Abstract
Neonates are severely compromised in the ability to generate an immune response to pathogens and thus rely heavily on maternally derived immunity that is acquired by transplacental and transmammary means. The passive transfer of maternal herpes simplex virus (HSV)-specific antibody is critical in determining the outcome of neonatal HSV infection. In adults, psychological stress alters immune responsiveness via the increased level of corticosterone that is produced as a result of hypothalamic-pituitary-adrenal axis activation. Although the behavioral and neuroendocrine effects of pre- and postnatal stress-induced increases in corticosterone are well documented, the effects of maternal stress on the efficacy of prenatally transferred and neonatally developed viral immunity has yet to be addressed. By using a well-established prenatal restraint-and-light stress mouse model, we investigated the effects of increased maternal corticosterone on the passive transfer of total and HSV-specific immunoglobulin G (IgG) antibody and subsequent neonatal susceptibility to HSV infection. Serum corticosterone levels in pregnant mice were significantly increased in response to restraint-and-light stress, and fetuses derived from these stressed mice had significantly elevated levels of corticosterone. Despite the increases in corticosterone, the passive transfer of total and HSV-specific IgG antibody persisted and, in turn, protected the neonate from systemic viral spread. Therefore, prenatal stress did not increase the susceptibility of neonates to HSV type 2-associated mortality. These findings demonstrate the resiliency of the passive transfer of protective HSV-specific immunity under conditions of acute psychological stress.
Neonates are severely deficient in the ability to generate an immune response to pathogens encountered both during and shortly after birth. Therefore, the ability of a neonate to resist infection is highly dependent on its capacity to acquire and utilize maternally derived immunity. In humans, this immunity is provided in part by antibody that is obtained by the fetus and neonate through both transplacental and transmammary means, respectively. This passive transfer of immunity from mothers to both fetuses and neonates protects neonates against a wide variety of pathogens, including herpes simplex virus (HSV).
It has been estimated that 1 in 3,000 to 7,000 human neonates per year is infected with HSV during vaginal delivery as a result of exposure to an HSV-infected genital tract (10). The probability of neonatal infection is a function of the type of HSV infection experienced by the mother at or near the time of delivery. For example, a primary HSV infection of the mother is associated with a 30 to 50% neonatal infection rate whereas a secondary or recurrent HSV infection is associated with only a 3% infection rate (10). This relatively low rate of occurrence of infection in neonates born to mothers with a recurrent infection suggests that high levels of pre-existing maternal HSV-specific antibody are transferred at or near the time of birth to the fetus and neonate at levels sufficient to mediate protection.
Indeed, this maternally derived protection of neonates is partially afforded by both transplacental transfer of HSV-specific immunoglobulin G (IgG) and transmammary transfer of HSV-specific secretory IgA. Such antibody confers protection by both viral neutralization and antibody-dependent, cell-mediated cytotoxicity (ADCC) activities (1, 13). Transplacental transfer of maternal IgG steadily increases throughout gestation and, in humans, is an active process that results in higher levels of IgG in the fetal compared to the maternal circulation (6). Although mice receive less maternal antibody by the transplacental route than do humans (8), previous studies with mice demonstrated that both prenatal and postnatal transmission of HSV-specific antibody protected neonates against a lethal HSV infection (8, 14).
Despite the overwhelming evidence that maternally derived antibody plays a protective role in neonatal susceptibility to infection, little is known about the factors that govern its transfer to and utilization by the fetus and neonate. One such factor of keen interest is psychological stress-induced activation of the hypothalamic-pituitary-adrenal (HPA) axis. Although the effects of psychological stress on a variety of immune functions in adult mice have been well established (reviewed in reference 3), those few studies that have sought to investigate the effects of prenatal stress on the passive transfer of immunity and subsequent immunocompetence of the offspring have been controversial. For example, administration of prenatal foot shock stress to rats and restraint of pregnant sows both resulted in a decrease in the total IgG levels in the offspring at birth (18, 19). In contrast, prenatal sound stress failed to alter the transfer of IgG in rhesus monkeys (5). However, chronic social stress did decrease the transplacental transfer of antibody (4). The latter finding likely reflects a reduction in maternal antibody transfer since neonates exhibit a lag in the ability to produce their own antibody (11) and possess antibody primarily of maternal origin. This reduction in the transfer of antibody from the mother to the offspring may be caused by psychological stress-induced activation of the maternal HPA axis and the subsequent increase in maternal serum corticosterone. While previous studies have investigated the effects of prenatal stress on the passive transfer of nonspecific immunity and the ensuing neonate response to nonpathogenic antigens, they have failed to determine the impact of stress on the transfer of virus-specific immunity. Therefore, we are the first to determine the effect of prenatal stress on the transplacental transfer of HSV-specific antibody and protection of neonates against HSV infection.
