Abstract
T-tropic (X4) and dualtropic (R5X4) human immunodeficiency virus type 1 (HIV-1) envelope glycoproteins kill primary and immortalized CD4+ CXCR4+ T cells by mechanisms involving membrane fusion. However, because much of HIV-1 infection in vivo is mediated by M-tropic (R5) viruses whose envelope glycoproteins use CCR5 as a coreceptor, we tested a panel of R5 and R5X4 envelope glycoproteins for their ability to lyse CCR5+ target cells. As is the case for CXCR4+ target cells, HIV-1 envelope glycoproteins expressed by single-round HIV-1 vectors killed transduced CD4+ CCR5+ cells in a membrane fusion-dependent manner. Furthermore, a CD4-independent R5 HIV-1 envelope glycoprotein was able to kill CD4-negative target cells expressing CCR5, demonstrating that CD4 is not intrinsically required for the induction of death. Interestingly, high levels of CD4 expression protected cells from lysis and syncytium formation mediated by the HIV-1 envelope glycoproteins. Immunoprecipitation experiments showed that high levels of CD4 coexpression inhibited proteolytic processing of the HIV-1 envelope glycoprotein precursor gp160. This inhibition could be overcome by decreasing the CD4 binding ability of gp120. Studies were also undertaken to investigate the ability of virion-bound HIV-1 envelope glycoproteins to kill primary CD4+ T cells. However, neither X4 nor R5X4 envelope glycoproteins on noninfectious virions caused death in primary CD4+ T cells. These results demonstrate that the interaction of CCR5 with R5 HIV-1 envelope glycoproteins capable of inducing membrane fusion leads to cell lysis; overexpression of CD4 can inhibit cell killing by limiting envelope glycoprotein processing.
Infection by human immunodeficiency virus type 1 (HIV-1) is characterized by the progressive loss of CD4+ T cells, resulting in AIDS (4, 15, 28, 33). Simian immunodeficiency virus (SIV) infection of some Old World monkeys also results in CD4+ T-cell loss and AIDS (19, 54). Infection by these viruses is persistent, with virus-producing cells contributing to a chronic viremia over the course of many years (23, 39, 41, 71). The cause of CD4+ T-cell depletion in vivo is still under debate (30). It has been suggested that generalized immune activation in HIV-1-infected individuals results in apoptosis of mostly uninfected CD4+ T lymphocytes (30a, 37, 38, 65). However, this apoptosis is also seen in CD8+ T cells and B lymphocytes (30, 37, 38, 65, 66), whose numbers are typically not decreased in HIV-1 infection (27, 28). Furthermore, viral loads do not correlate with levels of apoptosis (65, 66), but do correlate very tightly with CD4+ T-cell depletion and disease progression (11, 20, 59, 64, 78). Studies of virus and CD4+ T-cell turnover (41, 91) have further suggested that, compared with uninfected cells, HIV-1-producing cells exhibit very short half-lives (less than 3 days). The number of CD4+ T lymphocytes infected, destroyed, and replenished each day has been calculated to be on the order of 109 (41, 91). Plausible explanations for the loss of the virus-producing cells are immunologic clearance and cytopathicity of viral components.
Progressive immune system-mediated depletion of CD4+ T lymphocytes is unlikely to occur, based on several observations. First, all known cytolytic processes mediated by the immune system are dependent upon CD4+ helper T-cell activity. As these cells are depleted, immunologic lytic mechanisms should become less efficient. In contrast to this expectation, it has been shown that the rate of CD4+ T-cell depletion actually accelerates as the level of these cells drops below 200 per μl (92). Moreover, the turnover rate of virus-producing cells is not affected by the levels of CD4+ T cells (41, 91). In vivo research with animal models also disputes a major role for immunologic clearance in CD4+ T-cell destruction. Chimeric SIV-HIV (SHIV) viruses can dramatically deplete CD4+ T cells in rhesus macaques even when cytotoxic T-lymphocyte and antiviral antibody responses are undetectable (26, 45, 81). In fact, the pathogenic SHIVs are more resistant to antibody neutralization than nonpathogenic SHIVs (26, 45, 81). Poor antiviral immune responses are generally associated with more rapid CD4+ T-cell loss and disease induction in SHIV- and SIV-infected monkeys (19, 45, 54). These observations appear to be inconsistent with a model in which antiviral immune responses represent the primary mechanism underlying the loss of CD4+ T cells.
Many studies have suggested cytotoxic effects of HIV-1 proteins, including Tat, Vpr, Nef, and protease, in tissue culture systems (5, 12, 36, 44, 56, 69, 70, 73, 74, 80, 85, 86, 89, 93). However, vpr- or nef-deleted HIV-1 still exhibits significant cytotoxicity in tissue culture, and SIV mutants lacking these genes are still capable of causing AIDS in some monkeys (2, 35, 42, 49, 50, 63). In other tissue culture models, efficient cell killing has been observed in the absence of these HIV-1 gene products (9, 51, 53).
The HIV-1 envelope glycoproteins gp120 and gp41 have been consistently implicated in viral cytopathic effects in vitro and in CD4+ T-cell depletion in vivo. The HIV-1 envelope glycoproteins mediate viral entry by binding to CD4 and chemokine receptors (1, 13, 17, 18, 21, 22, 29, 46, 61). After receptor binding, the envelope glycoproteins mediate the fusion of the viral and target cell membrane (40, 50, 84). The high-affinity interaction between the gp120 glycoprotein and CD4 provides an attractive explanation for the specific loss of the CD4+ subset of T lymphocytes during HIV-1 infection. It has been proposed that soluble gp120, perhaps in conjunction with anti-gp120 antibodies, can cross-link CD4 and/or coreceptors and induce apoptotic signaling within the target cell (3, 6, 14, 16, 77). It has been further postulated that virion-bound HIV-1 envelope glycoproteins could kill cells by the same mechanism (24, 25). However, work ex vivo with lymphoid tissue has shown that infectious HIV-1 can deplete CD4+ cells but inactivated virions with functional and conformationally intact envelope glycoproteins are unable to do so (37a, 37b, 62a, 88).
Many studies have implicated the ability of the HIV-1 envelope glycoproteins to fuse membranes in viral cytopathic effects in tissue culture cells. One form of HIV-1 cytopathic effect is the formation of multinucleated giant cells termed syncytia (58, 82). Syncytia are formed when the surface envelope glycoproteins on infected cells mediate fusion with adjacent cells expressing CD4 and coreceptors. HIV-1 isolates have been classified as either syncytium inducing, which corresponds to the ability to use CXCR4 as a coreceptor, or non-syncytium inducing, indicating the utilization of CCR5 as a coreceptor (1a, 31, 49a, 79a, 89a). With the discovery of the coreceptors, it became clear that both syncytium-inducing and non-syncytium-inducing isolates of HIV-1 are able to form syncytia, provided that the target cells express CD4 and the appropriate coreceptor (8, 13).
Although syncytia can be identified in the tissues of HIV-1-infected individuals, they are not a prominent or universal histopathologic feature (55, 57, 62, 79). Nonetheless, the emergence of R5X4 and X4 syncytium-inducing viruses correlates with rapid disease progression in HIV-1-infected humans, and SHIVs with X4 HIV-1 envelope glycoproteins cause more rapid and dramatic CD4+ T-cell depletion than SHIVs with R5 envelope glycoproteins (10, 60, 67, 75). This may relate to the expression of CCR5 primarily on the typically small population of activated T lymphocytes (7). Infection of monkeys with R5 SIV or SHIV isolates results in the destruction of this CCR5-positive subset of CD4+ T lymphocytes, which are abundant in the gut-associated lymphoid tissue but less abundant in other lymphoid organs (39a, 52, 90, 90a). X4 and R5X4 isolates can infect a much larger proportion of the CD4+ T cells because CXCR4 is constitutively expressed even on resting T cells (7). This situation is mimicked in lymph node explants, in which R5 HIV-1 infects and causes destruction of the small population of CCR5+ CD4+ T cells, whereas X4 isolates infect and cause more profound loss of CD4+ T cells due to the abundance of CXCR4+ cells (37a, 37b, 62a, 72a). Thus, it appears that both R5 and X4 HIV-1 isolates can deplete only CD4+ lymphocytes that express the coreceptor that is able to be utilized by the virus. These observations imply that the interactions between CD4 and the virus and/or viral components are insufficient to result in the death of the T cell in vivo and in organ culture.
The expression of X4 and R5X4 HIV-1 envelope glycoproteins results in the lysis of single cells that are CD4+ and CXCR4+ (9, 46a, 46b, 51, 59a). Most primary CD4+ T lymphocytes from the peripheral blood die as single cells when wild-type X4 or R5X4 HIV-1 envelope glycoproteins are expressed in them (51). However, mutant HIV-1 envelope glycoproteins that can bind receptors but cannot mediate membrane fusion are not cytopathic in these circumstances (9, 51). In systems where intracellular expression of the HIV-1 envelope glycoproteins efficiently lysed the cells producing the viral glycoproteins, bystander CD4+ T cells were not affected when cultured at moderate density (51). Apparently, membrane fusion events mediated through the intracellular interaction of X4 and R5X4 envelope glycoproteins with CD4 and CXCR4 lead to cell lysis, probably through physical disruption of important cellular membranes.
Membrane fusion mediated by HIV-1 envelope glycoproteins has also been implicated in the rapid destruction of CD4+ T lymphocytes that accompanies infection of rhesus monkeys with R5X4 SHIVs. SHIVs differing in a single envelope glycoprotein residue that altered membrane-fusing capacity exhibited comparable levels of virus replication in monkeys but depleted CD4+ T lymphocytes with different levels of efficiency (26a, 45). These observations support a role for envelope glycoprotein-mediated membrane fusion events in the destruction of CD4+ T cells in this monkey model.
Here we explored the cytopathic effects induced by R5 HIV-1 envelope glycoproteins in both CD4+ CCR5+ and CD4− CCR5+ cells. The use of CD4+ CCR5+ cells expressing different levels of CD4 revealed an unexpected inhibitory effect of high CD4 expression on envelope glycoprotein-mediated cell lysis. We also included a CD4-independent R5 envelope glycoprotein in these studies to determine if CD4 is intrinsically necessary for the induction of death. We investigated the contribution of the membrane-fusing capacity of the R5 envelope glycoproteins to single-cell lysis. Finally, we examined whether the HIV-1 envelope glycoproteins in the context of virions might induce cytotoxic events in primary T cells.
MATERIALS AND METHODS
Generation and culture of stable cell lines.
The adherent 293T and Cf2Th cells (American Type Culture Collection) were grown in Dulbecco's modified Eagle's medium-10% fetal calf serum with antibiotics. Human CCR5 and CD4 genes were cloned into pcDNA3.1(+) and pcDNA3.1(+)ZEO (Invitrogen), respectively. To create stable CCR5-expressing cells, 20 μg of pCCR5 DNA was transfected into 5 × 106 Cf2Th cells and selected for stable integration by culture in medium supplemented with 500 μg of G418 per ml (Gibco-BRL). High-expressing CCR5+ cells were identified by antibody staining of cell surface CCR5 with monoclonal antibody 2D7 (BD Pharmingen) and sorting on a MoFlo cell sorter. CD4+ CCR5+ cells were generated by transfecting the above CCR5+ cell line with the CD4-Zeo construct and selecting for stable integration in medium supplemented with 500 μg of G418 and 300 μg of zeocin per ml (Invitrogen). CCR5+ cells expressing high levels of CD4 (Cf2Th-CD4hi/CCR5+ cells) or low levels of CD4 (Cf2Th-CD4lo/CCR5 cells) were sorted on a MoFlo cell sorter with the Q4120 anti-CD4 antibody (Sigma).
Primary cell culture and purification.
Primary human CD4+ T cells were isolated from fresh blood with the RosetteSep negative selection system (StemCell Technologies). Human peripheral blood mononuclear cells were isolated from fresh blood by Ficoll-Paque (Amersham Pharmacia) density centrifugation. All isolated primary cells were cultured (106 cells/ml) in RPMI-10% fetal calf serum with antibiotics. Activation of primary cells was achieved through an initial 3-day stimulation with 1 μg of purified phytohemagglutinin per ml (Murex Biotech) and subsequent culturing with 10 U of recombinant human interleukin-2 per ml (Collaborative Biomedical Products).
Viral vectors.
