Abstract
To determine the type of cell(s) that contain latent varicella-zoster virus (VZV) DNA, we prepared pure populations of neurons and satellite cells from trigeminal ganglia of 18 humans who had previously had a VZV infection. VZV DNA was present in 34 of 2,226 neurons (1.5%) and in none of 20,700 satellite cells. There was an average of 4.7 (range of 2 to 9) copies of VZV DNA per latently infected neuron. Latent VZV DNA was primarily present in large neurons, whereas the size distribution of herpes simplex virus DNA was markedly different.
Varicella-zoster virus (VZV) is present in a latent form in sensory ganglia of humans who have had a primary infection (varicella) with VZV (11). This is demonstrated by the reactivation of VZV in these individuals as herpes zoster and by the presence of VZV DNA and proteins in ganglia recovered at autopsy (2, 3, 5, 9, 10, 12, 13, 15, 16, 19, 20, 22-24, 26). Different laboratories have ascribed the site of latency to either neurons (12-14, 18, 22) or perineuronal satellite cells (5, 26); some laboratories suggest that neurons are the primary site of latency together with a smaller proportion of satellite cells containing the VZV genome (16, 20). The primary aim of the experiments described here was to determine the type of cell in trigeminal ganglia that harbors latent VZV and also to obtain data on the proportion of these cells that contain latent VZV. One reason why the site of VZV latency has remained an open question is that neurons and satellite-supporting cells are tightly associated in ganglia and the in situ methods used to locate VZV DNA did not clearly resolve the source of the hybridization signal. This resolution was easier for latent HSV because a strong hybridization signal to HSV facilitated its localization to the nucleus of the neuron. In contrast, the hybridization signal from VZV DNA is considerably weaker and dispersed over both nucleus and cytoplasm. While the hybridization methodology has improved, these considerations remain (21, 30). To reduce the ambiguities inherent in the in situ methods, we developed a procedure to separate cell types prior to analysis. The separation procedure has been verified with a study of HSV (1). This report presents data on the cellular localization of latent VZV, the frequency of latency, and the number of viral genomes present in latent cells.
MATERIALS AND METHODS
Cell separation.
Trigeminal ganglia were obtained at autopsy. The clinical history and time elapsed from death to autopsy were recorded. Trigeminal ganglia were processed as previously described in a study of HSV latency (1). Both ganglia from an autopsy were pooled, trimmed, weighed, and minced in 200 μl of phosphate-buffered saline (PBS) containing 3% bovine serum albumin (BSA). Eight milliliters of PBS-3% BSA was added to the minced tissue, and the number of neurons present was determined by direct microscopic count. Between 50 and 80% of the neurons were released by the mincing procedure, and 2 × 104 to 6 × 104 neurons were obtained from the pooled ganglia. A sample of the minced tissue was subjected to PCR analysis to determine the VZV status of the ganglia; 82% of available autopsies were VZV positive. VZV-positive ganglia were then rapidly processed. The cell suspension was passed from a syringe through a 60 μm-pore-size nylon net filter (NY6002500; Millipore) into a 15-ml tube. The tube containing the effluent was placed at 4°C for at least 6 h. The upper layer, containing small particle debris, was aspirated and discarded. The pellet was resuspended in 1 ml of PBS-3% BSA and placed in a 60-mm-diameter plastic dish so that the number of neurons and satellite cells could be determined. Cells were identified under a microscope by their morphology and retrieved with a micromanipulator (Leitz 833240) connected to a Hamilton Luer tip syringe (no. 81001). The syringe was fitted with a Fisher gel-loading tip that had the broad end cut back and the slender end cut on a slant to facilitate aspirating cells. Cells identified as neurons or satellite cell clumps were placed in separate dishes containing 6 ml of PBS-3% BSA. Isolated neurons or clumps of satellite cells free of neurons were washed three times in dishes containing autoclaved distilled water containing 0.01% Triton X-100. The desired number of cells was placed in MicroAmp tubes for PCR analysis. The purity of selected neurons and clumps of satellite cells was monitored by direct observation with Hoffman optics and after staining with 4′,6′-diamidino-2-phenylindol (DAPI) (VECTASHIELD; Vector Laboratories, Burlingame, Calif.).
VZV antibody determination.
Plasma from bloody fluid removed from the peritoneal cavity at autopsy was tested for VZV-specific antibody by an enzyme-linked immunosorbent assay method performed according to the manufacturer's instructions (no. 720-380; Diamedix).
VZV PCR.
The detection of VZV DNA was routinely carried out by using conditions previously described to amplify a segment of the HSV DNA polymerase gene (1). Selected neurons or satellite cells were placed in MicroAmp reaction tubes with 5 μl of distilled water. Five microliters of proteinase K solution (2 mg of proteinase K per ml of Tris-Cl buffer [10 mM {pH 9.5}, 0.2% Triton X-100]) was added, and the samples were subjected to a thermal cycle of 65°C for 10 min, 25°C for 2 min, 65°C for 2 min, and 97°C for 5 min and allowed to stand at 4°C. These conditions lysed the cells, making the DNA available for PCR, and inactivated the proteinase K. Forty microliters of PCR master mixture was added to each tube so that the final volume of 50 μl contained 10 mM KCl, 10 mM (NH4)2SO4, 20 mM Tris-Cl (pH 8.75), 2 mM MgSO4, 0.1% Triton X-100, 100 mg of BSA/ml, 200 mM concentrations of each of deoxynucleoside tripphosphate, 0.5 mM concentrations of each primer, and 1.25 U of DNA polymerase (cloned PFU of DNA polymerase, no. 600159; Stratagene). VZV genome open reading frame 29 (ORF29) (DNA-binding protein) was detected with a downstream primer sequence VZV29j of 5′-TACGGGTCTTGCCGGAGCTGGTAT-3′ and an upstream primer sequence VZV29k of 5′-AATGCCGTGACCACCAAGTATAAT-3′. PCR amplification with the VZV29j and VZV29k primers yielded a DNA fragment 273 nucleotides in length. In order to confirm that detection of the VZV DNA ORF29 accurately reflected the presence of the viral genome, PCR analysis was also carried out with a second VZV gene, ORF63. The downstream primer sequence was 5′-GCGAGATTCACGAAGATTGCG-3′, and the upstream primer sequence was 5′-CAATTACATCCGATGGCGTAG. PCR amplification with these primers yielded a DNA fragment of 293 nucleotides in length.
