Abstract
CD8+ T lymphocytes can inhibit human immunodeficiency virus type 1 (HIV-1) replication by secreting a soluble factor(s) known as CD8+ T-lymphocyte antiviral factor (CAF). One site of CAF action is inhibition of HIV-1 RNA transcription, particularly at the step of long terminal repeat (LTR)-driven gene expression. The inhibitory effect of CAF on HIV-1 LTR activation is mediated through STAT1 activation. A recent study reports that α-defensins 1 to 3 account for CAF activity against HIV-1. Here, we address whether α-defensins, particularly α-defensin-1, contribute to CAF-mediated inhibition of HIV-1 transcription. Both recombinant α-defensin-1 and CAF derived from herpesvirus saimiri (HVS)-transformed CD8+ cells inhibited HIV-1 infection and gene expression. For both factors, the inhibition of HIV-1 infection did not occur at the level of viral entry. Pretreatment of cells with α-defensin-1 followed by a washing out prior to infection blocked infection by HIV-1, indicating that direct inactivation of virions was not required for its inhibitory effect. In contrast to CAF, α-defensin-1 did not inhibit phorbol myristate acetate- or Tat-mediated HIV-1 LTR activation in a transient transfection system, nor did it activate STAT1 tyrosine phosphorylation. Furthermore, α-defensins 1 to 3 were below the level of detection in a panel of HVS-transformed CD8+ cells with potent HIV-1 inhibitory activity and a neutralizing antibody against α-defensins 1 to 3 did not reverse the inhibitory effect of CAF on HIV-1 gene expression in infected cells and on HIV-1 LTR activation in transfected cells. Taken together, our results suggest that α-defensin-1 inhibits HIV-1 infection following viral entry but that α-defensins 1 to 3 are not responsible for the HIV-1 transcriptional inhibition by CAF.
A soluble factor(s) from CD8+ T lymphocytes, designated CD8+ T-lymphocyte antiviral factor (CAF), is capable of inhibiting human immunodeficiency virus type 1 (HIV-1) replication in vitro (24, 43). CAF-associated antiviral activities have been reported to correlate with delayed disease progression in HIV-1-infected individuals (26). It is not clear what component(s) of CAF is responsible for its antiviral effect(s) (24). One contribution to the CAF activity is made by the β-chemokines macrophage inflammatory protein 1α, macrophage inflammatory protein 1β, and RANTES, which inhibit HIV-1 entry via CCR-5 (7). However, several studies have shown that these β-chemokines cannot entirely account for CAF-mediated antiviral effects, particularly since CAF can inhibit the replication of HIV-1 strains that use CXCR4 and not CCR5 for entry (1, 20, 28, 29, 36). Furthermore, CAF can prevent HIV-1 replication postentry (6, 10, 23, 25, 29, 41). In addition to the β-chemokines, a number of other soluble factors including interleukin-16, interferons, macrophage-derived chemokines, antithrombin III, and most recently α-defensins 1 to 3, may contribute to CAF activities (17, 49; reviewed in reference 40). However, the mechanisms of anti-HIV activity for many of these factors have not been determined. It seems probable that the antiviral action of CAF is achieved by one or more components that can act at different stages of the viral life cycle.
The molecular mechanism(s) by which CAF inhibits HIV-1 replication has been studied extensively. Several independent reports have shown that CAF can inhibit HIV-1 RNA transcription, particularly at the step of long terminal repeat (LTR)-driven gene expression (6, 10, 23, 25), although this is not the only step in the virus life cycle inhibited by CAF. Previously, we demonstrated that CAF inhibits HIV-1 replication and LTR activation through activation of STAT1 (signal transducers and activators of transcription) (3). CAF activities correlate with STAT1 activation. The inhibitory effect of CAF on HIV-1 replication and LTR activation is abolished in STAT1-deficient cells. Introduction of STAT1 proteins to STAT1-deficient cells restores CAF-mediated inhibition of LTR activation. Furthermore, the CAF inhibitory effect on LTR activation is diminished in cells expressing STAT1 dominant-negative proteins.