Replication-defective HIV-1 vectors that were capable of expressing R5 HIV-1 envelope glycoproteins and green fluorescent protein (GFP) were constructed by modifying previously reported plasmids (72). The psrHIVenvGFP vector (51), in addition to the envelope glycoproteins and GFP, expresses the HIV-1 Tat, Rev, and Vif proteins and a C-terminally truncated, functionally defective Vpr protein derived from the HXBc2 strain. The KpnI-BamHI fragments of the ADA and YU2 envelope glycoproteins, derived from pSVIIIenv plasmids (40, 87), were cloned into the corresponding sites of this vector. The ADAΔV1/V2 envelope expressor plasmid was generated by site-directed PCR-mediated deletion, as previously reported (47). Phenylalanine 522 in each of these envelope glycoproteins was changed to tyrosine by PCR site-directed mutagenesis to create the corresponding F/Y mutants. The D368R mutant of the ADAΔV1/V2 envelope glycoprotein was generated in a similar manner. The psrHIVΔenvGFP and 89.6, KB9, and corresponding F/Y constructs were reported previously (51).
Recombinant viruses were produced by cotransfection of 293T cells with psrHIVenvGFP, pCMVΔP1ΔenvpA (72), pHCMV-G (94), and a Rev-expressing plasmid in a 10:10:2:1 ratio. The GFP reporter virus was made by cotransfecting the HIVecGFP (43), pCMVΔP1ΔenvpA, and pSVIIIenv plasmids in a 10:10:2 ratio. Genomeless particles were made by cotransfecting pCMVΔP1ΔenvpA and pSVIIIenv plasmids in a 10:2 ratio. Virions containing the major histocompatibility complex (MHC) class II molecules were made by additionally cotransfecting MHCIIα1 and MHCIIβ7 plasmids (76). At 12 h following transfection, the cells were washed and cultured in RPMI-10% fetal calf serum with antibiotics. Twenty-four hours later, conditioned medium containing recombinant viruses was harvested and filtered (0.45-μm pore size). Virus was titered with reverse transcriptase assays.
Monoclonal antibody staining of receptor molecules.
Cell lines were grown to sufficient density and then detached with 5 mM EDTA in phosphate-buffered saline. Cells were pelleted, washed, and resuspended in 95 μl of phosphate-buffered saline containing 2% fetal calf serum and 5 μl of monoclonal antibody (final antibody concentration was 10 nM). The monoclonal antibodies (and the target proteins) were 12G5 (CXCR4) (BD Pharmingen), 2D7 (CCR5) (BD Pharmingen), and Q4120 (CD4) (Sigma Chemical). All antibodies were directly conjugated to the phycoerythrin (PE) fluorochrome. Samples were incubated for 20 min at 4°C, washed, and analyzed by flow cytometry (Becton Dickinson FACScan).
Immunoprecipitation of HIV-1 envelope glycoproteins.
Cf2Th cells were transduced with equivalent amounts of titered virus, washed 24 h after transduction, and incubated in [35S]methionine-labeling medium depleted of cysteine and methionine. At 48 h after transduction, the cells were removed from the plates with 5 mM EDTA in phosphate-buffered saline and pelleted, and the supernatant was discarded. Cell pellets were resuspended in NP-40 lysis buffer (0.5% NP-40, 0.15 M NaCl, 10 mM Tris, pH 7.5) and rocked at 4°C for 30 min. Samples were centrifuged for 30 min at 14,000 rpm to pellet cellular debris. Cellular lysates were precipitated in the presence of unlabeled cell lysate with anti-HIV-1 patient serum and protein G-Sepharose beads at 4°C overnight. Immunoprecipitation was followed by one wash with lysis buffer, one wash with wash buffer (0.5% NP-40 and 0.5 M NaCl in phosphate-buffered saline), and one wash with lysis buffer. Pellets were resuspended in Tris-acetate loading buffer containing 5% β-mercaptoethanol and boiled for 5 min. Samples were run on a 3 to 8% acrylamide gradient gel and exposed to film. Quantitation of bands was performed with a densitometer and ImageQuant software.
Transduction of cells and viability assay.
To assess the effects of HIV-1 envelope glycoprotein expression on cell viability, 2 × 104 cells were plated in duplicate in six-well plates in 1.5 ml of medium (without selection agents) and cultured overnight. When cells had adhered, 10,000 reverse transcriptase cpm of each recombinant HIV-1 expressing envelope glycoproteins was added. The virus-cell mixture was incubated at 37°C for 6 h and washed with new medium. Starting at 48 h posttransduction, samples were collected by spinning the plates at 2,400 rpm for 5 min, removing the medium, and trypsinizing the cells. Plates for subsequent time points were generated by adding ≈10% of the trypsinized cells to 1.5 ml of medium in new six-well plates. The remaining sample was collected, fixed in 3.7% formaldehyde, and analyzed for enhanced GFP (EGFP) expression by flow cytometry. At 48 h after transduction by the recombinant HIV-1 vectors, approximately 35 to 40% of the Cf2Th cells were positive for EGFP expression (data not shown).
Syncytium formation assay.
To assess syncytium-forming ability in the experiments shown in Fig. 2, 105 293T cells were transduced with equal amounts of recombinant HIV-1 vectors expressing HIV-1 envelope glycoproteins. Twenty-four hours later, these cells were split into two groups, and each was cultured with 105 Cf2Th-CCR5 or Cf2Th-CD4hi/CCR5 cells. Twenty-four hours later, syncytia were counted by visual inspection with a Nikon TE300 inverted microscope. For the experiments shown in Fig. 3, 105 Cf2Th-CCR5 or Cf2Th-CD4hi/CCR5 cells were directly transduced with equal amounts of recombinant HIV-1 vectors expressing envelope glycoproteins. Forty-eight hours later, syncytia were counted visually and photographed with a Roper Scientific charge-coupled device camera.
FIG. 2.
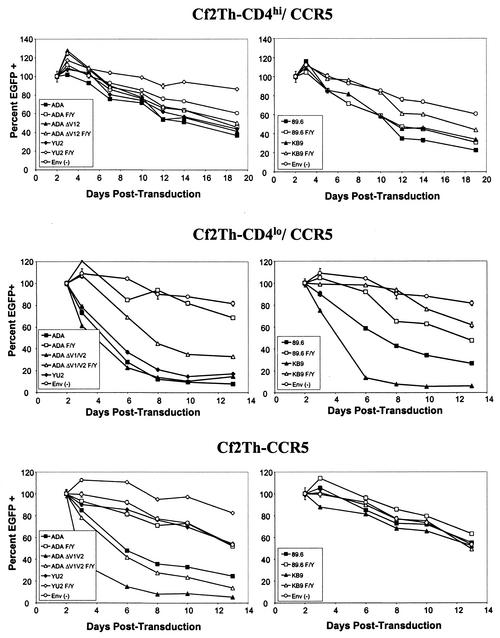
Effects of R5 and R5X4 HIV-1 envelope glycoprotein expression on viability of cells expressing CCR5 and different levels of CD4. The percentage of EGFP-positive cells in Cf2Th-CD4hi/CCR5 (upper row), Cf2Th-CD4lo/CCR5 (middle row), and Cf2Th-CCR5 (bottom row) cultures transduced by recombinant HIV-1 vectors expressing the indicated HIV-1 envelope glycoproteins or a control Env(−) vector is shown. The HIV-1 envelope glycoproteins in the experiments shown in the left column are R5 envelope glycoproteins, and those in the experiments shown in the right column are R5X4 envelope glycoproteins. The percentage of EGFP-positive cells in each culture 48 h after incubation with recombinant viruses was designated as 100%, and subsequent percentages were normalized to this value. The F522Y variants (designated F/Y) are also shown except for YU2 F/Y in the Cf2Th-CD4lo/CCR5 cultures, which had EGFP-positive percentages that exceeded the scale of the graph and were left out for the sake of clarity for the remaining samples.
FIG. 3.
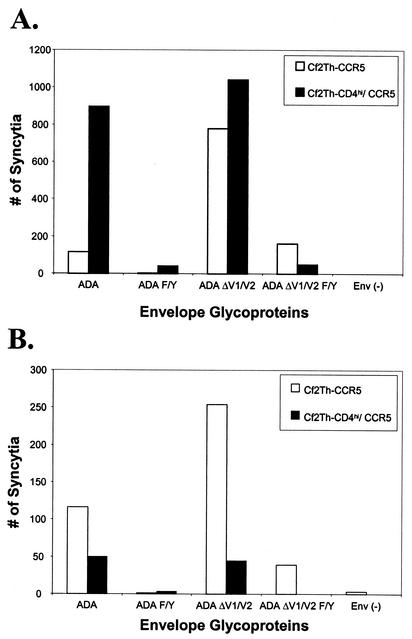
Ability of Cf2Th-CD4hi/CCR5 and Cf2Th-CCR5 cells to participate in syncytium formation. (A) The ability of Cf2Th-CD4hi/CCR5 (black bars) and Cf2Th-CCR5 cells (white bars) to serve as target cells for syncytium formation was examined. 293T cells were transduced with single-round HIV-1 vectors expressing the indicated envelope glycoproteins or with an Env(−) control vector. Twenty-four hours later, the 293T cells were split into two plates, and each was cocultivated at 37°C with either Cf2Th-CD4hi/CCR5 or Cf2Th-CCR5 cells. Twenty-four hours later, syncytia were counted by microscopy. (B) Syncytium formation resulting from HIV-1 envelope glycoprotein expression within Cf2Th-CD4hi/CCR5 and Cf2Th-CCR5 cells. Cf2Th-CD4hi/CCR5 and Cf2Th-CCR5 cells cultured at high density (105 cells in six-well plates) were transduced with recombinant HIV-1 vectors expressing the indicated HIV-1 envelope glycoproteins. The Env(−) control vector does not express HIV-1 envelope glycoproteins. Syncytia were scored 48 h later by light microscopy.
Apoptosis assay.
Recombinant HIV-1 vectors expressing EGFP were prepared as described above and inactivated by exposure to a UV source (GE G30T8 germicidal bulb [253.7-nm wavelength, 8-W UV output]) for 20 min at a distance of 2.5 ft. [ca. 75 cm]). Approximately 100,000 reverse transcriptase cpm of genomeless particles or UV-inactivated HIVecGFP virions were incubated in duplicate with 2 × 105 primary, activated CD4+ T cells in a total volume of 1 ml. This was equivalent to 100 ng of HIV-1 virus per ml as measured by the p24 enzyme-linked immunosorbent assay (New England Nuclear). Samples were collected at three time points and assayed for cytotoxic or apoptotic changes. The cells were spun down, and the supernatant was removed. The cells were incubated in 100 μl of phosphate-buffered saline with 250 ng of propidium iodide (Molecular Probes) for 15 min at room temperature and then washed. Cells were permeabilized with the Cytofix/Cytoperm kit (Pharmingen) for 15 min at 4°C and stained with the in situ cell death detection kit (Roche) at 37°C for 1 h. Apoptotic cells were quantified by fluorescence-activated cell sorting.
RESULTS
Ability of R5 and R5X4 HIV-1 envelope glycoproteins to kill CCR5+ cells.
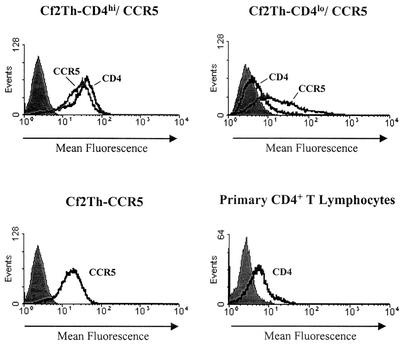
To test the capacity of R5 and R5X4 HIV-1 envelope glycoproteins to damage CCR5+ cells, we created three stable cell lines expressing HIV-1 receptors in Cf2Th dog thymocytes, which have no endogenous receptors that are usable by HIV-1 (13). Figure 1 shows the profiles of receptor expression in these cell lines; the expression of CD4 in activated primary human peripheral blood lymphocytes is shown for comparison. The Cf2Th-CD4hi/CCR5 and Cf2Th-CD4lo/CCR5 lines differed in their levels of CD4 expression by approximately 10-fold. The Cf2Th-CD4lo/CCR5 cells expressed CD4 at a level similar to that of primary blood lymphocytes. The Cf2Th-CD4hi/CCR5 and Cf2Th-CCR5 cells expressed comparable levels of CCR5 on their surface.
