cell proteins were transferred to nitrocellulose membranes and immunoblotted with polyclonal anti-TRAIL (H-257; 1:1,000) as described in Materials and Methods.
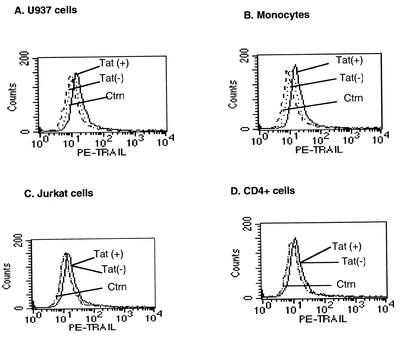
Tat induced little cell surface TRAIL on monocytes.
We used flow cytometry to determine whether HIV Tat induced the expression of TRAIL on the surface of human monocytes or U937 cells. Tat treatment for 20 h only slightly increased the membrane expression of TRAIL in U937 cells and monocytes in comparison to what was seen with controls (Fig. 4A and B). Tat did not affect surface expression of TRAIL on Jurkat cells or on primary CD4+ cells (Fig. 4C and D).
FIG. 4.
HIV Tat only slightly increases membrane-associated TRAIL expression in U937 cells and monocytes. U937 cells (A), human monocytes (B), Jurkat cells (C), and primary CD4+ cells (D) were stimulated with HIV Tat (100 ng/ml) for 20 h, stained with PE-conjugated mouse anti-human TRAIL or mouse IgG1 control monoclonal antibody as described in Materials and Methods, and then subjected to flow cytometry. The histograms shown are representative of three independent experiments.
Release of soluble TRAIL in the media from U937 cells and human monocytes upon stimulation with HIV Tat.
In activated human T cells, soluble TRAIL was released into the media in the form of micelles (43). We tested whether HIV Tat stimulation resulted in the majority of TRAIL being released from monocytes or T cells. Using a Western blotting assay, we clearly showed that Tat stimulation resulted in the appearance of TRAIL in the media from U937 cell (Fig. 5A) or human monocyte cultures (Fig. 5B). T cells did not release sufficient TRAIL after 20 h as detected by Western blotting or the cytotoxicity assay (Fig. 5C).
FIG. 5.
HIV Tat increases secretion of soluble TRAIL in media of U937 cells and monocytes. For this experiment, 3 × 106 U937 cells (A), human monocytes (B), and CD4+ cells (C) in 100 μl of medium were incubated with or without HIV Tat (10 to 1,000 ng/ml) at 37°C for 20 h, and then 20 μl of cell medium was separated by SDS-10% PAGE. Proteins were transferred to nitrocellulose membrane and incubated with polyclonal anti-TRAIL (H-257; 1:1,000). Lanes (from left), 1, no HIV Tat protein; 2 to 5, extracellular media exposed to 10 to 1,000 ng of Tat/ml. TRAIL secretion could not be detected from T-lymphocyte cultures after 20 h.
By comparing commercially available TRAIL protein with Tat-induced TRAIL in extracts, we estimated that our Western blots detected as little as 10 ng of TRAIL. Thus, in the weakest bands of our Western blots, we saw approximately 10 ng of TRAIL per 20 μl of medium or per cell lysate (6 × 104 cell equivalents), and in the strongest bands, we saw approximately 200 ng of TRAIL per 6 × 104 cell equivalents. At 20 h after Tat exposure, a little more TRAIL was found intracellularly than was found extracellularly (per cell) in the secreted form.
Uninfected T cells are more susceptible to TRAIL-mediated cell death than are HIV-infected T cells.