Amplification was performed with a GeneAmp PCR System 9700 Thermal Cycler. For both VZV genes, the initial denaturation step was 96°C for 60 s. There were 20 touchdown cycles with a denaturation step at 95°C for 15 s, annealing from 69 to 61°C (decrease of 0.4°C each cycle) for 30 s, and extension at 72°C for 30 s followed by 25 cycles of 95°C for 15 s, 61°C for 30 s, and 72°C for 30 s. The final extension was at 72°C for 6 min. The PCR products were separated on a 3% agarose gel and visualized by ethidium bromide staining.
For VZV ORF29, the PCR product was confirmed by Southern blot analysis. Blots were hybridized with a digoxigenin-labeled probe with the sequence 5′-CACGGAGGCAAACACGTTTACCCAGCTCC-3′ and detected with anti-digoxigenin alkaline phosphatase and chemiluminescent alkaline phosphatase substrate CDP-Star (no. 1093274 and 1685627; Boehringer Mannheim). To carry out nested primer PCR analysis of VZV ORF29, 1 μl of the initial PCR mixture was added to a second master mix reaction tube containing the downstream primer n-VZV29j (5′-ACTCACTACCAGTCATTTCTATC-3′) and the upstream primer n-VZV29k (5′-GTATTTTCTGGCTCTAATCCAAG-3′). The product obtained with the nested primers was 213 nucleotides in length.
The sensitivity of the VZV PCR assay was determined in reconstruction experiments with purified VZV plasmid DNA quantitated by optical density measurements and standard agarose gel analysis. The plasmid standard was diluted to give the desired number of DNA fragments per reaction tube prior to amplification. One copy of VZV DNA was detected in 4 of 12 samples (33%), 2 copies of VZV DNA were detected in 12 of 18 samples (67%), 4 copies of VZV DNA were detected in 14 of 18 samples (78%), and 6 copies of VZV DNA were detected in 17 of 18 samples (94%). Nested set PCR and Southern blot analyses generated strong signals in samples that had low VZV DNA copy numbers. These techniques confirmed that 1 to 2 copies of VZV DNA were detected 50% of the time but did not increase sensitivity significantly. As a control for DNA degradation, and as a measure of the relationship between gene dosage and cell number, the globin gene was measured by PCR in 1, 2, and 4 neurons by using primers and amplification conditions previously described (28). The globin gene was detected in 60% of the assays containing a single neuron and in 100% of those with two neurons.
Quantitative VZV PCR.
The number of copies of the VZV genome in latently infected neurons was estimated by quantitative competitive PCR in tubes containing known numbers of neurons and an internal control of a recombinant plasmid DNA containing a VZV ORF29 DNA fragment. VZV plasmid D-Vscript178 was constructed by using the primers of VZV29j and VZV29k as described by Forster (8). The 178-nucleotide product from the plasmid DNA is readily separated from the wild-type product by 3% agarose gel electrophoresis. PCR products from competitive reactions were stained with Vistra Green (RPN5786; Amersham) and quantitated with STORM 860 (Molecular Dynamics). A pooled sample of VZV-containing neurons was prepared, and the concentration of VZV DNA in this pool was determined by competitive PCR. Aliquots of this pool corresponding to 2, 4, 6, 8, and 10 copies of the latent VZV genome were mixed with 10 copies of the internal VZV plasmid standard in each tube. The reconstruction samples with the mixed DNA were subjected to PCR analysis, and the products were separated by electrophoresis and quantified. The ratio of the band intensity of latent VZV DNA and plasmid competitor DNA was plotted as a function of input latent VZV DNA. The number of VZV ORF29 DNA copies in neuronal cells in subsequent experiments was determined from this curve.
HSV PCR.
Herpes simplex virus (HSV) DNA was detected by using the PCR conditions previously reported (1).
Measurements of neurons size.
Neuron size was determined from digital photos of cells taken together with a calibration grid. Photos were analyzed with the Scion Image program (beta 4.0.2; Scion Corporation) to outline individual neurons and compute the area of neurons in terms of squared micrometers. Neurons from three autopsies (HG-98, HG-99, and HG-105) were analyzed in terms of area. Neurons were selected for PCR on the basis of cell diameters measured with the use of a microscope eyepiece containing a measuring bar. The neuron cell areas measured in photographs and the microscope measurements of cell diameter correlated.
RESULTS
Characterization of neurons and satellite cells.
