Abstract
The virus-encoded proteinase of Camberwell virus, a genogroup 2 norovirus, was synthesized in Escherichia coli. The purified proteinase had correct N and C termini and showed trans activity in cell-free assays. trans activity was also demonstrated in COS cells transfected with constructs encoding either the proteinase or a proteinase-polymerase fusion. The N-terminal protein of ORF1 was cleaved in COS cells, possibly at the site E194/S.
The family Caliciviridae contains single-stranded RNA viruses of positive sense. There are four genera, namely Vesivirus, Lagovirus, Norovirus, and Sapovirus, with respective type species of feline calicivirus (FCV), rabbit hemorrhagic disease virus (RHDV), Norwalk virus (NOR), and Sapporo virus (21, 23). Noroviruses and sapoviruses cause gastroenteritis in humans (2, 10, 17).
The genomes of the caliciviruses are organized into two or three open reading frames (ORFs). A large ORF, beginning at the 5′ end of the genome, encodes a polyprotein which contains motifs for a picornavirus 2C-like NTPase, a 3C-like proteinase, and a 3D-like polymerase. The capsid protein may be encoded by this same ORF (lagoviruses and sapoviruses) or by a separate ORF (vesiviruses and noroviruses). A small ORF located at the 3′ end of the genome encodes a minor structural protein ranging from 10 to 30 kDa (3, 4, 9). An established cell culture system is available only for FCV. There is no cell culture system and only a recent animal model (30) for investigating the human caliciviruses. More molecular details are known for the noroviruses than for the sapoviruses, based on genome sequencing and expression studies using cloned genes (4, 25).
The processing of the calicivirus polyprotein has been extensively studied by using in vitro transcription and translation (19, 33), synthesis in bacterial cells (20, 26, 32), synthesis in mammalian cells (18, 22, 25), and where a cell culture system is available, synthesis in infected cells (28, 29). Results obtained from these studies have shown that the virus-encoded proteinase is responsible for the processing of the polyprotein. The viral 3C-like proteinase belongs to the family of chymotrypsin-like serine proteinases in which the nucleophilic serine residue is replaced by a cysteine residue (11). The cysteine residue in the motif GDCG is critical for the activity of the proteinase, as mutagenesis of this residue leads to a loss of cleavage activity (1, 19, 25-27, 29). Recognition sites for the proteinase are E/A, E/G, and Q/G in the noroviruses (19, 20, 25). In RHDV, cleavage occurs at E/T, E/G, E/D, and Q/G; and in FCV, cleavage sites are E/A, E/D, E/N, and E/S (18, 22, 28). The proteins identified so far as a result of proteolytic processing of the norovirus ORF1 polyprotein (N terminus to C terminus) are the N-terminal protein of 37 to 48 kDa, the 2C-like NTPase protein of 40 kDa, the 3A-like protein of 20 kDa, the putative VPg of 16 kDa, the 3C-like proteinase of 19 kDa, and a 3D-like polymerase of 57 kDa (Fig. 1). For the noroviruses, a more complete profile of cleavage is observed by the expression of ORF1 in mammalian cells than following cell-free translation (13, 19, 25). An additional cleavage site within the N-terminal protein has been identified for RHDV and FCV (18, 22, 28). However, further processing of the N-terminal protein has not been shown for any human calicivirus.
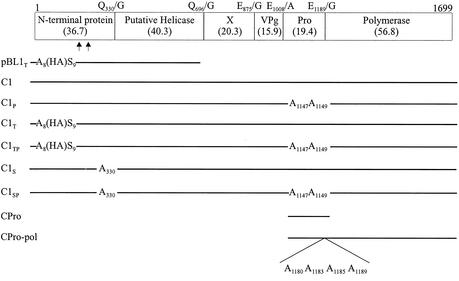
FIG. 1.
ORF1 map of CAM and the polypeptides encoded by the constructs used. The cleavage sites and their amino acid positions are shown above the box. Numbers in parentheses show the sizes (in kilodaltons) of the encoded proteins, calculated from the amino acid sequences. The HA tag was inserted between amino acids 8 and 9. Mutations were introduced into the proteinase and at Q330/G. Potential cleavage sites within the N-terminal protein are shown by arrowheads at E194/S and Q236/D. The construct CPro-pol encodes an uncleaved protein of 76 kDa.