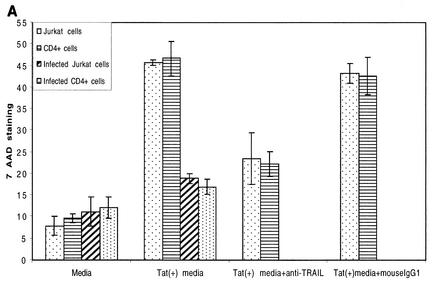
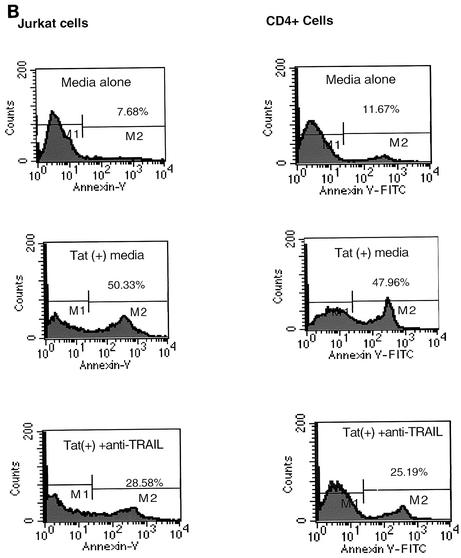
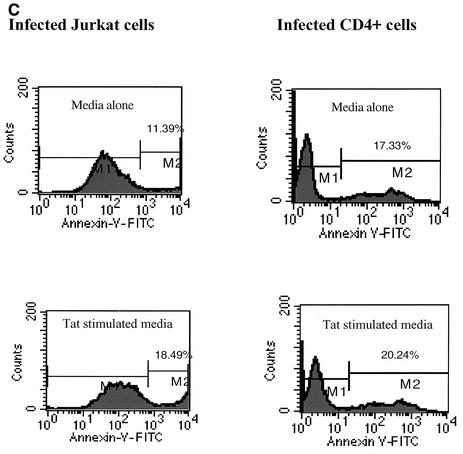
Initially, we used a 20-h 51Cr release assay to check the cytotoxicity of monocyte-associated TRAIL on Jurkat and primary CD4+ T cells (Fig. 6A and B). Cell-associated TRAIL has low cytotoxicity against these T-cell targets. At the same time, both uninfected and infected Jurkat and CD4+ cells were cultured with medium from monocytes that had been stimulated with 100 ng of Tat protein/ml (i.e., TSM medium). TSM medium caused significant cytotoxicity, approximately two- to threefold over the maximum cytotoxicity observed in the presence of Tat-treated monocytes (Fig. 6C). The cytotoxicity of TSM medium could be neutralized by anti-TRAIL monoclonal antibody but not by anti-human IgG1. This indicates that soluble TRAIL is released from Tat-stimulated monocytes. Evidence that target T cells underwent apoptosis was obtained by both annexin V staining for early apoptotic surface changes and by 7-AAD staining for later apoptotic DNA fragmentation. Figure 7 clearly shows that HIV TSM medium induced apoptosis in Jurkat cells and primary CD4+ T cells. This apoptosis decreased when TSM medium was preincubated with anti-TRAIL (N2B2). Jurkat cells and CD4+ cells infected with HIV-1 IIIB were exposed to TSM medium at 5 days after infection. Compared with uninfected cells, HIV-infected Jurkat cells and HIV-infected CD4+ cells were relatively resistant to the apoptotic effects of TSM medium (Fig. 6C and 7).
FIG. 6.
(A and B) Tat induces cytotoxic activity in monocytes. Human monocytes (effectors) were stimulated with 100 ng of Tat/ml for 24 h and then incubated with chromium-labeled targets, Jurkat (A) or CD4+ (B) cells, for a 20-h 51Cr-release assay. The percentage of specific lysis was observed at several effector-to-target cell (E:T) ratios. The data represent means ± standard errors of the means (SEM) from three independent experiments, each carried out in triplicate. (C) HIV Tat-stimulated human monocytes release functional soluble TRAIL. TSM medium was tested for cytotoxic activity against uninfected Jurkat cells, uninfected primary CD4+ cells, HIV-infected Jurkat cells, or infected CD4+ cells. These target cells were 51Cr-labeled and then incubated for 16 h with medium alone, 100 μl of TSM medium, TSMmedium preincubated with anti-TRAIL (N2B2; 100 ng/ml), or TSM medium preincubated with anti-mouse IgG1 (20 μg/ml). Bars represent means ± SEM from three independent experiments, each carried out in triplicate.
FIG. 7.
Apoptosis was detected by annexin V and 7-AAD staining. Jurkat cells and primary CD4+ cells were incubated with TSM media for 20 h and then stained with either annexin V (a marker for early stages of apoptosis) or 7-AAD (a marker for later stages of apoptosis) as described in Materials and Methods. (A) 7-AAD staining of infected and uninfected T cells. Bars represent the means ± SEM from three independent experiments, each carried out in triplicate. (B) Annexin V staining of uninfected Jurkat cells and CD4+ cells. (C) Annexin V staining of HIV-infected Jurkat and CD4+ cells. Data from three independent experiments were similar.
DISCUSSION
Extracellular HIV Tat has multiple effects on human monocytes, including upregulation of cytokine expression, upregulation of HIV coreceptor expression, promotion of chemotaxis, and promotion of microvascular invasion (19). We demonstrated that TRAIL expression by monocytes far exceeds TRAIL expression by T cells after a brief exposure to Tat. Fifty nanograms (or 5 nM) of Tat/ml initiates TRAIL production, which is within range of the 1 ng/ml found in HIV-infected sera (59), especially considering that Tat effects are more likely to occur in solid tissues than in circulation. Exposure to Tat only slightly increases membrane-bound TRAIL expression but significantly increases de novo transcription and secretion of TRAIL. The secreted TRAIL is highly toxic to uninfected T cells.