To elicit a stress response in pregnant mice, a widely used restraint-and-light stress procedure was used (9, 15, 16, 20, 22-24). This procedure has been used in numerous studies to administer psychological stress to pregnant rodents and has been shown to elevate serum corticosterone levels in both CF-1 mice (15) and rats (22). Restraint-and-light stress of pregnant C57BL/6 mice significantly elevated serum corticosterone levels and resulted in an increased transfer of corticosterone to the fetus by transplacental means. Despite the increased levels of maternal and fetal corticosterone, prenatal stress did not affect the transfer of total and HSV-specific IgG. In addition, the elevated levels of fetal corticosterone did not affect the ability of the neonate to utilize the maternally derived immunity, as there was limited viral spread and no increase in neonate mortality following HSV type 2 (HSV-2) infection.
MATERIALS AND METHODS
Mice.
Female and male C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, Maine) at 5 to 6 weeks of age and housed at five mice per cage until breeding pairs were established. Mice were maintained on a 12-h light-dark cycle (lights off from 1900 to 0700) and allowed 1 week to acclimate to these conditions prior to any experimental manipulations. Food and water were provided ad libitum, except during the restraint-and-light stress procedure. Mice were handled in accordance with American Association for Laboratory Animal Care and National Institutes of Health guidelines.
Virus.
HSV-2 strain 186 virus was propagated on Vero cells by infection at a multiplicity of infection of 0.01, and virus titers were determined by plaque assay on Vero cells. Virus stocks were stored at −70°C.
Maternal immunization and neonatal infection with HSV-2.
Similar to previous studies (8), female mice 7 to 8 weeks of age were immunized three times at 1-week intervals subcutaneously (s.c.) in both of the rear footpads with 105 (first two immunizations) and 106 (third immunization) PFU of HSV-2 in a volume of 50 μl (phosphate-buffered saline [PBS]-1% [vol/vol] fetal bovine serum [FBS]) per footpad. Two-day-old mice were infected intraperitoneally (i.p.) with 1 × 102, 5 × 102, 1 × 103, or 5 × 103 PFU of HSV-2 in a volume of 20 μl.
Mating.
One week following the final immunization, primiparous female mice at 10 to 11 weeks of age were housed singly with one male and monitored daily for the formation of a copulatory plug (day 0 of pregnancy). Male and female mice were separated on day 11 postcoitus.
Maternal restraint-and-light stress.
Individual mice were placed into a 4-ounce, wide-mouth, polypropylene bottle (Fisher Scientific, Pittsburgh, Pa.) containing approximately 90 0.5-cm-diameter holes for ventilation. The bottle was placed 30 cm from two 150-W lights three times a day for 45 min per session (0830 to 0915, 1230 to 1315, and 1630 to 1715) on days 12 to 17 postcoitus.
Serum collection.
Adult mice were sacrificed by cervical dislocation, and cardiac puncture was performed to obtain blood samples. Neonatal mice were anesthetized in a saturated atmosphere of isoflurane and decapitated, and trunk blood was collected. All samples were centrifuged at 16,000 × g for 2 min, and serum was collected and stored at −70°C.
Fetal homogenization.
Fetuses obtained by Caesarian section were blotted onto paper towels and frozen at −70°C in individual microcentrifuge tubes. Fetal tissue was homogenized in a volume of 500 μl of PBS-1% (vol/vol) FBS with a 1-ml ground-glass homogenizer. The homogenate was centrifuged at 100 × g for 3 min, transferred to a microcentrifuge tube, and centrifuged at 16,000 × g for 2 min. The supernatant was collected and stored at −70°C.
Fostering of neonates.
In a subset of studies, pregnant mice were monitored every 2 h for delivery of neonates beginning at day 18.5 of gestation. The day of delivery was designated day 0. Prior to nursing on their natural mothers, neonates were transferred to a non-HSV-immunized female who had delivered less than 24 h prior to the transfer.
Quantification of corticosterone.
Levels of corticosterone were determined with a commercially available radioimmunoassay kit (ICN, Orangeburg, N.Y.) that included standards containing 0 to 1,000 ng of corticosterone per ml.
Quantification of total and HSV-specific IgG levels.
Total and HSV-specific levels of IgG were determined by enzyme-linked immunosorbent assay (ELISA). For determination of HSV-specific IgG levels, 96-well flat-bottom MaxiSorp Nunc plates were coated overnight at 4°C with 50 μl of HSV-2 antigen in 0.1 M NaHCO3. The HSV-2 antigen was prepared from Vero cells infected with HSV-2 186. Briefly, infected cells were frozen and thawed three times and then sonicated for 1 min. Following centrifugation at 900 × g for 5 min, the supernatant was removed and saved and any remaining cells in the pellet were further dissociated with a 1-ml ground-glass homogenizer. Supernatant and homogenate were combined and centrifuged for 5 min at 900 × g. The resulting supernatant was exposed to short-wavelength (254-nm) UV light for 20 min to inactivate any infectious viral particles. Wells were coated overnight with this HSV-2 antigen preparation, washed with PBS (pH 7.0)-0.05% (vol/vol) Tween 20, and then blocked with PBS-10% (vol/vol) FBS for 2 h at room temperature (RT). Serial dilutions (in PBS-10% [vol/vol] FBS) of a pooled serum sample from female mice immunized three times with HSV-2 was used in each assay to generate a standard curve. Serum samples from mice were diluted 1:400 in PBS-10% (vol/vol) FBS, and 100 μl of each sample was assayed in triplicate. Following a 4-h incubation at RT, plates were washed and 100 μl of biotinylated anti-mouse IgG (eBioscience, San Diego, Calif.) was added at a concentration of 1 μg/ml. Plates were incubated at RT for 45 min. Following washing, 100 μl of avidin peroxidase (Sigma, St. Louis, Mo.) at a concentration of 2.5 μg/ml was added for 30 min of incubation at RT. Plates were washed, and 100 μl of 2,2′-azinobis(3-ethylbenzthiazoline sulfonic acid) (ABTS) substrate (Sigma) at a concentration of 0.03 mg/ml plus 0.03% H2O2 (Sigma) was added. The colorimetric reaction was allowed to develop in the dark at RT, and the optical density at 405 nm was determined after 10 and 40 min with an MRX microplate ELISA reader (Dynex, Chantilly, Va.) with Revelation 3.0 software. For determination of total IgG levels, the above protocol was followed, except that the plates were coated with 50 μl of a 0.5-mg/ml concentration of anti-mouse IgG (Fisher Scientific) and serial dilutions of an unlabeled mouse IgG (Fisher Scientific) were used in each assay to generate a standard curve. Biotinylated anti-mouse Ig (PharMingen; San Diego, Calif.) was used as the detecting antibody for determination of total-IgG levels.
