Abstract
Recombinant vaccinia viruses that express defective retroviral vectors upon a single infection event in normal host cells were constructed. The gag-pol and envelope genes and a retroviral vector unit were inserted as vaccinia virus promoter-controlled transcription units at three separate loci. The triple recombinant virus was used to infect such diverse cell types as monkey and rabbit kidney, human lung, and primary chicken cells, resulting in the production of transduction-competent defective retroviral vectors. Infection of Chinese hamster ovary cells, which are nonpermissive for vaccinia virus replication, also resulted in production of retroviral vectors and secondary permanent transduction of the host cells. Since vaccinia virus supports the expression of cytotoxic proteins, the vesicular stomatitis virus G glycoprotein could be chosen as the envelope allowing a broad host range of transduction. Functionality of particles was monitored by expression of the green fluorescent protein in transduced 3T3 cell clones. This is the first description of a single chimeric virus encoding and releasing functional retroviral vectors, providing proof of principle of the new concept. No replication-competent retrovirus was detectable by sensitive reverse transcriptase assays. Since vaccinia virus has a broad host range, is extremely robust, and can be obtained at high titers and safe nonreplicating vaccinia virus strains are available, the hybrid system may open new perspectives for gene delivery.
Retrovirus vectors have the almost unique ability to stably integrate into a host genome and are therefore frequently chosen for gene therapy approaches where long-lasting gene expression is required (17). Limiting factors of this technology are low titers and the instability of the transducing particles. Moreover, retroviral particles are known to be sensitive to complement lysis when produced in allogeneic cells (22, 30), which is the case for most of the packaging cell lines. Recent attempts with chimeric viral vectors focused on the combination of desirable properties of the retroviral system with those of other vector systems.
To reach this goal, a number of viral vectors that express retroviral vector components and give rise to transducing particles have been investigated. A herpes simplex virus expressing retroviral packaging functions that can mobilize vector genomes from a transduced cell line was reported (26). A chimeric vaccinia virus which produces functional particles in packaging cells, also allowing the construction of complex retroviral vectors transducing introns and internal polyadenylation sites, has been described by our group (10, 13). The generation of retroviral vectors by coinfection of three individual recombinant Semliki Forest viruses was also reported recently (14). In addition, a chimeric adenovirus-retrovirus system has been presented in which the retroviral components are split between two adenoviral vectors; transduction events could be demonstrated even in vivo when high-titer coinfection with the two adenoviruses was performed (5).
Because inclusion of all the required genetic information into a single vector is limited by the coding capacities of most viral vectors, the systems depend on the simultaneous infection of a cell with two ore more distinct viruses, which is obviously a rare event in vivo. Because vaccinia virus has the ability to incorporate over 25 kb of foreign DNA (19), it seemed to be an appropriate tool to encode all components of a complete retroviral vector. Therefore, we attempted to construct a recombinant vaccinia virus expressing simultaneously the gag-pol gene, an envelope gene, and a retroviral vector unit. In this report we describe the production of vesicular stomatitis virus (VSV)-G pseudotyped retroviral particles in several wild-type cell lines with a single chimeric vaccinia virus for infection.
MATERIALS AND METHODS
Virus and cell lines.
The monkey kidney cell lines CV-1 (ATCC CCL-70) and Vero (ATCC CCL-81), the NIH 3T3 cell line (ATCC CRL-1658), Chinese hamster ovary cells (ATCC CRL-9096), the thymidine kinase-negative cell line 143B (ATCC CRL-8303), human lung cell line MRC5 (CCL-171) and 293 kidney cell line (CRL-1573), and the Western Reserve (WR) strain of vaccinia virus (ATCC VR119) were obtained from the American Type Culture Collection. Cell line RK44.20 has been described (9). The PT67 cell line was obtained from Clontech Laboratories. Primary chicken embryo fibroblasts were prepared from 12-day chicken embryos and grown in tissue culture medium 199 (Gibco-BRL) supplemented with 5% fetal bovine serum.
Construction of plasmids. (i) pHA-MLVg.
Starting from the plasmid pgagpol-gpt (obtained from D. Klein and W. Günzburg), three vaccinia virus early transcription stop signals (defined by the nucleotide sequence TTTTTNT [5TNT]) (35), one within the gag region (located at nucleotide position 731; position 1 being the A of the start codon) and two in the pol region (at positions 2439 and 4411) of the Moloney murine leukemia virus (MLV) gag-pol open reading frame (ORF) were mutagenized (by nucleotide exchange not altering the protein sequence) with a PCR cloning strategy. Four sections of the gag-pol ORF were amplified by PCR with the primer pairs oRV-11 (5′-CCA TGG GCC AGA CTG TTA CCA CT-3′, introducing an NcoI restriction site) and oRV-12 (5′-CTG GAT CCT CAG AGA AAG AAG GGT T-3′, introducing a BamHI site), oRV-13 (5′-ATT AAC CCT TCT TTT TCT GAG GAT CCA GGT-3′, introducing a BamHI site) and oRV-14 (5′-CAT CCT TGA ATT CAA GCA CAG TGT ACC ACTG-3′, introducing an EcoRI site), oRV-15 (5′-TGA ATT CAA GGA TGC CTT CTT CTG CCT GA-3′, introducing an EcoRI site) and oRV-16 (5′-AAC TAG TAG ATA TTT ATA GCC ATAC-3′, introducing an SpeI site), and oRV-17 (5′-ATA TCT ACT AGT TTT CAT AGA TAC CT-3′, introducing an SpeI site) and oRV-18 (5′-GCG GCC GCT TAG GGG GCC TCG CGG GTTA-3′, introducing a NotI site).
The four parts of the gag-pol ORF were subcloned into the pCR2.1 vector (TA cloning kit; Invitrogen, Inc.), and subsequently, the gag-pol ORF was assembled with the above-named restriction enzymes and cloned downstream of the synthetic early/late promoter in the plasmid pTKgpt-selP (3, 23), resulting in the intermediate plasmid pTK-MLVg. This plasmid contains the modified selP-gag-pol gene cassette (selP, synthetic early/late promoter). In order to direct this gene cassette into the vaccinia virus hemagglutinin locus, plasmid pHA-MLVg was constructed by inserting the NsiI/SspI selP-gag-pol gene cassette into the NsiI- and SspI-cleaved pHA-vA(2)a (27), resulting in the final plasmid, pHA-MLVg.
(ii) pDR-P11Z5V.