In the processing of the calicivirus ORF1 polyprotein, a number of intermediates in addition to the final cleavage products have been observed. Of interest is the 3CD-like polypeptide that is detected in significant amount in cells expressing ORF1 (22, 25, 28). A multifunctional role for the calicivirus 3CD-like protein is likely. For FCV it is the active form of the RNA polymerase (31). For poliovirus in the family Picornaviridae, the 3CD and 3C proteins have proteolytic activity, with different cleavage specificities, and 3D is the polymerase (14, 34).
In this paper, new features of ORF1 processing for the human caliciviruses are reported. The proteinase encoded by Camberwell virus (CAM), a norovirus, was synthesized in bacterial cells. It was purified, possessed authentic N and C termini, and showed trans activity in cell-free assays. In further experiments, proteinase trans activity was shown in COS cells by cotransfection of constructs encoding a substrate polypeptide and either the proteinase (3C) or a proteinase-polymerase (3CD) fusion. For the first time, cleavage within the N-terminal protein of a norovirus is described.
Synthesis and purification of CAM proteinase.
The proteinase gene (nucleotides [nt] 3029 to 3571, GenBank accession no. AF145896) was amplified by PCR by use of the clone pCMC1 (25). In this paper, pCMC1 has been given the abbreviated name C1 (Fig. 1). The primer pair used was 13453 and 13454. The forward primer, 13453 (GTCATAGAATG3029CTCCACCAAGTATCTGG3046), contained a BsmI restriction enzyme site (underlined), while the reverse primer, 13454 (CATGACGAATTCTTAT3571TCAAGTG TAGCTTC3557), contained a stop codon (double underlined) and an EcoRI restriction site (underlined). The proteinase gene containing mutations in the motif GDC1147GC1149 was also amplified under similar conditions with C1P, previously named pCMC3 (25), used as the template. The modified motif was GDA1147GA1149. The PCR fragments were digested with BsmI and EcoRI and inserted into corresponding sites in the multiple cloning site of vector pTYB12 (NEB). This vector encodes a T7 promoter, a chitin binding domain, and the intein gene from Saccharomyces cerevisiae, followed by a multiple cloning site. The proteinase was synthesized in Escherichia coli as a fusion protein consisting of the chitin binding domain-intein-CAM proteinase (Fig. 2A). The fusion protein was immobilized on a chitin column, and upon induction of intein cleavage by dithiothreitol, the purified form of CAM proteinase was eluted. The protein yield was determined by using the Bradford assay. Both the active (Pro) and mutant (ProAA) forms of CAM proteinase of 19 kDa (Fig. 2B) had the authentic amino acid sequence at the N and C termini.
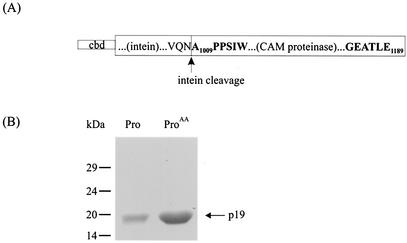
FIG. 2.
Synthesis of CAM proteinase. (A) CAM proteinase was synthesized as a fusion with the chitin binding domain (cbd) and intein. The vertical arrow indicates the intein cleavage site. (B) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of the CAM proteinase after intein cleavage. A Coomassie blue-stained gel of parental (Pro, 7 μg) and mutated (ProAA, 25 μg) proteins is shown. No additional bands were detected.
Analysis of proteinase activity in trans on translation products.