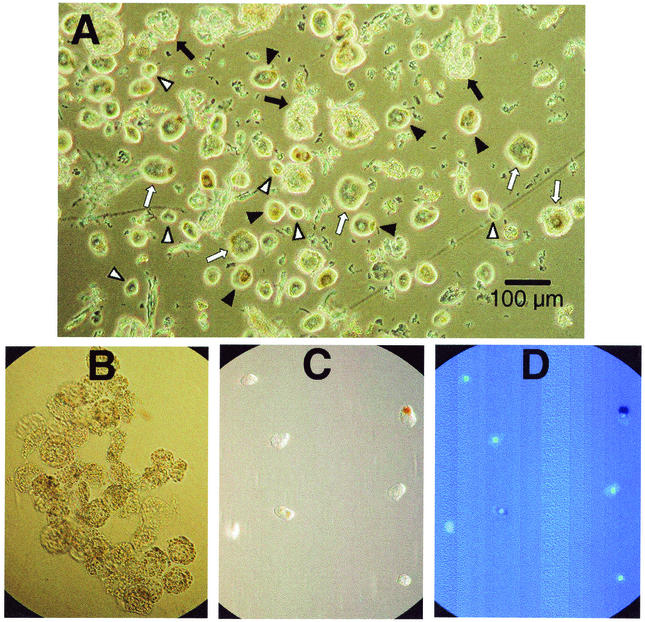
Neurons and satellite cells were cleanly separated from a trigeminal ganglion by a validated method (1). Neurons of various sizes are demonstrated in an unfractionated preparation of minced ganglia (Fig. 1A). The characteristic appearance of purified clumps of satellite cells without neurons is demonstrated in Fig. 1B. Isolated neurons in phase contrast (Fig. 1C) and DAPI staining of these same neurons demonstrates that they are free of satellite cells (Fig. 1D).
FIG. 1.
Photomicrographs of neurons and satellite cells. (A) Minced ganglia after filtration and sedimentation, showing small (▿), medium (▾), and large (⇒) neurons as well as clumps of satellite cells (➞). Magnification, ×20 (Hoffman optics). (B) Clumps of isolated satellite cells. Magnification, ×20 (Hoffman optics). (C) Single neurons viewed with phase contrast. Magnification, ×20. (D) Same field as panel C stained with DAPI, demonstrating that neurons are free of attached satellite cells. Magnification, ×20 (fluorescence).
Presence of VZV DNA solely in neurons.
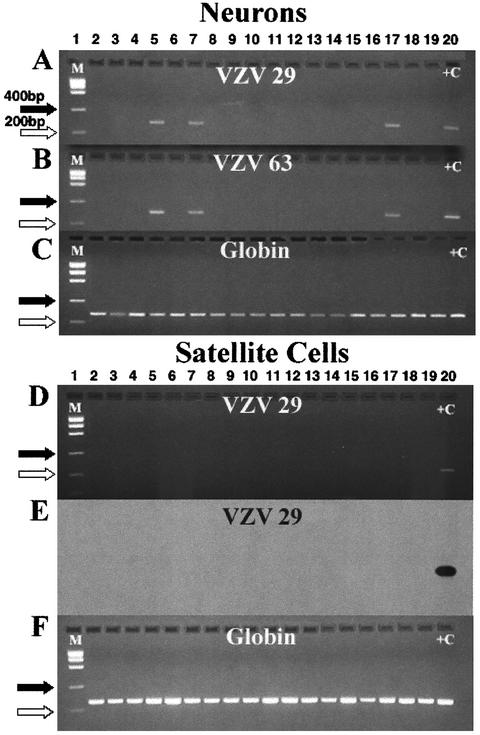
In order to determine the site of VZV latency in trigeminal ganglia, PCR analysis of isolated neurons and clumps of satellite cells was undertaken. Detection of globin DNA was used as a positive control for the PCR method and as a measure of sensitivity. From a single pair of trigeminal ganglia obtained at autopsy (HG-72), 18 samples, each containing 20 purified neurons, were prepared, lysed, and divided into three fractions. One-third of each sample was assayed for the presence of VZV gene 29, one-third was assayed for the presence of VZV gene 63, and the remainder were assayed for the presence of the globin gene. The results of the PCR analysis are shown in Fig. 2A and B. Three of the 18 samples, shown in lanes 5, 7, and 17 (Fig. 2A and B) were positive for both VZV genes. A Southern blot confirmed the identity of the gene 29 product (data not shown). All samples were positive for the globin gene (Fig. 2C).
FIG. 2.
PCR analysis of isolated neurons and satellite cells from ganglion HG-72. Panels A, B, and C show agarose gels of direct PCR products of DNA from isolated neurons. Panels D and F show gels of direct PCR products from DNA from satellite cells. Panels A and B show that the same three samples (lanes 5, 7, and 17) are positive for VZV genes 29 and 63. Panel C shows the detection of the globin gene in all samples. The direct PCR analysis for VZV gene 29 in satellite cells is shown in gel D, and the corresponding Southern blot is shown in gel E. Gel F shows that all satellite cell samples contain the globin gene. The primers used for VZV gene 29 were VZV 29J and VZV 29K. The primers used for VZV gene 63 are described in Materials and Methods. +C indicates the appropriate positive control for each panel. M indicates the molecular size markers. The sizes for marker bands of 200 (⇒) and 400 (➞) nucleotides are indicated.
Eighteen samples from the same ganglion, each containing 5 clumps of satellite cells, were prepared and divided in half and subjected to direct PCR analysis for the presence of VZV gene 29 or the globin gene. VZV DNA was not detected in satellite cells (approximately 56 cells/clump, total of 280 satellite cells) (Fig. 2D). Southern blot analysis confirmed the absence of VZV gene 29 DNA in satellite cells (Fig. 2E). Globin DNA was present in all samples of satellite cells (Fig. 2F). These results establish that VZV DNA was latent in neurons and not in satellite cells from ganglia from this autopsy. Because DNA from both VZV gene 29 and VZV gene 63 were present in the three samples of neurons, shown in lanes 5, 7, and 17, it appears that the VZV DNA was stable during isolation and that either gene is suitable for studying VZV latency.