FIG. 1.
Receptor expression on cell lines used in the study. Cf2Th cell lines and primary human CD4+ peripheral blood lymphocytes were stained for expression of the CD4 glycoprotein and/or the CCR5 glycoprotein. Cf2Th-CD4hi/CCR5 and Cf2Th-CD4lo/CCR5 cells were stained for CD4 and CCR5 expression. The Cf2Th-CCR5 cells were stained only for CCR5 expression, and the primary blood lymphocytes were stained only for CD4. Staining for CCR5 expression was performed with the 2D7 antibody, and staining for CD4 expression was performed with the Q4120 antibody. An isotype-matched antibody was used as a negative control (shaded peaks) and yielded staining similar to that observed for parental Cf2Th cells stained with the Q4120 antibody (data not shown).
Each cell type was transduced in duplicate with a single-round HIV-1 vector coexpressing an HIV-1 envelope glycoprotein and the enhanced green fluorescent protein (EGFP) marker. Previous work has shown that the expression of both proteins in cells transduced by these vectors is very tightly correlated (51). At 48 h after transduction, independently transduced duplicate cultures were trypsinized and assayed for EGFP expression. This percentage of EGFP-positive cells, which ranged from 35 to 40% of the Cf2Th cells, was used as the starting point for the number of envelope glycoprotein-expressing cells in each culture and designated 100%. At subsequent time points, cultures were collected and assayed for the percentage of EGFP-expressing cells. We expected that any cytotoxicity of the envelope glycoproteins expressed by the vector would be accompanied by a decrease in the percentage of EGFP-expressing cells in the culture. Cultures were split throughout the observation period to minimize cell-cell contact; under these low-density conditions, syncytium formation was prevented (data not shown).
R5 envelope glycoproteins caused only very minimal decreases in the viability of the Cf2Th-CD4hi/CCR5 cells compared to the cells transduced with the control vector lacking the ability to express HIV-1 envelope glycoproteins (Fig. 2, upper left panel). A CD4-independent R5 envelope glycoprotein, ADAΔV1/V2, also exerted minimal effects on the viability of the Cf2Th-CD4hi/CCR5 target cells. The small effects of these envelope glycoproteins on the viability of the Cf2Th-CD4hi/CCR5 cells were reduced in cells transduced with the F522Y variants of the envelope glycoproteins, which are less competent at mediating membrane fusion (5a, 9, 51). R5X4 envelope glycoproteins did not significantly decrease the viability of the Cf2Th-CD4hi/CCR5 cells (Fig. 2, upper right panel). We conclude that the expression of R5 and R5X4 HIV-1 envelope glycoproteins in the Cf2Th-CD4hi/CCR5 cells results in minimal decreases in cell viability.
The Cf2Th-CD4hi/CCR5 cell line expresses CD4 at a higher level than that expressed on the surface of most primary lymphocytes (Fig. 1). We therefore investigated the effects of intracellular expression of R5 and R5X4 HIV-1 envelope glycoproteins on the viability of Cf2Th-CD4lo/CCR5 cells, which express more physiologic receptor levels. The wild-type R5 and R5X4 envelope glycoproteins all induced dramatic decreases in the percentage of EGFP-positive Cf2Th-CD4lo/CCR5 cells (Fig. 2, middle row), in contrast to the minimal effects observed in Cf2Th-CD4hi/CCR5 cells (Fig. 2, top row). Interestingly, the KB9 envelope glycoproteins, which are derived from a pathogenic R5X4 SHIV, caused significantly more rapid cell loss than the 89.6 envelope glycoproteins, which are derived from the closely related but nonpathogenic SHIV-89.6. The apparent half-life of the cells expressing the ADA, YU2, or KB9 envelope glycoproteins was approximately 2 days, whereas that of the cells expressing the 89.6 envelope glycoproteins was approximately 5 days.
The expression of the CD4-independent ADAΔV1/V2 envelope glycoproteins also caused significant loss of EGFP-positive Cf2Th-CD4lo/CCR5 cells. No syncytia were observed in the cultures demonstrating dramatic decreases in EGFP-expressing cells (data not shown). The F522Y alteration greatly reduced the ability of all of the envelope glycoproteins to deplete the transduced cells, supporting a role for membrane fusion in the induction of cytopathic effects in this setting. Most of the Cf2Th-CD4lo/CCR5 cells expressing the F522Y variants survived similarly to the control cells not expressing HIV-1 envelope glycoproteins. However, the ADAΔV1/V2 F/Y envelope glycoproteins, although less cytopathic than the ADAΔV1/V2 envelope glycoproteins, still caused a significant decrease in the percentage of EGFP-positive cells in the culture. Notably, the percentage of EGFP-positive cells expressing the YU2 F/Y envelope glycoproteins increased dramatically with time; this increase was so dramatic that the results are not shown to allow easier visualization of the results for the remaining envelope glycoproteins.
To examine whether CD4 was absolutely essential for the induction of cytotoxicity by the HIV-1 envelope glycoproteins, the experiments were repeated in CD4− Cf2Th-CCR5 cells. Significant decreases in the percentage of EGFP-positive cells accompanied the expression of some of the R5 HIV-1 envelope glycoproteins in Cf2Th-CCR5 cells, which do not express CD4 (Fig. 2, bottom row). The CD4-independent ADAΔV1/V2 envelope glycoproteins, which can fuse membranes after binding directly to CCR5, rapidly depleted transduced cells from the culture. The wild-type ADA envelope glycoproteins also caused significant cell loss in this setting, whereas the wild-type YU2, 89.6, and KB9 envelope glycoproteins did not induce decreases in EGFP-expressing cells compared with the controls. No syncytia were observed in the cultures, even in those exhibiting significant decreases in EGFP-positive cells over time (data not shown).
To demonstrate directly that single-cell lysis contributed to the loss of EGFP-positive cells, Cf2Th-CCR5 cells were transduced with the vector expressing the ADAΔV1/V2 envelope glycoproteins or a control vector not expressing HIV-1 envelope glycoproteins. EGFP-positive cells were sorted by fluorescence-activated cell sorting, plated at low density, and intermittently stained with propidium iodide. At 3 days following incubation with the recombinant virus expressing the ADAΔV1/V2 envelope glycoproteins, 65% of the Cf2Th-CCR5 cells were positive for propidium iodide staining (data not shown). No syncytia were observed in these cultures, even in propidium iodide-positive cells (data not shown). Cf2Th-CCR5 cells transduced with the control vector not expressing HIV-1 envelope glycoproteins exhibited, on average, less than 15% propidium iodide-positive cells (data not shown). The absolute numbers of EGFP-positive cells in these control cultures ranged from 5 × 105 to 8 × 105 on days 2 to 8 following transduction, whereas the number of EGFP-positive cells in the population exposed to the ADAΔV1/V2 envelope glycoprotein-expressing vectors remained below 4 × 104 during this period. These results indicate that single-cell lysis accounts for the decrease in the percentage of EGFP-expressing cells that accompanies the expression of functional HIV-1 envelope glycoproteins in this system.
The membrane fusion-defective version of the CD4-independent envelope glycoprotein, ADAΔV1/V2 F/Y, depleted EGFP-positive Cf2Th-CCR5 cells less efficiently than the ADAΔV1/V2 envelope glycoproteins (Fig. 2, bottom row). The F522Y change completely abolished the ability of the wild-type ADA envelope glycoproteins to deplete transduced Cf2Th-CCR5 cells. These results indicate that both a wild-type and a CD4-independent R5 envelope glycoprotein can cause decreases in viability in cells lacking CD4. This cytotoxic effect is dependent, at least in part, on the membrane-fusing ability of the envelope glycoproteins.
Ability of Cf2Th-CCR5 and Cf2Th-CD4hi/CCR5 cells to form syncytia.
Our observation that expression of several of the HIV-1 envelope glycoproteins resulted in the lysis of Cf2Th-CD4lo/CCR5 and Cf2Th-CCR5 cells but not Cf2Th-CD4hi/CCR5 cells was unexpected. Because membrane fusion has previously been linked to HIV-1 envelope glycoprotein-mediated cell death (9, 51) and appears to contribute to the destruction of Cf2Th-CD4lo/CCR5 and Cf2Th-CCR5 cells, we postulated that the Cf2Th-CD4hi/CCR5 cells might be less susceptible to such membrane fusion. To examine this possibility, we compared the ability of the Cf2Th-CCR5+ and Cf2Th-CD4hi/CCR5 cells to act as fusion partners in a syncytium formation assay. We transduced 293T cells with recombinant HIV-1 vectors expressing the viral envelope glycoproteins and cocultured them with either Cf2Th-CCR5 or Cf2Th-CD4hi/CCR5 target cells.
The ADA and ADAΔV1/V2 envelope glycoproteins formed numerous syncytia with Cf2Th-CD4hi/CCR5 target cells (Fig. 3A). The ADAΔV1/V2 envelope glycoproteins induced slightly fewer syncytia when the Cf2Th-CCR5 target cells were used, consistent with the expectation that expression of CD4 in the target cells is not necessary but can contribute to the function of these envelope glycoproteins (48). The wild-type ADA envelope glycoproteins induced the formation of some syncytia in Cf2Th-CCR5 target cells. Thus, although virus entry mediated by the ADA envelope glycoproteins is CD4 dependent, in the context of the cell-cell fusion assay, these glycoproteins can mediate some membrane fusion in the absence of CD4. These results demonstrate that both Cf2Th-CD4hi/CCR5 and Cf2Th-CCR5 cells are fully capable of participating in membrane fusion reactions mediated by the HIV-1 envelope glycoproteins.
The results in Fig. 3A show that the change of phenylalanine 522 to tyrosine reduced the syncytium-forming ability of the wild-type ADA and ADAΔV1/V2 envelope glycoproteins. However, some residual membrane-fusing capacity was retained by the ADAΔV1/V2 F/Y mutant glycoproteins. It is noteworthy that the number of syncytia induced by the different HIV-1 envelope glycoproteins in the Cf2Th-CCR5 target cells correlated very well with the degree of cytotoxicity observed when these cells were transduced with vectors expressing these envelope glycoproteins.
CD4 coexpression inhibits syncytium formation mediated by the HIV-1 envelope glycoproteins.
The above studies indicate that Cf2Th-CD4hi/CCR5 cells are at least as competent as fusion partners as the Cf2Th-CCR5 cells. To examine syncytium formation in a different context, Cf2Th-CD4hi/CCR5 and Cf2Th-CCR5 cells were directly transduced with HIV-1 envelope glycoprotein-expressing vectors and cultured at high density to promote the formation of syncytia. Figure 3B shows that the ADA and ADAΔV1/V2 envelope glycoproteins efficiently induced the formation of syncytia when expressed in Cf2Th-CCR5 cells. Syncytium formation following expression of these envelope glycoproteins in Cf2Th-CD4hi/CCR5 cells was much less efficient than in Cf2Th-CCR5 cells. The syncytia formed by the ADAΔV1/V2 envelope glycoproteins in Cf2Th-CD4hi/CCR5 cells were smaller than those formed in Cf2Th-CCR5 cells (data not shown). Each of the F522Y mutants was less effective at inducing cell-cell fusion than the corresponding envelope glycoprotein with a wild-type gp41 sequence (Fig. 3B). The CD4-dependent wild-type YU2, 89.6, and KB9 envelope glycoproteins formed small and infrequent syncytia only when expressed in Cf2Th-CD4hi/CCR5 cells, not in Cf2Th-CCR5 cells (data not shown). The results obtained in the syncytium formation assay in which the HIV-1 envelope glycoproteins were expressed within receptor-bearing cells resemble those observed in the assay for single-cell lysis in Fig. 2. This supports the notion that syncytium formation and single-cell lysis involve similar functions of the HIV-1 envelope glycoproteins and that the type of cell death resulting from envelope glycoprotein expression depends on variables such as the likelihood of cell-cell contact.
HIV-1 envelope glycoprotein binding to CD4 inhibits cell killing.