Defensins are small cysteine-rich, cationic peptides found in leukocytes and epithelial cells (21, 22, 39, 44). The three types of mammalian defensins, alpha, beta, and circular, have mainly β-sheet structures stabilized by three disulfide bonds and differ in their distribution and connection of six cysteine residues (reviewed in reference 44). These peptides exhibit antimicrobial activities by permeabilizing the membranes of a broad spectrum of organisms including gram-positive and gram-negative bacteria, fungi, and enveloped viruses (reviewed in references 21 and 22). High concentrations (micromolar) of defensins are toxic to mammalian cells (42), whereas lower concentrations (nanomolar) of defensins may serve as potent mitogens for epithelial cells and fibroblasts (32). In addition to their direct antimicrobial effects, defensins display immunostimulatory activities (reviewed in references 21 and 44) including a chemotactic effect for T lymphocytes, monocytes, and immature dendritic cells (45, 46) and the induction of cytokine production (21, 44).
Inhibition of HIV replication by α-defensins has been reported since 1993 (34). The molecular mechanism of HIV inhibition remains unclear. It is known that defensins and peptides derived from HIV-1 gp41 (HIV Env 583 to 610) have similar structures and functions including binding to complement component C1q, complement activation, and protein kinase C (PKC) inhibition (4, 27, 35, 37, 38). Retrocyclin, a circular form of defensin, inhibits HIV-1 not through direct viral inactivation but at the steps prior to reverse transcription (8).
Recently, a report indicated that α-defensins 1 to 3 account for the CAF activity against HIV-1 (49) in activated CD8+ cells derived from patients classified as long-term nonprogressors. We investigated the effect of α-defensin-1 at various stages of HIV-1 infection and determined whether α-defensin-1 plays a role in CAF-mediated inhibition of HIV-1 transcription. We found that α-defensin-1 inhibited HIV-1 infection following viral entry but was not involved in the inhibition of HIV-1 gene expression and LTR activation attributed to CAF derived from HVS-transformed CD8+ cells.
MATERIALS AND METHODS
Reagents.
Recombinant human α-defensin-1 (HNP-1; produced in Escherichia coli with >95% purity by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and high-performance liquid chromatography analyses) was purchased from Chemicon International, Inc. (Temecula, Calif.). The endotoxin level is less than 0.1 ng per μg (1 EU/μg) of human α-defensin-1. AMD3100 was kindly provided by John P. Moore (Weill Medical College of Cornell University, New York, N.Y. [13]). Antibodies against phospho-STAT1 Y701 and STAT1 were purchased from Cell Signaling Technology (Beverly, Mass.). The monoclonal antibody against human neutrophil defensins 1 to 3 clone D21 and mouse immunoglobulin G1 (IgG1) control antibody were from Cell Science (Norwood, Mass.) and R&D systems (Minneapolis, Minn.), respectively.
Cell culture.
HeLa-CD4 cells were provided by David Kabat (University of Oregon, Portland). They were maintained in Dulbecco's minimal essential medium containing 10% fetal calf serum and glutamine. Peripheral blood mononuclear cells from normal healthy blood donors were isolated by Ficoll-Hypaque gradient centrifugation. Monocytes were selected by adherence to plastic and allowed to differentiate into macrophages between 7 and 14 days in culture. CD4+ T lymphocytes were isolated from peripheral blood mononuclear cells by negative selection by use of a CD4+ T-cell isolation kit from Miltenyi Biotech (Auburn, Calif.). The purity of cells is 98% based on flow cytometry analysis. CD4+ T lymphocytes were stimulated with phytohemagglutinin at 5 μg/ml and supplemented with interleukin-2 for 3 days at 37°C prior to viral infection.
HIV-1 Env pseudotype infection and gene expression assay.