Detection of HSV-2 in tissues.
On days 3 and 5 postinfection, neonates were anesthetized in a saturated atmosphere of isoflurane, decapitated, and frozen at −70°C. The brain, heart, lungs, spleen, and kidneys were dissected and frozen at −70°C in 0.5 ml of Iscove's medium. Organs were subsequently thawed and homogenized with an Omni TH homogenizer (Omni International, Warrenton, Va.). Homogenates were assayed for infectious virus by standard plaque assay on Vero cells. Cell monolayers were fixed with 5% formaldehyde, and plaques were visualized by staining with crystal violet.
Statistical analysis.
Statistical significance was assessed by analysis of variance with Statview 5.0.1 software (SAS Institute, Cary, N.C.). Post-hoc comparisons between groups were performed with the Tukey-Kramer test. The Kaplan-Meier method was used to generate survival curves for each group of mice, and the logrank test was used to test for differences between groups. P values of ≤0.05 are reported, and P values of >0.05 are considered not significant.
RESULTS
Effects of prenatal restraint-and-light stress on maternal serum and fetal corticosterone levels.
Before using this model to assess the effects of maternal stress on the passive transfer of HSV-specific immunity and neonatal susceptibility to HSV infection, it was important to demonstrate that restraint-and-light stress would indeed elevate serum corticosterone levels in pregnant C57BL/6 mice and their fetuses. Pregnant mice were subjected to three, 45-min sessions of restraint-and-light stress on days 12 to 17 postcoitus. Serum corticosterone levels were measured in these mice and their fetuses on days 13, 15, and 17 immediately following the first session of stress (0830 to 0915), when basal levels of corticosterone are typically at the lowest point of the day. This prenatal restraint-and-light stress procedure significantly increased the levels of maternal serum corticosterone on days 13 (P = 0.012), 15 (P = 0.0002), and 17 (P < 0.0001) compared to those of pregnant, nonstressed control mice (Table 1). On day 19, 2 days following the final session of stress, serum corticosterone levels were measured to determine if elevated levels of corticosterone persisted following the termination of the stressor. No significant differences were observed in maternal serum corticosterone levels between prenatally stressed and nonstressed control mice on this day, indicating that the effects of the restraint-and-light stress are short lived. Thus, the prenatal restraint-and-light stress model significantly elevates serum corticosterone levels in pregnant mice. To determine whether the increased levels of maternal corticosterone were being transferred to the fetus by the transplacental route, fetal corticosterone was also measured. As a sufficient amount of blood could not be collected from fetuses prior to gestational day 17, fetuses obtained from prenatally stressed and nonstressed control mice on gestational days 13, 15, and 17 were homogenized and the homogenate was assessed for levels of corticosterone. There was no elevation of corticosterone in 13-day-old fetuses; however, fetuses from prenatally stressed mice exhibited significantly elevated levels of corticosterone on days 15 (P < 0.001) and 17 (P < 0.0001) compared to those of fetuses obtained from nonstressed control mice on each of these days (Table 1). On day 19, the day of delivery and 2 days after the termination of stress, the serum corticosterone levels in neonates born to prenatally stressed and nonstressed mice were similar, indicating that prenatal stress does not result in persistently elevated levels of corticosterone in neonatal mice. These findings indicate that a stress-induced increase in maternal corticosterone results in increased levels of corticosterone in the fetus, thus allowing the use of this model for assessment of the effect of increased maternal corticosterone on neonatal susceptibility to HSV infection.
TABLE 1.