Frequency of neurons containing VZV DNA.
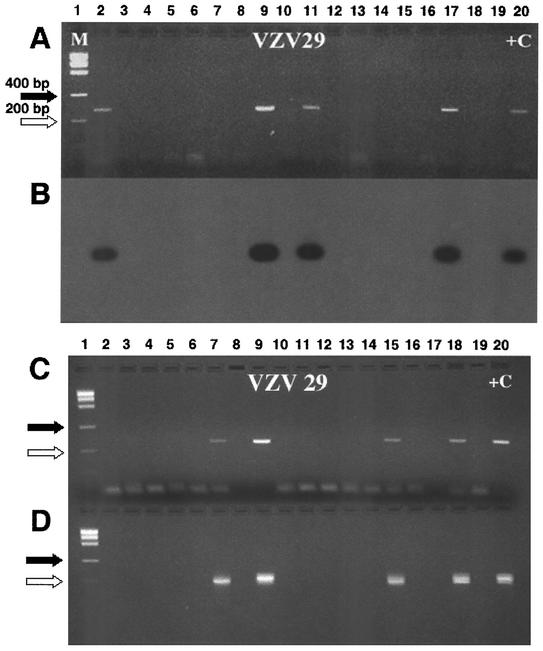
In order to determine the frequency of VZV latency, PCR analysis was carried out on individual neurons isolated from three cadavers (HG-70, HG-88, and HG-91). The proportion of neurons positive for VZV gene 29 was 1 of 15 cells, 3 of 100 cells, and 0 of 18 cells, respectively. This experiment indicates that, in trigeminal ganglia, the proportion of neurons that contain a VZV genome is low. Therefore, subsequent determinations utilized samples of 10 isolated neurons per PCR tube to establish the proportion of neurons in additional ganglia that contain VZV DNA. Table 1 contains information on the ganglia from 12 autopsies, all of which had the presence of VZV DNA detected prior to cell separation and were subsequently shown to be from VZV-seropositive cadavers. The age, sex, clinical history, and time from death to autopsy are listed. The number of neurons and satellite cell clumps analyzed for VZV gene 29 are indicated for each autopsy sample. Assuming that the VZV DNA in a PCR tube originated from a single VZV DNA-containing neuron, the data indicates that 34 of 2226 neurons contained VZV DNA. This assumption is based upon the low prevalence of VZV-positive neurons found in the analysis of single neurons. In the 12 individuals studied, the frequency of latency was consistently low, ranging from 0.8 to 3.8%, with a mean of 1.7% ± 0.86%. No tubes of satellite cell clumps, representing approximately 20,700 satellite cells, contained VZV DNA. To verify the authenticity of the VZV amplicon and test the sensitivity of the PCR method, Southern blots and nested-set PCRs were performed on samples from representative ganglia. A Southern blot of samples from ganglion HG-93 is shown in Fig. 3A and B. Nested PCRs of samples from ganglia HG-86 and HG-95 are shown in Fig. 3C and D. Nested PCRs of 139 samples of neurons from four autopsies detected only one additional positive sample not found by regular PCR. We conclude that Southern blots and nested PCR did not appreciably alter the conclusions from Table 1.
TABLE 1.
VZV PCR of separated neurons and satellite cells
| Specimena | Age (yr)/sexb | Cause of death | Time from death to autopsy (h)g | Results for:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Neurons
|
Satellite cells
|
||||||||||
| No. of tubes testedd | No. of cells tested | No. positive | Latent % of fractionh | No. of tubes tested | Total no. of clumps | Total no. of cellse | No. positive | ||||
| HG-70 | 71/M | CHFc | 6 | 2 | 26 | 1 | 3.8 | 13 | 46 | 2,484 | 0 |
| HG-76 | 90/F | Pneumonia | 3 | 34 | 340 | 4 | 1.2 | 18 | 38 | 2,052 | 0 |
| HG-79 | 49/F | Carcinoma | 14 | 22 | 220 | 6 | 2.7 | 18 | 90 | 4,860 | 0 |
| HG-82 | 85/M | Lymphoma | 16 | 22 | 220 | 2 | 0.9 | 6 | 30 | 1,620 | 0 |
| HG-83 | 64/M | Alzheimer's | Unknown | 12 | 120 | 1 | 0.8 | NDf | |||
| HG-88 | 66/M | Chronic leukemia | 15 | 18 | 180 | 3 | 1.7 | 18 | 90 | 4,860 | 0 |
| HG-91 | 72/M | Peritonitis | 21 | 22 | 220 | 3 | 1.4 | 18 | 90 | 4,860 | 0 |
| HG-93 | 28/M | Lymphoma | 18 | 18 | 180 | 4 | 2.2 | ND | |||
| HG-95 | 66/F | CHF | 6 | 18 | 180 | 2 | 1.1 | ND | |||
| HG-97 | 44/M | Cystic fibrosis | 22 | 18 | 180 | 3 | 1.7 | ND | |||
| HG-100 | 59/M | CHF/diabetes | 16 | 18 | 180 | 3 | 1.7 | ND | |||
| HG-101 | 61/M | Chronic leukemia | 20 | 18 | 180 | 2 | 1.1 | ND | |||
| Total | 222 | 2,226 | 34 | 91 | 384 | 20,736 | 0 | ||||
A fragment of each ganglion was demonstrated by PCR to contain VZV DNA prior to cell separation. Serum from each autopsy contained VZV-specific antibody.