The mechanism by which Tat induces monocyte TRAIL expression is not known. HIV Tat functions as a transcriptional activator when it arrives in the cell nucleus, so we first asked whether HIV Tat is taken up by monocytes. In contrast to previously reported results with T cells (57), Tat uptake could not be demonstrated with U937 cells or monocytes. Either very small amounts of intracellular Tat are required to induce TRAIL or Tat can induce TRAIL expression via extracellular signal transduction. Several previous studies have pointed to the role of Tat in signal transduction. The basic domain of Tat has homology with heparin-binding growth factors such as vascular endothelial growth factor, and Tat can use the vascular endothelial growth factor signaling pathways by its specific binding to cognate receptors (44). In addition, a classical RGD integrin-binding domain that can activate β integrins and bind to extracellular matrix proteins is found in the N terminus of Tat (6, 8, 66).
Recently, Martinez-Lorenzo et al. (42, 43) reported that bioactive TRAIL is released in the form of microvesicles from Jurkat cells. This group also observed that TRAIL-containing vesicles were released into media after PHA stimulation of PBMC (43, 46). More recently, Liabakk et al. (38) demonstrated soluble TRAIL in both human serum and plasma samples as well as in media from PHA-stimulated PBMC. Here we show that after stimulation with HIV Tat protein, human monocytes upregulate TRAIL mRNA within 2 h and release the soluble form of TRAIL into the medium. TRAIL can be released from the cell surface by shedding, which involves cleavage by cysteine proteases such as E64 and leupeptin (41), but not by metalloproteases as is the case for TNF-α and FasL (33). TRAIL is more similar to TNF in that both membrane-bound and soluble forms are biologically active, and it is dissimilar to FasL in that membrane-bound FasL is much more active than soluble FasL (sFasL) (52). We report here that TRAIL released from Tat-stimulated monocytes is the major cytotoxic agent responsible for killing T cells, since T cells treated with Tat for the same amount of time did not upregulate any detectable TRAIL mRNA or produce enough TRAIL to be detected by our immunoblotting or cytotoxicity assays.
Using flow cytometry, Zhang et al. (64) showed that HIV Tat upregulated TRAIL on the surface of monocyte-derived macrophages. In this study, we employed promonocytic U937 cells and human monocytes, but we failed to find a significant increase in cell surface TRAIL in these cells after Tat stimulation. Within the 24-h assay, we saw no cell surface increase in T-cell TRAIL. The monocyte-derived macrophages are less frequently found in circulating blood than the monocytes used in our work, and hence, our findings with fresh monocytes are more relevant to normal human PBMC.
To determine which portion of Tat was responsible for inducing TRAIL, we used overlapping synthetic peptides from all regions of Tat. Only one peptide, Tat16-35, containing six of the seven cysteines in Tat, was capable of inducing monocyte TRAIL. TRAIL induction required 50 ng (about 20 nM) of peptide/ml, so full-length Tat (inducing TRAIL at 5 nM) is at least fourfold more potent than peptide. Others have reported that a cysteine-rich peptide (Tat21-40, containing all seven cysteines) activated NF-κB and mediated transcriptional transactivation (9). Peptide Tat16-35 can induce TRAIL (this study) and can activate NF-κB but cannot mediate transcriptional transactivation (I. Tikhonov, unpublished results). Others have shown that Tat24-51, containing the same six cysteines, binds to monocytes and enhances chemotaxis (3). Thus, the cysteine-rich peptide we used is sufficient to induce TRAIL, and by extrapolation from the data of others (5), it can also induce NF-κB, a common transcription factor in monocyte activation. Although monocyte activation results in a cascade of events, we focused on the one factor, TRAIL, that has an overriding toxic effect on bystander T cells.
The predominant view of the role of apoptosis in AIDS is that uninfected bystander cells are depleted and that this contributes to immunodeficiency (17, 22, 25, 56). Bystander depletion is most apparent in lymphoid tissues, where in situ assays for apoptosis have revealed a substantial level of cell death that was not related directly to productive infection (17). A recent study of SCID mice transplanted with PBMC from HIV-infected humans also revealed a great deal more cell death in uninfected than in HIV-infected cells (45). In the murine study, splenocyte death could be inhibited by administering anti-TRAIL, thereby indicating that TRAIL is an important effector of cell death in vivo. In contrast to our results, that study demonstrated a prominent role for T-cell-associated TRAIL by showing dying cells juxtaposed to cells that stained positively for TRAIL as well as for CD3 or CD4. A possible explanation for this difference is that we are describing short-term TRAIL induction, whereas the murine results occurred 2 weeks after exposure to infected PBMC. It has long been known that T cells make TRAIL (28, 41-43, 56) and, especially in the environment of the spleen, may be important effectors of TRAIL-mediated apoptosis.
