Abstract
Here, we report that human immunodeficiency virus type 1 (HIV-1) Env glycoprotein is located mainly in the trans-Golgi network (TGN) due to determinants present in the cytoplasmic domain of the transmembrane gp41 glycoprotein (TMgp41). Internalization assays demonstrated that Env present at the cell surface returns to the TGN. We found that the cytoplasmic domain of TMgp41 binds to TIP47, a protein required for the transport of mannose-6-phosphate receptors from endosomes to the TGN. Overexpression of a mutant of TIP47 affected the transport of Env from endosomes to the TGN. Retrograde transport of Env to the TGN requires a Y802W803 diaromatic motif present in the TMgp41 cytoplasmic domain. Mutation of this motif abolished both targeting to the TGN as well as interaction with TIP47. These data support the view that binding of TIP47 to HIV-1 Env facilitates its delivery to the TGN. Lastly, we show that virus mutated in the Y802W803 motif is poorly infectious and presents a defect in Env incorporation, supporting a model in which retrograde transport of Env is implicated in the optimization of fully infectious HIV-1 production.
The envelope glycoprotein (Env) of the human immunodeficiency virus type 1 (HIV-1) is synthesized as a 160-kDa precursor and is processed during its passage through the secretory pathway by a host cell protease, furin, to yield the surface subunit (SUgp120) and the transmembrane (TM) subunit (TMgp41). The SUgp120 is responsible for binding to receptors and coreceptors, whereas the TMgp41 anchors the Env proteins at the membrane and induces membrane fusion during virus entry. The TM is comprised of an ectodomain, a single membrane-spanning domain, and a C-terminal cytoplasmic domain, containing more than 150 amino acids (aa) (17).
Newly synthesized HIV-1 Env undergoes endocytosis after its arrival at the cell surface (33). Internalization of HIV-1 Env is mediated by interaction of the AP-2 clathrin adaptor complexes with a GY712SPL membrane-proximal, tyrosine-based signal in the TMgp41 cytoplasmic domain (3, 5, 28). Additional determinants downstream of the proximal tyrosine-based sorting signal are implicated in HIV-1 Env endocytosis (3). The cytoplasmic domain of HIV-1 Env also interacts with AP-1 adaptor complexes (3, 37). We have shown that the dileucine motif at the C terminus of the cytoplasmic domain of TMgp41 is implicated in the recruitment of AP-1 complexes. This dileucine motif, together with the membrane-proximal tyrosine-based motif, helps to control expression of Env at the cell surface (37). Two other amino acid sequences (aa 750 to 763 and aa 764 to 785 of the TMgp41), which inhibited Env surface expression, have also been identified in the cytoplasmic domain of the TMgp41 (9).
AP-1 mediates the transport of a subset of protein from the trans-Golgi network (TGN) to endosomes (19). AP-1 may also mediate retrograde transport from early or recycling endosomes to the TGN (23, 25). This pathway is followed by TGN38 (24) and the B fragment of Shiga toxin after endocytosis (23). The recycling of mannose 6-phosphate receptors (MPRs) to the TGN occurs from late endosomes (LE). Recently, the protein TIP47 has been shown to be required for the retrograde transport of MPRs (12). TIP47 is present in the cytosol and on endosomes and binds selectively to the cytoplasmic domains of cation-dependent (CD) and cation-independent MPRs (20, 30). TIP47 also binds directly to the Rab9 guanosine triphosphatase, a Rab GTPase also required for endosome-to-Golgi transport (22, 32), in its active, GTP-bound conformation (10).
Given the importance of cell-associated viral glycoproteins in cytopathic effects, the levels of Env at the cell surface may well influence the pathogenesis of HIV in vivo. A recent report on simian immunodeficiency virus (SIV) supports this view since disruption of the tyrosine-sorting signal Y721RPV of the SIVmac239 Env resulted in in vivo attenuation of SIVmac239 retrovirus in Rhesus macaques (14). We have further investigated the intracellular trafficking of Env to evaluate its implication in HIV infectivity. We found that, at the steady state, Env is mostly concentrated in the TGN. This distribution is in fact dynamic, partly due to the retrograde transport of Env from the cell surface to the TGN. We show that Env interacts with TIP47 protein via a Y802W803 diaromatic motif in the cytoplasmic tail of gp41 that is responsible for the retrograde transport of Env to the TGN. These data suggest that recognition of TIP47 to Env via the Y802W803 motif drives the retrograde transport of Env to the TGN.
MATERIALS AND METHODS
Antibodies.
The following primary antibodies were used: monoclonal antibody (MAb) 100.3 anti-γ-adaptin (Sigma), MAb 3F10 anti-hemagglutinin (anti-HA) (Roche), MAb Leu2A anti-CD8-α (Becton Dickinson), MAbs 110H and 160A anti-Env (Hybridolab), MAb 41A anti-TMgp41 (Hybridolab), MAb 25A anti-p24 (Hybridolab), rabbit polyclonal anti-TIP47 (provided by S. Pfeffer), rabbit polyclonal anti-glycoprotein E (anti-gE) and anti-sialyltransferase (anti-ST) (provided by B. Hoflack), sheep polyclonal anti-TGN46 (Serotec), human MAb b12 anti-HIV-1 Env (provided by D. Burton and P. Parren), and MAb IAM2G12 (provided by H. Katinger and National Institute for Biological Standards and Control). All the secondary antibodies against the mouse, rabbit, rat, human, and sheep immunoglobulin G's coupled to Cy2, Cy3, or Cy5 were purchased from Jackson ImmunoResearch.
Mammalian expression vectors.
The vectors for expression of the wild type (wt) (pcEnv-wt) HIV-1 Env and the HIV-1 HXB2 molecular clone were provided by M. Thali (37). Constructs for the eukaryotic expression of TGN markers were provided by B. Hoflack (pSRα-STSVG for ST and pSFFV-gE for the varicella-zoster virus [VZV] gE) and by G. Banting (ΔpMEP4-TGN38-EGFP for TGN38 fused to green fluorescent protein [GFP]).
Cell culture.
HeLa, 293T, and HeLa P4-2 cells were grown in Dulbecco's modified Eagle's medium, and Jurkat cells were grown in RPMI 1640 (Gibco BRL). These media were supplemented with glutamine, antibiotics, and 10% fetal calf serum.
Subcellular localization of Env glycoprotein.
HeLa cells were spread on glass coverslips in 24-well plates (2 × 104 cells/well) 24 h before transfection. Vectors expressing the wt HIV-1 Env (pcEnv-wt) and TGN markers were cotransfected with FuGENE 6 reagent (Roche Diagnostics) according to the manufacturer's instructions. Forty-eight hours later, the transfected cells were washed twice with phosphate-buffered saline (PBS) and fixed for 20 min in 4% paraformaldehyde. They were then quenched and permeabilized for 30 min in 0.05% saponin-0.2% bovine serum albumin (BSA)-PBS. The cells were incubated for 45 min with anti-Env b12 MAb, washed, and stained with anti-human Cy3 or Cy2 antibody. The cells were then incubated for 30 min with each of the antibody mixtures, washed, and stained with corresponding secondary antibody coupled to Cy2 or Cy3. Immunofluorescence on HIV-1-infected P4-2 HeLa cells was performed 48 h after infection as described above. When indicated, the cells were treated before fixation with 50 μg of cycloheximide/ml for the indicated times. Confocal microscopy was performed with a Bio-Rad MRC1000 instrument. A series of optical sections at 0.5-μm intervals were recorded and mounted by using Adobe Photoshop software.
Subcellular localization of CD8-HIV chimera. (i) Generation of CD8-HIV chimera.
The pCD8-HIV chimera has been described previously (3). Deletions (pCD8-HIV707-725, pCD8-HIV707-767, pCD8-HIV707-780, pCD8-HIV707-801, pCD8-HIV707-809, pCD8-HIV707-819, and pCD8-HIV707-825) and point mutations (pCD8-HIVY802W803-AA and pCD8-HIV-A804/810) of CD8 were generated by PCR-directed mutagenesis with the appropriate primers. Deletions and mutations were verified by DNA sequencing.
(ii) Indirect immunofluorescence staining.
HeLa cells (8 × 106) underwent electroporation (37) with 7 μg of CD8-HIV chimera vectors and were spread on glass coverslips in 24-well plates (8 × 104 cells/well). They were then stained with CD8 Leu2A MAb for immunofluorescence as described above for Env. When indicated, the cells were treated with 50 μg of cycloheximide/ml for the indicated times before fixation.
Internalization and uptake assays.
Cells grown on coverslips and transiently transfected 2 days earlier were washed with PBS containing 0.2% BSA and incubated with human anti-Env MAb or mouse anti-CD8 MAb for 1 h at 0°C. The cells were washed and returned to 37°C for the desired amount of time, after which they were fixed directly and processed for immunofluorescence as described above. Cells used for uptake assays were incubated simultaneously with anti-Env b12 and anti-gE for 1 h at 37°C. They were then washed, fixed, permeabilized, and processed for immunofluorescence.