Numbers indicate age at death (average, 62.9 years). M, male; F, female.
CHF, congestive heart failure.
Contained 10 neurons per tube except for HG-70 (10 and 16 cells).
Each clump of satellite cells contained a mean of 54 cells (21).
ND, not done.
Average, 14.3 h.
Average ± standard deviation, 1.7% ± 0.86%.
FIG. 3.
Southern analyses and nested PCR of VZV gene 29. (A) Direct PCR of 18 samples containing 10 neurons prepared from ganglion HG-93. (B) A Southern blot of four PCR-positive samples (lanes 2, 9, 11, and 17) shown in panel A demonstrated corresponding positive bands. (C) Direct PCR of 10 neuron samples from ganglia HG-86 (lanes 2 to 11) and HG-95 (lanes 12 to 19). (D) A nested PCR from the direct PCR shown in panel C with primers n-VZV29j (5′-ACTCACTACCAGTCATTTCTATC-3′) and n-VZV29k (5′-GTATTTTCTGGCTCTAATCCAAG-3′) amplified DNA fragments of 213 nucleotides. +C indicates the appropriate positive control for each panel. M indicates the molecular size markers. The sizes for marker bands of 200 (⇒) and 400 (➞) nucleotides are indicated.
The VZV DNA detected by PCR was intracellular and did not reflect extracellular contamination, as demonstrated by the absence of detectable VZV DNA prior to cell lysis (1). This is further demonstrated by the absence of VZV DNA in most of the tubes from the single cell experiment, as well as by the absence of VZV DNA in satellite cells.
Amount of VZV DNA in single cells.
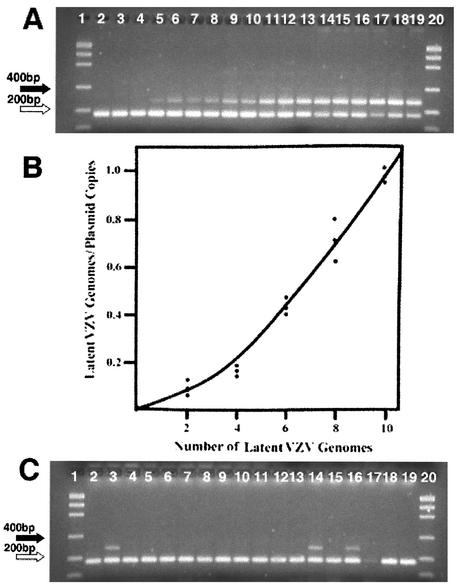
As was observed in the study of HSV latency, the band intensity for VZV DNA varied in 10 neuron samples analyzed by PCR (Fig. 3A) and by Southern blotting (Fig. 3B). These observations suggested that latently infected cells contained different amounts of viral DNA. The quantity of VZV DNA in single neuron samples was determined by quantitative competitive PCR. A calibration curve was determined with aliquots of latent VZV in neurons (Fig. 4A and B). A representative quantitative analysis (HG-100) is shown in Fig. 4C. These measurements show that the quantity of VZV DNA in a neuron is low. The variation observed within and between ganglia from four autopsies is narrow (Table 2).
FIG. 4.
Quantitative competitive PCR. A preliminary quantitative competitive PCR experiment was carried out to establish the amount of VZV gene 29 DNA in a pooled sample of VZV-positive neurons. This pooled sample was used as the latent VZV standard, and aliquots corresponding to 2, 4, 6, 8, and 10 copies of the latent VZV gene 29 were added to samples containing 10 copies of the internal VZV plasmid standard. The resulting competitive PCR is shown in panel A. The results from the quantitative scan of panel A were used to generate the curve shown in panel B. This curve was used to determine the content of latent VZV gene 29 in multiple samples of 10 neurons. A representative quantitative competitive PCR for 18 tubes containing 10 neuron samples is shown in panel C. Three tubes contained VZV DNA, and the ratio of the band intensities was utilized to determine the copy number. These data are included in Table 4 (as HG-100). The sizes for marker bands of 200 (⇒) and 400 (➞) nucleotides are indicated.
TABLE 2.
Quantitative VZV PCR on neurons from human trigeminal ganglia
| Ganglion | % Latencya | No. of genomes/neuronb |
|---|---|---|
| HG-88 | 1.7 | 6, 9, 6 (7) |
| HG-95 | 1.1 | 4, 4 (4) |
| HG-100 | 1.7 | 2, 4, 3 (3) |
| HG-101 | 1.1 | 5, 4 (4.5) |
| Avg | 1.4 | 4.7 |
Proportion of neurons with VZV DNA (Table 1).
Genome copy numbers in single neurons from the same ganglion are reported. The mean is in parentheses.
Presence of latent VZV and HSV in neurons as a function of neuronal size.
Neurons from 3 ganglia varied in size from 325 to 6,446 μm2. An arbitrary classification of neurons into small (325 to 2,502 μm2), medium (953 to 3,078 μm2), and large (2,031 to 6,446 μm2) classifications resulted in an average size distribution in minced ganglia from 4 autopsies of 19% small, 55% medium, and 26% large neurons. PCR analyses of ganglia from these three autopsies showed VZV DNA was present in 13 of 770 large neurons, 4 of 740 medium neurons, and 1 of 740 small neurons (Tables 3 and 4). PCR analysis of ganglia from one autopsy detected HSV DNA in 3 of 180 large neurons, 5 of 360 medium neurons, and 6 of 180 small neurons. The presence of HSV in neurons of all size classifications is in agreement with our earlier study (1), but there appears to be a tendency towards a higher frequency of HSV latency in neurons within the small classification.