Two 5TNT signals within the VSV-G ORF (at nucleotide positions 25 and 1402) were mutagenized. The first 5TNT was modified with the Quickchange PCR mutagenesis kit (Stratagene, Inc.) with the primers oRV-94 (5′-GCT CAA TTG CCT CTT TCT TCT TTA TCA TAG GG-3′) and oRV-95 (5′-CCC TAT GAT AAA GAA GAA AGA GGC AAT TGA GC-3′) according to the manufacturer's protocol. The second signal was modified simultaneously by PCR amplification with the primers oVSV-4 (5′-TAA TCA TGA AGT GCC TTT TGT ACT TAG CCT TCT TAT TCA-3′) and oVSV-2 (5′-ATA GTT TCT AGA AGA TCT TAC TCT CCA AGT CGG TTC ATC TC-3′). The PCR product was digested with NcoI and XbaI and cloned downstream of the mH5 promoter (a modified early/late promoter [33]), resulting in pD4-mH5-VSVg. The mH5-VSVg cassette from this construct was then inserted as an HpaI/NotI fragment into the multiple cloning site of pDR-MCS, an intermediate plasmid providing the correct cloning sites to assemble the final construct between the D4 and D5 flanking regions. The resulting plasmid, pDR-5V, contains the VSV-G gene cassette and provides the NsiI and HpaI sites into which a P11 promoter-lacZ selection cassette was inserted, yielding the final plasmid pDR-P11ZV5. This plasmid directs the VSV-G gene cassette together with the lacZ marker gene into the vaccinia virus D4/D5 intergenic region.
(iii) pR-XSNegfp.
The recombination plasmid pR-XSNegfp is derived from pR-XSN (10) and contains the enhanced green fluorescence protein (EGFP) ORF under the control of the retroviral long terminal repeat (LTR) promoter, cloned as an EcoRI/BamHI fragment and excised from plasmid pLXSNEGFP (a gift from W. Günzburg and D. Klein [11]). The resulting plasmid contains a complete retroviral vector genome and a gpt selection cassette flanked by vaccinia virus thymidine kinase gene sequences (12). The structure of this plasmid is depicted in Fig. 1C.
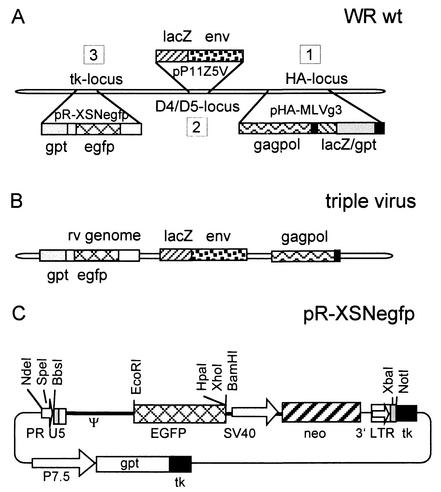
FIG. 1.
Schematic representation of the construction of the chimeric vaccinia viruses and structure of the plasmid pR-XSNegfp. (A) In step 1, the retroviral gag-pol sequences were inserted into the vaccinia virus hemagglutinin locus (HA locus). The lacZ and gpt markers were used transiently and are not present in the first construct, the virus vHA-MLVg. The second step was insertion of the VSV-G pseudo-env gene into the D4/D5 intergenic region (D4/D5 locus), resulting in the packaging virus. The last step consisted of insertion of a retroviral vector unit into the packaging virus, resulting in the triple virus. (B) Structure of the triple virus. (C) Structure of plasmid pR-XSNegfp. This plasmid transfers the retroviral vector genome together with a permanent gpt marker into the vaccinia virus thymidine kinase (tk) locus; PR, synthetic vaccinia virus early promoter R; U5, U5 region of the retroviral long terminal repeat; Ψ, packaging signal; EGFP, open reading frame of the EGFP gene; SV40, simian virus 40 early promoter; neo, neomycin resistance gene; 3′LTR, 3′ retroviral long terminal repeat; P7.5, vaccinia virus early/late promoter P7.5; gpt, selection marker gene encoding the Escherichia coli enzyme guanine phosphoribosyltransferase.
Construction of viruses. (i) gag-pol virus.
Three micrograms of plasmid pHA-MLVg was electroporated (0.4 kV, 125 μF, and 200 Ω, with a Bio-Rad electroporator and a 0.4-cm cuvette) into wild-type vaccinia virus Western Reserve strain (WR)-infected CV-1 cells at a multiplicity of infection of 0.5. After 72 h of growth in a petri dish (10-cm diameter), crude viral stocks were prepared and recombinant viruses were isolated with a transient selection protocol (27). With this procedure, three rounds of selecting lacZ/gpt-positive clones were followed by a further three rounds of screening white plaques in the absence of selective agents. In the final marker-free construct, the gag-pol ORF is controlled by the vaccinia virus synthetic early/late promoter (3) and the foreign gene is located in the vaccinia virus hemagglutinin (HA) locus.
(ii) Packaging virus.
Plasmid pDR-P11Z5V was transfected into CV-1 cells with Lipofectamine 2000 (Life Technologies, Inc.) prior to infection with the gag-pol virus. Screening for lacZ-positive viruses (2) over five rounds of plaque purification in CV-1 cells resulted in the packaging virus.
(iii) Triple virus.
Twenty-five micrograms of DNA of plasmid pR-XSNegfp was transfected into CV-1 cells with Lipofectamine 2000 prior to infection with the packaging virus. Two rounds of gpt selection (4) followed by two rounds of selection with bromodeoxyuridine in 143B thymidine kinase-negative cells (15) resulted in the triple virus. The genomic structure of the retroviral transcription unit in the viral context of the triple virus is shown in Fig. 7A.
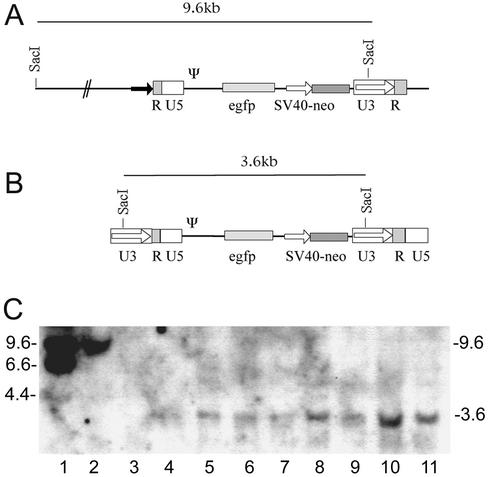
FIG. 7.
(A) Structure of the retroviral vector unit integrated in the triple virus and in virus vrR-XSNegfp. Since the 5′ LTR was modified (the U3 region was replaced with a vaccinia virus early promoter; black arrow), the SacI site usually present in the 5′ LTR was deleted. A SacI site is located in the vaccinia virus genome approximately 6 kb upstream of the integration site of the retroviral vector unit, resulting in the 9.6-kb viral SacI fragment. (B) Structure of the provirus in the transduced cell lines. During transduction, the 5′ LTR is reconstituted in the reverse transcription process, and the SacI site reappears, resulting in the 3.6-kb SacI fragment found in the transduced cell lines. (C) Genomic Southern blots of G418-positive NIH 3T3 clones transduced with cell culture supernatants obtained after infection of different cell types. Clones obtained after transduction with retroviral particles produced in CHO cells (lanes 4 and 5), CV-1 cells (lanes 6 and 7), Vero cells (lanes 8 and 9), and chicken cells (lanes 10 and 11) showed the characteristic 3.6-kb SacI band that indicates an integrated retroviral vector. Lane 1, marker DNA; lane 2, DNA of the virus vrR-XSNegfp harboring only the retroviral vector unit; lane 3, DNA of a nontransduced NIH 3T3 clone. Numbers at the left show the size of the marker bands; numbers at the right show size of the hybridizing bands in the samples (in kilobase pairs).