Levels of maternal serum and fetal corticosterone from prenatally stressed and nonstressed females and fetuses
| Day | Group | Mean corticosterone concn (ng/ml)a ± SEM (no. of mice)
|
|
|---|---|---|---|
| Maternal | Fetal | ||
| 13 | Control | 109 ± 33 (4) | 36 ± 4 (24) |
| Stress | 873 ± 68 (4)b | 23 ± 1 (20) | |
| 15 | Control | 173 ± 57 (4) | 31 ± 2 (22) |
| Stress | 1197 ± 114 (5)c | 87 ± 4 (32)c | |
| 17 | Control | 280 ± 41 (4) | 52 ± 5 (23) |
| Stress | 1177 ± 15 (4)d | 189 ± 13 (34)d | |
| 19 | Control | 190 ± 28 (5) | 87 ± 33 (12) |
| Stress | 386 ± 90 (4) | 54 ± 14 (14) | |
Statistical comparisons are between control and stress conditions within maternal and fetal groups on the indicated days.
P < 0.05.
P < 0.001.
P < 0.0001.
Maternally derived protection of neonates infected with HSV-2.
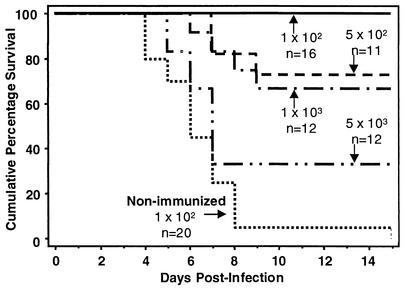
Before investigating the effects of maternal stress on neonatal susceptibility to HSV infection, it was important to determine in our model that neonates born to mothers previously immunized with HSV would indeed be protected against HSV infection in the absence of maternal stress. Female mice were immunized three times at 1-week intervals with HSV-2. One week following the final immunization, HSV-immune and nonimmunized mice were bred with naive C57BL/6 male mice. Neonates born to HSV-immune mice were infected i.p. at 2 days of age with 1 × 102, 5 × 102, 1 × 103, or 5 × 103 PFU and neonates born to nonimmunized mice were infected i.p. at 2 days of age with 102 PFU of HSV-2. Maternal HSV-specific immune-mediated protection resulted in 100% survival of neonates infected with 102 PFU and approximately 70% survival of neonates infected with either 5 × 102 or 1 × 103 PFU (Fig. 1). Infection of neonates with 5 × 103 PFU resulted in 33% survival. Neonates born to nonimmunized mice and infected with the lowest dose of HSV had a survival rate of only 5%. These results indicate that in this model, maternally derived HSV-specific immunity is transferable to the offspring and results in their protection against HSV infection in a dose-dependent manner.
FIG. 1.
Maternal immunization with HSV-2 protects progeny from HSV-2-induced mortality in a dose-dependent manner. Female mice 7 to 8 weeks of age were immunized s.c. in both of the rear footpads with HSV-2 three times at 1-week intervals. Two-day-old mice born to these HSV-immune mothers were infected i.p. with 1 × 102, 5 × 102, 1 × 103, or 5 × 103 PFU of HSV-2 in a volume of 20 μl. Neonates born to nonimmunized mothers were infected i.p. with 102 PFU of HSV-2. Neonatal mice were monitored daily for mortality until 15 days postinfection.
Effects of prenatal stress on passive transfer of total and HSV-specific antibody.
Previous studies had demonstrated a decrease in the transplacental transfer of IgG following prenatal stress (18, 19); however, they failed to determine the impact of prenatal stress on the transplacental transfer of a virus-specific antibody. Hence, we used our model to determine whether prenatal stress would decrease the transplacental transfer of both total and HSV-specific IgG.
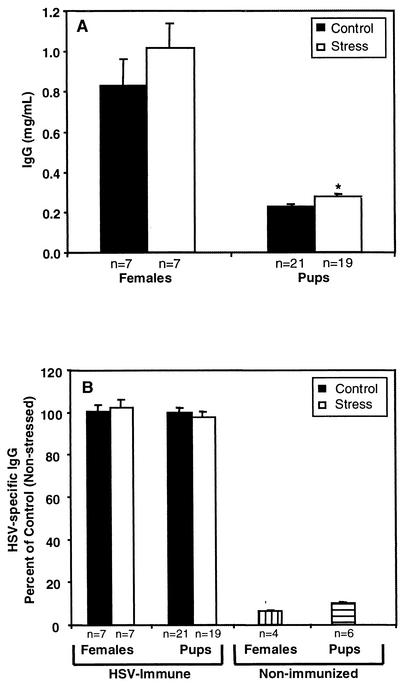
HSV-immune and nonimmunized mice were bred with naive C57BL/6 male mice. A subset of the pregnant mice was exposed to restraint-and-light stress on days 12 to 17 postcoitus. Serum was obtained from both the neonates and the mothers themselves immediately following delivery. The levels of total IgG (Fig. 2A) and HSV-specific IgG (Fig. 2B) were analyzed by ELISA. Prenatal restraint-and-light stress did not affect the levels of IgG in maternal serum, as prenatally stressed and nonstressed control mice had similar levels of total IgG (Fig. 2A). Prenatal restraint-and-light stress and the subsequent increase in maternal corticosterone also did not affect the transplacental transfer of IgG since neonates born to prenatally stressed and nonstressed control mice had comparable serum levels of IgG immediately following birth (0.28 versus 0.23 mg/ml). Although neonates from prenatally stressed mice had a statistically significant (P = 0.035) elevation of total IgG, the actual values are very similar; thus, the difference is most likely not biologically relevant in our model. Prenatal stress also did not affect the levels of HSV-specific IgG in maternal serum as prenatally stressed mice had levels of HSV-specific IgG similar to those found in nonstressed control mice (Fig. 2B). In addition, the transplacental transfer of HSV-specific IgG was not altered by maternal stress, as neonates born to prenatally stressed or nonstressed control mice had similar levels of HSV-specific IgG. As expected, nonimmunized females and their neonates had virtually no HSV-specific IgG in their serum. These findings indicate that transplacental transfer of total and HSV-specific IgG is preserved even under conditions of acute maternal stress.