HIV-1 HxB2 or vesicular stomatitis virus (VSV) Env-pseudotyped, replication-defective, luciferase-expressing reporter viruses were produced as described previously (5, 9, 14). Briefly, HEK 293T cells were cotransfected with a plasmid encoding the envelope-deficient HIV-1 NL4-3 virus with the luciferase reporter gene inserted into nef (pNL4-3.Luc.R-E-; provided by N. Landau, AIDS Research & Reference Reagent Program, National Institute of Allergy and Infectious Disease, National Institutes of Health) and a plasmid expressing the HIV-1-HxB2 (gift of D. Littman, New York University, New York) or VSV-G glycoprotein (gift of D. Trono, University of Geneva, Geneva, Switzerland). The supernatant medium was collected 48 h after transfection and filtered. Virus stocks were analyzed for HIV-1 p24 antigen concentration by enzyme-linked immunosorbent assay (ELISA) (SAIC Frederick, Frederick, Md.).
A single-cycle infection assay (5, 9, 14) was used to determine HIV-1 inhibition by α-defensin-1. Activated CD4+ T lymphocytes seeded at 106 per sample or HeLa-CD4 cells seeded at 5 × 104 per well were treated with α-defensin-1 or conditioned media (CAF) at the time of HIV-1 infection described in the text and figure legends. Viral infection was carried out by addition of viruses for 2 h at 37°C. Unbound virus was removed by washing, CAF or α-defensin-1 was added back to the media unless otherwise stated, and then the infected cells were incubated at 37°C before lysis with luciferase substrate buffer (Promega, Inc). Luciferase activity was measured (in relative light units [RLU]) on an EG & G (Berthold) MiniLumat LB9506 luminometer. When gene expression was analyzed, viral infection was carried out as described above. Infected cells were further incubated at 37°C for 16 h to allow viral integration to proceed prior to treatment with CAF or α-defensin-1 for 24 h.
HIV-1 infection in macrophages.
The effect of α-defensin-1 on a multiple-cycle HIV infection was determined by infecting macrophages with replication-competent HIV-1 BaL virus (Advanced Biotechnologies, Inc., Columbia, Md.) at a multiplicity of infection of 0.01 in the presence or absence of 10% CAF or 1 μg of α-defensin-1/ml for 2 h. After the virus was washed out, CAF or α-defensin-1 was added back and cells were incubated at 37°C. Supernatants were then harvested 7 days postinfection. p24 levels were measured by ELISA with the HIV-1 p24 antigen capture assay kit.
Transfection.
Transfections were performed by using the Lipofectamine Plus reagent (Invitrogen) as described previously (3). The pCH101 reporter plasmid expressing the β-galactosidase gene under the control of the simian virus 40 promoter was used as a control. The transfected cells were incubated at 37°C for 16 h and then treated with α-defensin-1 or conditioned media (CAF) for an additional 16 to 24 h before addition of phorbol myristate acetate (PMA) (20 ng/ml) for 8 h. To analyze Tat-dependent HIV-1 LTR activation, the cells were cotransfected with the pSVtat plasmid expressing Tat proteins that activate the HIV-1 LTR-luciferase gene. Luciferase activity was measured as described above, and β-galactosidase activity was analyzed with the Promega assay system.
Western blot analysis.
Whole-cell extracts (WCE) were prepared by lysis of cells in 20 mM HEPES buffer (pH 7.9) with 0.2% NP-40, 10% glycerol, 200 mM NaCl, 0.1 mM EDTA, 1 mM dithiothreitol, 1 mM sodium orthovanadate, 0.5 mM phenylmethylsulfonyl fluoride and protease inhibitor cocktail (Boehringer Mannheim Inc.). The protein concentration in WCE was determined by the Bradford method with the Bio-Rad (Hercules, Calif.) protein assay. Proteins (30 μg per well) were separated by SDS-PAGE. Following electrophoresis, proteins were transferred to polyvinylidene fluoride membranes (Millipore, Bedford, Mass.). The membranes were blocked with 5% milk in rinse buffer (10 mM Tris, 150 mM NaCl, 1 mM EDTA, 0.1% Triton X-100) for 30 min at room temperature and then incubated overnight at 4°C with the appropriate primary antibodies in rinse buffer with 5% bovine serum albumin fraction V. Following three washes in rinse buffer for 15 min, the blots were incubated with horseradish peroxidase-linked secondary antibody (KPL, Gaithersburg, Md.) at a 1:10,000 dilution for 1 h at room temperature and then washed three times with rinse buffer for 15 min. The immunoblotted proteins were visualized by using the chemiluminescent substrate according to the manufacturer's specifications (ECL; Amersham Biosciences, Newark, N.J.). To reprobe blots, membranes were incubated in stripping buffer (100 mM beta-mercaptoethanol, 2% SDS, 62.5 mM Tris-HCl [pH 6.7]) at 55°C for 1 h and rinsed with phosphate-buffered saline (PBS) several times prior to a second Western blot analysis.