(iv) vrR-XSNegfp.
vrR-XSNegfp was constructed by transfection of pR-XSNegfp into wild-type virus (WR)-infected CV-1 cells followed by gpt and thymidine kinase-negative selection as described above. vrR-XSNegfp contains the same retroviral vector unit as the triple virus but has no packaging functions. Final isolates of the resulting viruses were grown to large scale in CV-1 cells, and cell-bound virus was prepared by trypsinization and centrifugation through a 36% sucrose cushion.
Genomic characterization of viruses.
To characterize the genomic structure of the viruses, 2 μg of DNA of each of the recombinant viruses (the gag-pol, packaging, and triple viruses) and of the wild-type WR strain (negative control) were digested with the appropriate restriction enzymes, separated on 1% agarose gels, and transferred to nylon membranes (Hybond N; Amersham Biotech) by capillary transfer. For hybridization DNA fragments containing the sequences corresponding to the gag-pol, VSV-G, and EGFP gene were α-32P-radiolabeled by random priming (High Prime system; Roche Molecular Biochemicals). Correct integration of the gag-pol cassette into the HA locus was revealed by two fragments of 6.9 kb and 4.5 kb after digestion with MunI. Integration of the VSV-G gene in the intergenic D4/D5 region is shown by a characteristic 5.5-kb Asp718 fragment, and integration of the EGFP gene in the thymidine kinase locus leads to a 7.2-kb XhoI fragment.
Western blots.
The Western blot analyses were performed according to standard methods (31). To detect gag-pol-encoded proteins, the filters were incubated with a polyclonal goat antiserum against disrupted MLV (1:1,000 dilution; serum ID 81S000044; Quality Biotech, Camden, N.J.) followed by incubation with alkaline phosphatase-conjugated rabbit anti-goat IgG (Bio-Rad Inc., LS1706518) in a 1:2,000 dilution. The VSV-G protein bands were detected with a mouse monoclonal (Roche, Inc.; P5D4, IgG1, 1667360) in a 1:5,000 dilution, followed by incubation with a goat anti-mouse IgG (Bio-Rad Inc.; LS1706520 RevB).
Colony-forming assay.
Cell lines (see Table 1) were infected with the triple virus and the wild-type virus (WR) at a multiplicity of infection of 5 for 12 h. Supernatants were filtered through 0.1-μm sterile filters (Schleicher & Schuell) to remove the vaccinia virus vector. Polybrene was added to a final concentration of 4 μg/ml. NIH 3T3 cells were infected with retroviral particles essentially as described in the user manual of the RetroXpress System (Clontech, Inc.). Selection was performed with 500 μg of G418 per ml (PAA Laboratories, Linz, Austria). For titer determination, cell colonies were stained with crystal violet after 14 days. For further analysis, cell clones were picked and grown to large scale under G418 selection.
TABLE 1.
Production of retroviral particles in cell lines
| Cell line | CFU/ml
|
|
|---|---|---|
| Wild-type virus | Triple virus | |
| CV-1 | 0 | 2.3 × 105 |
| Vero | 0 | 2.3 × 104 |
| RK44.20 | 0 | 7.5 × 102 |
| CHO | 0 | 2.3 × 102 |
| MRC5 | 0 | 1.3 × 102 |
| 293 | 0 | 7.0 × 102 |
| CEC | 0 | 5.0 × 101 |
| PT67 | 0 | 6.0 × 104 |
Southern blotting of DNA of transduced cell clones.
Total DNAs from the transduced cell clones and from NIH 3T3 cells (negative control) were isolated by standard methods. Twenty micrograms of DNA was digested with SacI, separated on 1% agarose gels by field-inversed gel electrophoresis, and transferred to nylon membranes (Hybond N; Amersham Biotech Inc.) by capillary transfer. The positive control was NIH 3T3 cell DNA spiked with 500 pg of vrR-XSNegfp viral DNA cleaved with SacI. For hybridization, an NdeI/SacI fragment from plasmid pR-XSNegfp containing most of the retroviral transduction cassette was α-32P-radiolabeled by random priming.
PERT assays.
PCR enhanced reverse transcriptase (PERT) assays were done essentially as described before (24). For the preparation of the lysates, a volume of 500 μl of the sample was filtered through a 0.2-μm sterile, hydrophilic filter and centrifuged for 20 min at 213,000 × g. The pellet was resuspended in 50 μl of lysis buffer, and 5 μl of an appropriate dilution was assayed for reverse transcriptase activity with bacteriophage MS2 RNA as the template. Purified reverse transcriptase (avian myeloblastosis virus reverse transcriptase; Promega), serially diluted in lysis buffer to a concentration of 1 nU per 5 μl, was used as the positive control. PCR products were separated by polyacrylamide gel electrophoresis, followed by detection of laser-induced fluorescence (7). A detailed protocol will be provided upon request.
RESULTS
Design of chimeric vaccinia virus vectors.
Vaccinia virus is a cytoplasmic DNA virus and has evolved its own transcriptional regulatory signals, including early transcription stop signals consisting, on the DNA level, of the sequence 5′-TTTTTNT-3′, which on the transcriptional level results in termination of early transcripts (35). To allow efficient early expression of the packaging components and to avoid premature termination of the retroviral vector unit by the vaccinia virus transcription apparatus, the three sequences for the retroviral vector components (gag-pol, env, and the vector unit) were modified by mutagenizing all vaccinia virus early transcription stop signals into nonfunctional ones (preserving the amino acid sequences) and placing them under control of vaccinia virus promoters. The components were inserted into separate loci on the vaccinia virus genome. This strategy should prevent the formation of replication-competent retrovirus by recombination events.
The construction steps and the arrangement of the genetic elements in the final vaccinia virus vector are outlined in Fig. 1A. First, the gag-pol gene cassette was inserted into the hemagglutinin (HA) locus of wild-type vaccinia virus (step 1), resulting in the gag-pol virus. Next, the env component consisting of the VSV-G glycoprotein gene was inserted into the gag-pol virus, resulting in the packaging virus (step 2). For this insertion, the nonessential intergenic region downstream of the vaccinia virus D4R gene (D4/D5 locus) was chosen as the integration site. The packaging virus expresses the retroviral packaging functions and is suited to accommodate a retroviral vector unit of choice after simple recombination into the vaccinia virus thymidine kinase locus (step 3) with standard selection techniques (4, 15). The virus carrying all retroviral components, termed the triple virus, is shown in Fig. 1B.
Construction and genomic characterization of viruses.
First, the gag-pol expression cassette was inserted into the vaccinia virus HA locus of the vaccinia virus WR strain. For this purpose, plasmid pHA-MLVg, which contains the gag-pol ORF of Moloney MLV controlled by the vaccinia virus synthetic early/late promoter (3) flanked by vaccinia virus sequences derived from the viral HA locus, was constructed. An additional gene cassette, consisting of the marker genes lacZ and gpt flanked by direct repeats, was cloned into the plasmid. Transient selection (27) resulted in a marker-free intermediate virus, the gag-pol virus that had incorporated the gag-pol sequences, as shown by Southern blot analysis (Fig. 2A, lane 2). The wild-type virus was negative in this analysis (lane 1).