FIG. 2.
Passive transfer of total and HSV-specific IgG is preserved in the presence of prenatal stress. Female mice 7 to 8 weeks of age were immunized s.c. in both of the rear footpads with HSV-2 three times at 1-week intervals. A subset of these HSV-immune mice was subjected to restraint-and-light stress on days 12 to 17 postcoitus. Serum was obtained from both the neonates and the mothers immediately following birth and analyzed for total IgG (A) and HSV-specific IgG (B) by ELISA. Values represent the mean ± the standard error of the mean.
Effects of prenatal stress on the susceptibility of neonates to HSV-2 infection.
Although there was no alteration in the transplacental transfer of HSV-specific antibody, elevated levels of corticosterone were being transferred to the fetus. Such elevated levels of corticosterone may have an impact on the developing fetal immune system, specifically by influencing the ability of the neonate to utilize the transferred maternal antibody to generate an antiviral ADCC response (12, 13). Since a diminished capacity to utilize maternal antibody could increase neonatal susceptibility to HSV infection, we examined whether prenatal stress affects viral spread and neonate mortality following HSV-2 infection.
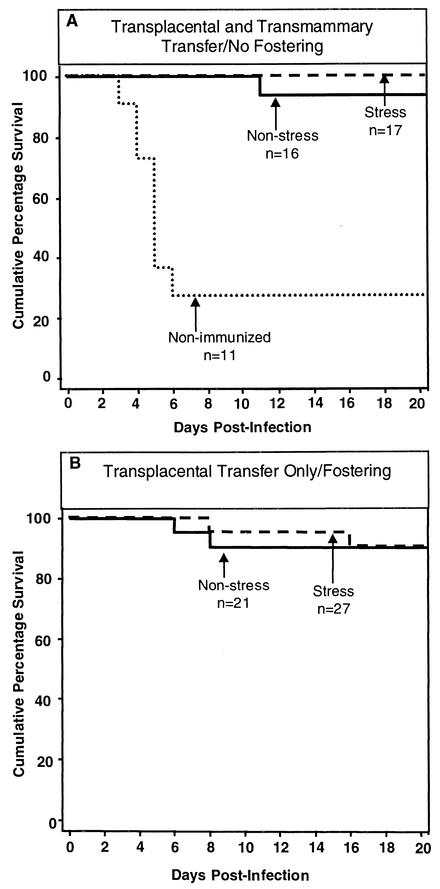
HSV-immune and nonimmunized female mice were bred with naive C57BL/6 male mice. A subset of the pregnant mice was exposed to restraint-and-light stress on days 12 to 17 postcoitus. A subset of mice born to HSV-immune, prenatally stressed and nonstressed mice was fostered immediately following delivery to nonimmunized mice. In this manner, neonates could obtain HSV-specific antibody by only transplacental means. Another subset of neonates remained with their HSV-immune, prenatally stressed or nonstressed mothers or with their nonimmunized mothers. These neonates could obtain HSV-specific antibody by both transplacental and transmammary means. At 2 days of age, all neonates were challenged i.p. with 102 PFU of HSV-2. A subset of neonates was sacrificed at 3 and 5 days postinfection for determination of HSV spread to the brain, spleen, lungs, kidneys, and heart. A second subset was monitored daily for mortality. Those neonates born to nonimmunized mice succumbed rapidly to the viral infection (Fig. 3A) and had infectious HSV in organs examined on day 3 postinfection (Table 2). In the absence of fostering, neonates born to prenatally stressed or nonstressed HSV-immune mice were protected against HSV-associated mortality by transplacental and transmammary transfer of antibody (Fig. 3A). Likewise, neonates born to HSV-immune, prenatally stressed or nonstressed mice that were fostered to nonimmunized mice, thereby receiving HSV-specific antibody by only transplacental means, showed no increase in mortality (Fig. 3B). Virus was detected in only a few of the neonates born to HSV-immune, prenatally stressed or nonstressed mice on day 3 postinfection with a minimal increase in virus in some of the organs on day 5 postinfection (Table 2). Together, the limited amount of virus and mortality indicate that transplacental transfer of HSV-specific antibody is sufficient to protect neonates against HSV infection. In addition, the elevated levels of corticosterone transferred from stressed mothers to the fetus do not affect the ability of the neonate to utilize maternally derived antibody to inhibit the progression of HSV infection.
FIG. 3.