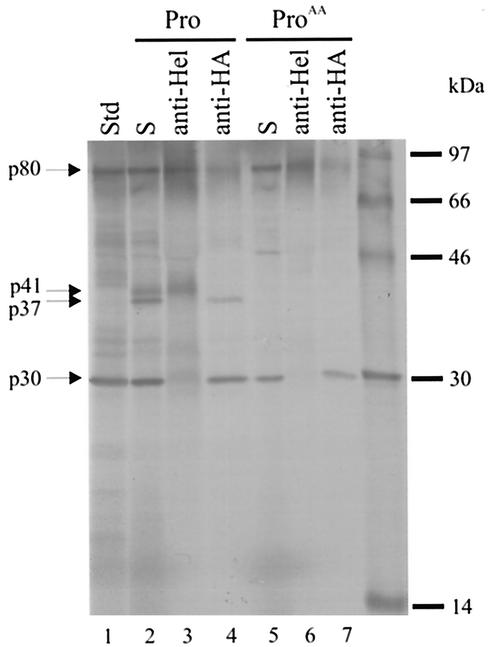
The ORF1 cDNA of CAM (nt 5 to 5383) was inserted between the EcoRI and SmaI sites in the vector pBluescript SK(+) (Stratagene). The sequence encoding an epitope of nine amino acids (YPYDVPDYA) from influenza virus hemagglutinin (HA tag), was inserted by PCR between nt 28 and 29 of ORF1 (amino acids 8 and 9). The resulting construct was further truncated by digestion with SmaI (nt 2043) and BamHI (nt 3398), the BamHI site was filled in, and the plasmid was religated to form plasmid pBL1T (Fig. 1). This plasmid encodes the first 681 amino acids of the ORF1 polyprotein followed by 11 amino acids and a stop signal. The RiboMAX T7 system (Promega) was used to transcribe in vitro pBL1T linearized with XbaI (present in the multiple cloning site of the vector). This was followed by translation (90 min at 30°C) using a rabbit reticulocyte lysate (Promega) in the absence of added proteinase (Fig. 3, lane 1) or in the presence of 2 μg of either Pro (Fig. 3, lanes 2 to 4) or ProAA (Fig. 3, lanes 5 to 7). A polypeptide p80 was detected, corresponding to the full-length translation product. It was immunoprecipitated by antiserum directed against either the putative helicase (NTPase) or the HA tag (Roche). When translation was carried out in the presence of the proteinase (Pro), two additional products, p41 and p37, were observed (Fig. 3, lane 2). The protein p41 was immunoprecipitated by anti-Hel antiserum (Fig. 3, lane 3) and therefore corresponded to the 2C-like NTPase protein. The anti-HA antiserum precipitated the protein p37 (Fig. 3, lane 4). This was close to the anticipated size for the N-terminal protein (36.7 kDa) with the HA tag (1.1 kDa). The designation p37 is used below for the protein with and without tag. In contrast, when translation was carried out in the presence of ProAA, p37 and p41 were not detected (Fig. 3, lanes 5 to 7). This result was consistent with the active Pro cleaving in trans at the Q330/G site (Fig. 1 and Table 1) that has been identified previously (19, 25). A protein, p30, was also observed following all translation reactions and was precipitated by antiserum against the HA tag. Possible explanations for its presence are premature termination of translation and autoprocessing of the p80 product. At present it is not possible to distinguish between the alternatives, although the first explanation may be more likely since (i) there are no recognizable proteinase motifs in the amino acid sequence of p80, (ii) the motif Asn-Pro-Gly-Pro that is found at the 2A/B autoprocessing site of aphthoviruses and cardioviruses (24) is absent, and (iii) p30 is not detected in transfected COS cells (see below).
FIG. 3.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of 35S-labeled polypeptides after in vitro transcription and translation of pBL1T in the absence (Std, lane 1) or presence of either Pro (lanes 2 to 4) or ProAA (lanes 5 to 7). Incubation conditions were 90 min at 30°C. Lanes labeled S were not immunoprecipitated. Immunoprecipitates were prepared with antiserum directed against either a segment (ORF1, amino acids 551 to 696) of the putative helicase (Hel) or the HA tag as indicated.
TABLE 1.