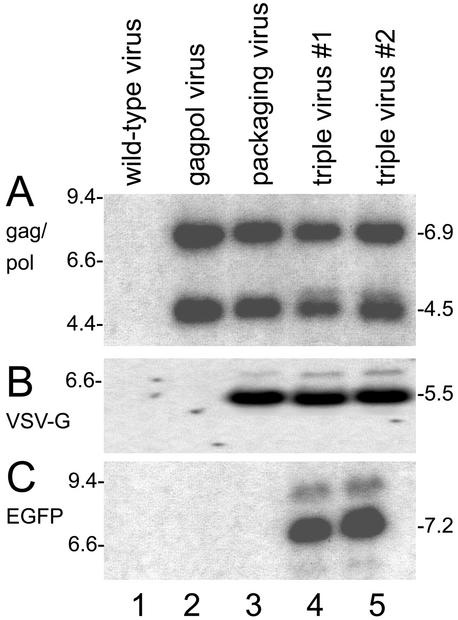
FIG. 2.
Characterization of recombinant vaccinia viruses by Southern blotting. Genomic DNA of the different viruses was digested with the respective restriction endonuclease, transferred to a filter, and hybridized to the indicated radioactively labeled probes. (A) Viral DNAs hybridized to the gag-pol probe showed the expected MunI fragments of 6.9 and 4.5 kb in the gag-pol, packaging, and triple viruses (lanes 2 to 5). (B) Hybridization with the VSV-G probe revealed the characteristic 5.5-kb Asp718 fragments in the packaging and triple viruses (lanes 3 to 5). (C) The 7.2-kb XhoI fragment hybridizing with the EGFP probe was found only in the triple viruses (lanes 4 and 5). The wild-type virus (lanes 1) did not show these signals. The numbers at the left show the marker bands; those at the right show the sizes of the fragments (in kilobase pairs).
The next step was to include an envelope (env) gene into this virus. Because retroviruses with envelopes consisting of the VSV-G glycoprotein are stabler and have a broadened host range (1), the VSV-G glycoprotein, which is usually difficult to express in packaging cell lines (34), was used. Plasmid pDR-P11Z5V contains all sequence elements necessary to direct the env gene cassette, together with a permanent lacZ marker gene, into the D4/D5 locus, a useful insertion site for stable recombinant viruses (20). The VSV-G env is driven by the vaccinia virus mH5 promoter (33). Plasmid pDR-P11Z5V was transfected into gag-pol virus-infected cells. The resulting packaging virus was selected with lacZ screening (2). This virus had incorporated both the gag-pol and the env sequences, as shown by Southern blotting (Fig. 2A and B, lanes 3), while the control viruses were negative for env sequences (lanes 1 and 2).
The construction of the final vaccinia virus vector, termed the triple virus, was completed by insertion of a vaccinia virus promoter-controlled retroviral vector unit together with the gpt selection marker into the vaccinia virus thymidine kinase locus. The retroviral vector unit consisted of an LTR promoter-driven EGFP gene and a simian virus 40 promoter-neomycin gene cassette to allow in vivo detection and titration of neomycin-positive colonies, respectively (11). Plasmid pR-XSNegfp was used to construct this virus. The structure of this plasmid is shown in Fig. 1C. The presence of all three inserts in the triple virus was shown by hybridization with a gag-pol, a VSV-G, and an EGFP probe in two independent triple virus clones (Fig. 2, lanes 4 and 5). The DNAs from wild-type virus (Fig. 2, lane 1) and from the precursor constructs (Fig. 2, lanes 2 and 3) served as controls.
Viruses express retroviral packaging components.
To determine whether the transgenes were expressed, Western blot analyses were performed (Fig. 3). The viruses were used to infect CV-1 cells, and lysates were probed with antibodies against MLV gag-pol (Fig. 3A). The gag-pol virus (Fig. 3A, lane 2), the packaging virus (Fig. 3A, lane 4), and two isolates of the triple virus (Fig. 3A, lanes 5 and 6) showed the expected gag-pol bands, including the Pr65 gag precursor and the p30 capsid protein, while the negative control did not show these bands (Fig. 3A, lane 3). Next, the blot was incubated with VSV-G env-specific antibodies (Fig. 3B). A single vaccinia virus expressing VSV-G (12) showed the expected band in the 55-kDa range characteristic of VSV-G env (Fig. 3B, lane 2). The packaging and triple viruses were also positive (Fig. 3B, lanes 4 to 6), confirming expression of the retroviral packaging components.
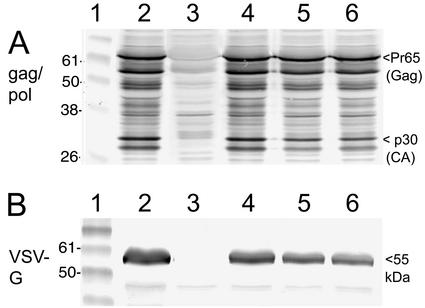
FIG. 3.
Expression of retroviral packaging components by recombinant vaccinia viruses shown by Western blotting. CV-1 cells were infected with the indicated viruses, and total cellular extracts were prepared and subjected to the Western blot procedure. (A) Incubation of the blots with a gag-pol antiserum revealed a characteristic pattern of protein bands, including the Pr65 gag-specific band and the p30 capsid (CA) band (arrowhead, right side) in the packaging virus and two clones of the triple viruses. Lane 1, protein markers. Lane 2, positive control, lysate of cells infected with the virus vHA-MLVg, which has a single insert of the gag-pol sequences (see text). Lane 3, negative control, lysate of cells infected with WR wild-type virus. Lane 4, lysate of cells infected with the packaging virus. Lane 5, lysate of cells infected with triple virus clone 1. Lane 6, lysate of cells infected with triple virus clone 2. (B) Incubation of blots with VSV-G antibodies revealed the typical 55-kDa band of the VSV-G glycoprotein in the packaging and triple viruses. Lane 1, protein markers. Lane 2, positive control, lysate of cells infected with the virus vDD4-mH5-VSVg, a recombinant WR-based virus with a single VSV-G gene insert. Lane 3, negative control, lysate of cells infected with wild-type WR virus. Lane 4, lysate of cells infected with the packaging virus. Lane 5, lysate of cells infected with triple virus clone 1. Lane 6, lysate of cells infected with triple virus clone 2. Numbers at the left indicate the size of the protein markers, and those at the right show the size of characteristic bands (in kilodaltons).
Single vaccinia virus vector mediates efficient generation of retroviral particles in wild-type cell lines.