The above experiments suggest that CD4 expression is the inhibiting factor in the HIV-1 envelope glycoprotein's poor ability to lyse Cf2Th-CD4hi/CCR5 cells in the experiment shown in Fig. 2. To confirm this, we altered the CD4-binding site in the gp120 subunit of the ADAΔV1/V2 envelope glycoproteins. Specifically, the aspartic acid at position 368 of gp120, which interacts with arginine 59 of CD4, was changed to an arginine to ablate CD4 binding (47, 48). As the ADAΔV1/V2 envelope glycoproteins are CD4 independent, they retain the capacity to mediate membrane fusion even though CD4-binding ability is attenuated. Cf2Th-CCR5 and Cf2Th-CD4hi/CCR5 cells were transduced with recombinant HIV-1 vectors expressing EGFP and either the ADAΔV1/V2 or ADAΔV1/V2 D368R envelope glycoproteins or with a control vector containing an env deletion [Env(−)]. The cells were cultured at low density, and the percentage of EGFP-positive cells was used as an indicator of the viability of the transduced cells.
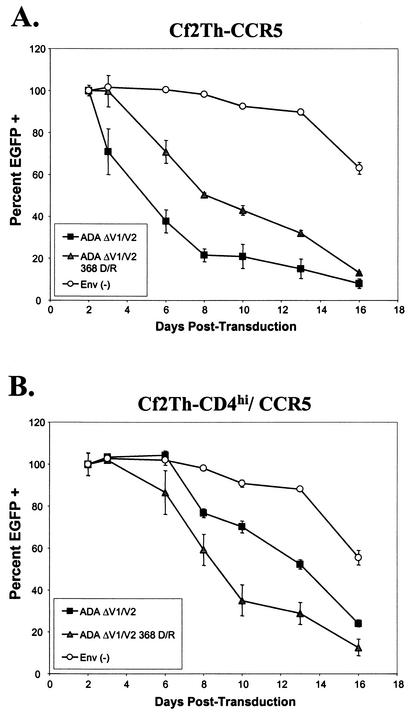
As seen previously, the expression of the ADAΔV1/V2 envelope glycoproteins in Cf2Th-CCR5 cells resulted in a rapid decrease in cell viability (Fig. 4A). The Cf2Th-CCR5 cells expressing the ADAΔV1/V2 D368R envelope glycoproteins were lysed at a rate slightly lower than that seen in the cells expressing the ADAΔV1/V2 envelope glycoproteins. Thus, the D368R change does not eliminate the cytotoxic capacity of the ADAΔV1/V2 envelope glycoproteins but may slightly reduce the efficiency of envelope glycoprotein functions relevant to the induction of cytopathic effects.
FIG. 4.
Increase in cytotoxicity of a CD4-independent HIV-1 envelope glycoprotein in CD4-expressing cells due to a decrease in CD4-binding ability. Cf2Th-CCR5 cells (A) and Cf2Th-CD4hi/CCR5 cells (B) were infected with recombinant HIV-1 vectors expressing either ADAΔV1/V2 or ADAΔV1/V2 D368R envelope glycoproteins or with a control vector that does not express HIV-1 envelope glycoproteins [Env(−)]. All of the vectors expressed EGFP. The percentage of EGFP-positive cells in the culture 48 h after transduction was considered 100%, and the percentages of EGFP-positive cells in each culture at subsequent time points were normalized to this value.
In Cf2Th-CD4hi/CCR5 cells, moderate decreases in cell viability were observed in cells expressing the ADAΔV1/V2 envelope glycoproteins compared with the control cells (Fig. 4B). In contrast to the results seen in the Cf2Th-CCR5 cells, the ADAΔV1/V2 D368R envelope glycoproteins exerted greater cytopathic effects than the ADAΔV1/V2 envelope glycoproteins in Cf2Th-CD4hi/CCR5 cells. Thus, a gp120 change that reduced CD4-binding affinity enhanced cell killing by a CD4-independent envelope glycoprotein only in the cells that expressed high levels of CD4. This is consistent with the hypothesis that CD4 expression exerts an inhibitory effect on HIV-1 envelope glycoproteins expressed in the same cell.
CD4 coexpression inhibits proteolytic cleavage of the HIV-1 gp160 envelope glycoprotein precursor.
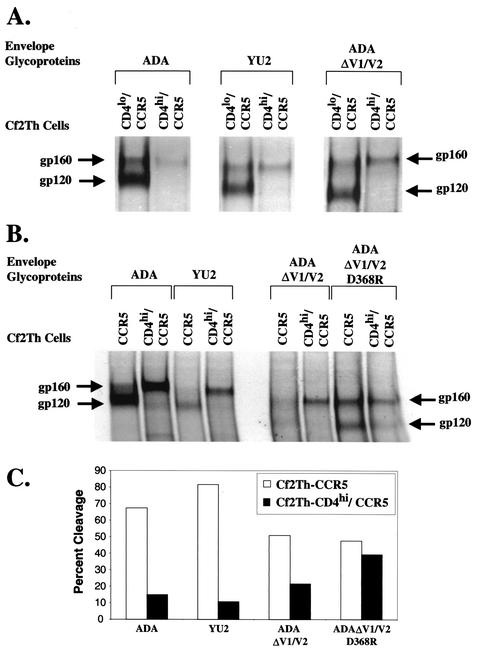
To understand the mechanism of CD4 inhibition of HIV-1 envelope glycoprotein-mediated cytotoxicity and syncytium formation, we immunoprecipitated the HIV-1 envelope glycoproteins that were expressed in Cf2Th-CD4hi/CCR5 cells. Figure 5A shows that ADA and YU2 envelope glycoproteins, when expressed in Cf2Th-CCR5 cells, were found mainly in the mature, proteolytically processed state, with a high ratio of gp120 to the gp160 precursor. However, when the same envelope glycoproteins were expressed in Cf2Th-CD4hi/CCR5 cells, the uncleaved, gp160 precursor form predominated. This was also true for the CD4-independent ADAΔV1/V2 envelope glycoproteins. The efficiency of proteolytic processing of the ADAΔV1/V2 envelope glycoprotein precursor was decreased in Cf2Th-CD4hi/CCR5 cells compared with that in Cf2Th-CCR5 cells (Fig. 5A and B). The alteration of aspartic acid 368 in the ADAΔV1/V2 D368R envelope glycoprotein, which reduces CD4-binding affinity, improved the level of precursor processing in the Cf2Th-CD4/CCR5 cells. These results suggest that the inhibitory effect of high levels of CD4 expression on HIV-1 envelope glycoprotein-mediated induction of cytopathic effects is due, at least in part, to a decrease in envelope precursor processing.
FIG. 5.
Decrease in HIV-1 envelope glycoprotein precursor processing by CD4 binding. Cf2Th-CD4lo/CCR5, Cf2Th-CD4hi/CCR5, and Cf2Th-CCR5 cells were infected with recombinant HIV-1 vectors expressing the indicated HIV-1 envelope glycoproteins. Twenty-four hours later, the cells were washed and labeled with [35S]methionine. Twenty-four hours later, the cells were lysed, and the lysates were used for immunoprecipitation by pooled sera from HIV-1-infected individuals. Precipitates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (A and B). The gp160 envelope glycoprotein precursor and the mature gp120 envelope glycoprotein are indicated. (C) The gel image in B was analyzed with ImageQuant software. The background present in each lane was determined and subtracted from the values obtained for each band of interest. Cleavage was calculated as [gp120/(gp120 + gp160)] × 100. Repetition of this experiment yielded similar results.
Induction of apoptosis by virion-bound HIV-1 envelope glycoproteins.
In addition to the ability of HIV-1 envelope glycoproteins to induce the lysis of cells in which they are expressed, it has been postulated that envelope glycoproteins may induce apoptosis when added exogenously to receptor-bearing cells. There is considerable disagreement in the literature on whether the death of “innocent bystander” cells occurs, either in HIV-1-infected cultures or in response to treatment of cells with HIV-1 envelope glycoproteins (3, 16, 24, 30, 34, 38, 51, 53, 77). Our previous studies showed that, in primary CD4-positive T lymphocytes expressing HIV-1 envelope glycoproteins, although considerable cell lysis was documented in the envelope glycoprotein-expressing cells, no toxic effects on bystander cells were observed under typical culture conditions (51). Only when cells were cultured at high density were bystander cells incorporated into syncytia and killed (51).
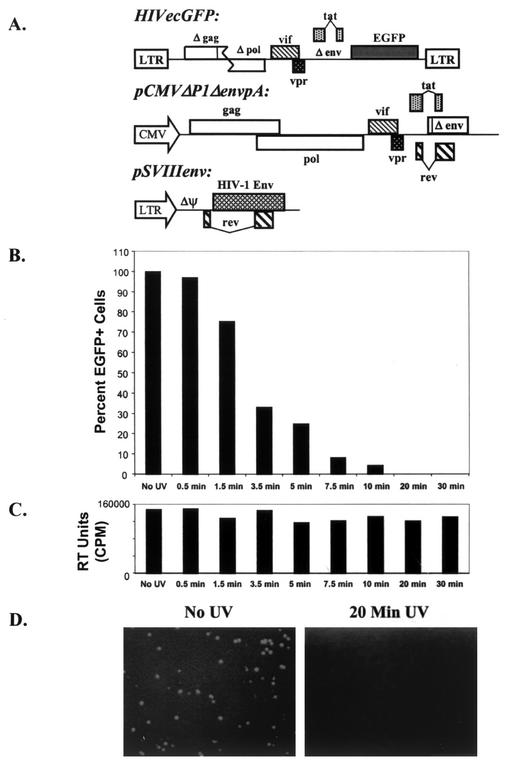
It is possible that the HIV-1 envelope glycoproteins could exert more significant toxic effects on bystander cells expressing receptors if the viral envelope glycoproteins were bound to virions. To examine this possibility, we used two approaches to produce replication-defective HIV-1 virions. In one approach, recombinant HIV-1 vectors expressing EGFP and containing HIV-1 envelope glycoproteins were produced by transfection and inactivated by UV irradiation. The plasmids used to produce these vectors are shown in Fig. 6A. The effects of UV irradiation on the ability of these vector preparations to transduce the EGFP gene into Jurkat T lymphocytes are shown in Fig. 6B and 6D. A 20-min exposure of the virus preparation to UV radiation resulted in complete loss of infectivity, as measured by EGFP expression in the target cells. The reverse transcriptase activity measured in pelleted virions following UV irradiation was preserved (Fig. 6C), indicating that the defects in UV-inactivated viruses were selective. This is consistent with the expectation that the integrity of nucleic acid is more sensitive to UV-induced damage than that of protein or lipid structures. In fact, addition of DNA repair enzymes to many UV-inactivated viruses can rescue their activity (32, 83). Thus, UV irradiation can generate noninfectious virion particles.
FIG. 6.
Generation of inactivated HIV-1 virions. (A) Virions that incorporated the HXBc2, HXBc2-P, 89.6, or KB9 envelope glycoproteins as well as the F522Y fusion-defective variants of these envelope glycoproteins were made by cotransfecting the three plasmids shown. The recombinant viruses were inactivated by UV irradiation. (B) Effect of the duration of UV treatment on the number of EGFP-positive cells that resulted from incubation of the viruses with Jurkat T lymphocytes is shown. (C) Effect of duration of UV irradiation on the reverse transcriptase (RT) activity measured in pelleted virions is shown. (D) Jurkat T lymphocytes were incubated with the recombinant HIV-1 vector containing the vesicular stomatitis virus G protein envelope glycoproteins either after no UV irradiation or after 20 min of UV irradiation. The EGFP-positive cells were visualized. Equivalent numbers of Jurkat cells were present in both fields (data not shown). Cells incubated with the recombinant HIV-1 vector expressing HXBc2 envelope glycoprotein showed similar results (data not shown).
In a second approach, replication-defective HIV-1 virions lacking the viral genome were generated. These particles were produced by transfection of 293T cells with the packaging plasmid pCMVΔP1ΔenvpA and the pSVIIIenv plasmid expressing the HIV-1 envelope glycoproteins. As neither plasmid contains a packaging signal, the virions produced will not efficiently package either RNA product. We expected that these virions would interact with receptor-bearing cells in a manner identical to that of wild-type HIV-1 but would not initiate infection.
The potential cytotoxicity of the defective HIV-1 virions was monitored by two methods. Apoptosis was measured by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick-end labeling (TUNEL), in which DNA degradation, a hallmark of apoptosis, is detected by the incorporation of fluorescent nucleotides into the DNA ends. Propidium iodide was also used to identify cells with a permeable membrane; such cells may represent necrotic cells or end-stage apoptotic cells. Thus, cell populations can be divided into TUNEL-negative/propidium iodide-negative (viable cells), TUNEL-positive/propidium iodide-negative (apoptotic cells), TUNEL-negative/propidium iodide-positive (necrotic cells), and TUNEL-positive/propidium iodide-positive (late-stage apoptotic cells).