Prenatal restraint-and-light stress does not affect the susceptibility of neonatal mice to HSV infection. Female mice 7 to 8 weeks of age were immunized s.c. in both of the rear footpads with HSV-2 three times at 1-week intervals. A subset of these HSV-immune mice was subjected to restraint-and-light stress on days 12 to 17 postcoitus. A subset of neonates remained with their HSV-immune, prenatally stressed or nonstressed mothers or with their nonimmunized mothers (A). A second subset of mice born to HSV-immune, prenatally stressed and nonstressed mice was fostered to nonimmunized mice (B). All neonates were infected i.p. at 2 days of age with 102 PFU of HSV-2 in a volume of 20 μl. Neonatal mice were monitored daily for mortality until 20 days postinfection.
TABLE 2.
Detection of infectious HSV in neonates born to nonimmunized or HSV-immune females
| Organ | Day postinfection | No. of mice with infectious HSV as determined by plaque assay/total (%)
|
|
|---|---|---|---|
| Nonimmunized female | Immunized femalea | ||
| Brain | 3 | 8/11 (73) | 0/15 (0) |
| 5 | NDb | 4/15 (27) | |
| Spleen | 3 | 10/11 (91) | 0/15 (0) |
| 5 | ND | 3/15 (20) | |
| Lung | 3 | 11/11 (100) | 4/15 (27) |
| 5 | ND | 5/15 (33) | |
| Kidney | 3 | 9/11 (82) | 2/15 (13) |
| 5 | ND | 1/15 (7) | |
| Heart | 3 | 3/11 (27) | 0/15 (0) |
| 5 | ND | 3/15 (20) | |
Includes both stressed and nonstressed immune females.
ND, not determined; all neonates died by day 5 postinfection.
DISCUSSION
The studies described herein used a well-established restraint-and-light stress mouse model to assess the effects of elevated levels of maternal and fetal corticosterone on the passive transfer of virus-specific immunity and the protective capacity of this immunity against HSV-2 infection in neonatal mice. Pregnant mice subjected to restraint-and-light stress exhibited significantly elevated levels of serum corticosterone. We determined that elevated levels of maternal corticosterone were transferred to the fetus by the transplacental route. We further examined whether these elevated levels of corticosterone could affect the transplacental transfer of maternal antibody and the developing immune system of the fetus. We found that despite the elevated levels of maternal and fetal corticosterone, the passive transfer of total and HSV-specific IgG from the mother to the fetus was preserved. In addition, the elevated levels of corticosterone in the fetus did not hinder the ability of the neonate to utilize the maternal antibody as there was no effect of prenatal stress on the progression of HSV infection or the survival of neonates infected with HSV-2.
Restraint stress has been widely used in biomedical research to elicit a stress response in a variety of research animals (reviewed in reference 7). Restraint stress reproducibly activates the HPA axis, resulting in elevated levels of serum corticosterone. These elevated levels of corticosterone have been repeatedly shown to affect both the innate and adaptive immune responses to viral infection in adult mice (reviewed in reference 3). Recently, the deleterious effects of increased levels of maternal corticosterone during pregnancy on the developing nervous and endocrine systems of the fetus were recognized (reviewed in reference 21). Since bidirectional communication exists among the nervous, endocrine, and immune systems, it was important to consider the effect of increased maternal corticosterone on the developing immune system of the fetus. Maternal stress during gestation had previously been reported to alter immunological development and function in the offspring (9, 17-19). However, many of these studies simply focused on the passive transfer of nonspecific immunity and on the ensuing lymphocyte responses to nonviral antigens. In addition, no studies had investigated the impact of maternal stress on neonatal susceptibility to an infectious pathogen. Hence, our studies are the first to determine the effects of prenatal stress on the passive transfer of HSV-specific immunity and subsequent neonatal susceptibility to HSV infection.
Previous studies have used a similar restraint-and-light stress procedure to elevate serum corticosterone levels in pregnant CF-1 mice (15). However, since the CF-1 mice were stressed only twice daily and since C57BL/6 mice were used in our study, it was important to determine the impact of three daily exposures to restraint-and-light stress on the latter strain of mice. Pregnant mice exposed to restraint-and-light stress had corticosterone levels that were significantly elevated above those of pregnant, nonstressed control mice (Table 1). These serum corticosterone levels were notably lower than the levels reported in pregnant CF-1 mice (15); however, in the present study, serum samples were obtained in the morning immediately following the first session of restraint-and-light stress, when levels of corticosterone are typically at their lowest of the day. Corticosterone levels were slightly elevated in the pregnant, nonstressed control mice compared to those of nonpregnant, nonstressed adult mice; however, previous studies have shown that pregnant mice can have elevated basal levels of corticosterone (2).
Since the levels of serum corticosterone were increased in pregnant mice subjected to restraint-and-light stress, it was important to determine if such increases resulted in higher levels of corticosterone in the fetus. Previous studies have shown elevated levels of fetal serum corticosterone on day 17 following prenatal stress (15). We determined that fetuses obtained from prenatally stressed mice had significantly elevated levels of corticosterone on days 15 and 17 postcoitus (Table 1). The corticosterone levels in fetuses obtained from both prenatally stressed and nonstressed mice were highest on day 17 postcoitus, most likely because of the approach of parturition. On day 19, the day of delivery, corticosterone levels in neonates from prenatally stressed and nonstressed mice were similar, indicating that the corticosterone levels of the offspring did not remain elevated following the termination of maternal stress.