Electron microscopy immunocytochemistry.
HeLa cells were transfected with 20 μg of pcEnvwt vector. Forty-eight hours later, cells were treated with cycloheximide for 3 h and fixed with 4% paraformaldehyde-0.1% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) (PB). Cells were prepared as described previously (31). Briefly, after fixation, cells were centrifuged at 1,000 × g for 10 min, embedded in 10% gelatin for 30 min on ice, and cut into small blocks that were infiltrated with 2.3 M sucrose and frozen in liquid nitrogen. Ultrathin cryosections were performed in a Leica Ultracut E cryoultramicrotome with a cryochamber attachment. For immunolabeling, the sections were incubated with anti-Env 2G12 MAb (1/1,000) (8) overnight at 4°C after preincubation in PB-10 mM NH4Cl-1% BSA for 20 min. Then, they were incubated with protein A gold (10-nm diameter; purchased from J. W. Slot, University Utrecht, Utrecht, The Netherlands) for 45 min, washed with PB, fixed in 1% glutaraldehyde for 5 min, rinsed in H2O, and prestained with 2% neutral uranyl acetate. The sections were stained in a mixture of 2% methyl cellulose with 3% aqueous uranyl and examined with a Philips Tecnai 12 electron microscope operating at 80 Kv.
Two-hybrid assay.
The ΤΙP47 open reading frame (ORF) (12), the full-length cytoplasmic domain of TMgp41, and the deleted fragments of TMgp41 (aa 707 to 768, 751 to 856, and 786 to 824) were obtained by PCR and fused to the Gal4 activator domain and to the LexA binding domain (BD), respectively, in pGAD and pLex vectors. Y802W803-AA-mutated pLex-TMgp41786-824 was generated by PCR-directed mutagenesis with the appropriate primers. The yeast reporter strains L40 containing the HIS3 and β-galactosidase (β-Gal) LexA-inducible genes were cotransformed and plated on selective medium lacking tryptophan and leucine (3). L40 double transformants were patched on the same media and then analyzed for histidine auxotrophy by replica plating on selective medium lacking tryptophan, leucine, and histidine (−His) and for qualitative and quantitative β-Gal (35).
TIP47 binding assays.
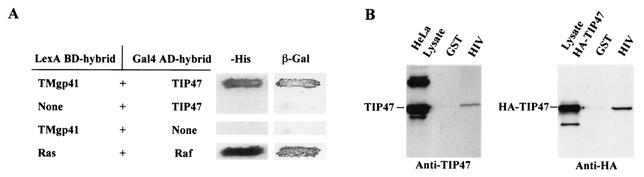
HA-TIP47 was obtained following direct subcloning of the TIP47 ORF in pAS1B vector (35). HeLa cells were transfected by electroporation with 10 μg of pAS1B-TIP47. Forty-eight hours later, HA-TIP47-transfected cells or untransfected HeLa cells were lysed in 50 mM Tris, 150 mM NaCl, 5 mM EDTA, and 1% Triton X-100 (pH 8). Lysates corresponding to 5 × 106 cells were incubated overnight at 4°C with either 5 μg of purified glutathione S-transferase (GST), wt GST-HIV (3), GST-HIV-Y802W803-AA, or GST-HIV-S804QELKNS810-A804/810 protein. The beads were then washed 6 times with 50 mM Tris, 300 mM NaCl, and 5 mM EDTA (pH 8). Bound cellular proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and revealed by Western blotting with anti-HA or anti-TIP47 antibody.
TIP47 transdominant assay.
Mutant HA-TIP47Ala167AlaAla was obtained following direct subcloning of the TIP47Ala167AlaAla (10) in pAS1B vector. Plasmids encoding HA-TIP47 or HA-TIP47Ala167AlaAla were cotransfected with pcEnvwt and pSRα-STSVG vectors with FuGENE 6 reagent according to the manufacturer's instructions. HeLa cells grown on coverslips and transiently transfected 2 days earlier were washed with PBS containing 0.2% BSA and incubated with anti-Env b12 MAb for 1 h at 0°C. The cells were washed and returned to 37°C for the desired amount of time, after which they were fixed directly and processed for immunofluorescence.
Quantitative syncytium formation assay.
The vector used for the quantitative assay of syncytium formation of the wt HIV-1 Env contained the ORF of the HIV-1 HXB2 Env and those of HIV-1 Tat and Rev proteins driven by the cytomegalovirus promoter (pcEnv-wt). pcEnv-Y802W803-SL was obtained by site-directed mutagenesis of the pcEnv-wt vector with the QuikChange system (Stratagene) according to the manufacturer's instructions. The following primers were used: Y802W803-SL, 5′-GGAATCTCCTACAGTCCTTGAGTCAGGAACTAAAG-3′ and 5′-CTTTAGTTCCTGACTCAAGGACTGTAGGAGATTCC-3′.The introduced mutations respected the ORF and the amino acid sequence of Rev protein. Mutations were confirmed by DNA sequencing.
The P4 HeLa-derived cell line, obtained from M. Alizon, is susceptible to fusion induced by the HIV-1 HXB2 Env protein. Both cell lines contain the β-Gal reporter gene under the control of the HIV-1 long terminal repeat. 293T cells (1 × 105) were plated in six-well plates and transfected with 3 μg of Env expression vectors by the calcium phosphate method. These vectors express the Env glycoproteins as well as Tat and Rev. HeLa-P4 reporter cells (2 × 105) were added to each well 24 h later. The presence of viral Env on the surface of transfected cells caused the formation of syncytia with indicator cells, allowing Tat-dependent synthesis of β-Gal. Coculture was continued for 48 h, after which the cells were fixed and stained for β-Gal activity (11). Syncytia containing three or more blue-stained nuclei were counted. The syncytium formation index is the number of syncytia obtained with the mutated glycoproteins divided by that obtained with the wt.
Flow cytometry.
The Env and CD8-HIV hybrid at the cell surface was monitored by flow cytometry as described previously (3). Env labeling at the cell surface was performed by using anti-Env 110H MAb. The overall synthesis of Env in transfected cells was monitored by Western blotting with anti-Env 110H MAb.
Viral replication kinetics.
To generate Y802W803-SL mutant HXB2 proviral clones, the NheI-XhoI fragment (nucleotides 4520 to 6158) from the pcEnv-Y802W803-SL construct was cloned into HXB2 digested by NheI and XhoI. Mutation was verified by sequencing. Viral stocks of wt or Y802W803-SL mutant HXB2 HIV-1 were obtained by transfecting 293T cells (1.5 × 106) with 20 μg of corresponding HXB2 proviral DNA by using the calcium phosphate procedure. Forty-eight hours later, transfected cell supernatants were harvested, filtered through 0.22-μm-pore-size filters, quantified for HIV-1 p24 antigen by using a Coulter HIV-1 p24 antigen assay (Beckman Coulter), and used in infections as described below. Virus-containing supernatants (50 ng of p24) were used to infect 106 Jurkat cells for 2 h. Cells were washed and cultured for 23 days. Every 2 or 3 days, supernatant samples were collected and stored at −80°C, and cells were counted and split to maintain the same number of cells for all viruses (106 cells/ml). All collected supernatants were analyzed for p24 quantity.
Env incorporation assay.
To generate the vesicular stomatitis virus G protein (VSV-G) pseudotype, 293T cells (1.5 × 106) were transfected with 10 μg of VSV-G expression vector and either 10 μg of wt or Y802W803-SL mutant HXB2 HIV-1 provirus by using the calcium phosphate procedure. Forty-eight hours later, supernatants of transfected cells were harvested, filtered through 0.45-μm-pore-size filters, quantified for HIV-1 p24 antigen by using a Coulter HIV-1 p24 antigen assay, normalized, and used in infections as described below.
Jurkat cells (15 × 106) were infected overnight with 3 ml of VSV-G-pseudotyped HIV-1. Cells were washed three times with PBS buffer and cultured for 2 days in 15 ml of RPMI medium. To block subsequent spread of virions bearing the HIV-1 env glycoprotein following VSV-G-mediated infection, the CXCR4 inhibitor AMD3100 (100 ng/ml) was added to the infected cultures. Supernatants were collected, filtered through 0.45-μm-pore-size filters, and ultracentrifuged at 90,000 rpm for 30 min in the rotor TLA 100.3. The pellet were resuspended in 80 μl of Laemmli buffer. Cell and virions lysates were subjected to SDS-PAGE and transferred to polyvinylidene difluoride membranes (Amersham). Membranes were incubated with either the anti-SUgp120 110H, or the anti-TMgp41 41A or anti-p24 25A antibody (Hybridolab). Subsequently, membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Dako), and the antibody-bound proteins were detected with an ECL+ kit (Amersham).