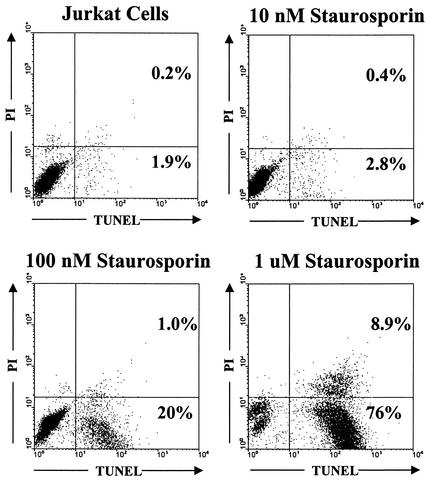
Figure 7 demonstrates the ability of the assay to detect apoptosis induced by treatment of Jurkat T lymphocytes with staurosporine, a kinase inhibitor that induces apoptosis (68). Staurosporine treatment resulted in a large increase in the percentage of TUNEL-positive cells in the culture, whereas the percentage of TUNEL-negative/propidium iodide-positive cells (representing necrotic cells) did not change. This assay was used to determine the effect of virion-bound envelope glycoproteins on the viability of primary CD4+ T cells. CD4+ T cells were isolated from human blood by negative selection (51) and activated with phytohemagglutinin and interleukin-2. Approximately 100,000 reverse transcriptase cpm (≈100 ng of p24) of defective virions were added to these primary CD4+ T cells. Duplicate cultures were collected and stained at three time points after the addition of virions. As negative controls, supernatants collected from cells transfected with the pCMVΔP1ΔenvpA plasmid alone or with the pSVIIIenv plasmid alone were used. Medium from untransfected 293T cells was used as an additional control. Additionally, vesicular stomatitis virus G protein-pseudotyped virions were included to determine whether any effects seen were HIV-1 envelope glycoprotein specific. Incubation with defective HIV-1 virions lacking the viral genome and containing either fusion-competent or fusion-defective HIV-1 envelope glycoproteins did not result in toxic effects in primary CD4+ T cells above those observed in the negative controls (Fig. 8). Likewise, UV-inactivated HIV-1 particles did not induce toxic effects beyond those seen in negative controls (data not shown).
FIG. 7.
Assay for detecting cytotoxicity. Jurkat T lymphocytes were stained with propidium iodide (PI) and by TUNEL and analyzed by fluorescence-activated cell sorting. The results with untreated Jurkat cells are shown in the upper left panel. The other panels show the results obtained with Jurkat cells treated for 16 h with the indicated concentrations of staurosporine.
FIG. 8.
Effect of recombinant HIV-1 virions lacking genomic RNA on primary CD4+ T cells. To produce virions lacking genomic RNA, 293T cells were transfected with the pCMVΔP1ΔenvpA plasmid and, in some cases, with the pSVIIIenv plasmid expressing the indicated HIV-1 envelope glycoproteins or a plasmid expressing the vesicular stomatitis virus G protein glycoprotein. Virions lacking envelope glycoproteins (Gag/Pol), medium from cells transfected with plasmids expressing envelope glycoproteins (HXBc2 Env and vesicular stomatitis virus G protein Env), or medium from untransfected 293T cells (medium) were used as negative controls. CD4+ T cells were isolated by negative selection from human peripheral blood mononuclear cells and stimulated with phytohemagglutinin and interleukin-2 for 3 days. The cells were then incubated with recombinant HIV-1 virions lacking genomic RNA at 37°C. On the indicated days, a TUNEL assay was performed, with the results shown.
It has been shown that various cellular proteins can be incorporated into HIV-1 virions as they bud from the cell surface. These include complement receptors, adhesion molecules, and MHC class II molecules (25, 76). It has been reported that inclusion of MHC II proteins in HIV-1 virions augmented the ability to induce apoptotic changes in CD4+ T cells (25). Therefore, we cotransfected plasmids expressing MHC II α and β chains with our virion-expressing plasmids to generate virus particles that contain both envelope glycoproteins and MHC II proteins. We tested the ability of these virions to induce cell death in primary CD4+ T cells.
UV-inactivated virions produced from cells expressing high levels of HIV-1 envelope glycoproteins and MHC II proteins did not cause an increase in the percentage of TUNEL-positive or propidium iodide-positive cells in primary CD4+ T-cell cultures compared with the negative controls (data not shown). Similar results were obtained with virus particles lacking a genome (data not shown). In summary, under the conditions employed in our assay, virion-like particles did not cause significant levels of cell death in primary CD4+ T lymphocytes.
DISCUSSION
Studies on T-cell turnover in HIV-1-infected humans (41, 91) indicate that the virus-producing cells are short-lived. The half-life of these infected cells is independent of the immune status of the host (41) or the antiviral cytotoxic T-cell response (67a). Thus, virus-induced cytopathic effects likely contribute to the loss of virus-expressing CD4+ T cells in vivo. Studies of monkeys infected with SHIV variants demonstrate that the composition and membrane-fusogenic function of the viral envelope glycoproteins determine the efficiency with which CD4+ T cells are depleted, at a given level of virus replication (26a, 45). The expression of the HIV-1 envelope glycoproteins in tissue-cultured cells can result in the induction of both forms of viral cytopathic effect, syncytium formation and single-cell lysis (9). Both X4 and R5 HIV-1 envelope glycoproteins can induce the formation of syncytia in cells bearing CD4 and the appropriate receptor (13). Syncytia are not prominent features in the lymphoid organs of most HIV-1-infected individuals, so their role in CD4+ T-cell depletion in vivo is unknown (55, 57, 62, 79). Expression of wild-type X4 and R5X4 HIV-1 envelope glycoproteins in primary human CD4+ T lymphocytes results almost exclusively in the lysis of single cells (51). Only when these primary CD4+ cells are cultured at very high densities are syncytia induced (51). The ability of X4 and R5X4 HIV-1 envelope glycoproteins to lyse primary CD4+ T lymphocytes is dependent on the membrane-fusing capacity of these viral glycoproteins (51).
R5 isolates predominate early in the course of HIV-1 infections of humans and, in some patients, are found exclusively throughout infection (18a, 79a). Both the in vivo pathogenesis and tissue culture growth properties of R5 HIV-1 isolates are thought to be less robust than those of X4 or R5X4 viruses. The lower percentage of CCR5-expressing cells compared with those expressing CXCR4 in primary T-cell populations probably contributes to these phenotypes (1a, 89a). R5 HIV-1 envelope glycoproteins have been classified as non-syncytium-inducing based on their inability to fuse cells that express CD4 and CXCR4. However, R5 HIV-1 isolates kill CD4+ CCR5+ T cells in tissue culture (95) and in lymph node explants (37a, 37b, 62a). Here we examined the cytopathic properties of several R5X4 and R5 HIV-1 envelope glycoproteins in CCR5+ cells that expressed various levels of CD4.
In CCR5+ target cells that expressed low levels of CD4 (Cf2Th-CD4lo/CCR5), expression of the wild-type R5 and R5X4 HIV-1 envelope glycoproteins by the vector resulted in efficient single-cell lysis. A CD4-independent R5 envelope glycoprotein (ADAΔV1/V2) also lysed these target cells efficiently. The apparent half-lives of the cells expressing the wild-type and ΔV1/V2 envelope glycoproteins was approximately 2 days, which is similar to the estimated half-life of virus-producing cells in HIV-1-infected humans (41, 91). The lytic ability of all of the envelope glycoproteins was reduced by a single, conservative amino acid change (phenylalanine 522 to tyrosine) in the gp41 “fusion peptide.” This change significantly attenuates membrane-fusing capacity without affecting other HIV-1 envelope glycoprotein functions, including receptor binding (5a, 9). The impact of the F522Y change on single-cell lysis indicates that receptor binding and any consequent signaling events are not sufficient to kill the envelope glycoprotein-expressing cell. Of note, the KB9 envelope glycoproteins from a pathogenic SHIV caused more rapid cell lysis than the closely related 89.6 envelope glycoproteins, which are derived from a nonpathogenic SHIV (75). These envelope glycoproteins differ in the capacity to fuse cell membranes (26, 26a, 45), an observation consistent with the difference in single-cell lytic capacity and the deduced relationship of this property to cytopathic potential.
The F522Y change in the fusion peptide did not completely eliminate the membrane-fusing capacity of the ADAΔV1/V2 envelope glycoproteins. These envelope glycoproteins are CD4 independent, are particularly prone to neutralization by antibodies and soluble CD4, and initiate entry by directly binding CCR5 (47, 48). These properties led to the suggestion that the ADAΔV1/V2 envelope glycoproteins spontaneously negotiate the conformational transitions normally promoted by CD4 binding. The efficiency with which the ADAΔV1/V2 F/Y envelope glycoproteins are triggered by viral contact with target cell CCR5 may partially compensate for the inefficiency of the subsequent membrane fusion events. The resultant retention of some membrane-fusing capacity can therefore account for the observed ability of this envelope glycoprotein to lyse Cf2Th-CD4lo/CCR5 and CD4-negative Cf2Th-CCR5 cells. Lysis of the CD4-negative cells by the CD4-independent envelope glycoproteins demonstrates that CD4 is not absolutely necessary for single-cell killing. However, in a natural context, as CD4-independent HIV-1 envelope glycoproteins are thought to be rare, CD4 assists in virus attachment and serves as a trigger of events leading to membrane fusion and cell lysis.
The wild-type ADA envelope glycoproteins exhibited some ability to fuse CD4-negative, CCR5-positive membranes and to lyse CCR5-positive cells lacking CD4. This may indicate that these glycoproteins are slightly more prone to bypassing CD4 than some of the other primary R5 HIV-1 envelope glycoproteins.
When high levels of CD4 were coexpressed with the HIV-1 envelope glycoproteins, single-cell lysis was decreased compared with that mediated by the same envelope glycoproteins expressed in cells with lower CD4 levels. This unexpected observation apparently resulted from CD4 binding intracellularly to the envelope glycoproteins and limiting the efficiency of their proteolytic maturation, which is needed for membrane fusogenicity (63a). As proteolytic processing of the HIV-1 envelope glycoproteins occurs in the Golgi apparatus (84a), one mechanism by which high levels of CD4 could inhibit envelope glycoprotein function is prevention of movement along the secretory pathway from the endoplasmic reticulum to the Golgi network. The nascent HIV-1 envelope glycoproteins and CD4 first become competent for binding each other in the endoplasmic reticulum, and complexes of these proteins in the endoplasmic reticulum have been documented (16a, 43a, 46a, 46b). An ADAΔV1/V2 variant, D368R, which is significantly attenuated in its ability to bind CD4 (70a) lysed Cf2Th-CD4hi/CCR5 cells more efficiently than the parental ADAΔV1/2 envelope glycoproteins. This observation is consistent with a model in which high-affinity binding and saturation of HIV-1 envelope glycoprotein complexes by high levels of CD4 leads to retention in the endoplasmic reticulum.
The CD4 expression level in primary T lymphocytes is lower than that in the Cf2Th-CD4hi/CCR5 cell line, and thus, HIV-1 does not typically encounter receptor-mediated trapping of envelope glycoproteins to the degree seen in this cell line. The HIV-1 Vpu protein, which downregulates the level of CD4 by increasing its turnover in the endoplasmic reticulum, also helps to minimize this problem for viral envelope glycoprotein processing (45a, 92a). The HIV-1 Vpu protein was not expressed in the target cells by the vectors used in our study.
The available data allow the following picture of the sequence of events leading to HIV-1 envelope glycoprotein-mediated cell lysis to be assembled. After synthesis and folding in the endoplasmic reticulum, the envelope glycoprotein trimers and CD4 can bind to each other. If the level of CD4 expression is not extremely high, the complexes can be transported to the Golgi apparatus. Here, two important posttranslational modifications occur. First, sulfation of N-terminal tyrosines in CCR5 allows high-affinity binding to the envelope glycoprotein-CD4 complexes (26b). Second, proteolytic cleavage of the envelope glycoproteins occurs, activating their fusogenic potential (63a). Next, a process dependent on membrane fusion occurs that ultimately results in the necrotic lysis of the cell. The most plausible model would involve the creation of numerous pores in the membranes of the Golgi apparatus and, as exocytic transport occurs, of the plasma membrane as well. As membrane damage exceeds the repair capacity of the cell, death ensues.