Cleavage activity of Pro and Pro-pol within the N-terminal protein p37 and between p37 and NTPase (p41)
Previous in vitro translation studies using ORF1 of NOR (13) and Southampton (SOU) viruses (19) also showed processing of the ORF1 polyprotein at Q/G sites by the viral proteinase. Cleavage at other sites was not detected. The products were p48 (N-terminal protein), p41 (NTPase), and p113 (the remaining C-terminal region of the polyprotein). The proteins p48 and p41 correspond to p37 and p41 of CAM.
Analysis of proteins in transfected mammalian cells.
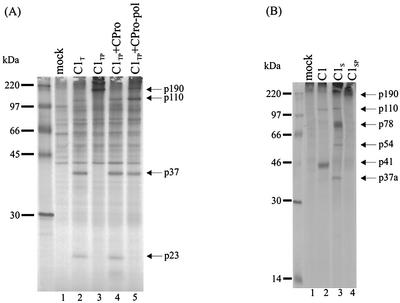
A more complete processing of the ORF1 polyprotein in mammalian cells than in cell-free systems has been demonstrated (13, 19, 25). Therefore, cleavage of CAM p37 in COS cells was further assessed. The plasmids C1 and C1P (Fig. 1) were previously described with designations of pCMC1 and pCMC3 (25). They contain CAM ORF1 cDNA in pCMV5. The vector pCMV5 has the cytomegalovirus immediate-early promoter upstream of a multiple cloning site, followed by the simian virus 40 polyadenylation signal and the origin of replication. DNA encoding the HA epitope tag was inserted into ORF1 of C1 and C1P between nt 28 and 29 (ORF1-encoded amino acids 8 and 9) by PCR to produce the plasmids C1T and C1TP, respectively (Fig. 1). To construct plasmid CPro, cDNA encoding the proteinase gene (nt 3029 to 3571) was amplified by PCR using the plasmid C1 as a template and primer pair 16550 and 16552. The forward primer 16550 (TCGATTGAATTCACCATGG3029CCCCACCAAG3039) contained an EcoRI site (underlined) and initiation codon (double underlined). The reverse primer 16552 (CAGGTCGACTCTAGATTAT3571TCAAGTGTAGCTTC3557) contained a stop codon (double underlined) and an XbaI site (underlined). To construct CPro-pol, the cDNA encoding the proteinase-polymerase (3CD-like) sequence was amplified by using pCMC5 (25) as the template. The plasmid pCMC5 encodes the CAM ORF1 polyprotein with the amino acids Q1180, E1183, E1185, and E1189 replaced by alanine. The primer pair 16550 and 16551 was used. The reverse primer 16551 (CAGGTCGACTCTAGAT5103CACTCGACGCCATCTTC5087) contained an XbaI site (underlined). Both cDNA fragments obtained after PCR were digested with EcoRI and XbaI and cloned into corresponding identical sites in the vector pCMV5 to form CPro and CPro-pol. Plasmids C1P and C1TP were used in cotransfection with either CPro or CPro-pol. Conditions for the transfection of COS-M6 cells and labeling of the proteins with [35S]methionine for 4 h at 25 h posttransfection were similar to those described previously (25). Three proteins, p110, p37, and p23, were precipitated by anti-HA antiserum from lysates of cells transfected with C1T (Fig. 4A, lane 2) but not from mock-transfected cells. The protein p37 corresponded to the N-terminal region of the ORF1 polyprotein produced by cleavage at Q330/G. The protein p23 had the HA tag and was therefore a product of processing within p37. A protein of 22 kDa (p23 with the HA tag) is larger than the corresponding proteins for FCV (5.6 kDa) (28) and RHDV (16 kDa) (18, 22). When the proteinase was inactivated by mutagenesis (C1TP), p190 corresponding to the complete ORF1 polyprotein and no cleaved viral polypeptides were detected (Fig. 4A, lane 3). Provision of the proteinase in trans by cotransfection with a separate plasmid encoding only the proteinase restored the cleavage pattern to that seen with C1T (Fig. 4A, lanes 2 and 4). The protein p23 was, however, not detected in lysates of cells that coexpressed C1TP and CPro-pol (Fig. 4A, lane 5). This demonstrated that the proteinase, but not the proteinase-polymerase fusion, was capable of processing within p37.