RESULTS
The HIV-1 Env glycoprotein is in the TGN at steady state.
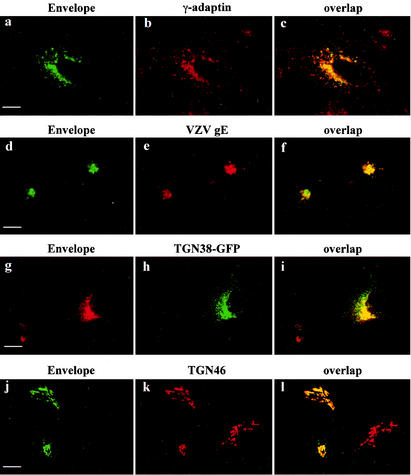
It was shown previously that the HIV-1 Env glycoprotein interacts with AP-1 and AP-2 adaptor complexes (3, 37) and is present in a perinuclear region of the cell. To further analyze the intracellular distribution and trafficking of HIV-1 Env, the protein was transiently expressed in HeLa cells and its intracellular distribution was compared with that of γ-adaptin, the large specific subunit of AP-1 adaptor complexes that is present on TGN. We also coexpressed the HIV-1 Env with VZV gE, TGN38 fused to GFP, or ST, three markers of the TGN (1, 16). Cells were analyzed 48 h after transfection and treated with cycloheximide (50 μg/ml) for 3 h to eliminate the newly synthesized Env from the early secretory pathway.
Env staining was concentrated in a perinuclear region, with some faint staining in intracellular vesicles scattered throughout the cytoplasm (Fig. 1a, d, g, and j). Env was rarely detected at the cell surface, although there was some labeling at the surface of the extensions of the transfected cell. Laser confocal immunofluorescence studies showed that Env colocalized with γ-adaptin in the perinuclear region (Fig. 1c). Colocalization in perinuclear area was also observed with the VZV gE (Fig. 1f), TGN38-GFP (Fig. 1i), and ST (not shown). Moreover, in HeLa P4-2 cells infected by HIV-1 virus, Env staining in the perinuclear region overlapped the labeling of TGN46, an endogenous marker of the TGN (Fig. 1j to l). Thus, HIV-1-infected cells and Env-transfected HeLa cells revealed that Env is mainly concentrated in a perinuclear region, in a compartment that is very like that of TGN, as judged by our simultaneous labeling.
FIG. 1.
Localization of HIV-1 Env glycoprotein by confocal microscopy. HeLa cells were transfected with pcEnv-wt (a to c) or cotransfected with pcEnv-wt and pSFFI-gE (d to f), or pcEnv-wt and ΔpMEP4-TGN38EGFP (g to i). P4-2 HeLa cells were infected with HXB2 HIV-1 (j to l). The transfected and infected cells were fixed and processed for immunofluorescence with the b12 anti-Env MAb (a, d, g, and j) and either the anti-γ-adaptin MAb (b) or antibodies against VZV gE (e) or TGN46 (k). Cells were treated with 50 μg of cycloheximide/ml for 3 h before immunofluorescence. Colocalization was examined by confocal microscopy. A series of optical sections at 0.5-μm intervals was recorded. A representative medial section is shown. Scale bar, 20 μm.
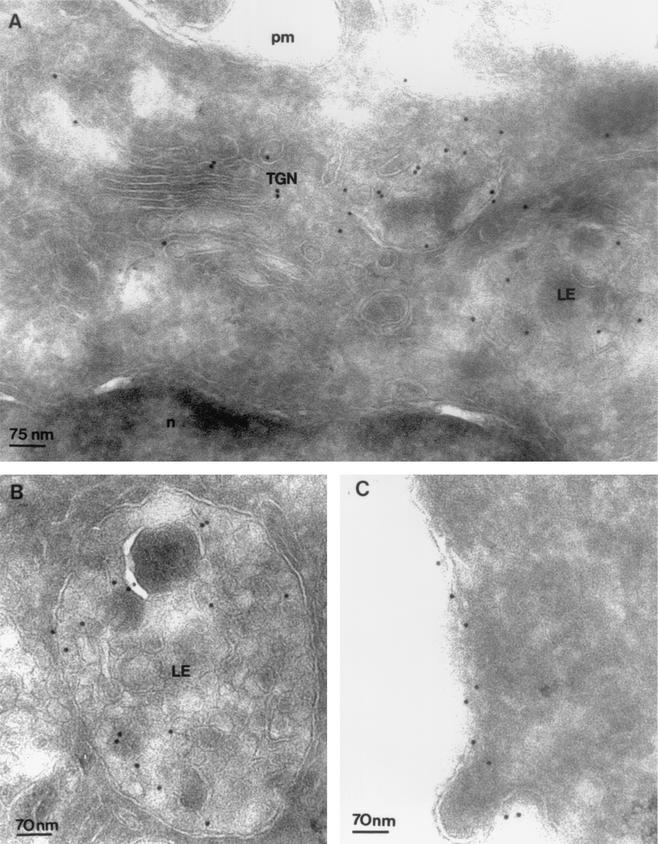
Electron microscopy-immunogold labeling of Env confirmed and extended these results. Ultrathin cryosections of HeLa cells expressing Env were labeled with anti-Env 2G12 MAb (8). At the steady state, labeling of 2G12 was observed on the Golgi cisternae and tubulo-vesicular structures of TGN (Fig. 2A). 2G12 was also localized on multivesicular LE (Fig. 2B). Futhermore, 2G12 MAb reveals labeling of the plasma membrane in some transfected cells (Fig. 2C). Other intracellular organelles remained unlabeled, confirming the specificity of Env labeling (Fig. 2A).
FIG. 2.
Localization of Env by immunoelectron microscopy. Ultrathin cryosections of HeLa cells expressing Env immunogold-labeled with anti-Env 2G12 (10-nm-diameter gold particles). (A) At the steady state, Env was detected in the Golgi cisternae and tubulo-vesicular structures of the TGN (TGN). n, nucleus. On the right, a multivesicular LE was labeled with 2G12 MAb. (B) Multivesicular LE labeled with 2G12 MAb. (C) At the steady state, immunogold labeling of Env appears scattered along the plasma membrane (pm).
The cytoplasmic domain of TMgp41 governs TGN distribution.
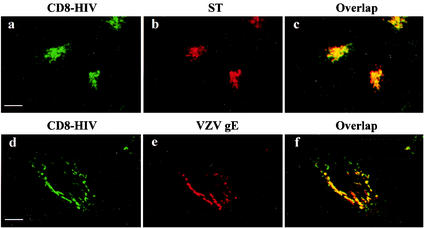
We constructed a chimeric molecule composed of the cytoplasmic domain of TMgp41, fused to the extracellular and transmembrane domains of the CD8-α chain antigen (pCD8-HIV) (3), to determine whether this cytoplasmic domain contains the sorting information required to direct Env to the TGN. The CD8-HIV chimera was compared with a control construct, the CD8-stop chimera, lacking a cytoplasmic domain, after expression in HeLa cells. The CD8-stop chimera was found only at the cell surface (3). In contrast, fusion with the TMgp41 cytoplasmic domain led to the appearance of the chimera in a perinuclear compartment as well as in some vesicles dispersed throughout the cytoplasm (Fig. 3a).
FIG. 3.
Localization of CD8-HIV chimeric molecules by confocal microscopy. HeLa cells were cotransfected with pCD8-HIV with either pSRα-STVSVG (a to c) or pSFFI-gE (d to f). The transfected cells were fixed and processed for immunofluorescence with the CD8-Leu2A MAb (a and d) and antibodies against ST (b) or VZV gE (e). Before fixation, all the cells were treated with 50 μg of cycloheximide/ml for 3 h. Colocalization was examined by confocal microscopy as described in the legend to Fig. 1. Scale bar, 20 μm.
The CD8-HIV chimera staining could be superimposed onto that obtained for γ-adaptin in the perinuclear area (data not shown). HeLa cells were also cotransfected with CD8-HIV chimera and vectors expressing Golgi apparatus markers such as ST (Fig. 3b), VZV gE (Fig. 3e), or TGN38-GFP (data not shown). The CD8 staining was found mostly in the perinuclear region, where it overlapped the labeling of ST (Fig. 3c) or VZV gE (Fig. 3f). A part of CD8-HIV chimera was also in vesicles dispersed in cytoplasm where ST and VZV gE are not. These results corroborated those obtained with the full-length Env and support the view that the cytoplasmic domain of HIV-1 Env is sufficient to direct it to the TGN.
Cycling of Env between the TGN and the cell surface.