Extracellular HIV-1 envelope glycoprotein binding to cell surface receptors has been proposed to trigger cytotoxic events through as yet unclear mechanisms (24, 25). We investigated this with inactivated virions containing functional HIV-1 envelope glycoproteins. Adding high concentrations of virions, more than are present in vivo even during acute HIV-1 viremia, to primary CD4+ T cells was not sufficient to induce any level of apoptosis over that of the negative controls. Incorporation of MHC II molecules into the virions has been suggested to increase the cytotoxicity of HIV-1 (25), but no specific effect of MHC II proteins was evident in our system. In summary, we found no evidence that noninfectious HIV-1 virions induce cytotoxic changes in innocent bystander cells. Therefore, all of our studies support a model in which death occurs exclusively in cells expressing the HIV-1 envelope glycoproteins and not in innocent bystanders, unless these cells are incorporated into lethal syncytia. These studies provide a basic understanding of a process that efficiently and specifically lyses CD4+ T lymphocytes in culture and provide a rationale for examining the in vivo role of this process in AIDS pathogenesis.
Acknowledgments
We thank Sheri Farnum and Yvette McLaughlin for manuscript preparation, Michelle LaBonte for helpful discussions, and Greg Babcock for technical assistance with immunoprecipitations. We also acknowledge Chengsheng Zhang and Dana Gabuzda for reagents and Geoffrey Holm for technical assistance with the TUNEL assay.
This work was supported by grants from the National Institutes of Health (AI24755 and a Center for AIDS Research award) and the Bristol-Myers Squibb Foundation. We also acknowledge the support of the late William F. McCarty-Cooper.
REFERENCES
- 1.Alkhatib, G., C. Combadiere, C. C. Broder, Y. Feng, P. E. Kennedy, P. M. Murphy, and E. A. Berger. 1996. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955-1958. [DOI] [PubMed] [Google Scholar]
- 1a.Asjo, B., L. Morfeldt-Manson, J. Albert, G. Biberfeld, A. Karlsson, K. Lidman, and E. M. Fenyo. 1986. Replicative capacity of human immunodeficiency virus from patients with varying severity of HIV infection. Lancet ii:660-662. [PubMed] [Google Scholar]
- 2.Baba, T. W., V. Liska, A. H. Khimani, N. B. Ray, P. J. Dailey, D. Penninck, R. Bronson, M. F. Greene, H. M. McClure, L. N. Martin, and R. M. Ruprecht. 1999. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat. Med. 5:194-203. [DOI] [PubMed] [Google Scholar]
- 3.Banda, N. K., J. Bernier, D. K. Kurahara, R. Kurrle, N. Haigwood, R. P. Sekaly, and T. H. Finkel. 1992. Crosslinking CD4 by human immunodeficiency virus gp120 primes T cells for activation-induced apoptosis. J. Exp. Med. 176:1099-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barre-Sinoussi, F., J. C. Chermann, F. Rey, M. T. Nugeyre, S. Chamaret, J. Gruest, C. Dauguet, C. Axler-Blin, F. Vezinet-Brun, C. Rouzioux, W. Rozenbaum, and L. Montagnier. 1983. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220:868-871. [DOI] [PubMed] [Google Scholar]
- 5.Bartz, S. R., and M. Emerman. 1999. Human immunodeficiency virus type 1 Tat induces apoptosis and increases sensitivity to apoptotic signals by up-regulating FLICE/caspase-8. J. Virol. 73:1956-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.Bergeron, L., N. Sullivan, and J. Sodroski. 1992. Target cell-specific determinants of membrane fusion within the human immunodeficiency virus type 1 gp120 third variable region and gp41 amino terminus. J. Virol. 66:2389-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berndt, C., B. Mopps, S. Angermuller, P. Gierschik, and P. H. Krammer. 1998. CXCR4 and CD4 mediate a rapid CD95-independent cell death in CD4(+) T cells. Proc. Natl. Acad. Sci. USA 95:12556-12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bleul, C. C., L. Wu, J. A. Hoxie, T. A. Springer, and C. R. Mackay. 1997. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc. Natl. Acad. Sci. USA 94:1925-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broder, C. C., D. S. Dimitrov, R. Blumenthal, and E. A. Berger. 1993. The block to HIV-1 envelope glycoprotein-mediated membrane fusion in animal cells expressing human CD4 can be overcome by a human cell component(s). Virology 193:483-491. [DOI] [PubMed] [Google Scholar]
- 9.Cao, J., I. W. Park, A. Cooper, and J. Sodroski. 1996. Molecular determinants of acute single-cell lysis by human immunodeficiency virus type 1. J. Virol. 70:1340-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cayabyab, M., G. B. Karlsson, B. A. Etemad-Moghadam, W. Hofmann, T. Steenbeke, M. Halloran, J. W. Fanton, M. K. Axthelm, N. L. Letvin, and J. G. Sodroski. 1999. Changes in human immunodeficiency virus type 1 envelope glycoproteins responsible for the pathogenicity of a multiply passaged simian-human immunodeficiency virus (SHIV-HXBc2). J. Virol. 73:976-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakrabarti, L., M. C. Cumont, L. Montagnier, and B. Hurtrel. 1994. Variable course of primary simian immunodeficiency virus infection in lymph nodes: relation to disease progression. J. Virol. 68:6634-6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, M., R. T. Elder, M. Yu, M. G. O'Gorman, L. Selig, R. Benarous, A. Yamamoto, and Y. Zhao. 1999. Mutational analysis of Vpr-induced G2 arrest, nuclear localization, and cell death in fission yeast. J. Virol. 73:3236-3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choe, H., M. Farzan, Y. Sun, N. Sullivan, B. Rollins, P. D. Ponath, L. Wu, C. R. Mackay, G. LaRosa, W. Newman, N. Gerard, C. Gerard, and J. Sodroski. 1996. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 85:1135-1148. [DOI] [PubMed] [Google Scholar]
- 14.Cicala, C., J. Arthos, A. Rubbert, S. Selig, K. Wildt, O. J. Cohen, and A. S. Fauci. 2000. HIV-1 envelope induces activation of caspase-3 and cleavage of focal adhesion kinase in primary human CD4(+) T cells. Proc. Natl. Acad. Sci. USA 97:1178-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clavel, F., D. Guetard, F. Brun-Vezinet, S. Chamaret, M. A. Rey, M. O. Santos-Ferreira, A. G. Laurent, C. Dauguet, C. Katlama, C. Rouzioux, et al. 1986. Isolation of a new human retrovirus from West African patients with AIDS. Science 233:343-346. [DOI] [PubMed] [Google Scholar]
- 16.Cloyd, M. W., J. J. Chen, P. Adeqboyega, and L. Wang. 2001. How does HIV cause depletion of CD4 lymphocytes? A mechanism involving virus signaling through its cellular receptors. Curr. Mol. Med. 1:545-550. [DOI] [PubMed] [Google Scholar]
- 16a.Crise, B., L. Buonocore, and J. K. Rose. 1990. CD4 is retained in the endoplasmic reticulum by the human immunodeficiency type 1 glycoprotein precursor. J. Virol. 64:5585-5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng, H., R. Liu, W. Ellmeier, S. Choe, D. Unutmaz, M. Burkhart, P. Di Marzio, S. Marmon, R. E. Sutton, C. M. Hill, C. B. Davis, S. C. Peiper, T. J. Schall, D. R. Littman, and N. R. Landau. 1996. Identification of a major coreceptor for primary isolates of HIV-1. Nature 381:661-666. [DOI] [PubMed] [Google Scholar]
- 18.Deng, H. K., D. Unutmaz, V. N. KewalRamani, and D. R. Littman. 1997. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature 388:296-300. [DOI] [PubMed] [Google Scholar]
- 18a.de Roda Husman, A. M., R. P. van Rij, H. Blaak, S. Broersen, and H. Schuitemaker. 1999. Adaptation to promiscuous usage of chemokine receptors is not a prerequisite for human immunodeficiency virus type 1 disease progression. J. Infect. Dis. 180:1106-1115. [DOI] [PubMed] [Google Scholar]
- 19.Desrosiers, R. C. 1990. The simian immunodeficiency viruses. Annu. Rev. Immunol. 8:557-578. [DOI] [PubMed] [Google Scholar]
- 20.Desrosiers, R. C., J. D. Lifson, J. S. Gibbs, S. C. Czajak, A. Y. Howe, L. O. Arthur, and R. P. Johnson. 1998. Identification of highly attenuated mutants of simian immunodeficiency virus. J. Virol. 72:1431-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doranz, B. J., J. Rucker, Y. Yi, R. J. Smyth, M. Samson, S. C. Peiper, M. Parmentier, R. G. Collman, and R. W. Doms. 1996. A dualtropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell 85:1149-1158. [DOI] [PubMed] [Google Scholar]
- 22.Dragic, T., V. Litwin, G. P. Allaway, S. R. Martin, Y. Huang, K. A. Nagashima, C. Cayanan, P. J. Maddon, R. A. Koup, J. P. Moore, and W. A. Paxton. 1996. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381:667-673. [DOI] [PubMed] [Google Scholar]
- 23.Embretson, J., M. Zupancic, J. L. Ribas, A. Burke, P. Racz, K. Tenner-Racz, and A. T. Haase. 1993. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature 362:359-362. [DOI] [PubMed] [Google Scholar]
- 24.Esser, M. T., J. W. Bess, Jr., K. Suryanarayana, E. Chertova, D. Marti, M. Carrington, L. O. Arthur, and J. D. Lifson. 2001. Partial activation and induction of apoptosis in CD4+ and CD8+ T lymphocytes by conformationally authentic noninfectious human immunodeficiency virus type 1. J. Virol. 75:1152-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esser, M. T., D. R. Graham, L. V. Coren, C. M. Trubey, J. W. Bess, Jr., L. O. Arthur, D. E. Ott, and J. D. Lifson. 2001. Differential incorporation of CD45, CD80 (B7-1), CD86 (B7-2), and major histocompatibility complex class I and II molecules into human immunodeficiency virus type 1 virions and microvesicles: implications for viral pathogenesis and immune regulation. J. Virol. 75:6173-6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Etemad-Moghadam, B., Y. Sun, E. K. Nicholson, M. Fernandes, K. Liou, R. Gomila, J. Lee, and J. Sodroski. 2000. Envelope glycoprotein determinants of increased fusogenicity in a pathogenic simian-human immunodeficiency virus (SHIV-KB9) passaged in vivo. J. Virol. 74:4433-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a.Etemad-Moghadam, B., D. Rhone, T. Steenbeke, Y. Sun, J. Manola, R. Gelman, J. W. Fanton, P. Racz, K. Tenner-Racz, M. Axthelm, N. Letvin, and J. Sodroski. 2001. Membrane-fusing capacity of the human immunodeficiency virus (HIV-1) envelope proteins determines the efficiency of CD4+ T-cell depletion in macaques infected by a simian-human immunodeficiency virus. J. Virol. 75:5646-5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26b.Farzan, M., T. Mirzabekov, P. Kolchinsky, R. Wyatt, M. Cayabyab, N. P. Gerard, C. Gerard, J. Sodroski, and H. Choe. 1999. Tyrosine sulfation of the amino-terminus of CCR5 facilitates HIV-1 entry. Cell 96:667-676. [DOI] [PubMed] [Google Scholar]
- 27.Fauci, A. S. 1988. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science 239:617-622. [DOI] [PubMed] [Google Scholar]
- 28.Fauci, A. S., A. M. Macher, D. L. Longo, H. C. Lane, A. H. Rook, H. Masur, and E. P. Gelmann. 1984. NIH conference. Acquired immunodeficiency syndrome: epidemiologic, clinical, immunologic, and therapeutic considerations. Ann. Intern. Med. 100:92-106. [DOI] [PubMed] [Google Scholar]
- 29.Feng, Y., C. C. Broder, P. E. Kennedy, and E. A. Berger. 1996. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 272:872-877. [DOI] [PubMed] [Google Scholar]
- 30.Finkel, T. H., and N. K. Banda. 1994. Indirect mechanisms of HIV pathogenesis: how does HIV kill T cells? Curr. Opin. Immunol. 6:605-615. [DOI] [PubMed] [Google Scholar]
- 30a.Finkel, T. H., G. Tudor-Williams, N. K. Banda, M. F. Cotton, T. Curiel, C. Monks, T. W. Baba, R. M. Ruprecht, and A. Kupfer. 1995. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat. Med. 1:129-134. [DOI] [PubMed] [Google Scholar]
- 31.Freed, E. O., and M. A. Martin. 1995. The role of human immunodeficiency virus type 1 envelope glycoproteins in virus infection. J. Biol. Chem. 270:23883-23886. [DOI] [PubMed] [Google Scholar]
- 32.Friedberg, E. C., G. C. Walker, and W. Siede. 1995. DNA repair and mutagenesis. American Society for Microbiology, Washington, D.C.