FIG. 4.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of 35S-labeled polypeptides immunoprecipitated from lysates of transfected COS cells. The antisera used for immunoprecipitation were directed against the HA tag (A) or the putative helicase protein (B). The DNA constructs used in transfection are shown in Fig. 1. At 24 h after transfection, cells were incubated in medium lacking Met and Cys and then labeled for 4 h with Trans35S-label (ICN Pharmaceuticals).
The protein p110 also had the HA tag (Fig. 4A, lanes 2, 4, and 5); in addition to p37 it was likely to contain the NTPase (40.3 kDa)-X (20.3 kDa)-VPg (15.9 kDa).
Mutation at Q330/G.
In order to confirm the size of the C-terminal cleavage fragment of p37 (predicted to be 15 kDa), we initially inserted the HA tag into the C-terminal region of p37. However, when the anti-HA antiserum failed to detect proteins in lysates of transfected cells (results not shown), we chose instead to mutagenize the Q330/G site to A330/G (Fig. 1) and search for the fragment linked to p41. Mutagenesis was carried out by overlap PCR (16) by using C1 as the template. In lysates of cells transfected with C1S, the antiserum directed against the putative helicase detected p110, p78, p54, and p37a (Fig. 4B). The protein p110 (Fig. 4B, lane 3) corresponded to that observed in Fig. 4A (p37-NTPase-X-VPg) and was also detected in lysates of cells transfected with C1 (Fig. 4B, lane 2). The strong band representing p78 matched the estimated size of p37-NTPase, the result of lack of cleavage at Q330/G. The accumulation of a similar p80 for NOR was also reported when amino acids around the NOR Q398/G site were mutated (13). The protein p54 corresponded to that expected for the C-terminal cleavage product of p37 (p15) linked to the NTPase protein (p41). The origin of p37a is unknown but was possibly due to cleavage at an alternative site within p41 when Q330/G was mutated. The proteins p37a and p37 were not identical. They were precipitated by different antisera, and a functional cleavage site that allowed the formation of the N-terminal protein p37 would also give rise to the NTPase protein p41, which was not observed in Fig. 4B (lane 3). A possibly related protein of 30 kDa was detected following in vitro translation of the NOR ORF1 containing a mutation in the corresponding Q398/G site (13). Only the full-length ORF1 polyprotein p190 was observed when the proteinase gene was mutated in C1SP (Fig. 4B, lane 4).
An alignment of the amino acid sequences of the N-terminal proteins of the noroviruses (Fig. 5) revealed potential cleavage sites that would yield a protein the size of p23 (p22 without the HA tag). The sites were chosen after consideration of the sequences that have been identified in RHDV (E/D, E/G, E/T, and Q/G) (18, 22), FCV (E/A, E/D, E/N, and E/S) (28), and CAM (Q/G, E/A, and E/G) (25). Cleavage at E194/S in CAM would produce proteins of 22 and 15 kDa. The E/S sequence is a cleavage site in FCV and is conserved in the genogroup 2 noroviruses CAM, Lordsdale, and Hawaii (Fig. 5). In the genogroup 1 noroviruses SOU, NOR, Chiba, and BS5, the corresponding possible site is E/N (Fig. 5). E/N has also been identified as one of the cleavage sites in RHDV. A second potential site is located at Q236/D. Cleavage at this site would yield products of 27 kDa (without the HA tag) and 10 kDa. Cleavage after Q residues occurs for both the picornavirus 3C proteinase and the calicivirus proteinases of RHDV, CAM, SOU, and NOR as listed above (7, 13, 19, 22, 25).
FIG. 5.
Partial amino acid sequence alignment of the N-terminal protein for the noroviruses. Amino acids are numbered according to CAM. Potential cleavage sites are highlighted. The accession numbers of the sequences (top to bottom) are AF145896, X86557, U07611, M87661, L07418, AB042808, and AF093797.