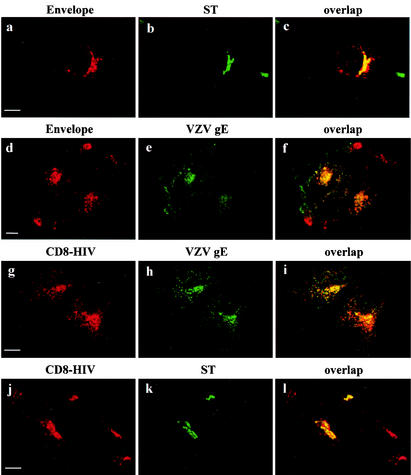
Furin, TGN38, and gE cycle between the TGN and the cell surface (1, 7, 26). Internalization and uptake assays were performed to determine whether the location of Env results from its cell surface-to-TGN transport after internalization. HeLa cells expressing Env and ST proteins were labeled for 1 h with anti-Env antibodies at 4°C and subjected to internalization for 1 h at 37°C. Env staining after internalization was concentrated around the nucleus (Fig. 4a). This perinuclear labeling overlapped with the bulk of ST labeling (Fig. 4b and c). We confirmed this by carrying out uptake experiments on Env- and gE-transfected HeLa cells. Transfected cells were incubated with anti-gE and anti-Env antibodies at 37°C for 1 h. We observed a strong perinuclear signal, together with some vesicular staining throughout the cytosol, for Env (Fig. 4d) and gE staining (Fig. 4e). Distribution of the two glycoproteins overlapped intracellularly (Fig. 4f). But they did not colocalize at the surface of the transfected cell: gE was restricted to some area of the cell surface, whereas Env was absent. These results suggest that some of the Env that gets to the cell surface returns to the TGN after internalization, like gE.
FIG. 4.
Internalization of HIV-1 Env glycoprotein and CD8-HIV chimeric molecules. HeLa cells expressing either Env and ST (a and b), CD8-HIV chimera and VZV gE (g and h), or CD8-HIV chimera and ST (j and k) were incubated at 0°C with the b12 anti-Env (a) or Leu2A anti-CD8 (g and j) MAbs for 1 h and then returned to 37°C for 1 h. The cells were fixed, permeabilized, and labeled with antibodies against either ST (b and k) or VZV gE (h). For uptake experiments (d), HeLa cells expressing Env and VZV gE (d to f) were incubated for 1 h at 37°C with the b12 anti-Env (d) and anti-VZV gE (e) antibodies. The cells were fixed, permeabilized, and stained with the appropriate secondary antibodies (d to f). Colocalization was examined by confocal microscopy as described in the legend to Fig. 1. Scale bar, 20 μm.
Similar internalization assays were performed on HeLa cells transfected with the CD8-HIV chimera. The bulk of the internalized CD8-HIV chimera was present in the perinuclear region (Fig. 4g and j) and colocalized with gE (Fig. 4h and i) and ST (Fig. 4k and l). These results show that the cytoplasmic domain of TMgp41 is able to mediate the return of Env to the TGN.
Env binds to TIP47.
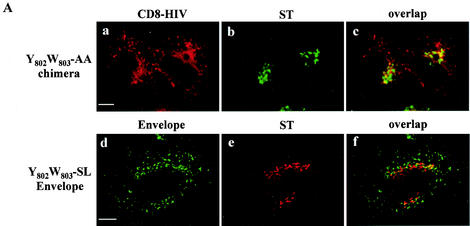
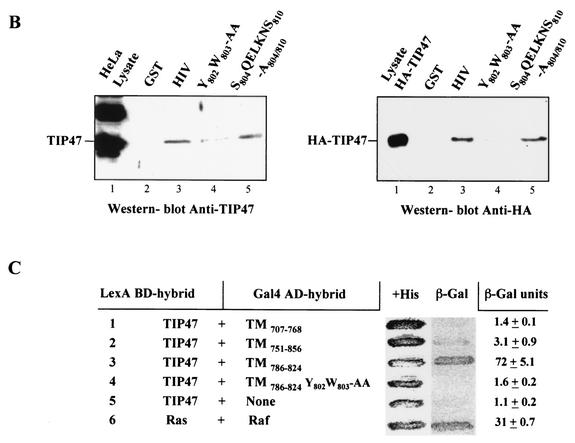
Recently, the protein TIP47 has been implicated in the retrograde transport of MPRs (12). We used a two-hybrid system to reveal interactions between Env and TIP47 molecules. The cytoplasmic domain of TMgp41 was fused to the LexA BD, and interaction with a hybrid of TIP47 with the Gal4 activator domain was assayed by using the L40 reporter strain. The cytosolic tail of TMgp41 bound efficiently to TIP47, as indicated by expression of the reporter genes β-Gal and HIS3 (Fig. 5A). This interaction was confirmed by using the TIP47 binding assay. Endogenous TIP47 and HA-TIP47 bound to the cytosolic tail of TMgp41 fused to GST (GST-HIV) but not to GST (Fig. 5B). Independent binding experiments revealed that the gp41 cytoplasmic domain bound to TIP47 with a Kd of 1 μM (J. Krise and S. Pfeffer, personal communication), comparable to the affinity of TIP47 for the MPR cytoplasmic domain. Altogether, these data demonstrate that TIP47 binds to the HIV gp41 cytoplasmic domain.
FIG. 5.
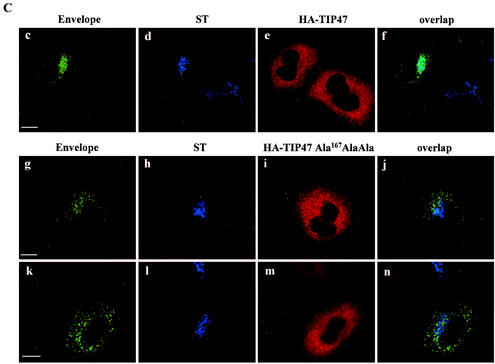
Binding of the cytosolic tail of Env to TIP47 (A) Interaction of the cytosolic tail of Env with TIP47 in yeast two-hybrid assay. The yeast reporter strain L40 expressing the indicated hybrid protein pairs was analyzed for histidine auxotrophy (−His) and β-Gal expression. The full-length cytosolic tail of TMgp41 was fused to the LexA BD. The TIP47 ORF was fused to the Gal4 activation domain (Gal4AD). Ras and Raf proteins, which bind to each other efficiently, were used as positive controls. (B) Binding of the cytosolic tail of Env to TIP47 in HeLa cell lysate binding assay. HeLa cell lysates expressing endogenous TIP47 (left panel) or HA-TIP47 (right panel) were incubated with equal amounts of purified GST or GST fused to the cytoplasmic tail of HIV-1 TMgp41 (GST-HIV). Bound materials were analyzed by SDS-PAGE, and TIP47 binding was analyzed by Western blotting with either anti-TIP47 (left panel) or anti-HA MAb (right panel). Crude lysates corresponding to 2 × 105 cells were also run as a control to detect endogenous TIP47 (lane HeLa lysate in left panel) or HA-TIP47 (lane lysate HA-TIP47 in right panel). (C) Transdominant effect of TIP47Ala167AlaAla on Env retrograde transport. HeLa cells expressing Env, ST, and HA-TIP47 (upper panel) or HA-TIP47Ala167AlaAla mutant (lower panels) were incubated at 0°C with the b12 anti-Env MAb for 1 h and then returned to 37°C for 1 h. The cells were fixed, permeabilized, and labeled with antibodies directed against ST and the HA epitope. Colocalization was examined by confocal microscopy as described in the legend to Fig. 1. Overlaps between Env and ST labeling were presented in panels f, j, and n. Scale bar, 20 μm.
TIP47 and retrograde transport of Env to the TGN.
TIP47 function required TIP47's capacity to bind Rab9 GTPase efficiently. When TIP47 residues Ser167ValVal were mutated to Ala167AlaAla, the ability of TIP47 to bind Rab9 was decreased. Expression of this mutant TIP47 inhibited MPR retrograde transport to the TGN (10). Env internalization assays in the presence of HA-TIP47 and of the HA-TIP47Ala167AlaAla mutant were performed to determine the implication of TIP47 in Env retrograde transport to the TGN. Expression of HA-TIP47 did not alter the retrograde transport of Env to the TGN (Fig. 5C, panels c to f). Env staining after internalization was concentrated around the nucleus. This perinuclear labeling overlapped with the bulk of ST labeling. By contrast, in cells transfected with the HA-TIP47Ala167AlaAla mutant (Fig. 5C, panels g to n), most of the Env labeling was found in vesicular structures dispersed throughout the cytoplasm and colocalization of Env with ST was lost. Altogether, these results suggest that TIP47 through this binding to the cytosolic domain of TMgp41 is implicated in retrograde transport of Env to the TGN.
Amino acid residues 802 to 809 control the localization and retrograde transport of Env to the TGN.