- 33.Gallo, R. C., S. Z. Salahuddin, M. Popovic, G. M. Shearer, M. Kaplan, B. F. Haynes, T. J. Palker, R. Redfield, J. Oleske, B. Safai, et al. 1984. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science 224:500-503. [DOI] [PubMed] [Google Scholar]
- 34.Gandhi, R. T., B. K. Chen, S. E. Straus, J. K. Dale, M. J. Lenardo, and D. Baltimore. 1998. HIV-1 directly kills CD4+ T cells by a Fas-independent mechanism. J. Exp. Med. 187:1113-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gibbs, J. S., A. A. Lackner, S. M. Lang, M. A. Simon, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1995. Progression to AIDS in the absence of a gene for vpr or vpx. J. Virol. 69:2378-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glushakova, S., J. Munch, S. Carl, T. C. Greenough, J. L. Sullivan, L. Margolis, and F. Kirchhoff. 2001. CD4 down-modulation by human immunodeficiency virus type 1 Nef correlates with the efficiency of viral replication and with CD4+ T-cell depletion in human lymphoid tissue ex vivo. J. Virol. 75:10113-10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gougeon, M. L., S. Garcia, J. Heeney, R. Tschopp, H. Lecoeur, D. Guetard, V. Rame, C. Dauguet, and L. Montagnier. 1993. Programmed cell death in AIDS-related HIV and SIV infections. AIDS Res. Hum. Retroviruses 9:553-563. [DOI] [PubMed] [Google Scholar]
- 37a.Grivel, J. C., and L. B. Margolis. 1999. CCR5- and CXCR4-tropic HIV-1 are equally cytopathic for their T-cell targets in human lymphoid tissue. Nat. Med. 5:592-593. [DOI] [PubMed] [Google Scholar]
- 37b.Grivel, J. C., M. L. Penn, D. A. Eckstein, B. Schramm, R. F. Speck, N. W. Abbey, B. Herndier, L. Margolis, and M. A. Goldsmith. 2000. Human immunodeficiency virus type 1 coreceptor preferences determine target T-cell depletion and cellular tropism in human lymphoid tissue. J. Virol. 74:5347-5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Groux, H., G. Torpier, D. Monte, Y. Mouton, A. Capron, and J. C. Ameisen. 1992. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J. Exp. Med. 175:331-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haase, A. T. 1994. The role of active and covert infections in lentivirus pathogenesis. Ann. N. Y. Acad. Sci. 724:75-86. [DOI] [PubMed] [Google Scholar]
- 39a.Harouse, J. M., A. Gettie, R. C. Tan, J. Blanchard, and C. Cheng-Mayer. 1999. Distinct pathogenic sequela in rhesus macaques infected with CCR5- or CXCR4-utilizing SHIVs. Science 284:816-819. [DOI] [PubMed] [Google Scholar]
- 40.Helseth, E., M. Kowalski, D. Gabuzda, U. Olshevsky, W. Haseltine, and J. Sodroski. 1990. Rapid complementation assays measuring replicative potential of human immunodeficiency virus type 1 envelope glycoprotein mutants. J. Virol. 64:2416-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho, D. D., A. U. Neumann, A. S. Perelson, W. Chen, J. M. Leonard, and M. Markowitz. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373:123-126. [DOI] [PubMed] [Google Scholar]
- 42.Hoch, J., S. M. Lang, M. Weeger, C. Stahl-Hennig, C. Coulibaly, U. Dittmer, G. Hunsmann, D. Fuchs, J. Muller, S. Sopper, et al. 1995. vpr deletion mutant of simian immunodeficiency virus induces AIDS in rhesus monkeys. J. Virol. 69:4807-4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hofmann, W., D. Schubert, J. LaBonte, L. Munson, S. Gibson, J. Scammell, P. Ferrigno, and J. Sodroski. 1999. Species-specific, postentry barriers to primate immunodeficiency virus infection. J. Virol. 73:10020-10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43a.Hoxie, J. A., J. D. Alpers, J. L. Rackowski, K. Huebner, B. S. Haggarty, A. J. Cedarbaum, and J. C. Reed. 1986. Alterations in T4 (CD4) protein and mRNA synthesis in cells infected with HIV. Science 234:1123-1127. [DOI] [PubMed] [Google Scholar]
- 44.Kaplan, A. H., and R. Swanstrom. 1991. Human immunodeficiency virus type 1 Gag proteins are processed in two cellular compartments. Proc. Natl. Acad. Sci. USA 88:4528-4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karlsson, G. B., M. Halloran, D. Schenten, J. Lee, P. Racz, K. Tenner-Racz, J. Manola, R. Gelman, B. Etemad-Moghadam, E. Desjardins, R. Wyatt, N. P. Gerard, L. Marcon, D. Margolin, J. Fanton, M. K. Axthelm, N. L. Letvin, and J. Sodroski. 1998. The envelope glycoprotein ectodomains determine the efficiency of CD4+ T lymphocyte depletion in simian-human immunodeficiency virus-infected macaques. J. Exp. Med. 188:1159-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45a.Kimura, T., M. Nishikawa, and A. Ohyama. 1994. Intracellular membrane traffic of human immunodeficiency virus type 1 envelope glycoproteins: vpu liberates Golgi-targeted gp160 from CD4-dependent retention in the endoplasmic reticulum. J. Biochem. (Tokyo) 115:1010-1020. [DOI] [PubMed] [Google Scholar]
- 46.Klatzmann, D., E. Champagne, S. Chamaret, J. Gruest, D. Guetard, T. Hercend, J. C. Gluckman, and L. Montagnier. 1984. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature 312:767-768. [DOI] [PubMed] [Google Scholar]
- 46a.Koga, Y., M. Sasaki, K. Nakamura, G. Kimura, and K. Nomoto. 1990. Intracellular distribution of the envelope glycoprotein of human immunodeficiency virus and its role in the production of cytopathic effect in CD4+ and CD4− human cell lines. J. Virol. 64:4661-4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46b.Koga, Y., K. Nakamura, M. Sasaki, G. Kimura, and K. Nomoto. 1994. The difference in gp160 and gp120 of HIV type 1 in the induction of CD4 downregulation preceding single-cell killing. Virology 201:137-141. [DOI] [PubMed] [Google Scholar]
- 47.Kolchinsky, P., E. Kiprilov, P. Bartley, R. Rubinstein, and J. Sodroski. 2001. Loss of a single N-linked glycan allows CD4-independent human immunodeficiency virus type 1 infection by altering the position of the gp120 V1/V2 variable loops. J. Virol. 75:3435-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kolchinsky, P., T. Mirzabekov, M. Farzan, E. Kiprilov, M. Cayabyab, L. J. Mooney, H. Choe, and J. Sodroski. 1999. Adaptation of a CCR5-with, primary human immunodeficiency virus type 1 isolate for CD4-independent replication. J. Virol. 73:8120-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Konvalinka, J., M. A. Litterst, R. Welker, H. Kottler, F. Rippmann, A. M. Heuser, and H. G. Krausslich. 1995. An active-site mutation in the human immunodeficiency virus type 1 proteinase (PR) causes reduced PR activity and loss of PR-mediated cytotoxicity without apparent effect on virus maturation and infectivity. J. Virol. 69:7180-7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49a.Koot, M. R. van Leeuwen, R. E. de Goede, I. P. Keet, S. Danner, J. K. Eeftinck Schattenkerk, P. Reiss, M. Tersmette, J. M. Lange, and H. Schuitemaker. 1999. Conversion rate towards a syncytium-inducing (syncytium-inducing) phenotype during different stages of human immunodeficiency virus type 1 infection and prognostic value of syncytium-inducing phenotype for survival after AIDS diagnosis. J. Infect. Dis. 179:254-258. [DOI] [PubMed] [Google Scholar]
- 50.Kowalski, M., L. Bergeron, T. Dorfman, W. Haseltine, and J. Sodroski. 1991. Attenuation of human immunodeficiency virus type 1 cytopathic effect by a mutation affecting the transmembrane envelope glycoprotein. J. Virol. 65:281-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.LaBonte, J. A., T. Patel, W. Hofmann, and J. Sodroski. 2000. Importance of membrane fusion mediated by human immunodeficiency virus envelope glycoproteins for lysis of primary CD4−positive T cells. J. Virol. 74:10690-10698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lackner, A. A., M. Schiodt, G. C. Armitage, P. F. Moore, R. J. Munn, P. A. Marx, M. B. Gardner, and L. J. Lowenstine. 1989. Mucosal epithelial cells and Langerhans cells are targets for infection by the immunosuppressive type D retrovirus simian AIDS retrovirus serotype 1. J. Med Primatol. 18:195-207. [PubMed] [Google Scholar]
- 53.Laurent-Crawford, A. G., B. Krust, Y. Riviere, C. Desgranges, S. Muller, M. P. Kieny, C. Dauguet, and A. G. Hovanessian. 1993. Membrane expression of HIV envelope glycoproteins triggers apoptosis in CD4 cells. AIDS Res. Hum. Retroviruses 9:761-773. [DOI] [PubMed] [Google Scholar]
- 54.Letvin, N. L., M. D. Daniel, P. K. Sehgal, R. C. Desrosiers, R. D. Hunt, L. M. Waldron, J. J. MacKey, D. K. Schmidt, L. V. Chalifoux, and N. W. King. 1985. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science 230:71-73. [DOI] [PubMed] [Google Scholar]
- 55.Lewin-Smith, M., S. M. Wahl, and J. M. Orenstein. 1999. Human immunodeficiency virus-rich multinucleated giant cells in the colon: a case report with transmission electron microscopy, immunohistochemistry, and in situ hybridization. Mod. Pathol. 12:75-81. [PubMed] [Google Scholar]
- 56.Li, C. J., D. J. Friedman, C. Wang, V. Metelev, and A. B. Pardee. 1995. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science 268:429-431. [DOI] [PubMed] [Google Scholar]
- 57.Li, S. L., E. E. Kaaya, H. Feichtinger, P. Putkonen, C. Parravicini, D. Bottiger, G. Biberfeld, and P. Biberfeld. 1991. Monocyte/macrophage giant cell disease in SIV-infected cynomolgus monkeys. Res Virol. 142:173-182. [DOI] [PubMed] [Google Scholar]
- 58.Lifson, J. D., M. B. Feinberg, G. R. Reyes, L. Rabin, B. Banapour, S. Chakrabarti, B. Moss, F. Wong-Staal, K. S. Steimer, and E. G. Engleman. 1986. Induction of CD4-dependent cell fusion by the HTLV-III/LAV envelope glycoprotein. Nature 323:725-728. [DOI] [PubMed] [Google Scholar]
- 59.Lifson, J. D., M. A. Nowak, S. Goldstein, J. L. Rossio, A. Kinter, G. Vasquez, T. A. Wiltrout, C. Brown, D. Schneider, L. Wahl, A. L. Lloyd, J. Williams, W. R. Elkins, A. S. Fauci, and V. M. Hirsch. 1997. The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J. Virol. 71:9508-9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59a.Lu, Y. Y., Y. Koga, K. Tanaka, M. Sasaki, G. Kimura, and K. Nomoto. 1994. Apoptosis induced in CD4+ cells expressing gp160 of human immunodeficiency virus type 1. J. Virol. 68:390-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luciw, P. A., C. P. Mandell, S. Himathongkham, J. Li, T. A. Low, K. A. Schmidt, K. E. Shaw, and C. Cheng-Mayer. 1999. Fatal immunopathogenesis by SIV/HIV-1 (SHIV) containing a variant form of the HIV-1SF33 env gene in juvenile and newborn rhesus macaques. Virology 263:112-127. [DOI] [PubMed] [Google Scholar]
- 61.Maddon, P. J., A. G. Dalgleish, J. S. McDougal, P. R. Clapham, R. A. Weiss, and R. Axel. 1986. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell 47:333-348. [DOI] [PubMed] [Google Scholar]
- 62.Maier, H., H. Budka, H. Lassmann, and P. Pohl. 1989. Vacuolar myelopathy with multinucleated giant cells in the acquired immune deficiency syndrome (AIDS). Light and electron microscopic distribution of human immunodeficiency virus (HIV) antigens. Acta Neuropathol. 78:497-503. [DOI] [PubMed] [Google Scholar]