We have shown that TRAIL is more toxic for uninfected cells than for infected cells, thereby providing a molecular basis for observations of bystander cell death. We have not yet identified a mechanism for the differential sensitivity to TRAIL, although in another system, it has been shown that B lymphocytes acquired resistance to TRAIL by downregulating receptors TRAIL-R1 and TRAIL-R2 (40). Since infected Jurkat cells have higher levels of TRAIL receptors than do uninfected cells (39), TRAIL resistance is more likely due to alteration of intracellular signaling. The resistance of monocytes to HIV- and Tat-mediated apoptosis has been attributed to upregulation of Bcl-2, a known intracellular inhibitor of apoptosis (2, 65; Y.Yang, unpublished data). Others have shown that Jurkat cells stably transfected with HIV-tat are less susceptible to TRAIL-mediated cell death than are untransfected cells (20). Unfortunately, the process of making stably transfected cells often selects for cell death resistance that might alter the outcome. Nevertheless, our comparisons of HIV-infected and noninfected cell cultures are similar to the results with transfected cells. Our results confirm that HIV-infected cells are relatively resistant to TRAIL-mediated cell death, a mechanism that might contribute to viral persistence or establishment of the latent reservoir.
This study focused on CD4+ cell death because it represents a large proportion of the cell death in AIDS. However, it will be interesting in future studies to determine whether TRAIL mediates any selective depletion of CD8+ T cells, since they play a critical role in AIDS pathogenesis.
In summary, we described the rapid induction and secretion of TRAIL in HIV Tat-stimulated monocytes, and its ability to selectively eliminate uninfected T cells. This finding provides important insight into the mechanism of lymphocyte cell death during HIV infection.
Acknowledgments
This study was supported by Public Health Service grants AI49805 (C.D.P.) and AI46244 (M.S.S.).
We thank Robert Gallo for critical comments on the manuscript and Robert Powell and Christine Grassel of the Microquant Core Facility for providing fresh human monocytes, lymphocytes, and HIV-infected cultures.
REFERENCES
- 1.Adams, D. O., and T. A. Hamilton. 1984. The cell biology of macrophage activation. Annu. Rev. Immunol. 2:283-318. [DOI] [PubMed] [Google Scholar]
- 2.Aillet, F., H. Masutani, C. Elbim, H. Raoul, L. Cene, M.-T. Nugeyre, C. Paya, F. Barre-Sinoussi, M.-A. Gougerot-Pocidalo, and N. Israel. 1998. Human immunodeficiency virus induces a dual regulation of Bcl-2, resulting in persistent infection of CD4+ T- or monocytic cell lines. J. Virol. 72:9698-9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albini, A., S. Ferrini, R. Benelli, S. Sforzini, D. Giunciuglio, and M. G. Aluigi. 1998. HIV-1 Tat protein mimicry of chemokines. Proc. Natl. Acad. Sci. USA 95:13153-13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashkenazi, A., R. C. Pai, S. Fong, S. Leung, D. A. Lawrence, S. A. Marsters, C. Blackie, L. Chang, A. E. McMurtrey, A. Hebert, L. DeForge, I. L. Koumenis, D. Lewis, L. Harris, J. Bussiere, H. Koeppen, Z. Shahrokh, and R. H. Schwall. 1999. Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. Investig. 104:155-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badou, A., Y. Bennasser, M. Moreau, C. Leclerc, M. Benkirane, and E. Bahraoui. 2000. Tat protein of human immunodeficiency virus type 1 induces interleukin-10 in human peripheral blood monocytes: implication of protein kinase C-dependent pathway. J. Virol. 74:10551-10562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barillari, G., R. Gendelman, R. C. Gallo, and B. Ensoli. 1993. The Tat protein of human immunodeficiency virus type 1, a growth factor for AIDS Kaposi sarcoma and cytokine-activated vascular cells, induces adhesion of the same cell types by using integrin receptors recognizing the RGD amino acid sequence. Proc. Natl. Acad. Sci. USA 90:7941-7945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartz, S. R., and M. Emerman. 