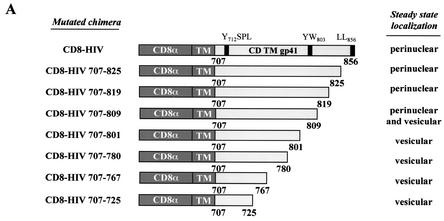
We constructed 7 CD8-HIV proteins with truncated Env cytoplasmic domains to determine the sorting determinants involved in TGN localization of Env. The steady-state distributions and retrograde transport of these mutants were analyzed by immunofluorescence (Fig. 6). The mutant proteins lacking 31 aa (CD8-HIV707-825) or 37 aa (CD8-HIV707-819) at the C terminus of TMgp41 were present in the perinuclear area, similarly to the wt CD8 chimera (Fig. 6A). However, removal of the last 47 aa (CD8-HIV707-809) resulted in a mixed distribution between the perinuclear area and some vesicles in the cytoplasm. The most dramatic effect was observed when 55 (HIV707-801), 76 (HIV707-780), 89 (HIV707-767), or 131 (HIV707-725) aa were removed from the cytoplasmic domain. All these mutants were found in vesicles scattered throughout the cytoplasm (Fig. 6A). These findings suggest that, downstream of the proximal tyrosine-based motif, there are other signals within the cytoplasmic tail of Env that are necessary for the proper trafficking of this glycoprotein.
FIG. 6.
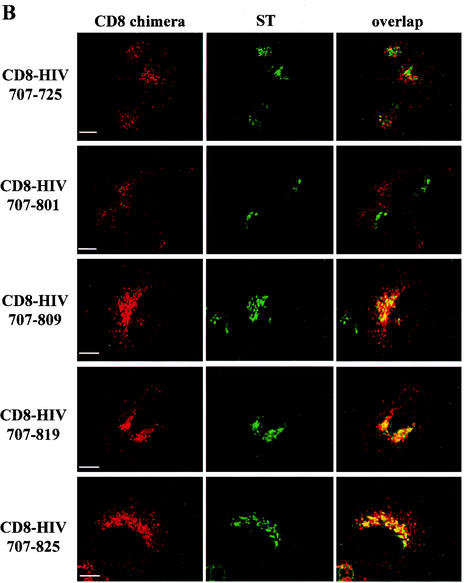
Representation of the cytoplasmic tail of wt HIV-1 Env and of the mutants used in this study. (A) The external domain and the anchor peptide (TM) of the human CD8α chain antigen are indicated. Numbering corresponds to the position of the beginning and end of the HIV-1 TMgp41 cytoplasmic domain (CD TMgp41). The steady-state locations of the different mutants are judged by immunofluorescence analysis. (B) Effect of deletion of the TMgp41 cytoplasmic domain on return of the CD8-HIV chimeric molecule to the TGN. HeLa cells were transfected with pSRα-STVSVG and either pCD8-HIV707-725, pCD8-HIV707-801, pCD8-HIV707-809, pCD8-HIV707-819, or pCD8-HIV 707-825 vector. The transfected cells were incubated at 0°C with the CD8-Leu2A MAb for 1 h and then returned to 37°C for 1 h. The cells were fixed, permeabilized, and labeled with anti-ST antibodies. Colocalization was examined by confocal microscopy as described in the legend to Fig. 1. Scale bar, 20 μm.
We revealed that residues 802 to 809 were important for the retrograde transport of Env from the cell surface to the TGN by using internalization assays with truncated CD8-HIV chimera. Deletion of the last 31, 37, or 47 aa of TMgp41 did not affect the return of the chimera to the TGN, since the corresponding constructs were present in TGN, as illustrated by the colocalization with ST after internalization, and in endosomal compartments (pCD8-HIV707-825, pCD8-HIV707-819, and pCD8-HIV707-809) (Fig. 6B). In contrast, removal of the last 55 or 131 residues caused these chimeras to be located in punctate vesicles scattered throughout the cytoplasm with a loss of the colocalization with the ST marker (pCD8-HIV707-801 and pCD8-HIV707-725) (Fig. 6B). These results show that the last 51 aa of the TMgp41 cytoplasmic tail are essential for the steady-state localization and retrograde transport of Env to the TGN.
Retrograde transport of Env to the TGN requires a Y802W803 diaromatic motif.
We used site-directed mutagenesis to investigate the role of the residues between positions 802 and 809 in the Env. The sequence 802YWSQELKN809 contains two aromatic residues at positions 802 and 803 that are conserved in the different classes of HIV-1 (see Fig. 9C). We constructed a double point mutant in which the Y802 and the W803 residues were mutated to alanine. The HIV-1 Env S804QELKNS810 region was also changed to a stretch of alanine residues (pCD8-HIV-A804/810). We measured the return of the mutated CD8 chimera to the TGN by using an internalization assay. Mutation of the region S804QELKNS810 did not alter the distribution of the CD8 chimera (data not shown). In contrast, colocalization between ST and the wt CD8 chimera was diminished by mutation of the Y802W803 residues (compare Fig. 7A, panels a to c, to 4, panels j to l). CD8 staining in the perinuclear area of cells transfected with YW-mutated CD8 chimera was significantly reduced. These results suggest that the Y802W803 residues are important for the retrograde transport of the Env to the TGN.
FIG. 9.
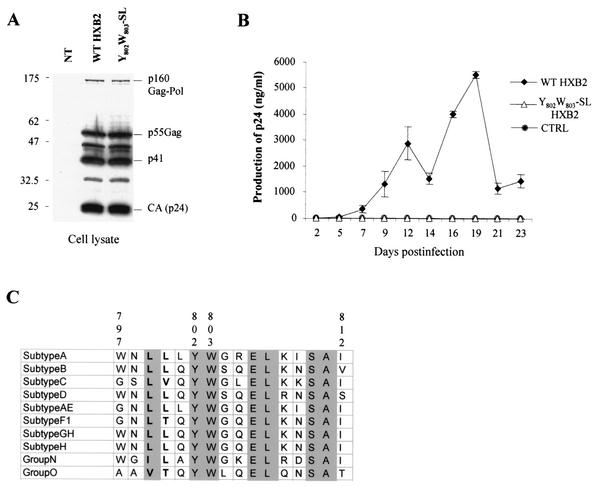
Replication in Jurkat T cells of wt and Y802W803-SL mutant HIV-1 virus. (A) Expression of Gag (p160Gag-Pol, p41, p55Gag, or CAp24) proteins of wt and Y802W803-SL mutant HXB2 HIV-1 provirus in transfected 293T cells was verified by Western blot analysis with anti-p24 antibodies. NT, untransfected cells. (B) Replication kinetics. The Jurkat T-cell line was infected in parallel with a low inoculum of wt and Y802W803-SL mutant HXB2 HIV-1 (50 ng of p24/106 cells). Cells were split every 2 or 3 days; culture supernatant samples were collected at each point for p24 antigen quantification. Values are the averages of 3 independent experiments ± standard deviations. CTRL, noninfected cells. (C) Sequence comparison for residues 797 to 812 of the Env cytosolic domain of the different HIV-1 subgroups. The amino acid sequences of representative isolates of different clades (21) (A, U455; B, HXB2R; C, ETH2220; D, ELI; AE, 92TH011A; F1, 93BR020.1; GH, AG-VI525A2; H, 90CR056; O, ANT70) of the HIV-1 groups M, N, and O were aligned. YW is shown in boldface.
FIG.7.
Effect of mutations in the Y802W803 aromatic doublet on internalization of CD8-HIV chimera and Env glycoprotein. (A) HeLa cells expressing either mutated chimera CD8-HIV Y802W803-AA and ST (a to c) or Y802W803-SL-mutated Env and ST (d to f) were incubated at 0°C with the anti-CD8 Leu2A (a) or the b12 anti-Env (d) MAbs for 1 h and then returned to 37°C for 1 h. The cells were fixed, permeabilized, and labeled with anti-ST antibodies (b and e). Colocalization was examined by confocal microscopy (f). (B) Binding of the mutated cytosolic tail of Env to TIP47. HeLa cell lysates expressing endogenous TIP47 (left panel) or HA-TIP47 (right panel) were incubated with equal amounts of purified GST, GST-HIV, Y802W803-AA, and S804QELKNS810-A804/810 mutated GST-HIV. Bound materials were separated by SDS-PAGE, and TIP47 binding was analyzed by Western blotting with anti-TIP47 (left panel) or with anti-HA MAb (right panel). Crude lysates corresponding to 2 × 105 cells were also run as a control to detect TIP47 or HA-TIP47. (C) Analysis of the ability of the TMgp41 deletion mutant to interact with TIP47 in the two-hybrid assay. The yeast reporter strain L40 expressing the indicated hybrid protein pairs was analyzed for β-Gal expression. The TMgp41 deletion mutants indicated were fused to the Gal4 activation domain (Gal4AD). The TIP47 ORF was fused to the LexA BD. Ras and Raf proteins, which bind to each other efficiently, were used as positive controls.