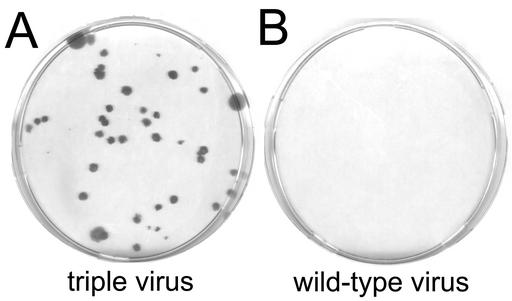
To test the functionality of the viral construct, a cell line usually used to grow vaccinia virus (cell line CV-1) was infected with 5 PFU of the triple virus per cell. After 12 h of growth, supernatants were filtered through 0.1-μm filters to remove cell debris and residual vaccinia virus, diluted, and used to transduce NIH 3T3 cells as indicator cells. The 0.1-μm filtration procedure resulted in vaccinia virus-free supernatants of defective retroviral particles. Surprisingly, CV-1 cells turned out to efficiently release transduction-competent retroviral particles. Neomycin-resistant 3T3 cell clones growing in the presence of the antibiotic G418 (500 μg/ml) could be observed upon titration, showing for the first time that retroviral vector production induced by a single large DNA virus in a wild-type cell line is feasible (Fig. 4A). The negative control, a supernatant obtained by infection of CV-1 cells with wild-type vaccinia virus, did not induce clones (Fig. 4B).
FIG. 4.
Colony-forming assays in NIH 3T3 cells. The kidney cell line CV-1 was infected with the indicated viruses, and the vaccinia virus-free supernatants were used to perform colony-forming assays in NIH 3T3 cells with the transduced neomycin resistance marker. Colonies were selected at 500 μg of the antibiotic G418 per ml and stained with crystal violet after 2 weeks. (A) Supernatants of CV-1 cells (the 1:1,000 dilution is shown) infected with the vaccinia virus triple virus gave rise to G418-resistant NIH 3T3 colonies. (B) Supernatants of the same cell line infected with wild-type virus did not result in colonies of the indicator cell line.
To determine the potential to produce retroviral vectors, additional cell lines were infected, and supernatants were collected and used to transduce 3T3 indicator cells (Table 1). The most productive cell lines were those that also efficiently supported the growth of vaccinia virus, like the kidney cell lines CV-1 and Vero. Chinese hamster ovary (CHO) cells, which are nonpermissive for vaccinia virus, were also able to release functional retroviral vectors upon infection with the triple virus, showing that abortive infection and early gene expression are sufficient for the release of retroviral vector particles. The human cell lines MRC5 and 293 and the rabbit kidney cell line RK44.20 (9) were also able to release transduction-competent vectors, as were primary chicken cells, albeit in small amounts. Triple virus-infected PT67 packaging cells induced a titer of 6.0 × 104 CFU/ml (Table 1). As a positive control, PT67 cells infected with a vaccinia virus with a single insert of the retroviral vector unit having no packaging functions (vrR-XSNegfp) released retroviral particles in the range of 5 × 105 CFU/ml (not shown in Table 1). Therefore, despite the additional gag-pol expression by the triple virus, no enhancing effect was seen by infecting the packaging cell line PT67 with the triple virus compared to the control virus lacking packaging components.
In summary, retroviral vectors were produced in diverse cell lines, including primary chicken cells. The highest productivity was observed with CV-1 cells, in which average titers of 105 CFU/ml were obtained. The triple virus stock was grown and titered in CV-1 cells, which may explain the good results obtained in the monkey cell lines. Growing the stock in human cells such as MRC-5 may improve vector production in human cell lines because adaptation by passaging may improve infectivity and yields. The supernatants from cells infected with the control viruses had no transducing potential. The technology allowed permanent retroviral transduction of host cells by infection with the hybrid vaccinia virus without classical packaging cell lines.
Infected cell cultures and transduced cell clones express EGFP.
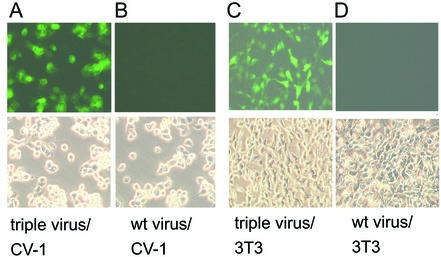
Next, the triple virus was used to study EGFP expression in infected CV-1 cells and in transduced NIH 3T3 clones. The CV-1 cell line was infected with 5 PFU per cell, and cytopathic effect and EGFP fluorescence were monitored. As expected, a strong cytopathic effect but surprisingly also EGFP expression could be observed in primary infections with the triple virus (Fig. 5A). The strong EGFP expression in the vaccinia virus-infected cells was unexpected because the EGFP gene is not controlled by a vaccinia virus promoter in the triple virus. The single-insert virus vrR-XSNegfp induced only barely detectable fluorescence. Therefore, unintended readthrough and expression of EGFP cannot fully explain the strong fluorescence induced by the triple virus. Since all retroviral components are controlled by vaccinia virus early or early/late promoters, one might speculate that retroviral transduction and proviral EGFP expression already occur in the vaccinia virus-infected cell, resulting in the strong EGFP fluorescence. The wild-type vaccinia virus infection induced the same type of cytopathic effect but no green fluorescence (Fig. 5B).
FIG. 5.
Induction of green fluorescence by infections with triple virus or transduction with retroviral particles induced by triple virus. CV-1 cells directly infected (1 PFU per cell) with the triple virus showed a strong fluorescence 12 h after infection (A), while the wild-type (wt) virus did not induce a signal (B). The characteristic cytopathic effects could be observed in the phase contrast picture (lower panels). Supernatants of CV-1 cells infected with the triple virus were used to transduce NIH 3T3 cells. Green fluorescence of NIH 3T3 clones after 6 days is shown (C). A parallel experiment with the same cells transduced with control supernatants (wild-type virus-infected CV-1 cells) did not result in a signal (D).
Next, transduction experiments in NIH 3T3 cells with the filtered CV-1 cell supernatants were performed. At 24 to 60 h after transferring the supernatants, increasing EGFP fluorescence was detectable in the NIH 3T3 target cells, which stayed stable thereafter (Fig. 5C). No signs of vaccinia virus infection such as cytopathic effect or plaque formation could be detected. As vaccinia virus grows well in NIH 3T3 cells, eventually destroying the whole culture, the continued growth of the transduced culture under nonselective conditions is a proof of the absence of vaccinia virus. The control experiment, transduction with supernatants from wild-type virus-infected cells, did not result in EGFP expression (Fig. 5D). Thus, the functionality of the system and the particles was confirmed by the second marker present in the retroviral vector.
Secondary transduction after abortive infection of CHO cells.
In order to achieve permanent transduction of cells by infection with the triple virus, a cell line that does not support replication of vaccinia virus was chosen for an infection-transduction experiment. The CHO cell line is nonpermissive for vaccinia virus (28); early vaccinia virus protein synthesis proceeds, but intermediate and late protein synthesis does not proceed after infection, and apoptosis is induced shortly after entry of the virus into the cells (25). A low multiplicity of infection with the triple virus should therefore result in infection of a minority of cells' releasing transduction-competent particles before undergoing apoptosis. The majority of cells, however, should proceed to grow and, if transduced with the EGFP retrovirus released by the apoptotic cells, become permanently growing green fluorescent cells.