TABLE 3.
Size distribution of neurons
| Ganglion | % of neurons of sizea (area [μm2]):
|
||
|---|---|---|---|
| Small (325-1,502) | Medium (983-3,078) | Large (2,031-6,446) | |
| HG-98 | 21 | 46 | 31 |
| HG-99 | 20 | 47 | 33 |
| HG-105 | 14 | 68 | 18 |
| HG-108 | 20 | 58 | 22 |
Area ranges were generated from size measurements made on HG-98, HG-99, and HG-105.
TABLE 4.
Size and frequency of VZV-positive neurons
| Ganglion | % (no. of PCR-positive cells/total no. of cells) of neurons of size (area [μm2]):
|
||
|---|---|---|---|
| Small (325-1,502) | Medium (983-3,078) | Large (2,031-6,446) | |
| VZV-positive ganglia | |||
| HG-99 | 0 (0/320) | 0.3 (1/320) | 1.3 (4/320) |
| HG-100 | 0.6 (1/180) | 0.6 (1/180) | 2.2 (4/180) |
| HG-108 | 0 (0/240) | 0.4 (1/240) | 1.9 (5/270) |
| Total | 0.1 (1/740) | 0.5 (4/740) | 1.7 (13/770)a |
| HSV-positive ganglion HG-108 | 3.3 (6/180)b | 1.4 (5/360)c | 1.7 (3/180)c |
The proportion of large neurons with VZV DNA is significantly different from the proportions of medium or large neurons with VZV DNA (by analysis of variance, P = 0.0025).
The proportion of small neurons with HSV DNA is significantly different from the proportion of small neurons with VZV DNA (by chi-square test, P = 0.0044).
The proportion of medium and large neurons with HSV DNA is not significantly different from the proportion of medium and large neurons with VZV DNA (P > 0.2).
Relationship of latent VZV and HSV in neurons.
Thirty-eight samples, each containing 10 isolated neurons, were lysed, and one-half were analyzed for the presence of VZV DNA and one-half were analyzed for the presence of HSV DNA. The VZV genome was present in 6 of the tubes, consistent with 6 neurons of 380 (1.6%) containing latent VZV. Similarly, 7 of the tubes of neurons contained HSV DNA, suggesting that 1.8% of neurons contained latent HSV. One of these tubes had both HSV and VZV DNA. This is the expected result in a tube with 10 neurons if the presence of an HSV genome in a neuron does not significantly increase the likelihood that a VZV genome would also be present in that neuron.
DISCUSSION
While primary infection with two members of the alphaherpesvirus family, HSV and VZV, leads to different diseases, both viruses establish latency in neurons (7, 30, 31). The site of HSV latency is exclusively in the neuron (7, 31, 32). Previous publications based on semiquantitative evaluation of human ganglia have determined the proportion of neurons containing VZV DNA to be <0.3% (13), 2 to 7% (14, 16), or the majority (12), and using calculations based on estimates of numbers of neurons, the number of VZV copies per neuron has been calculated to be 2 to 16 (4, 18, 27). While recent publications indicate that neurons are the predominant site of VZV latency, the close association between satellite supporting cells and neurons makes it difficult to completely eliminate satellite cells as a secondary site of latency (16, 21, 30). Our experiments address this question directly by using a fractionation procedure that yields highly purified populations of neurons and satellite cells. Our sensitive PCR assay detected VZV DNA in 1.7% ± 0.86% of the isolated neurons and did not detect VZV DNA in >20,700 nonneuronal cells. These data indicate that VZV, like HSV, is latent only in neurons and provide an incentive for identifying neuronal subtypes containing VZV genomes.
The proportion of latently infected neurons varied slightly among ganglia, but the mean was similar to that determined by in situ PCR (16), by an alternative fractionation procedure based on selective filtration (18), and by calculation from real-time PCR analysis of nucleic acids extracted from ganglionic tissue (4, 27). The procedure utilized in this paper is distinct from the methods listed above, since it is based on individual neurons studied in the absence of other types of cells and makes no assumptions about the frequency of viral latency.
Using the same fractionation method, we reported previously that the proportion of HSV latently infected neurons ranged from 2 to 7% with a mean value of 3% (1). The proportion of VZV-positive neurons is lower than that determined for HSV-positive neurons, but should be considered a minimal value, since neurons that contain one or two VZV genome copies would not give a positive PCR signal in 50% of the assays. However, nested-set PCR and Southern blot analyses gave the same frequency of VZV latency as that found with direct PCR analyses. We observed that the proportion of VZV latently infected neurons varied slightly among ganglia, with the range of 0.8 to 3.8% and a mean value of 1.7% ± 0.86%. As with HSV, it is possible that the latency burden of VZV varies with the severity of the primary illness (29). Consistent with this possibility is the reported variation in virus load during the viremic stage of acute VZV infection (6, 25).