The results were confirmed by mutation of the Y802W803 motif in the vector encoding the full-length Env (pcEnv-wt). Tyrosine (Y802) and tryptophan (W803) residues were changed to serine and leucine residues to conserve the amino acid sequence of the overlapping HIV-1 Rev protein. HeLa cells were transfected with this construct and analyzed as above. Like the YW-mutated chimera, the Y802W803-SL-mutated Env did not colocalize with ST after internalization (Fig. 7A, panels d to f). Cells transfected with this construct showed no perinuclear staining for Env, and most of the labeling was found in vesicular structures dispersed throughout the cytoplasm (compare Fig. 7A, panels d to f, to 4, panels a to c). Thus, these results indicate that the YW diaromatic motif is an important determinant within the cytoplasmic tail of TMgp41 that controls the retrograde transport of Env to the TGN.
The role of the YW motif in the binding of TIP47 to Env was assessed by introducing a mutated Y802W803-AA motif into a full-length HIV-1 cytoplasmic domain fused to GST. The Y802W803-AA mutation abolished or strongly diminished the binding to HA-TIP47 or to endogenous TIP47 (Fig. 7B, lane 4) whereas the change of S804QELKNS810 region to a stretch of alanine residues (Fig. 7B, lane 5) did not alter the Env-TIP47 interaction. The impact of Y802W803-AA mutation on the Env-TIP47 interaction was also evaluated in a two-hybrid system. Whereas TIP47 interacted efficiently with the residues 786 to 824 of Env cytoplasmic domain, the Y802W803-AA mutation abolished the binding of TIP47 to this region of Env (Fig. 7C). Altogether, these data suggest that TIP47 binds to Env via the YW motif and thereby facilitates the retrograde transport to the TGN.
Cell surface expression of mutated HIV-1 Env glycoproteins.
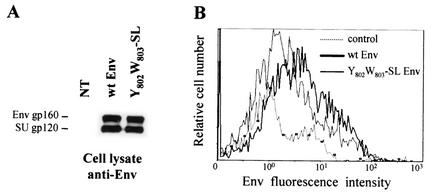
The effect of Y802W803-SL mutation on the level of HIV-1 Env protein present at the cell surface was analyzed by using flow cytometry. We checked that wt and mutated Env were expressed to similar degrees by Western blot analysis with an anti-Env MAb (Fig. 8A). Replacement of the Y802 and W803 residues by serine and leucine residues significantly diminished the percentage of GFP+ Env+ cells (wt, 34.2 ± 1.2 GFP+ Env+ cells; Y802W803-SL, 24.4 ± 2.2 GFP+ Env+ cells) as well as the mean Env fluorescence intensity of these cells (Fig. 8B). This decrease was also observed in the context of CD8 chimera (not shown).
FIG. 8.
Expression of Env mutated in Y802W803 at the cell surface. (A) Identical quantities of lysates from transfected cells were analyzed by Western blotting with anti-Env MAb. NT, untransfected control cells. (B) 293T cells (2 × 106) were cotransfected by the calcium phosphate method with 8 μg of wt (thick line) or Y802W803-SL (thin line) Env expression vector and 0.5 μg of pRSV-GFP vector. The amount of Env at the surface of GFP+ cells was analyzed by flow cytometry 48 h later. Dotted line, cells transfected with the pRSV-GFP vector alone, as the negative control (ctrl). Data are representative of three independent experiments.
To confirm this decrease of YW-mutated Env cell surface expression, we used a functional quantitative syncytium-forming assay. This assay measures Env at the surface of transfected cells as it causes these cells to form syncytia with CD4+ indicator cells (HeLa P4-2). In our conditions, 719 ± 49 syncytia were formed per well with the wt HIV-1 Env (pEnv-wt) (Table 1). The Y802W803-SL Env mutant caused a 24% decrease in syncytium formation with respect to the wt HIV Env. By contrast, the HIV-1 Env with the Y712-A mutation in the well-defined tyrosine-based signal resulted in a 59% increase in the number of syncytia (1,149 ± 74 syncytia). These results are in agreement with the results of flow cytometry and indicate that less Env was present at the cell surface and available for fusion.
TABLE 1.
Effect of Y802W803 substitution on the fusogenic capacity of HIV-1 Env glycoprotein
| Mutant | No. of syncytia/well (293T/HeLa P4 cocultivation)a | % of wtb | Pc |
|---|---|---|---|
| NTd | 0 | NDe | ND |
| pcEnv-wt | 719 ± 49 | 100 | ND |
| pcEnv-Y802W803-SL | 546 ± 88 | 76 | <0.02 |
| pcEnv-Y712-A | 1,149 ± 74 | 159 | <0.001 |
Values shown are the numbers of syncytia obtained per well and are the averages of three independent experiments performed in triplicate.
Data are numbers of syncytia divided by the number obtained with wt pEnv-wt (719 ± 49 syncytia/well).
The results for the mutant Env glycoproteins were compared to those for the wt by means of the t test for unpaired samples.
NT, cells transfected with the pcDNA3 vector alone.
ND, not determined.
The Y802W803-SL mutation in the cytoplasmic domain of TMgp41 impairs HIV-1 replication.
We examined whether the Y802W803-SL change in the cytoplasmic domain of TMgp41, introduced in the HIV-1 HXB2 virus, had any effect on the HIV-1 replication in a 23-day replication kinetic. We generated wt and Y802W803-SL mutant HIV-1 viral stocks by transfecting 293T cells with 20 μg of corresponding HXB2 proviral DNA. Expression of wt and Y802W803-SL mutant HXB2 HIV-1 provirus in 293T cells was verified by Western blot analysis by using anti-p24 antibodies (Fig. 9A). The same level of Gag-Pol and Env products was observed. The replication kinetics of these two viruses in Jurkat T cells were compared by measuring the level of HIV p24 antigen in cell culture supernatant at 23 days. HIV-1 wt virus established a productive infection in Jurkat cells (Fig. 9B). On the other hand, the Y802W803-SL mutant HIV-1 failed to replicate in Jurkat cells since the p24 production was reduced to a very low level and no peak of HIV production was observed compared to the wt virus (Fig. 9B). These results indicate that the Y802W803 motif not only is required for Env retrograde transport but, more importantly, also plays an essential role in optimal HIV-1 production.
The Y802W803-SL mutation in the cytoplasmic domain of TMgp41 impairs HIV-1 Env incorporation into virions.
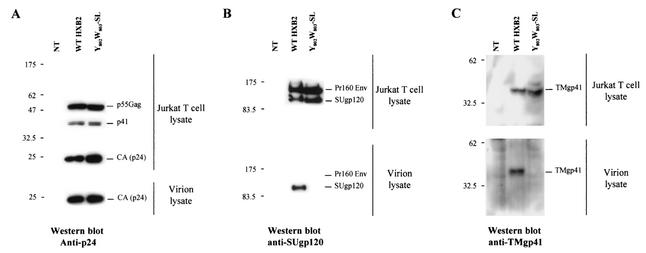
We determined the effect of Y802W803-SL mutation on Env incorporation in T-cell lines. We utilized a high-level, transient HIV-1 expression system based on pseudotyping with VSV-G (27). 293T cells were cotransfected with a VSV-G expression vector and either wt HXB2 or a derivative expressing the TMgp41 Y802W803-SL mutant. VSV-G-pseudotyped virus stocks were harvested and used to infected Jurkat T cells. Two days postinfection, cells were harvested and virions were pelleted by ultracentrifugation. The expression of wt and Y802W803-SL mutant HXB2 HIV-1 proteins in productive Jurkat T cells was verified by Western blot analysis with anti-p24 and anti-SUgp120 antibodies (Fig. 10). The same level of expression and processing of Gag (Fig. 10A) and Env products (Fig. 10B and C) was observed intracellularly in Jurkat T cells. Analysis of Env and Gag protein content of T-cell-produced virion in supernatants of infected cells showed wt levels of Gag products (Fig. 10A) but strong reduction in the amount of SUgp120 proteins incorporated into virions (Fig. 10B). To determine that the reduced level of gp120 present in Y802W803-SL-mutated virions was not the result of increased shedding from virions following Env incorporation, we evaluated the level of TMgp41 on wt and YW-mutated virus. Consistent with the gp120 data, Y802W803-SL-mutated virion showed a similar strong reduction of TMgp41 incorporated in virions (Fig. 10C). These results demonstrate that the altered replication kinetic of the Y802W803-SL-mutated virus is presumably due to impaired incorporation of Env in T-cell-produced virions.
FIG. 10.