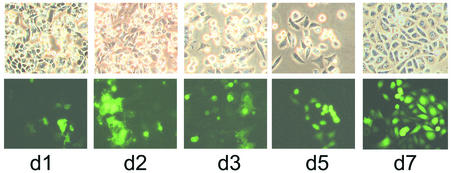
To test this scenario, CHO cells were infected at a low multiplicity of infection (0.5 PFU per cell) and observed for 7 days. After day 1, the monolayer showed the typical cytopathic effect (Fig. 6, d1, upper panel), and some of them showed green fluorescence. At day 2, the green color of the directly infected cells intensified and the cytopathic effect was more pronounced. At days 3 and 5, many of the infected cells began to detach from the surface, and transduced cells started growing. At day 7, finally, the monolayer of live CHO cells had recovered and contained many fluorescing islands of transduced clones. This indicates that infection of cells with a nonreplicating vaccinia virus releasing transduction-competent particles results in permanently transduced secondary cells. This would also mimic the situation after infection of animals with a triple virus based on nonreplicating vaccinia virus strains such as modified vaccinia virus Ankara (29) or defective vaccinia virus vectors (9).
FIG. 6.
Infection of nonpermissive Chinese hamster ovary cells with triple virus results in secondary transductions. CHO cells were infected with 0.5 PFU of the triple virus, and green fluorescence of the culture was monitored over 7 days (d1 to d7). Phase contrast pictures (upper panel) and EGFP fluorescence (lower panel) are shown. In the first days after infection with the vaccinia virus hybrid virus (d1 to d3), infected CHO cells showed a strong cytopathic effect and also showed green fluorescence. The vaccinia virus-infected cells underwent apoptosis and detached, and after 5 days (d5), the noninfected but transduced cells recovered and formed confluent monolayers with islands of fluorescing cells (d7).
Transduced clones have the correct genomic inserts.
Several neomycin-resistant NIH 3T3 clones from the titrations of the different cell lines were amplified and screened for EGFP expression. All of the clones examined displayed stable EGFP transgene expression. Integration of the retroviral vector into the genome of the target cell was verified by Southern blotting of genomic cellular DNA. Total cellular DNA of transduced cell lines and controls was digested with the enzyme SacI. This enzyme cuts once in the U3 region of the retroviral transcription unit and therefore, after reverse transcription and integration, releases a distinct fragment of 3.6 kb including most of the proviral sequences in the transduced clones.
The structure of the retroviral vector unit in the vaccinia virus and in the transduced cells is depicted in Fig. 7A and B, and the Southern blot is shown in Fig. 7C. The positive control, consisting of total DNA of wild-type NIH 3T3 cells spiked with 500 pg of vR-XSNegfp vaccinia virus viral DNA, shows a larger fragment resulting from a second SacI site in the vaccinia virus genome that is positioned about 6 kb upstream of the retroviral transcription unit (Fig. 7C, lane 2). In the negative control, total DNA from NIH 3T3 cells, this signal was not detectable (Fig. 7C, lane 3). The fragment of 3.6 kb was found in all cell lines transduced with particles obtained from CHO (Fig. 7C, lanes 4, 5), CV-1 (Fig. 7C, lanes 6 and 7), Vero (Fig. 7C, lanes 8 and 9), and primary chicken cells (Fig. 7C, lanes 10 and 11). This analysis demonstrates integration of the provirus and confirms the reconstitution of the U3 region by the presence of a new upstream SacI site and shows that all cell lines examined produced functional retroviral particles upon infection with the triple virus.
Attempts to detect replication-competent retrovirus.
We then asked whether recombination events in cells infected with the poxvirus-retrovirus vectors would result again in replication-competent retroviruses. The gag-pol gene inserted in the vaccinia virus hemagglutinin locus shares a short homologous segment of approximately 400 bp with the defective provirus located in the vaccinia virus thymidine kinase locus. This region of homology is an overlap of the packaging signal that extends into the gag ORF. Formation of replication-competent retrovirus would require a series of precise recombination events. Without further homologies, recombinations resulting in functional replication-competent retrovirus, although theoretically possible, are an improbable event because the gag-pol and env genes lack their own retroviral promoters and are dispersed as separate transcription units in the vaccinia virus vector.
In order to detect replication-competent retrovirus, ultrasensitive product-enhanced reverse transcriptase (PERT) assays (24) were performed. Cell cultures (CV-1, NIH 3T3, and PT67) were infected with the triple virus, resulting in the production of defective retroviruses (for titers, see Table 1). The undiluted supernatants (2 × 104 to 2 × 105 CFU, depending on the cell line), passed twice through 0.1-μm filters to remove vaccinia virus, were plated onto NIH 3T3 cells (six-well plate, 3-cm diameter), grown to confluency, split once, and grown (without selection) again to confluency. This passaging should result in amplification of replication-competent retrovirus, if present in infected cells, or in multiplication of residual vaccinia virus, resulting in false-positive PERT activities. The supernatants of these cultures were subjected to the PERT assay. The positive controls consisted of triple virus-infected, filtered supernatants and supernatants obtained with the packaging cell line PT67 infected with the virus vrR-XSNegfp.
High reverse transcriptase activities in the range of 103 to 105 nU were found in the positive controls (Table 2). The negative controls consisted of supernatants of the same cell cultures infected with the wild-type vaccinia virus. Only the PT67 packaging cells, which actively produce gag-pol particles, gave a positive PERT signal (Table 2). The wild-type-infected cell supernatants of the CV-1 production cells did not induce reverse transcriptase activity (below the detection limit of 2 nU), and the NIH 3T3 supernatants of the passaged cells were all negative. If replication-competent retrovirus had formed in one of the production cells after infection with the triple virus, it should have replicated after transduction and passage in 3T3 cells. Since the PERT assay did not reveal any reverse transcriptase activity in the passaged supernatants, the hybrid viral system is not prone to replication-competent retrovirus formation.
TABLE 2.
PERT assays for detection of replication-competent retrovirusa
| Group | Sample | Reverse transcriptase (nU/ml) | Titer (CFU/ml) |
|---|---|---|---|
| Positive and negative controls | Lys CV-1 mock | <2 | 0 |
| Sup CV-1 mock | <2 | 0 | |
| Sup CV-1/wt virus | <2 | 0 | |
| Sup CV-1/triple virus#9 | 211,046 | 104 | |
| Lys PT67/mock | 4,405 | 0 | |
| Sup PT67/mock | 86,234 | 0 | |
| Sup PT67/wt virus | 338,517 | 0 | |
| Sup PT67/triple virus | 6,584 | 103 | |
| Transduced 3T3 supernatants | Sup 3T3 (CV-1/triple virus 9) | <2 | ND |
| Sup 3T3 (CV-1/wt virus) | <2 | ND | |
| Lys 3T3 mock | 13 | 0 | |
| Sup 3T3 mock | <2 | 0 |
wt, wild type; lys, cell lysate; sup, supernatant; ND, not determined.
Retrovirus vector preparations and test for replicating vaccinia virus.