The number of VZV genome copies per latently infected neuron was 2 to 9 genome copies (mean 4.7). These values are similar to, but somewhat lower than, those determined by other methods and also lower than those found for HSV by using isolated neurons (1). The low abundance and narrow range in VZV genome copy number suggest that VZV replication is limited at the time that latency is established. Our results differ from the reports of Pevenstein et al. (27) and Cohrs et al. (4), who found a wider range of VZV DNA concentration per ganglion based on DNA measurements. Assuming 1% of the cells in ganglia are neurons (17) and 2% of neurons are latent for VZV, the data in the paper by Pevenstein et al. indicate that the VZV DNA content per latently infected cell ranges from 2 to 50 genome copies, with a mean value of 13.2 ± 2 copies per cell (27). Using the same assumptions, the data presented by Cohrs et al. for VZV gene 63 indicate a range of 14 to 1,390 copies per cell, with a mean value of 225 copies per cell (4). The differences between the data reported in this paper and the earlier reports could be due to the methods of analysis or our small sample size. The quantitative PCR method used to obtain the data shown in Table 2 and the real-time PCR method used by Cohrs et al. are equally sensitive (data not shown). Our preliminary analyses of 8 additional ganglia indicate that the VZV copy number remains low, at an average of 5.3 copies per neuron.
As pointed out by Cohrs et al., the high values found in a few samples could be due to a high frequency of latency or virus reactivating from latency. However, Table 1 indicates that the proportion of VZV-containing neurons were similar, regardless of the cause of death or the interval from death to autopsy.
The present procedure allows fractionation of neurons on the basis of size. Microscopic measurements show that neurons in filtered and sedimented preparations range from 325 to 6,446 μm2 and that filtration did not lead to the loss of the larger neurons (data not shown). Analysis of neurons on the basis of size shows that the VZV genome is present predominantly in the large (2,031 to 6,446 μm2) neurons. This observation suggests that the establishment of VZV latency is not random and occurs in a subset of neurons. We did not observe this preference for large neurons in our study of HSV latency. This difference between the two alphaherpesviruses is consistent with our observation that the HSV and VZV genomes are rarely latent in the same neuron and argues against a unique neuronal type that is used by both alphaherpesviruses to establish latency at the time of primary infection. Moreover, the predilection of VZV for large neurons (representing 26% of trigeminal ganglion neurons) suggests that these neurons may have unique properties such as a higher density of VZV-tropic surface receptors or genetic elements that facilitate latency.
The frequency of recurrence is different between the two alphaherpesviruses. This may reflect the type of neuron in which latency is maintained, the number of neurons infected, or the expression of viral genes in latently infected cells. Our data indicate that different types of neurons are preferentially infected by each of these viruses and that the proportions of neurons that establish latency are somewhat different.
Acknowledgments
We thank Stephanie Fischer for excellent editorial assistance and preparation of the manuscript, Sharon Kleibenstein, Department of Pathology, for help with autopsy material, and Steven Straus and Randall Cohrs for critical readings of the manuscript and thoughtful comments.
These studies were supported by the Louis and Sidelle Bruckner Memorial Fund.
REFERENCES
- 1.Cai, G. Y., L. I. Pizer, and M. J. Levin. 2002. Fractionation of neurons and satellite cells from human sensory ganglia in order to study herpesvirus latency. J. Virol. Methods 104:21-32. [DOI] [PubMed] [Google Scholar]
- 2.Cohrs, R. J., M. Barbour, and D. H. Gilden. 1996. Varicella-zoster virus (VZV) transcription during latency in human ganglia: detection of transcripts mapping to genes 21, 29, 62, and 63 in a cDNA library enriched for VZV RNA. J. Virol. 70:2789-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohrs, R. J., M. B. Barbour, R. Mahalingam, M. Wellish, and D. H. Gilden. 1995. Varicella-zoster virus (VZV) transcription during latency in human ganglia: prevalence of VZV gene 21 transcripts in latently infected human ganglia. J. Virol. 69:2674-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohrs, R. J., J. Randall, J. Smith, D. H. Gilden, C. Dabrowski, H. van Der Keyl, and R. Tal-Singer. 2000. Analysis of individual human trigeminal ganglia for latent herpes simplex virus type 1 and varicella-zoster virus nucleic acids using real-time PCR. J. Virol. 74:11464-11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Croen, K. D., J. M. Ostrove, L. J. Dragovic, and S. E. Straus. 1988. Patterns of gene expression and sites of latency in human nerve ganglia are different for varicella-zoster and herpes simplex viruses. Proc. Natl. Acad. Sci. USA 85:9773-9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Jong, M. D., J. F. Weel, T. Schuurman, P. M. Wertheim-van Dillen, and R. Boom. 2000. Quantitation of varicella-zoster virus DNA in whole blood, plasma, and serum by PCR and electrochemiluminescence. J. Clin. Microbiol. 38:2568-2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Efstathiou, S., A. C. Minson, H. J. Field, J. R. Anderson, and P. Wildy. 1986. Detection of herpes simplex virus-specific DNA sequences in latently infected mice and in humans. J. Virol. 57:446-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forster, E. 1994. An improved general method to generate internal standards for competitive PCR. BioTechniques 16:18-20. [PubMed] [Google Scholar]