1999. Human immunodeficiency virus type 1 Tat induces apoptosis and increases sensitivity to apoptotic signals by up-regulating FLICE/caspase-8. J. Virol. 73:1956-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benelli, R., R. Mortarini, A. Anichini, D. Giunciuglio, D. M. Noonan, S. Montalti, and A. Albini. 1998. Monocyte-derived dendritic cells and monocytes migrate to HIV-Tat RGD and basic peptides. AIDS 12:261-265. [DOI] [PubMed] [Google Scholar]
- 9.Boykins, R. A., R. Mahieux, U. T. Shankavaram, Y. S. Gho, S. F. Lee, I. K. Hewlett, L. M. Wahl, H. K. Kleinman, J. N. Brady, K. M. Yamada, and S. Dhawan. 1999. A short polypeptide domain of HIV-1-Tat protein mediates pathogenesis. J. Immunol. 163:15-20. [PubMed] [Google Scholar]
- 10.Cohen, S. S., C. Li, L. Ding, A. B. Pardee, E. M. Shevach, and D. I. Cohen. 1999. Pronounced acute immunosuppression in vivo mediated by HIV Tat challenge. Proc. Natl. Acad. Sci. USA 96:10842-10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cullen, B. R. 1986. Transcription of human immunodeficiency virus occurs via a bimodal mechanism. Cell 46:937-982. [DOI] [PubMed] [Google Scholar]
- 12.Ding, A. H., C. F. Nathan, and D. J. Stuehr. 1988. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J. Immunol. 141:2407-2412. [PubMed] [Google Scholar]
- 13.Djavani, M., J. Rodas, I. S. Lukashevich, D. Horejsh, P. P. Pandolfi, K. L. Borden, and M. S. Salvato. 2001. Role of the promyelocytic leukemia protein PML in the interferon sensitivity of lymphocytic choriomeningitis virus. J. Virol. 75:6204-6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dockrell, D. H., A. D. Badley, J. S. Villacian, C. J. Heppelmann, A. Algeciras, S. Ziesmer, H. Yagita, D. H. Lynch, P. C. Roche, P. J. Leibson, and C. V. Paya. 1998. The expression of Fas ligand by macrophages and its upregulation by human immunodeficiency virus infection. J. Clin. Investig. 101:2394-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ensoli, B., G. Barillari, S. Salhuddin, R. C. Gallo, and F. Wong-Staal. 1990. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature 345:84-87. [DOI] [PubMed] [Google Scholar]
- 16.Fanales-Belasio, E., S. Moretti, F. Nappi, G. Barillari, F. Michetti, A. Cafaro, and B. Ensoli. 2002. Native HIV-1 Tat protein targets monocyte-derived dendritic cells and enhances their maturation, function, and antigen-specific T cell responses. J. Immunol. 168:197-206. [DOI] [PubMed] [Google Scholar]
- 17.Finkel, T. H., G. Tudor-Williams, N. K. Banda, M. F. Cotton, T. Curiel, C. Monks, T. W. Baba, R. M. Ruprecht, and A. Kupfer. 1995. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat. Med. 1:129-132. [DOI] [PubMed] [Google Scholar]
- 18.Frankel, A. D., and C. O. Pabo. 1988. Cellular uptake of the Tat protein from human immunodeficiency virus. Cell 55:1189-1193. [DOI] [PubMed] [Google Scholar]
- 19.Gallo, R. C. 1999. Tat as one key to HIV-induced immune pathogenesis and Tat toxoid as an important component of a vaccine. Proc. Natl. Acad. Sci. USA 96:8324-8326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibellini, D., M. C. Re, C. Ponti, C. Maldini, C. Celeghini, A. Cappellini, M. La Placa, and G. Zauli. 2001. HIV-1 Tat protects CD4+ Jurkat T lymphoblastoid cells from apoptosis mediated by TNF-related apoptosis-inducing ligand. Cell. Immunol. 207:89-99. [DOI] [PubMed] [Google Scholar]
- 21.Gosselin, E. J., K. Wardwell, D. R. Gosselin, N. Alter, J. L. Fisher, and P. M. Guyre. 1992. Enhanced antigen presentation using human Fc gamma receptor (monocyte/macrophage)-specific immunogens. J. Immunol. 149:3477-3481. [PubMed] [Google Scholar]
- 22.Gougeon, M. L., H. Lecour, A. Dulioust, M.-G. Enouf, M. Crouvoisier, C. Goujard, T. Dehord, and L. Montagnier. 1996. Programmed cell death in peripheral lymphocytes from HIV-infected persons. J. Immunol. 156:3509-3515. [PubMed] [Google Scholar]