We have established in our model that prenatal stress increases both maternal and fetal levels of corticosterone. We further used this model to investigate the effects of such stress on the transplacental transfer of HSV-specific antibody. Previous studies have demonstrated that both prenatal and postnatal transfers of HSV-specific antibody protect 2-day-old neonatal mice against infection with 102 PFU of HSV-2 (8, 14). Neonate survival was shown to correlate with the levels of neutralizing antibody present in maternal serum (8). The data presented in Fig. 1 demonstrate that in our model, maternal immunization with HSV protects neonatal mice against increasing doses of HSV-2 in a dose-dependent manner.
Since neonates rely heavily on maternally derived immunity, it was important to determine the effects of prenatal stress on the transfer of HSV-specific antibody to the fetus and the subsequent utilization of this antibody by the neonate. We determined that prenatal stress did not alter the levels of total and HSV-specific IgG in maternal serum and did not affect the transplacental transfer of total and HSV-specific IgG to the fetus (Fig. 2A and B). The latter finding was unexpected since previous studies had demonstrated a decrease in total IgG levels following prenatal stress (4, 18, 19). However, these studies used more severe forms of psychological stress, such as foot shock (18) and chronic social stress (4). We consider our restraint-and-light stress model to be an acute stressor since our data show that corticosterone levels decrease to the baseline in 2 days and previous studies showed decreases within 4 h following the termination of such a stressor (15). Studies are in progress to determine whether exposure of pregnant females to chronically elevated levels of corticosterone affects the transplacental transfer of HSV-specific antibody.
To determine whether prenatal stress affects the ability of the neonate to utilize the maternal, HSV-specific antibody, we determined the effect of maternal stress on the spread of virus and neonate mortality following HSV infection. In order to determine the impact of prenatal stress on only transplacental transfer of HSV-specific antibody, we performed fostering studies in which newborn mice were transferred from their HSV-immune mothers to nonimmunized mice (Fig. 3A and B). Studies in our laboratory have demonstrated that the HSV-specific, transplacentally acquired maternal antibody is relatively short lived in neonatal serum and is significantly diminished by 7 days of age (data not shown). Prenatal stress did not diminish the protective capacity of the passively transferred, HSV-specific antibody since there was little viral spread within neonates born to HSV-immune, prenatally stressed or nonstressed females (Table 2) and no increase in neonate mortality (Fig. 3A and B).
To date, this study is the first to investigate the effects of prenatal stress on the transplacental transfer of virus-specific immunity. Our findings show that stress-induced increases in corticosterone do not influence the transplacental transfer and subsequent utilization of HSV-specific antibody and emphasizes the resiliency of the passive transfer of immunity from mother to offspring. This resiliency may be largely dictated by the high levels of HSV-specific antibody that are present in the female serum near the time of birth. In the present study, female mice were immunized three times with HSV and thus had high levels of HSV-specific antibody. Because high levels of HSV-specific antibody were transferred to the fetus, the maternal antibody may have neutralized the virus, therefore not requiring the neonate to generate its own ADCC response. Female mice immunized once with HSV have significantly lower levels of HSV-specific antibody; however, in the absence of stress, even this lower level of antibody protects neonates against HSV-associated mortality (data not shown). Studies are in progress to determine whether prenatal stress affects the passive transfer of HSV-specific antibody when only limiting amounts of antibody are available.
Acknowledgments
We acknowledge Crystal Anglen, Elizabeth Traister, and Heidi Young for technical support and helpful discussions. We thank Mary Ellen Truckenmiller for critically reviewing the manuscript.
This work was supported by Public Health Service research grant NICHD 39262 and in part by NIH Training Grant 5 T32 CA60395.
REFERENCES
- 1.Arvin, A. M. 1991. Relationships between maternal immunity to herpes simplex virus and the risk of neonatal herpesvirus infection. Rev. Infect. Dis. 13(Suppl. 11):S953-S956. [DOI] [PubMed]
- 2.Barlow, S. M., P. J. Morrison, and F. M. Sullivan. 1975. Effects of acute and chronic stress on plasma corticosterone levels in the pregnant and non-pregnant mouse. J. Endocrinol. 66:93-99. [DOI] [PubMed] [Google Scholar]
- 3.Bonneau, R. H., D. A. Padgett, and J. F. Sheridan. 2001. Psychoneuroimmune interactions in infectious disease: studies in animals, p. 483-497. In R. Ader, D. Felten, and N. Cohen (ed.), Psychoneuroimmunology, 3rd ed. Academic Press, San Diego, Calif.