Analysis of wt and Y802W803-SL mutant Env incorporation into virions. Analysis of Gag (p160Gag-Pol, p41, p55Gag, or CAp24) and Env (Pr160 env, SUgp120, or TMgp41) content was analyzed by Western blotting. Cell and virion lysates were prepared from Jurkat T cells infected with wt and Y802W803-SL virus stocks pseudotyped with VSV-G. Samples were transferred to polyvinylidene difluoride membranes and blotted with either an anti-p24 (CA) (A), an anti-SUgp120 (B), or an anti-TMgp41 (C) MAb (Materials and Methods). These results are representative of duplicate experiments. NT, untransfected cells.
DISCUSSION
We have shown that the HIV-1 Env glycoprotein is located primarily in the TGN at the steady state and arrives in that compartment after internalization from the cell surface. Mutagenesis analysis of the cytoplasmic domain indicated that the proper distribution and retrograde transport of HIV-1 Env to the TGN depends on a Y802W803 diaromatic motif located in the cytoplasmic domain of TMgp41.
Env colocalizes in a perinuclear area with multiple, well-defined TGN markers, AP-1 adaptor complexes (6), the TGN38 and TGN46 glycoproteins (7), the VZV gE (1), and with a marker of the Golgi apparatus, ST. Immunoelectron microscopy reveals Env in the Golgi cisternae and tubulo-vesicular structures of the TGN and in multivesicular LE. Internalization and antibody uptake assays showed that Env at the cell surface is able to return to the TGN via the endosomal compartment (data not shown). Other proteins, such as TGN38, VZV gE, and furin also undergo retrograde transport from the cell surface to the TGN. These proteins cycle continuously between the cell surface, endosomes, and the TGN and are found predominantly in the TGN at the steady state (1, 7, 26).
Both the TGN location and the intracellular routing of furin or gE require a sorting signal in their cytosolic domain. The furin sorting signals include canonical tyrosine-based and dileucine signals as well as an acidic cluster containing casein kinase II phosphorylation sites (18). The cytoplasmic domain of VZV gE contains an acidic cluster that is implicated in the cycling of this glycoprotein between the cell surface and the TGN (1). Our present study shows that localization and retrograde transport of Env to the TGN depend on an essential Y802W803 diaromatic motif present in the TMgp41 cytoplasmic domain. The CD MPR, which is responsible for delivering acid hydrolases to lysosomes, contains several sorting signals within its cytoplasmic tail, notably a pair of aromatic amino acids, FW. This FW signal is required for the correct sorting of the CD MPR to endosomes and prevents delivery of this receptor to lysosomes (34). Likewise, the Y802W803 motif of HIV-1 Env is required for correct endosomal sorting and targeting to the TGN. Thus, a diaromatic motif appears to be a general determinant for endosomal trafficking.
There are at least two pathways from the endosomes to the TGN. Studies on TGN38 (24) and on the endocytosed B fragment of Shiga toxin (23) indicate that these proteins reach the TGN via recycling endosomes, whereas MPRs and furin return to the TGN via another pathway involving one or two classes of LE (22, 24, 32). Antisense depletion of TIP47 in living cells (12), generation of a TIP47 mutant interfering in MPR transport, and also demonstration that overexpression of TIP47 enhances MPR retrograde transport (10) show that TIP47 is required for MPR transport from endosomes to the TGN, although TIP47 is related to a lipid droplet-associated protein (2, 36). The implication of TIP47 in retrograde transport of Env and the detection of Env in LE by immunoelectron microscopy support the view that HIV-1 Env follows the same path as MPRs to reach the TGN involving the LE. Moreover, binding of TIP47 to Env via its cytoplasmic domain could facilitate the retrograde transport and the location of Env to the TGN. Env retrograde transport is the second example of TGN retrograde transport mediated by TIP47. The interaction between Env and TIP47 underlines the importance of the TMgp41 cytoplasmic domain in HIV-1 Env trafficking, as it had been previously shown in the studies on the Env sorting signal (33) and the interaction of Env with AP-1 and AP-2 adaptor complexes (3, 5, 28, 37) and with prenylated Rab acceptor (13).
The HIV-1 Env must be present at the cell surface to be incorporated into nascent virions. The presence of an endocytic signal such as the Y712SPL tyrosine-based sorting signal could limit the amount of Env at the surface of infected cells and the susceptibility of these infected cells to the humoral response. Flow cytometry and syncytium-forming assays show that mutation of the YW motif reduces the amount of Env at the cell surface. Moreover, endocytosis assays show that the internalization rate of the Y802W803-mutated chimera is not significantly modified (not shown). Thus, these data suggest that the targeting of Env to the TGN participates, like the Env endocytosis, to the control of the level of Env at the cell surface.
Sorting and trafficking of the Env via different endosomal pathways could control where the HIV-1 Env are located, thereby ensuring optimal incorporation of the Env into infectious virions. Several studies have reported the presence of budding HIV-1 particles in a vacuolar compartment of intracytoplasmic origin in monoblastoid cells differentiated into macrophage (15, 29) or dendritic cells (4). Hence, the intracellular trafficking of Env through the endosomal compartment and the presence of Env in multivesicular LE (see immunoelectron microscopy) (Fig. 2) could allow production and accumulation of infectious HIV-1 virions in these intracytoplasmic compartments. This could enable the virus to persist in vivo. Moreover, the trafficking of Env through the endosomal and TGN compartments could be required for correct assembly of the Env with the HIV-1 Gag precursor and for the production of infectious HIV-1 virions. Mutation of Y802W803 motif drastically affected the replication of HIV-1 HXB2 virus in a 23-day replication kinetic assay in T-cell-line culture. This impairment of infectivity resulting from the Y202W203-SL mutation is not due to defects in synthesis or in processing of the Gag or Env precursors (Fig. 9 and 10). It is indeed due to the disruption of Env incorporation into virions produced from infected Jurkat T cells. Very low amounts of SUgp120 and TMgp41 Env proteins were found incorporated, although the same level of p24 was present in the Y802W803-mutated virion (Fig. 10). This impaired incorporation certainly results in a very low infectivity of the particle and thus has a strong impact on HIV-1 replication. Our results are consistent with a recent report showing that deletion of residues 802 to 806 of TMgp41, which contains the Y802W803 motif, markedly impairs virus infectivity and Env incorporation (27). Thus, the YW motif, which abolishes both TGN localization and interaction with TIP47 and decreases Env cell surface expression, is crucial for Env incorporation into virions to ensure full infectivity of viruses produced in T cells. Altogether, these data obtained with Y802W803-mutated HIV-1 support the view that retrograde transport of Env via its binding to TIP47 is important for optimization of HIV-1 replication. Lastly, the striking conservation of the diaromatic motif (21) in all HIV-1 isolates (Fig. 9C) suggests that it could be important in the pathogenesis of these viruses in vivo. Further studies are required to determine how trafficking and retrograde transport of Env via this interaction with TIP47 molecules contribute to the HIV-1 infectivity.
Acknowledgments
We thank I. Bouchaert, F. Letourneur, and N. Lebrun for technical assistance and S. Pfeffer, M. Thali, I. Dunia, B. Hoflack, J. Dubuisson, L. Johannes, G. Banting, N. Heveker, S. Lebigot, H. Katinger, the NIBSC, D. Burton, and P. Parren for kind gifts of reagents, helpful discussions, and critical reading of the manuscript. The English text was edited by O. Parkes and S. Pfeffer.
This work was supported by grants from the ANRS, FRM, SIDACTION, and Ligue Nationale Contre le Cancer.