In order to evaluate the hybrid system for the production of retroviral vector supernatants, 12 large flasks of CV-1 cells (a total of 2.1 × 108 cells) were infected with the triple virus (5 PFU/cell) and grown for 12 h. The cell culture supernatants (170 ml) were harvested, filtered once through 0.1-μm filters, and concentrated by ultracentrifugation. The pellet was resuspended in 1 ml of medium, and the number of retroviral particles was determined. The retrovirus titer before ultracentrifugation was 1.65 × 105 CFU/ml; after the pelleting step, a titer of 2.6 × 107 CFU/ml was achieved. Thus, a 158-fold concentration of defective retroviral particles was achieved, and the yield was 92%. The experiment was repeated with similar results, showing that the pseudotyped particles could be concentrated without significant loss of retroviral titer.
Replicating vaccinia virus was also determined. The titers of the supernatants before filtration were approximately 106 PFU/ml (a total of 1.7 × 108 PFU); after the 0.1-μm filtration step, no virus was detectable in the undiluted supernatant. After ultracentrifugation, however, a total of 3.8 × 102 PFU of vaccinia virus were detectable, resulting in a calculated titer after filtration and before ultracentrifugation of around 2 PFU/ml. A single 0.1-μm filtration step decreased the titer by more than five orders of magnitude but did not totally remove vaccinia virus. A second 0.1-μm filtration step on the level of the concentrated supernatant, though presumably reducing the retroviral particle yield, may be used to fully eliminate vaccinia virus. In some critical experiments such as replication-competent retrovirus determination (see above), the retrovirus-containing supernatants were filtered twice to exclude any replicating vaccinia virus. Contamination by vaccinia virus was not observed in the colony-forming assays (where supernatants are usually diluted) or when culturing or passaging transduced cell lines.
DISCUSSION
This report describes a large DNA virus, vaccinia virus, that releases transducing retroviral vectors upon infection of normal host cells. Packaging functions are encoded by vaccinia virus, allowing retroviral transduction of host cells by simple vaccinia virus infection. Interestingly, large DNA viruses may naturally contain functional retroviral inserts. A nearly full-length avian provirus integrated into the DNA of a fowlpox virus strain was infectious in chickens, and herpesviruses with retroviral inserts have been documented (8). In contrast, the triple viruses described here do not contain functional proviruses but defective retroviral vector units encoding foreign genes. The packaging functions are dispersed as separate vaccinia virus promoter-driven transcription units in the vaccinia virus genome, practically excluding the formation of replicating retroviruses.
An advantage of the hybrid system is the ability to produce pseudotyped vectors with env genes that are usually cytotoxic in packaging cell lines. To improve transduction and stability, retroviral vectors that have incorporated more favorable nonretroviral envelope proteins, such as the VSV-G envelope, have been constructed (1). Because of the fusogenic properties of the VSV-G protein, it has been difficult to establish stable cell lines expressing this protein (6, 34). Only inducible expression of the VSV-G protein with the tetracycline-regulatable expression system allowed the construction of a packaging cell line (21). Since vaccinia virus expression in host cells is transient, there were no difficulties in using VSV-G in the triple virus, allowing the construction of pseudotyped retroviruses in any vaccinia virus-permissive cell line.
In addition, concentration of culture supernatants to high titers without loss of retroviral infectivity was feasible. Vaccinia virus is one of the largest animal viruses, having a diameter of 200 to 400 nm. Therefore, vaccinia virus was removed by nanofiltration with 0.1-μm filters prior to ultracentrifugation. Nanofiltration was quite effective, reducing titers by several orders of magnitude. Low levels of vaccinia virus could, however, be detected upon ultracentrifugation of large amounts of supernatant, an issue that may be overcome by a second nanofiltration step.
For safety reasons, replicating vaccinia virus vectors are not the vectors of choice to perform vaccinations or gene therapy. Nonreplicating vectors such as modified vaccinia virus Ankara (16) or defective vaccinia virus vectors (9, 20) are safer and may be used as future hybrid vectors. In fact, defective vaccinia virus-based vectors efficiently produce transduction-competent particles in classical packaging cell lines (10), suggesting that triple viruses based on the defective vaccinia virus vector technology would be as efficient as replicating viruses in retrovirus particle production, eliminating problems with replicating vaccinia virus in concentrated retroviral supernatants. Moreover, infection with the replicating triple virus of nonpermissive CHO cells, which mimics nonreplicating vectors, resulted in production of retroviral particles and led to permanent transduction of the recovering cell culture. Cells infected with vaccinia virus usually undergo apoptosis and die. Especially in the case of nonreplicating vectors, infection within a host organism is self-limiting and provides a safe system to achieve transduction in vivo.
The immunogenic properties of vaccinia virus vectors preclude the use of these vectors in classical gene therapy, for instance, in the replacement of defective with intact genes. However, immunogenic properties and transducing capacity may be useful properties in applications such as tumor immunotherapy. Direct intratumoral injection of vectors that in part lyse the cells and transduce neighboring cells with therapeutic genes or genes that increase the immune response may be a promising concept. In preliminary in vivo experiments in a nude mouse tumor model, the triple virus induced strong green fluorescence of the tumor mass; however, vaccinia virus was not cleared, and high vaccinia virus titers were still detectable 2 weeks after injection (S. Coulibaly, unpublished data). Thus, infection could not be differentiated from transduction in this model. Therefore, the construction of defective triple vaccinia virus vectors may be the next step to show transduction of tumor cells in vivo.
Retroviral vectors based on MLV transduce only dividing cells, and therefore an improved system based on lentiviral vectors would be desirable. Lentiviral vectors also transduce a range of nondividing cells, improving gene transfer in some nondividing tissues (18, 32). The wide host range of vaccinia virus, the stability of the virus, and the availability of safe nonreplicating vaccinia virus strains may therefore open new perspectives in gene delivery.
Acknowledgments
We thank V. Wieser and W. Gritschenberger for expert technical assistance, D. Klein and W. Günzburg (University of Veterinary Medicine, Vienna) for the EGFP and gag-pol plasmids, and I. Livey for critically reading the manuscript.