- 4.Coe, C. L., and H. R. Crispen. 2000. Social stress in pregnant squirrel monkeys (Saimiri boliviensis peruviensis) differentially affects placental transfer of maternal antibody to male and female infants. Health Psychol. 19:554-559. [PubMed] [Google Scholar]
- 5.Coe, C. L., J. W. Kemnitz, and M. L. Schneider. 1993. Vulnerability of placental antibody transfer and fetal complement synthesis to disturbance of the pregnant monkey. J. Med. Primatol. 22:294-300. [PubMed] [Google Scholar]
- 6.de Moraes-Pinto, I., and C. A. Hart. 1997. Transplacental antibody transfer and neonatal immunity. Br. J. Hosp. Med. 58:317-319. [PubMed] [Google Scholar]
- 7.Glavin, G. B., W. P. Pare, T. Sandbak, H. K. Bakke, and R. Murison. 1994. Restraint stress in biomedical research: an update. Neurosci. Biobehav. Rev. 18:223-249. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi, Y., T. Wada, and R. Mori. 1983. Protection of newborn mice against herpes simplex virus infection by prenatal and postnatal transmission of antibody. J. Gen. Virol. 64:1007-1012. [DOI] [PubMed] [Google Scholar]
- 9.Klein, S. L., and D. R. Rager. 1995. Prenatal stress alters immune function in the offspring of rats. Dev. Psychobiol. 28:321-336. [DOI] [PubMed] [Google Scholar]
- 10.Kohl, S. 1997. Neonatal herpes simplex virus infection. Clin. Perinatol. 24:129-150. [PubMed] [Google Scholar]
- 11.Kohl, S. 1989. The neonatal human's immune response to herpes simplex virus infection: a critical review. Pediatr. Infect. Dis. J. 8:67-74. [PubMed] [Google Scholar]
- 12.Kohl, S. 1991. Role of antibody-dependent cellular cytotoxicity in neonatal infection with herpes simplex virus. Rev. Infect. Dis. 13(Suppl. 11):S950-S952. [DOI] [PubMed] [Google Scholar]
- 13.Kohl, S., M. S. West, C. G. Prober, W. M. Sullender, L. S. Loo, and A. M. Arvin. 1989. Neonatal antibody-dependent cellular cytotoxic antibody levels are associated with the clinical presentation of neonatal herpes simplex virus infection. J. Infect. Dis. 160:770-776. [DOI] [PubMed] [Google Scholar]
- 14.Kohl, S., and L. S. Loo. 1984. The relative role of transplacental and milk immune transfer in protection against lethal neonatal herpes simplex virus infection in mice. J. Infect. Dis. 149:38-42. [DOI] [PubMed] [Google Scholar]
- 15.Montano, M. M., M. H. Wang, M. D. Even, and F. S. vom Saal. 1991. Serum corticosterone in fetal mice: sex differences, circadian changes, and effect of maternal stress. Physiol. Behav. 50:323-329. [DOI] [PubMed] [Google Scholar]
- 16.Montano, M. M., M. H. Wang, and F. S. vom Saal. 1993. Sex differences in plasma corticosterone in mouse fetuses are mediated by differential placental transport from the mother and eliminated by maternal adrenalectomy or stress. J. Reprod. Fertil. 99:283-290. [DOI] [PubMed] [Google Scholar]
- 17.Sobrian, S. K., V. T. Vaughn, W. K. Ashe, B. Markovic, V. Djuric, and B. D. Jankovic. 1997. Gestational exposure to loud noise alters the development and postnatal responsiveness of humoral and cellular components of the immune system in offspring. Environ. Res. 73:227-241. [DOI] [PubMed] [Google Scholar]
- 18.Sobrian, S. K., V. T. Vaughn, E. F. Bloch, and L. E. Burton. 1992. Influence of prenatal maternal stress on the immunocompetence of the offspring. Pharmacol. Biochem. Behav. 43:537-547. [DOI] [PubMed] [Google Scholar]
- 19.Tuchscherer, M., E. Kanitz, W. Otten, and A. Tuchscherer. 2002. Effects of prenatal stress on cellular and humoral immune responses in neonatal pigs. Vet. Immunol. Immunopathol. 86:195-203. [DOI] [PubMed] [Google Scholar]
- 20.vom Saal, F. S., M. D. Even, and D. M. Quadagno. 1991. Effects of maternal stress on puberty, fertility and aggressive behavior of female mice from different intrauterine positions. Physiol. Behav. 49:1073-1078. [DOI] [PubMed] [Google Scholar]
- 21.Welberg, L. A., and J. R. Seckl. 2001. Prenatal stress, glucocorticoids and the programming of the brain. J. Neuroendocrinol. 13:113-128. [DOI] [PubMed] [Google Scholar]
- 22.Williams, M. T., H. N. Davis, A. E. McCrea, S. J. Long, and M. B. Hennessy. 1999. Changes in the hormonal concentrations of pregnant rats and their fetuses following multiple exposures to a stressor during the third trimester. Neurotoxicol. Teratol. 21:403-414. [DOI] [PubMed] [Google Scholar]
- 23.Williams, M. T., M. B. Hennessy, and H. N. Davis. 1995. CRF administered to pregnant rats alters offspring behavior and morphology. Pharmacol. Biochem. Behav. 52:161-167. [DOI] [PubMed] [Google Scholar]
- 24.Williams, M. T., M. B. Hennessy, and H. N. Davis. 1998. Stress during pregnancy alters rat offspring morphology and ultrasonic vocalizations. Physiol. Behav. 63:337-343. [DOI] [PubMed] [Google Scholar]