- 9.Furuta, Y., T. Takasu, S. Fukuda, K. C. Sato-Matsumura, Y. Inuyama, R. Hondo, and K. Nagashima. 1992. Detection of varicella-zoster virus DNA in human geniculate ganglia by polymerase chain reaction. J. Infect. Dis. 166:1157-1159. [DOI] [PubMed] [Google Scholar]
- 10.Furuta, Y., T. Takasu, S. Suzuki, S. Fukuda, Y. Inuyama, and K. Nagashima. 1997. Detection of latent varicella-zoster virus infection in human vestibular and spiral ganglia. J. Med. Virol. 51:214-216. [DOI] [PubMed] [Google Scholar]
- 11.Gilden, D. H., B. K. Kleinschmidt-DeMasters, J. J. LaGuardia, R. Mahalingam, and R. J. Cohrs. 2000. Neurologic complications of the reactivation of varicella-zoster virus. N. Engl. J. Med. 342:635-645. [DOI] [PubMed] [Google Scholar]
- 12.Gilden, D. H., Y. Rozenman, R. Murray, M. Devlin, and A. Vafai. 1987. Detection of varicella-zoster virus nucleic acid in neurons of normal human thoracic ganglia. Ann. Neurol. 22:377-380. [DOI] [PubMed] [Google Scholar]
- 13.Hyman, R. W., J. R. Ecker, and R. B. Tenser. 1983. Varicella-zoster virus RNA in human trigeminal ganglia. Lancet 2:814-816. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy, P. G., E. Grinfeld, and J. E. Bell. 2000. Varicella-zoster virus gene expression in latently infected and explanted human ganglia. J. Virol. 74:11893-11898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kennedy, P. G., E. Grinfeld, and J. W. Gow. 1999. Latent Varicella-zoster virus in human dorsal root ganglia. Virology 258:451-454. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy, P. G., E. Grinfeld, and J. W. Gow. 1998. Latent varicella-zoster virus is located predominantly in neurons in human trigeminal ganglia. Proc. Natl. Acad. Sci. USA 95:4658-4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaGuardia, J. J., R. J. Cohrs, and D. H. Gilden. 2000. Numbers of neurons and non-neuronal cells in human trigeminal ganglia. Neurol. Res. 22:565-566. [DOI] [PubMed] [Google Scholar]
- 18.LaGuardia, J. J., R. J. Cohrs, and D. H. Gilden. 1999. Prevalence of varicella-zoster virus DNA in dissociated human trigeminal ganglion neurons and nonneuronal cells. J. Virol. 73:8571-8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lungu, O., P. W. Annunziato, A. Gershon, S. M. Staugaitis, D. Josefson, P. LaRussa, and S. J. Silverstein. 1995. Reactivated and latent varicella-zoster virus in human dorsal root ganglia. Proc. Natl. Acad. Sci. USA 92:10980-10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lungu, O., C. A. Panagiotidis, P. W. Annunziato, A. A. Gershon, and S. J. Silverstein. 1998. Aberrant intracellular localization of Varicella-Zoster virus regulatory proteins during latency. Proc. Natl. Acad. Sci. USA 95:7080-7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahalingam, R., P. G. Kennedy, and D. Gilden. 1999. The problems of latent varicella zoster virus in human ganglia: precise cell location and viral content. J. Neurovirol. 5:445-448. [DOI] [PubMed] [Google Scholar]
- 22.Mahalingam, R., M. Wellish, R. Cohrs, S. Debrus, J. Piette, B. Rentier, and D. H. Gilden. 1996. Expression of protein encoded by varicella-zoster virus open reading frame 63 in latently infected human ganglionic neurons. Proc. Natl. Acad. Sci. USA 93:2122-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahalingam, R., M. Wellish, D. Lederer, B. Forghani, R. Cohrs, and D. Gilden. 1993. Quantitation of latent varicella-zoster virus DNA in human trigeminal ganglia by polymerase chain reaction. J. Virol. 67:2381-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahalingam, R., M. Wellish, W. Wolf, A. N. Dueland, R. Cohrs, A. Vafai, and D. Gilden. 1990. Latent varicella-zoster viral DNA in human trigeminal and thoracic ganglia. N, Engl. J. Med. 323:627-631. [DOI] [PubMed] [Google Scholar]
- 25.Mainka, C., B. Fuss, H. Geiger, H. Hofelmayr, and M. H. Wolff. 1998. Characterization of viremia at different stages of varicella-zoster virus infection. J. Med. Virol. 56:91-98. [DOI] [PubMed] [Google Scholar]
- 26.Meier, J. L., R. P. Holman, K. D. Croen, J. E. Smialek, and S. E. Straus. 1993. Varicella-zoster virus transcription in human trigeminal ganglia. Virology 193:193-200. [DOI] [PubMed] [Google Scholar]
- 27.Pevenstein, S. R., R. K. Williams, D. McChesney, E. K. Mont, J. E. Smialek, and S. E. Straus. 1999. Quantitation of latent varicella-zoster virus and herpes simplex virus genomes in human trigeminal ganglia. J. Virol. 73:10514-10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saiki, R. K., S. Scharf, F. Faloona, K. B. Mullis, G. T. Horn, H. A. Erlich, and N. Arnheim. 1985. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 230:1350-1354. [DOI] [PubMed] [Google Scholar]
- 29.Sawtell, N. M., D. K. Poon, C. S. Tansky, and R. L. Thompson. 1998. The latent herpes simplex virus type 1 genome copy number in individual neurons is virus strain specific and correlates with reactivation. J. Virol. 72:5343-5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silverstein, S. J., and S. E. Straus. 2000. Pathogenesis of latency and reactivation, p. 123-141. In A. M. Arvin and A. A. Gershon (ed.), Varicella-zoster virus. Cambridge University Press, New York, N.Y.
- 31.Steiner, I., and P. G. Kennedy. 1995. Herpes simplex virus latent infection in the nervous system. J. Neurovirol. 1:19-29. [DOI] [PubMed] [Google Scholar]
- 32.Wagner, E. K., and D. C. Bloom. 1997. Experimental investigation of herpes simplex virus latency. Clin. Microbiol. Rev. 10:419-443. [DOI] [PMC free article] [PubMed] [Google Scholar]