REFERENCES
- 1.Burns, J. C., T. Friedmann, W. Driever, M. Burrascano, and J. K. Yee. 1993. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc. Natl. Acad. Sci. USA 90:8033-8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakrabarti, S., K. Brechling, and B. Moss. 1985. Vaccinia virus expression vector: coexpression of β-galactosidase provides visual screening of recombinant virus plaques. Mol. Cell. Biol. 5:3403-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakrabarti, S., J. R. Sisler, and B. Moss. 1997. Compact, synthetic, vaccinia virus early/late promoter for protein expression. BioTechniques 23:1094-1097. [DOI] [PubMed] [Google Scholar]
- 4.Falkner, F. G., and B. Moss. 1988. Escherichia coli gpt gene provides dominant selection for vaccinia virus open reading frame expression vectors. J. Virol. 62:1849-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng, M., W. H. Jackson, Jr., C. K. Goldman, C. Rancourt, M. Wang, S. K. Dusing, G. Siegal, and D. T. Curiel. 1997. Stable in vivo gene transduction via a novel adenoviral/retroviral chimeric vector. Nat. Biotechnol. 15:866-870. [DOI] [PubMed] [Google Scholar]
- 6.Florkiewicz, R. Z., and J. K. Rose. 1984. A cell line expressing vesicular stomatitis virus glycoprotein fuses at low pH. Science 225:721-723. [DOI] [PubMed] [Google Scholar]
- 7.Haemmerle, T., M. Himmelspach, F. Dorner, and F. G. Falkner. 1997. A sensitive PCR assay system for the quantitation of viral genome equivalents: human immunodeficiency virus type 1 (HIV-1) and hepatitis B virus (HBV). Arch. Virol. 142:1297-1306. [DOI] [PubMed] [Google Scholar]
- 8.Hertig, C., B. E. Coupar, A. R. Gould, and D. B. Boyle. 1997. Field and vaccine strains of fowlpox virus carry integrated sequences from the avian retrovirus, reticuloendotheliosis virus. Virology 235:367-376. [DOI] [PubMed] [Google Scholar]
- 9.Holzer, G. W., and F. G. Falkner. 1997. Construction of a vaccinia virus deficient in the essential DNA repair enzyme uracil DNA glycosylase by a complementing cell line. J. Virol. 71:4997-5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holzer, G. W., J. A. Mayrhofer, W. Gritschenberger, F. Dorner, and F. G. Falkner. 1999. Poxviral/retroviral chimeric vectors allow cytoplasmic production of transducing defective retroviral particles. Virology 253:107-114. [DOI] [PubMed] [Google Scholar]
- 11.Klein, D., S. Indraccolo, K. von Rombs, A. Amadori, B. Salmons, and W. H. Gunzburg. 1997. Rapid identification of viable retrovirus-transduced cells with the green fluorescent protein as a marker. Gene Ther. 4:1256-1260. [DOI] [PubMed] [Google Scholar]
- 12.Konetschny, C. 2002. Studien an poxviralen-retroviralen Hybridvektoren. Ph.D. thesis. Technische Universität Wien, Vienna, Austria.
- 13.Konetschny, C., G. W. Holzer, and F. G. Falkner. 2002. Retroviral vectors produced in the cytoplasmic vaccinia virus system transduce intron-containing genes. J. Virol. 76:1236-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li, K. J., and H. Garoff. 1998. Packaging of intron-containing genes into retrovirus vectors by alphavirus vectors. Proc. Natl. Acad. Sci. USA 95:3650-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackett, M., G. L. Smith, and B. Moss. 1982. Vaccinia virus: a selectable eukaryotic cloning and expression vector. Proc. Natl. Acad. Sci. USA 79:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayr, A., H. Stickl, H. K. Müller, K. Danner, and H. Singer. 1978. The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defence mechanism. Zentbl. Bakteriol. Hyg. I Abt. Orig. B 167:375-390. [PubMed] [Google Scholar]
- 17.Miller, A. D. 1997. Development and applications of retroviral vectors, p. 437-473. In J. M. Coffin, S. H. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [PubMed]
- 18.Mitrophanous, K., S. Yoon, J. Rohll, D. Patil, F. Wilkes, V. Kim, S. Kingsman, A. Kingsman, and N. Mazarakis. 1999. Stable gene transfer to the nervous system with a non-primate lentiviral vector. Gene Ther. 6:1808-1818. [DOI] [PubMed] [Google Scholar]
- 19.Moss, B. 1996. Poxviridae: the viruses and their replication, p. 2637-2671. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 20.Ober, B. T., P. Bruhl, M. Schmidt, V. Wieser, W. Gritschenberger, S. Coulibaly, H. Savidis-Dacho, M. Gerencer, and F. G. Falkner. 2002. Immunogenicity and safety of defective vaccinia virus Lister: comparison with modified vaccinia virus Ankara. J. Virol. 76:7713-7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ory, D. S., B. A. Neugeboren, and R. C. Mulligan. 1996. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc. Natl. Acad. Sci. USA 93:11400-11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pensiero, M. N., C. A. Wysocki, K. Nader, and G. E. Kikuchi. 1996. Development of amphotropic murine retrovirus vectors resistant to inactivation by human serum. Hum. Gene Ther. 7:1095-1101. [DOI] [PubMed] [Google Scholar]
- 23.Pfleiderer, M., F. G. Falkner, and F. Dorner. 1995. Requirements for optimal expression of secreted and nonsecreted recombinant proteins in vaccinia virus systems. Protein Expr. Purif. 6:559-569. [DOI] [PubMed] [Google Scholar]
- 24.Pyra, H., J. Boni, and J. Schupbach. 1994. Ultrasensitive retrovirus detection by a reverse transcriptase assay based on product enhancement. Proc. Natl. Acad. Sci. USA 91:1544-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramsey-Ewing, A., and B. Moss. 1998. Apoptosis induced by a postbinding step of vaccinia virus entry into Chinese hamster ovary cells. Virology 242:138-149. [DOI] [PubMed] [Google Scholar]
- 26.Savard, N., F. L. Cosset, and A. L. Epstein. 1997. Defective herpes simplex virus type 1 vectors harboring gag, pol, and env genes can be used to rescue defective retrovirus vectors. J. Virol. 71:4111-4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheiflinger, F., F. Dorner, and F. G. Falkner. 1998. Transient marker stabilisation: a general procedure to construct marker-free recombinant vaccinia virus. Arch. Virol. 143:467-474. [DOI] [PubMed] [Google Scholar]
- 28.Spehner, D., S. Gillard, R. Drillien, and A. Kirn. 1988. A cowpox virus gene required for multiplication in Chinese hamster ovary cells. J. Virol. 62:1297-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutter, G., and B. Moss. 1992. Nonreplicating vaccinia virus vector efficiently expresses recombinant genes. Proc. Natl. Acad. Sci. USA 89:10847-10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeuchi, Y., F. L. Cosset, P. J. Lachmann, H. Okada, R. A. Weiss, and M. K. Collins. 1994. Type C retrovirus inactivation by human complement is determined by both the viral genome and the producer cell. J. Virol. 68:8001-8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vigna, E., and L. Naldini. 2000. Lentiviral vectors: excellent tools for experimental gene transfer and promising candidates for gene therapy. J. Gene Med. 2:308-316. [DOI] [PubMed] [Google Scholar]
- 33.Wyatt, L. S., S. T. Shors, B. R. Murphy, and B. Moss. 1996. Development of a replication-deficient recombinant vaccinia virus vaccine effective against parainfluenza virus 3 infection in an animal model. Vaccine 14:1451-1458. [DOI] [PubMed] [Google Scholar]
- 34.Yee, J. K., T. Friedmann, and J. C. Burns. 1994. Generation of high-titer pseudotyped retroviral vectors with very broad host range. Methods Cell Biol. 43:99-112. [DOI] [PubMed] [Google Scholar]
- 35.Yuen, L., and B. Moss. 1987. Oligonucleotide sequence signaling transcriptional termination of vaccinia virus early genes. Proc. Natl. Acad. Sci. USA 84:6417-6421. [DOI] [PMC free article] [PubMed] [Google Scholar]