In the experiments described above, we obtained a purified form of CAM proteinase with authentic N and C termini. The proteinase was capable of cleaving in trans at Q330/G in reticulocyte lysates to produce p37 and p41. These proteins represented the N-terminal protein and the NTPase of CAM (Table 1; Fig. 3, lanes 4 to 6). No further processing of p37 was observed. Similarly, no further processing in vitro of the N-terminal protein p48 of NOR and SOU was detected (13, 19). However, for RHDV and FCV, the corresponding respective proteins of p39 and p37.6 were cleaved by the virus-encoded proteinase during in vitro translation (28, 33). To determine if p37 of CAM is further processed, the HA-tagged ORF1 polypeptide was synthesized in COS cells, since in a previous report it was shown that cleavage was more complete in mammalian cells than in a cell-free system (13, 19, 25). The results obtained here demonstrated that p37 is in fact cleaved by the virus-encoded proteinase in COS cells. In addition, trans activity of the proteinase on p37 was clearly shown (Table 1; Fig. 4A, lane 4). The difference in the processing of CAM p37 observed between COS cells and reticulocyte lysates suggested that cellular factors or components present only in intact cells are required for cleavage within p37.
The proteins of RHDV (p39) and FCV (p37.6) are similar in size but share only 18% identity in amino acid sequence with CAM p37. The products of cleavage vary widely in size: for CAM, p22 and p15; for RHDV, p16 and p23 (18, 22); and for FCV, p5.6 and p32 (28). Proteolysis of FCV p37.6 in infected cells is required for virus replication, since virus could not be recovered when the site between p5.6 and p32 was mutated (28). Based on their location in the genome, it is hypothesized that these two proteins of the caliciviruses correspond to the 2A- and 2B-like proteins of the picornaviruses (18, 33). Among the viruses belonging to the picornavirus supergroup, the 2A proteins vary in sequence and size (24), as reflected here in the difference in the sizes of the proteins of CAM (p22), RHDV (p16), and FCV (p5.6).
The amino acid sequence alignment of the noroviruses and a comparison with RHDV and FCV suggested two potential sites of cleavages within CAM p37 (Fig. 5). They were E194/S and Q236/D. Mutagenesis of the downstream site Q330/G led to the detection of p54 (Fig. 4B, lane 3), consistent with cleavage occurring at E194/S. The greater accumulation of p78 over p54 indicated that cleavage at Q330/G may be a prerequisite for efficient cleavage at E194/S.
Proteolytic activity in the human caliciviruses is not confined to the 3C-like proteinase. In these and earlier experiments (25), the 3CD-like polypeptide of CAM was active in transfected COS cells. However, unlike the proteinase alone, the proteinase-polymerase fusion did not cleave within p37 (Table 1). Proteolysis with differing substrate specificity by the 3C and 3CD proteins of picornaviruses has also been demonstrated (5, 6, 14, 34). The 3CD protein of poliovirus is multifunctional. In addition to cleavage, its activities include RNA binding, repression of translation, and promotion of negative-strand synthesis (8, 15). The full extent to which the 3CD-like protein of caliciviruses is multifunctional is not fully known. However, for FCV it is the active form of viral polymerase, and it is found to be associated with the replication complex in infected cells (12, 31).
In summary, we purified the viral proteinase of a human calicivirus CAM and demonstrated proteolysis in vitro. A possible cleavage site at E194/S within the N-terminal protein p37 was identified. The site was not cleaved by purified proteinase in vitro, but it was recognized in transfected COS cells. Further studies on the structure and substrate specificity of the proteinase are in progress.
Acknowledgments
This work was supported by a project grant from the National Health and Medical Research Council of Australia.