- 62a.Malkevich, N., C. Womack, P. Pandya, J. C. Grivel, A. S. Fauci, and L. Margolis. 2001. Human immunodeficiency virus type 1 (HIV-1) non-B subtypes are similar to HIV-1 subtype B in that coreceptor specificity is a determinant of cytopathicity in human lymphoid tissue infected ex vivo. J. Virol. 75:10520-10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCloskey, T. W., M. Ott, E. Tribble, S. A. Khan, S. Teichberg, M. O. Paul, S. Pahwa, E. Verdin, and N. Chirmule. 1997. Dual role of HIV Tat in regulation of apoptosis in T cells. J. Immunol. 158:1014-1019. [PubMed] [Google Scholar]
- 63a.McCune, J. M., L. B. Rabin, M. B. Feinberg, M. Lieberman, J. C. Kosek, G. R. Reyes, and I. L. Weissman. 1988. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell 53:55-67. [DOI] [PubMed] [Google Scholar]
- 64.Mellors, J. W., C. R. Rinaldo, Jr., P. Gupta, R. M. White, J. A. Todd, and L. A. Kingsley. 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272:1167-1170. [DOI] [PubMed] [Google Scholar]
- 65.Meyaard, L., S. A. Otto, R. R. Jonker, M. J. Mijnster, R. P. Keet, and F. Miedema. 1992. Programmed death of T cells in HIV-1 infection. Science 257:217-219. [DOI] [PubMed] [Google Scholar]
- 66.Muro-Cacho, C. A., G. Pantaleo, and A. S. Fauci. 1995. Analysis of apoptosis in lymph nodes of HIV-infected persons. Intensity of apoptosis correlates with the general state of activation of the lymphoid tissue and not with stage of disease or viral burden. J. Immunol. 154:5555-5566. [PubMed] [Google Scholar]
- 67.Narayan, S. V., S. Mukherjee, F. Jia, Z. Li, C. Wang, L. Foresman, C. McCormick-Davis, E. B. Stephens, S. V. Joag, and O. Narayan. 1999. Characterization of a neutralization-escape variant of SHIVKU-1, a virus that causes acquired immune deficiency syndrome in pig-tailed macaques. Virology 256:54-63. [DOI] [PubMed] [Google Scholar]
- 67a.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 68.Ojeda, F., H. Folch, M. I. Guarda, B. Jastorff, and H. A. Diehl. 1995. Induction of apoptosis in thymocytes: new evidence for an interaction of PKC and PKA pathways. Biol. Chem. Hoppe Seyler 376:389-393. [PubMed] [Google Scholar]
- 69.Okada, H., R. Takei, and M. Tashiro. 1997. HIV-1 Nef protein-induced apoptotic cytolysis of a broad spectrum of uninfected human blood cells independently of CD95(Fas). FEBS Lett. 414:603-606. [DOI] [PubMed] [Google Scholar]
- 70.Okada, H., R. Takei, and M. Tashiro. 1997. Nef protein of HIV-1 induces apoptotic cytolysis of murine lymphoid cells independently of CD95 (Fas) and its suppression by serine/threonine protein kinase inhibitors. FEBS Lett. 417:61-64. [DOI] [PubMed] [Google Scholar]
- 70a.Olshevsky, U., E. Helseth, C. Furman, J. Li, W. A. Haseltine, and J. G. Sodroski. 1990. Identification of individual HIV-1 gp120 amino acids important for CD4 receptor binding. J. Virol. 64:5701-5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pantaleo, G., C. Graziosi, J. F. Demarest, L. Butini, M. Montroni, C. H. Fox, J. M. Orenstein, D. P. Kotler, and A. S. Fauci. 1993. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature 362:355-358. [DOI] [PubMed] [Google Scholar]
- 72.Parolin, C., B. Taddeo, G. Palu, and J. Sodroski. 1996. Use of cis- and trans-acting viral regulatory sequences to improve expression of human immunodeficiency virus vectors in human lymphocytes. Virology 222:415-422. [DOI] [PubMed] [Google Scholar]
- 72a.Penn, M. L., J. C. Grivel, B. Schramm, M. A. Goldsmith, and L. Margolis. 1999. CXCR4 utilization is sufficient to trigger CD4+ T-cell depletion in HIV-1-infected human lymphoid tissue. Proc. Natl. Acad. Sci. USA 96:663-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Purvis, S. F., J. W. Jacobberger, R. M. Sramkoski, A. H. Patki, and M. M. Lederman. 1995. HIV type 1 Tat protein induces apoptosis and death in Jurkat cells. AIDS Res. Hum. Retroviruses 11:443-450. [DOI] [PubMed] [Google Scholar]
- 74.Rasola, A., D. Gramaglia, C. Boccaccio, and P. M. Comoglio. 2001. Apoptosis enhancement by the HIV-1 Nef protein. J. Immunol. 166:81-88. [DOI] [PubMed] [Google Scholar]
- 75.Reimann, K. A., J. T. Li, R. Veazey, M. Halloran, I. W. Park, G. B. Karlsson, J. Sodroski, and N. L. Letvin. 1996. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J. Virol. 70:6922-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rizzuto, C. D., and J. G. Sodroski. 1997. Contribution of virion ICAM-1 to human immunodeficiency virus infectivity and sensitivity to neutralization. J. Virol. 71:4847-4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roggero, R., V. Robert-Hebmann, S. Harrington, J. Roland, L. Vergne, S. Jaleco, C. Devaux, and M. Biard-Piechaczyk. 2001. Binding of human immunodeficiency virus type 1 gp120 to CXCR4 induces mitochondrial transmembrane depolarization and cytochrome c-mediated apoptosis independently of Fas signaling. J. Virol. 75:7637-7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ruprecht, R. M., T. W. Baba, R. Rasmussen, Y. Hu, and P. L. Sharma. 1996. Murine and simian retrovirus models: the threshold hypothesis. AIDS 10:S33-40. [DOI] [PubMed] [Google Scholar]
- 79.Ryzhova, E. V., P. Crino, L. Shawver, S. V. Westmoreland, A. A. Lackner, and F. Gonzalez-Scarano. 2002. Simian immunodeficiency virus encephalitis: analysis of envelope sequences from individual brain multinucleated giant cells and tissue samples. Virology 297:57-67. [DOI] [PubMed] [Google Scholar]
- 79a.Schuitemaker, H., M. Koot, N. A. Kootstra, M. W. Dercksen, R. E. de Goede, R. P. van Steenwijk, J. M. Lange, J. K. Schattenkerk, F. Miedema, and M. Tersmette. 1992. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J. Virol. 66:1354-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shoeman, R. L., B. Honer, T. J. Stoller, C. Kesselmeier, M. C. Miedel, P. Traub, and M. C. Graves. 1990. Human immunodeficiency virus type 1 protease cleaves the intermediate filament proteins vimentin, desmin, and glial fibrillary acidic protein. Proc. Natl. Acad. Sci. USA 87:6336-6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Si, Z., M. Cayabyab, and J. Sodroski. 2001. Envelope glycoprotein determinants of neutralization resistance in a simian-human immunodeficiency virus (SHIV-HXBc2P 3.2) derived by passage in monkeys. J. Virol. 75:4208-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sodroski, J., W. C. Goh, C. Rosen, K. Campbell, and W. A. Haseltine. 1986. Role of the HTLV-III/LAV envelope in syncytium formation and cytopathicity. Nature 322:470-474. [DOI] [PubMed] [Google Scholar]
- 83.Srinivasan, V., W. M. Schnitzlein, and D. N. Tripathy. 2001. Fowlpox virus encodes a novel DNA repair enzyme, CPD-photolyase, that restores infectivity of UV light-damaged virus. J. Virol. 75:1681-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stein, B. S., S. D. Gowda, J. D. Lifson, R. C. Penhallow, K. G. Bensch, and E. G. Engleman. 1987. pH-independent HIV entry into CD4−positive T cells via virus envelope fusion to the plasma membrane. Cell 49:659-668. [DOI] [PubMed] [Google Scholar]
- 84a.Stein, B. S., and E. G. Engleman. 1990. Intracellular processing of the gp160 HIV-1 envelope precursor. Endoproteolytic cleavage occurs in a cis or medial compartment of the Golgi complex. J. Biol. Chem. 265:2640-2649. [PubMed] [Google Scholar]
- 85.Stewart, S. A., B. Poon, J. B. Jowett, and I. S. Chen. 1997. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J. Virol. 71:5579-5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stewart, S. A., B. Poon, J. Y. Song, and I. S. Chen. 2000. Human immunodeficiency virus type 1 vpr induces apoptosis through caspase activation. J. Virol. 74:3105-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sullivan, N., Y. Sun, J. Li, W. Hofmann, and J. Sodroski. 1995. Replicative function and neutralization sensitivity of envelope glycoproteins from primary and T-cell line-passaged human immunodeficiency virus type 1 isolates. J. Virol. 69:4413-4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sylwester, A. W., J. C. Grivel, W. Fitzgerald, J. L. Rossio, J. D. Lifson, and L. B. Margolis. 1998. CD4+ T-lymphocyte depletion in human lymphoid tissue ex vivo is not induced by noninfectious human immunodeficiency virus type 1 virions. J. Virol. 72:9345-9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tomasselli, A. G., J. O. Hui, L. Adams, J. Chosay, D. Lowery, B. Greenberg, A. Yem, M. R. Deibel, H. Zurcher-Neely, and R. L. Heinrikson. 1991. Actin, troponin C, Alzheimer amyloid precursor protein and pro-interleukin 1 beta as substrates of the protease from human immunodeficiency virus. J. Biol. Chem. 266:14548-14553. [PubMed] [Google Scholar]
- 89a.van't Wout, A. B., H. Blaak, L. J. Ran, M. Brouwer, C. Kuiken, and H. Schuitemaker. 1998. Evolution of syncytium-inducing and non-syncytium-inducing biological virus clones in relation to replication kinetics during the course of human immunodeficiency virus type 1 infection. J. Virol. 72:5099-5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T-cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 90a.Veazey, R. S., K. G. Mansfield, I. C. Tham, A. C. Carville, D. E. Shvetz, A. E. Forand, and A. A. Lackner. 2000. Dynamics of CCR5 expression by CD4+ T cells in lymphoid tissues during simian immunodeficiency virus infection. J. Virol. 74:11001-11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wei, X., S. K. Ghosh, M. E. Taylor, V. A. Johnson, E. A. Emini, P. Deutsch, J. D. Lifson, S. Bonhoeffer, M. A. Nowak, B. H. Hahn, et al. 1995. Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373:117-122. [DOI] [PubMed] [Google Scholar]
- 92.White, D. O., and F. J. Fenner. 1994. Medical virology, 4th ed., p. 548-552. Academic Press, San Diego, Calif.
- 92a.Willey, R. L., F. Maldarelli, M. A. Martin, and K. Strebel. 1992. Human immunodeficiency virus type 1 Vpu protein regulates the formation of intracellular gp160-CD4 complexes. J. Virol. 66:226-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yao, X. J., A. J. Mouland, R. A. Subbramanian, J. Forget, N. Rougeau, D. Bergeron, and E. A. Cohen. 1998. Vpr stimulates viral expression and induces cell killing in human immunodeficiency virus type 1-infected dividing Jurkat T cells. J. Virol. 72:4686-4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yee, J. K., A. Miyanohara, P. LaPorte, K. Bouic, J. C. Burns, and T. Friedmann. 1994. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc. Natl. Acad. Sci. USA 91:9564-9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yu, X., M. F. McLane, W. O'Brien, R. Collman, M. Essex, and T. H. Lee. 1994. Killing of primary CD4+ T cells by nonsyncytium-inducing macrophage-tropic human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 91:10237-10241. [DOI] [PMC free article] [PubMed] [Google Scholar]