- 23.Griffith, T. S., W. A. Chin, G. C. Jackson, D. H. Lynch, and M. Z. Kubin. 1998. Intracellular regulation of TRAIL-induced apoptosis in human melanoma cells. J. Immunol. 6:2833-2840. [PubMed] [Google Scholar]
- 24.Griffith, T. S., and C. T. Lynch. 1998. TRAIL: a molecule with multiple receptors and control mechanisms. Curr. Opin. Immunol. 10:559-563. [DOI] [PubMed] [Google Scholar]
- 25.Groux, H., G. Torpier, D. Monte, Y. Mouton, A. Capron, and J. C. Amieson. 1992. Activation-induced death by apoptosis in CD4+ T cells from HIV-infected asymptomatic individuals. J. Exp. Med. 175:331-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halaas, O., R. Vik, A. Asikenaza, and T. Espevik 2000. Lipopolysaccharide induces expression of Apo2L/TRAIL in human monocytes and macrophages. Scand. J. Immunol. 51:244-250. [DOI] [PubMed] [Google Scholar]
- 27.Huang, L., I. Bosch, W. Hoffmann, J. Sodroski, and A. B. Pardee. 1998. Tat protein induces human immunodeficiency virus type 1 (HIV-1) coreceptors and promotes infection with both macrophage-tropic and T-lymphotropic HIV-1 strains. J. Virol. 72:8952-8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeremias, I., I. Herr, T. Boehler, and K. M. Debatin. 1998. TRAIL/Apo-2-ligand-induced apoptosis in human T cells. Eur. J. Immunol. 1:143-152. [DOI] [PubMed] [Google Scholar]
- 29.Jewett, A., J. V. Giorgi, and B. Bonavida. 1990. Antibody-dependent cellular cytotoxicity against HIV-coated target cells by peripheral blood monocytes from HIV seropositive asymptomatic patients. J. Immunol. 145:4065-4071. [PubMed] [Google Scholar]
- 30.Jo, M., T. H. Kim, D. W. Seol, J. E. Esplen, K. Dorko, T. R. Billiar, and S. Strom. 2000. Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat. Med. 6:564-567. [DOI] [PubMed] [Google Scholar]
- 31.Johnsen, A. C., J. Haux, B. Steinkjer, U. Nonstad, K. Egeberg, A. Sundan, A. Ashkenazi, and T. Espevik. 1999. Regulation of APO-2 ligand/trail expression in NK cells—involvement in NK cell-mediated cytotoxicity. Cytokine 11:664-672. [DOI] [PubMed] [Google Scholar]
- 32.Katsikis, P. D., M. E. Garcia-Ojeda, J. F. Torres-Roca, I. M. Tijoe, C. A. Smith, L. A. Herzenberg, and L. A. Herzenberg. 1997. Interleukin-1β converting enzyme-like protease involvement in Fas-induced and activation-induced peripheral blood T cell apoptosis in HIV infection. TNF-related apoptosis-inducing ligand can mediate activation-induced T cell death in HIV infection. J. Exp. Med. 186:1365-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kayagaki, N., A. Kawazaki, T. Ebata, H. Ohmato, S. Inoue, K. Yoshino, K. Okumura, and H. Yagita. 1995. Metalloproteinase-mediate release of human Fas ligand. J. Exp. Med. 182:1777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kothny-Wilkes, G., D. Kulms, B. Poppelmann, T. A. Luger, M. Kubin, and T. Schwarz. 1998. Interleukin-1 protects transformed keratinocytes from tumor necrosis factor-related apoptosis-inducing ligand. J. Biol. Chem. 44:29247-29252. [DOI] [PubMed] [Google Scholar]
- 35.Le Buanec, H., and B. Bizzini. 2000. Procedures for preparing biologically inactive, but immunogenic HIV-1 Tat protein (Tat toxoid) for human use. Biomed. Pharmacother. 54:41-44. [DOI] [PubMed] [Google Scholar]
- 36.Lecoeur, H., E. Ledru, M. C. Prevost, and M. L. Gougeon. 1997. Strategies for phenotyping apoptotic peripheral human lymphocytes comparing ISNT, annexin-V and 7-AAD cytofluorometric staining methods. J. Immunol. Methods 209:111-123. [DOI] [PubMed] [Google Scholar]
- 37.Li, C. J., D. J. Friedman, C. Wang, V. Metelev, and A. B. Pardee. 1995. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat proteins. Science 268:429-431. [DOI] [PubMed] [Google Scholar]
- 38.Liabakk, N. B., A. Sundan, S. Torp, P. Aukrust, S. S. Froland, and T. Espevik. 2002. Development, characterization and use of monoclonal antibodies against sTRAIL: measurement of sTRAIL by ELISA. J. Immunol. Methods 259:119-128. [DOI] [PubMed] [Google Scholar]