REFERENCES
- 1.Boniotti, B., C. Wirblich, M. Sibilia, G. Meyers, H. J. Thiel, and C. Rossi. 1994. Identification and characterization of a 3C-like protease from rabbit hemorrhagic disease virus, a calicivirus. J. Virol. 68:6487-6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiba, S., S. Nakata, K. Numata-Kinoshita, and S. Honma. 2000. Sapporo virus: history and recent findings. J. Infect. Dis. 181(Suppl. 2):S303-S308. [DOI] [PubMed] [Google Scholar]
- 3.Clarke, I. N., and P. R. Lambden. 1997. The molecular biology of caliciviruses. J. Gen. Virol. 78:291-301. [DOI] [PubMed] [Google Scholar]
- 4.Clarke, I. N., and P. R. Lambden. 2000. Organization and expression of calicivirus genes. J. Infect. Dis. 181(Suppl. 2):S309-S316. [DOI] [PubMed] [Google Scholar]
- 5.Cornell, C. T., and B. L. Semler. 2002. Subdomain specific functions of the RNA polymerase region of poliovirus 3CD polypeptide. Virology 298:200-213. [DOI] [PubMed] [Google Scholar]
- 6.Davis, G. J., Q. M. Wang, G. A. Cox, R. B. Johnson, M. Wakulchik, C. A. Dotson, and E. C. Villarreal. 1997. Expression and purification of recombinant rhinovirus 14 3CD proteinase and its comparison to the 3C proteinase. Arch. Biochem. Biophys. 346:125-130. [DOI] [PubMed] [Google Scholar]
- 7.Dougherty, W. G., and B. L. Semler. 1993. Expression of virus-encoded proteinases: functional and structural similarities with cellular enzymes. Microbiol. Rev. 57:781-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gamarnik, A. V., and R. Andino. 1998. Switch from translation to RNA replication in a positive-stranded RNA virus. Genes Dev. 12:2293-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glass, P. J., L. J. White, J. M. Ball, I. Leparc-Goffart, M. E. Hardy, and M. K. Estes. 2000. Norwalk virus open reading frame 3 encodes a minor structural protein. J. Virol. 74:6581-6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glass, R. I., J. Bresee, B. Jiang, J. Gentsch, T. Ando, R. Fankhauser, J. Noel, U. Parashar, B. Rosen, and S. S. Monroe. 2001. Gastroenteritis viruses: an overview. Novartis Found. Symp. 238:5-19. [DOI] [PubMed] [Google Scholar]
- 11.Gorbalenya, A. E., A. P. Donchenko, V. M. Blinov, and E. V. Koonin. 1989. Cysteine proteases of positive strand RNA viruses and chymotrypsin-like serine proteases. A distinct protein superfamily with a common structural fold. FEBS Lett. 243:103-114. [DOI] [PubMed] [Google Scholar]
- 12.Green, K. Y., A. Mory, M. H. Fogg, A. Weisberg, G. Belliot, M. Wagner, T. Mitra, E. Ehrenfeld, C. E. Cameron, and S. V. Sosnovtsev. 2002. Isolation of enzymatically active replication complexes from feline calicivirus-infected cells. J. Virol. 76:8582-8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardy, M., T. Crone, J. Brower, and K. Ettayebi. 2002. Substrate specificity of the Norwalk virus 3C-like proteinase. Virus Res. 89:29.. [DOI] [PubMed] [Google Scholar]
- 14.Harris, K. S., S. R. Reddigari, M. J. Nicklin, T. Hammerle, and E. Wimmer. 1992. Purification and characterization of poliovirus polypeptide 3CD, a proteinase and a precursor for RNA polymerase. J. Virol. 66:7481-7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris, K. S., W. Xiang, L. Alexander, W. S. Lane, A. V. Paul, and E. Wimmer. 1994. Interaction of poliovirus polypeptide 3CDpro with the 5′ and 3′ termini of the poliovirus genome. Identification of viral and cellular cofactors needed for efficient binding. J. Biol. Chem. 269:27004-27014. [PubMed] [Google Scholar]
- 16.Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51-59. [DOI] [PubMed] [Google Scholar]
- 17.Kapikian, A. Z., M. K. Estes, and R. M. Chanock. 1996. Norwalk group of viruses, p. 783-810. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven, Philadelphia, Pa.