REFERENCES
- 1.Alconada, A., U. Bauer, and B. Hoflack. 1996. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO J. 15:6096-6110. [PMC free article] [PubMed] [Google Scholar]
- 2.Barbero, P., E. Buell, S. Zulley, and S. R. Pfeffer. 2001. Tip47 is not a component of lipid droplets. J. Biol. Chem. 276:24348-24351. [DOI] [PubMed] [Google Scholar]
- 3.Berlioz-Torrent, C., B. L. Shacklett, L. Erdtmann, L. Delamarre, I. Bouchaert, P. Sonigo, M. C. Dokhelar, and R. Benarous. 1999. Interactions of the cytoplasmic domains of human and simian retroviral transmembrane proteins with components of the clathrin adaptor complexes modulate intracellular and cell surface expression of envelope glycoproteins. J. Virol. 73:1350-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blom, J., C. Nielsen, and J. M. Rhodes. 1993. An ultrastructural study of HIV-infected human dendritic cells and monocytes/macrophages. APMIS 101:672-680. [DOI] [PubMed] [Google Scholar]
- 5.Boge, M., S. Wyss, J. S. Bonifacino, and M. Thali. 1998. A membrane-proximal tyrosine-based signal mediates internalization of the HIV-1 envelope glycoprotein via interaction with the AP-2 clathrin adaptor. J. Biol. Chem. 273:15773-15778. [DOI] [PubMed] [Google Scholar]
- 6.Bonifacino, J. S., and E. C. Dell'Angelica. 1999. Molecular bases for the recognition of tyrosine-based sorting signals. J. Cell Biol. 145:923-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bos, K., C. Wraight, and K. K. Stanley. 1993. TGN38 is maintained in the trans-Golgi network by a tyrosine-containing motif in the cytoplasmic domain. EMBO J. 12:2219-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchacher, A., R. Predl, K. Strutzenberger, W. Steinfellner, A. Trkola, M. Purtscher, G. Gruber, C. Tauer, F. Steindl, A. Jungbauer, et al. 1994. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res. Hum. Retrovir. 10:359-369. [DOI] [PubMed] [Google Scholar]
- 9.Bultmann, A., W. Muranyi, B. Seed, and J. Haas. 2001. Identification of two sequences in the cytoplasmic tail of the human immunodeficiency virus type 1 envelope glycoprotein that inhibit cell surface expression. J. Virol. 75:5263-5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroll, K. S., J. Hanna, I. Simon, J. Krise, P. Barbero, and S. R. Pfeffer. 2001. Role of Rab9 GTPase in facilitating receptor recruitment by TIP47. Science 292:1373-1376. [DOI] [PubMed] [Google Scholar]
- 11.Chackerian, B., N. L. Haigwood, and J. Overbaugh. 1995. Characterization of a CD4-expressing macaque cell line that can detect virus after a single replication cycle and can be infected by diverse simian immunodeficiency virus isolates. Virology 213:386-394. [DOI] [PubMed] [Google Scholar]
- 12.Diaz, E., and S. R. Pfeffer. 1998. TIP47: a cargo selection device for mannose 6-phosphate receptor trafficking. Cell 93:433-443. [DOI] [PubMed] [Google Scholar]
- 13.Evans, D. T., K. C. Tillman, and R. C. Desrosiers. 2002. Envelope glycoprotein cytoplasmic domains from diverse lentiviruses interact with the prenylated Rab acceptor. J. Virol. 76:327-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fultz, P. N., P. J. Vance, M. J. Endres, B. Tao, J. D. Dvorin, I. C. Davis, J. D. Lifson, D. C. Montefiori, M. Marsh, M. H. Malim, and J. A. Hoxie. 2001. In vivo attenuation of simian immunodeficiency virus by disruption of a tyrosine-dependent sorting signal in the envelope glycoprotein cytoplasmic tail. J. Virol. 75:278-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gendelman, H. E., J. M. Orenstein, M. A. Martin, C. Ferrua, R. Mitra, T. Phipps, L. A. Wahl, H. C. Lane, A. S. Fauci, D. S. Burke, et al. 1988. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J. Exp. Med. 167:1428-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girotti, M., and G. Banting. 1996. TGN38-green fluorescent protein hybrid proteins expressed in stably transfected eukaryotic cells provide a tool for the real-time, in vivo study of membrane traffic pathways and suggest a possible role for rat TGN38. J. Cell Sci. 109:2915-2926. [DOI] [PubMed] [Google Scholar]
- 17.Hunter, E., and R. Swanstrom. 1990. Retrovirus envelope glycoproteins. Curr. Top. Microbiol. Immunol. 157:187-253. [DOI] [PubMed] [Google Scholar]
- 18.Jones, B. G., L. Thomas, S. S. Molloy, C. D. Thulin, M. D. Fry, K. A. Walsh, and G. Thomas. 1995. Intracellular trafficking of furin is modulated by the phosphorylation state of a casein kinase II site in its cytoplasmic tail. EMBO J. 14:5869-5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirchhausen, T., J. S. Bonifacino, and H. Riezman. 1997. Linking cargo to vesicle formation: receptor tail interactions with coat proteins. Curr. Opin. Cell Biol. 9:488-495. [DOI] [PubMed] [Google Scholar]
- 20.Krise, J. P., P. M. Sincock, J. G. Orsel, and S. R. Pfeffer. 2000. Quantitative analysis of TIP47-receptor cytoplasmic domain interactions: implications for endosome-to-trans Golgi network trafficking. J. Biol. Chem. 275:25188-25193. [DOI] [PubMed] [Google Scholar]
- 21.Kuiken, C., B. Foley, B. Hahn, P. Marx, F. McCutchan, J. Mellors, J. Mullins, S. Wolinsky, and B. Korber. 1999. Human retroviruses and AIDS, vol. 1. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, N.M.
- 22.Lombardi, D., T. Soldati, M. A. Riederer, Y. Goda, M. Zerial, and S. R. Pfeffer. 1993. Rab9 functions in transport between late endosomes and the trans Golgi network. EMBO J. 12:677-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mallard, F., C. Antony, D. Tenza, J. Salamero, B. Goud, and L. Johannes. 1998. Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of Shiga toxin B-fragment transport. J. Cell Biol. 143:973-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mallet, W. G., and F. R. Maxfield. 1999. Chimeric forms of furin and TGN38 are transported with the plasma membrane in the trans-Golgi network via distinct endosomal pathways. J. Cell Biol. 146:345-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer, C., D. Zizioli, S. Lausmann, E. L. Eskelinen, J. Hamann, P. Saftig, K. von Figura, and P. Schu. 2000. mu1A-adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J. 19:2193-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molloy, S. S., L. Thomas, J. K. VanSlyke, P. E. Stenberg, and G. Thomas. 1994. Intracellular trafficking and activation of the furin proprotein convertase: localization to the TGN and recycling from the cell surface. EMBO J. 13:18-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murakami, T., and E. O. Freed. 2000. Genetic evidence for an interaction between human immunodeficiency virus type 1 matrix and alpha-helix 2 of the gp41 cytoplasmic tail. J. Virol. 74:3548-3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohno, H., R. C. Aguilar, M. C. Fournier, S. Hennecke, P. Cosson, and J. S. Bonifacino. 1997. Interaction of endocytic signals from the HIV-1 envelope glycoprotein complex with members of the adaptor medium chain family. Virology 238:305-315. [DOI] [PubMed] [Google Scholar]
- 29.Orenstein, J. M., M. S. Meltzer, T. Phipps, and H. E. Gendelman. 1988. Cytoplasmic assembly and accumulation of human immunodeficiency virus types 1 and 2 in recombinant human colony-stimulating factor-1-treated human monocytes: an ultrastructural study. J. Virol. 62:2578-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orsel, J. G., P. M. Sincock, J. P. Krise, and S. R. Pfeffer. 2000. Recognition of the 300-kDa mannose 6-phosphate receptor cytoplasmic domain by 47-kDa tail-interacting protein. Proc. Natl. Acad. Sci. USA 97:9047-9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raposo, G., M. J. Kleijmeer, G. Posthuma, J. W. Slot, and H. J. Geuze. 1997. Immunogold labelling of ultrathin cryosections: application in immunology, p. 1-4. In I. Blakwell (ed.), Handbook of experimental immunology. Elsevier, Cambridge, Mass.
- 32.Riederer, M. A., T. Soldati, A. D. Shapiro, J. Lin, and S. R. Pfeffer. 1994. Lysosome biogenesis requires Rab9 function and receptor recycling from endosomes to the trans-Golgi network. J. Cell Biol. 125:573-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rowell, J. F., P. E. Stanhope, and R. F. Siliciano. 1995. Endocytosis of endogenously synthesized HIV-1 envelope protein. Mechanism and role in processing for association with class II MHC. J. Immunol. 155:473-488. [PubMed] [Google Scholar]
- 34.Schweizer, A., S. Kornfeld, and J. Rohrer. 1997. Proper sorting of the cation-dependent mannose 6-phosphate receptor in endosomes depends on a pair of aromatic amino acids in its cytoplasmic tail. Proc. Natl. Acad. Sci. USA 94:14471-14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selig, L., J. C. Pages, V. Tanchou, S. Preveral, C. Berlioz-Torrent, L. X. Liu, L. Erdtmann, J. Darlix, R. Benarous, and S. Benichou. 1999. Interaction with the p6 domain of the gag precursor mediates incorporation into virions of Vpr and Vpx proteins from primate lentiviruses. J. Virol. 73:592-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolins, N. E., B. Rubin, and D. L. Brasaemle. 2001. TIP47 associates with lipid droplets. J. Biol. Chem. 276:5101-5108. [DOI] [PubMed] [Google Scholar]
- 37.Wyss, S., C. Berlioz-Torrent, M. Boge, G. Blot, S. Höning, R. Benarous, and M. Thali. 2001. Highly conserved C-terminal dileucine motif in the cytosolic domain of the human immunodeficiency virus type 1 envelope glycoprotein is critical for its association with the AP-1 clathrin adapter. J. Virol. 75:2982-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]