- 39.Lum, J. J., P. A. Andre, J. Sanchez-Dardon, B. N. Phenix, J. E. Kim, J. Mihowhich, K. Janison, N. Hawley-Foss, D. H. Lynch, and A. D. Badley. 2001. Induction of cell death in human immunodeficiency virus-infected macrophages and resting memory CD4 T cells by TRAIL/Apo2L. J. Virol. 75:11128-11136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacFarlane, M., N. Harper, R. T. Snowden, M. J. Dyer, G. A. Barnett, J. H. Pringle, and G. M. Cohen. 2002. Mechanisms of resistance to TRAIL-induced apoptosis in primary B cell chronic lymphocytic leukaemia. Oncogene 21:6809-6818. [DOI] [PubMed] [Google Scholar]
- 41.Mariani, S. M., and P. H. Krammer. 1998. Surface expression of TRAIL/Apo-2 ligand in activated mouse T and B cells. Eur. J. Immunol. 5:1492-1498. [DOI] [PubMed] [Google Scholar]
- 42.Martinez-Lorenzo, M. J., M. A. Alava, S. Gamen, K. J. Kim, A. Chuntharapai, A. Pineiro, J. Naval, and A. Anel. 1998. Involvement of APO2 ligand/TRAIL in activation-induced death of Jurkat and human peripheral blood T cells. Eur. J. Immunol. 28:2714-2725. [DOI] [PubMed] [Google Scholar]
- 43.Martinez-Lorenzo, M. J., A. Anel, S. Gamen, I. Monle, P. Lasierra, L. Larrad, A. Pineiro, M. A. Alava, and J. Naval. 1999. Activated human T cells release bioactive Fas ligand and APO2 ligand in microvesicles. J. Immunol. 163:1274-1281. [PubMed] [Google Scholar]
- 44.Mitola, S., S. Sozzani, W. Luini, L. Primo, A. Borsatti, H. Weich, and F. Bussolino. 1997. Tat-human immunodeficiency virus-1 induces monocyte chemotaxis by activation of vascular endothelial growth factor receptor-1. Blood 90:1365-1372. [PubMed] [Google Scholar]
- 45.Miura, Y., N. Misawa, N. Maeda, Y. Inagaki, Y. Tanaka, M. Ito, N. Kayagaki, N. Yamamoto, H. Yagita, H. Mizusawa, and Y. Koyanagi. 2001. Critical contribution of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) to apoptosis of human CD4+ T cells in HIV-1-infected hu-PBL-NOD-SCID mice. J. Exp. Med. 193:651-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monleon, I., M. J. Martinez-Lorenzo, L. Monteagudo, P. Lasierra, M. Taules, M. Iturralde, A. Pineiro, L. Larrad, M. A. Alava, J. Naval, and A. Anel. 2001. Differential secretion of Fas ligand- or APO2 ligand/TNF-related apoptosis-inducing ligand-carrying microvesicles during activation-induced death of human T cells. J. Immunol. 167:6736-6742. [DOI] [PubMed] [Google Scholar]
- 47.Old, T. J. 1985. Tumor necrosis factor (TNF). Science 230:630-635. [DOI] [PubMed] [Google Scholar]
- 48.Pauza, C. D., P. Trivedi, M. Wallace, T. J. Ruckwardt, H. Le Buanec, W. Lu, B. Bizzini, A. Burny, D. Zagury, and R. C. Gallo. 2000. Vaccination with Tat toxoid attenuates disease in simian/HIV-challenged macaques. Proc. Natl. Acad. Sci. USA 97:3515-3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfeifer, J. D., M. J. Wick, R. L. Roberts, K. Findlay, S. J. Normark, and C. V. Harding. 1993. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature 361:359-362. [DOI] [PubMed] [Google Scholar]
- 50.Pollok, K. E., Y. J. Kim, J. Hurtado, Z. Zhou, K. K. Kim, and B. S. Kwon. 1994. 4-1BB T-cell antigen binds to mature B cells and macrophages, and costimulates anti-μ-primed splenic B cells. Eur. J. Immunol. 24:367-370. [DOI] [PubMed] [Google Scholar]
- 51.Rieger, J., U. Naumann, T. Glaser, A. Ashkenazi, and M. Weller. 1998. APO2 ligand: a novel lethal weapon against malignant glioma? FEBS Lett. 427:124-128. [DOI] [PubMed] [Google Scholar]
- 52.Schneider, P., N. Holler, J. L. Bodmer, M. Hahne, K. Frei, A. Fontana, and J. Tschopp. 1998. Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J. Exp. Med. 187:1205-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Secchiero, P., D. Zella, S. Capitani, R. C. Gallo, and G. Zauli. 1999. Extracellular HIV-1 Tat protein up-regulates the expression of surface CXC-chemokine receptor 4 in resting CD4+ T cells. J. Immunol. 162:2427-2431. [PubMed] [Google Scholar]