- 18.Konig, M., H. J. Thiel, and G. Meyers. 1998. Detection of viral proteins after infection of cultured hepatocytes with rabbit hemorrhagic disease virus. J. Virol. 72:4492-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu, B., I. N. Clarke, and P. R. Lambden. 1996. Polyprotein processing in Southampton virus: identification of 3C-like protease cleavage sites by in vitro mutagenesis. J. Virol. 70:2605-2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, B. L., G. J. Viljoen, I. N. Clarke, and P. R. Lambden. 1999. Identification of further proteolytic cleavage sites in the Southampton calicivirus polyprotein by expression of the viral protease in E. coli. J. Gen. Virol. 80:291-296. [DOI] [PubMed] [Google Scholar]
- 21.Mayo, M. A. 2002. A summary of taxonomic changes recently approved by ICTV. Arch. Virol. 147:1655-1663. [DOI] [PubMed] [Google Scholar]
- 22.Meyers, G., C. Wirblich, H. J. Thiel, and J. O. Thumfart. 2000. Rabbit hemorrhagic disease virus: genome organization and polyprotein processing of a calicivirus studied after transient expression of cDNA constructs. Virology 276:349-363. [DOI] [PubMed] [Google Scholar]
- 23.Pringle, C. R. 1998. Virus taxonomy-San Diego 1998. Arch. Virol. 143:1449-1459. [DOI] [PubMed] [Google Scholar]
- 24.Ryan, M. D., and M. Flint. 1997. Virus-encoded proteinases of the picornavirus super-group. J. Gen. Virol. 78:699-723. [DOI] [PubMed] [Google Scholar]
- 25.Seah, E. L., J. A. Marshall, and P. J. Wright. 1999. Open reading frame 1 of the Norwalk-like virus Camberwell: completion of sequence and expression in mammalian cells. J. Virol. 73:10531-10535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Someya, Y., N. Takeda, and T. Miyamura. 2000. Complete nucleotide sequence of the Chiba virus genome and functional expression of the 3C-like protease in Escherichia coli. Virology 278:490-500. [DOI] [PubMed] [Google Scholar]
- 27.Someya, Y., N. Takeda, and T. Miyamura. 2002. Identification of active-site amino acid residues in the Chiba virus 3C-like protease. J. Virol. 76:5949-5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sosnovtsev, S. V., M. Garfield, and K. Y. Green. 2002. Processing map and essential cleavage sites of the nonstructural polyprotein encoded by ORF1 of the feline calicivirus genome. J. Virol. 76:7060-7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sosnovtseva, S. A., S. V. Sosnovtsev, and K. Y. Green. 1999. Mapping of the feline calicivirus proteinase responsible for autocatalytic processing of the nonstructural polyprotein and identification of a stable proteinase-polymerase precursor protein. J. Virol. 73:6626-6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subekti, D. S., P. Tjaniadi, M. Lesmana, J. McArdle, D. Iskandriati, I. N. Budiarsa, P. Walujo, I. H. Suparto, I. Winoto, J. R. Campbell, K. R. Porter, D. Sajuthi, A. A. Ansari, and B. A. Oyofo. 2002. Experimental infection of Macaca nemestrina with a Toronto Norwalk-like virus of epidemic viral gastroenteritis. J. Med. Virol. 66:400-406. [DOI] [PubMed] [Google Scholar]
- 31.Wei, L., J. S. Huhn, A. Mory, H. B. Pathak, S. V. Sosnovtsev, K. Y. Green, and C. E. Cameron. 2001. Proteinase-polymerase precursor as the active form of feline calicivirus RNA-dependent RNA polymerase. J. Virol. 75:1211-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wirblich, C., M. Sibilia, M. B. Boniotti, C. Rossi, H. J. Thiel, and G. Meyers. 1995. 3C-like protease of rabbit hemorrhagic disease virus: identification of cleavage sites in the ORF1 polyprotein and analysis of cleavage specificity. J. Virol. 69:7159-7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wirblich, C., H. J. Thiel, and G. Meyers. 1996. Genetic map of the calicivirus rabbit hemorrhagic disease virus as deduced from in vitro translation studies. J. Virol. 70:7974-7983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ypma-Wong, M. F., P. G. Dewalt, V. H. Johnson, J. G. Lamb, and B. L. Semler. 1988. Protein 3CD is the major poliovirus proteinase responsible for cleavage of the P1 capsid precursor. Virology 166:265-270. [DOI] [PubMed] [Google Scholar]