- 54.Smith, C. A., H. J. Gruss, T. Davis, D. Anderson, T. Farrah, E. Baker, G. R. Sutherland, C. I. Brannan, N. G. Copeland, and N. A. Jenkins. 1993. CD30 antigen, a marker for Hodgkin's lymphoma, is a receptor whose ligand defines an emerging family of cytokines with homology to TNF. Cell 73:1349-1360. [DOI] [PubMed] [Google Scholar]
- 55.Sodroski, J., R. Patarca, C. Rosen, F. Wong-Staal, and W. Haseltine. 1985. Location of the transactivating region on the genome of human T-cell lymphotropic virus type III. Science 229:74-79. [DOI] [PubMed] [Google Scholar]
- 56.Tan, K. B., J. Harrop, M. Reddy, P. Young, J. Terrett, J. Emery, G. Moore, and A. Truneh. 1997. Characterization of a novel TNF-like ligand and recently described TNF ligand and TNF receptor superfamily genes and their constitutive and inducible expression in hematopoietic and non-hematopoietic cells. Gene 204:35-39. [DOI] [PubMed] [Google Scholar]
- 57.Tikhonov, I., T. J. Ruckwardt, G. S. Hatfield, and C. D. Pauza. 2003. Tat-neutralizing antibodies in vaccinated macaques. J. Virol. 77:3157-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wallace, M., P. M. Waterman, J. L. Mitchen, M. Djavani, C. Brown, P. Trivedi, D. Horejsh, M. Dykhuizen, M. Kitabwalla, and C. D. Pauza. 1999. Lymphocyte activation during acute simian/human immunodeficiency virus SHIV89.6 PD infection in macaques. J. Virol. 73:10236-10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Westendorp, M. O., R. Frank, C. Ochsenbauer, K. Stricker, J. Dhein, H Walczak, K. M. Debatin, and P. H. Krammer. 1995. Sensitization of T cells to CD95-mediated apoptosis by HIV-Tat and gp120. Nature 375:497-500. [DOI] [PubMed] [Google Scholar]
- 60.Yin, C., M. Djavani, A. R. Schenkel, D. S. Schmidt, C. D. Pauza, and M. S. Salvato. 1998. Dissemination of lymphocytic choriomeningitis virus from the gastric mucosa requires G protein-coupled signaling. J. Virol. 72:8613-8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yin, C., M. S. Wu, C. D. Pauza, and M. S. Salvato. 1999. High major histocompatibility complex-unrestricted lysis of simian immunodeficiency virus envelope-expressing cells predisposes macaques to rapid AIDS progression. J. Virol. 73:3692-3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zagury, D., A. Lachgar, V. Chams, L. S. Fall, J. Bernard, J. F. Zagury, B. Bizzini, A. Gringeri, E. Santagostino, and J. Rappaport. 1998. Interferon alpha and Tat involvement in the immunosuppression of uninfected T cells and C-C chemokine decline in AIDS. Proc. Natl. Acad. Sci. USA 95:3851-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zauli, G., M. la Placa, M. Vignoli, M. C. Re, D. Gibellini, G. Furlini, D. Milani, M. Marchisio, M. Mazzoni, and S. Capitani. 1995. An autocrine loop of HIV-1 Tat protein responsible for the improved survival/proliferation capacity of permanently Tat-transfected cells and required for optimal human immunodeficiency virus type 1 long terminal repeat transactivating activity. J. Acquir. Immune Defic. Syndr. 10:306-316. [PubMed] [Google Scholar]
- 64.Zhang, M., X. Li, X. Pang, L. Ding, O. Wood, K. Clouse, I. Hewlett, and A. I. Dayton. 2001. Identification of a potential HIV-induced source of bystander-mediated apoptosis in T cells: upregulation of TRAIL in primary human macrophages by HIV-1 Tat. J. Biomed. Sci. 8:290-296. [DOI] [PubMed] [Google Scholar]
- 65.Zhang, M., X. Li, X. Pang, L. Ding, O. Wood, K. Clouse, I. Hewlett, and A. I. Dayton. 2002. Bcl-2 upregulation by HIV-1 Tat during infection of primary human macrophages in culture. J. Biomed. Sci. 9:133-139. [DOI] [PubMed] [Google Scholar]
- 66.Zocchi, M. R., A. Poggi, and A. Rubartelli. 1997. The RGD-containing domain of exogenous HIV Tat inhibits the engulfment of apoptotic bodies by dendritic cells. AIDS 11:1227-1232. [DOI] [PubMed] [Google Scholar]