ELISA for α-defensins 1, 2, and 3.
α-Defensins were measured in CAF by using the Hbt human HNP1-3 ELISA kit (Hycult Biotechnology b.v., Uden, The Netherlands), which had a minimum limit for detection of 50 pg/ml.
RESULTS
α-Defensin-1 inhibits HIV-1 infection in activated CD4+ T lymphocytes, in primary macrophages, and in HeLa-CD4 cells.
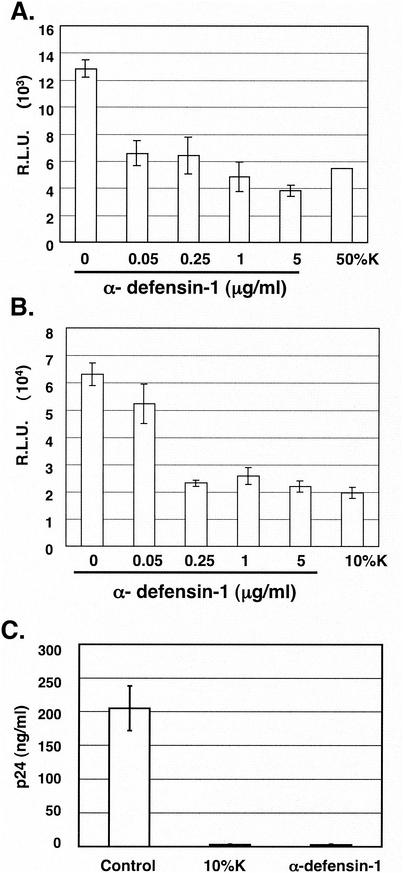
To examine the effect of α-defensin-1 on HIV-1 infection during a single viral life cycle, activated CD4+ T lymphocytes and HeLa-CD4 cells were infected with HIV-1HxB2 pseudotyped replication-defective virus containing a luciferase reporter gene for 2 h prior to treatment with recombinant α-defensin-1 at different concentrations. Cells were also treated with CAF at 10 or 50% (vol/vol) in conditioned media derived from the HVS-transformed CD8+ T-cell line K #1 50K (3, 29), hereafter referred to as K in this paper. Using the same lot of conditioned media from cell line K, similar degrees of inhibitory effect on HIV-1 infection were obtained with HeLa-CD4 cells treated with 10 or 50% (vol/vol) CAF, whereas 50% CAF is required to achieve >50% inhibition in T cells (data not shown). When luciferase activity was measured at 48 h postinfection (p.i.), 0.05 μg of α-defensin-1/ml inhibited HIV-1HxB2 Env-pseudotyped viral infection by 49% in activated CD4+ T lymphocytes (Fig. 1A). The maximal inhibitory effect (approximately 60 to 70% reduction) was observed in both activated CD4+ T lymphocytes and HeLa-CD4 cells with treatment of α-defensin-1 at concentrations of 1 to 5 μg/ml (Fig. 1A and B). In addition, α-defensin-1 at a concentration of 0.25 μg/ml in the single-cycle infection assay and 1 μg/ml in the primary macrophage infection assay (Fig. 1C) inhibited viral infection at a level similar to that observed with CAF at a concentration of 10 to 50% (vol/vol). No cytotoxicity was observed when cells were treated with α-defensin-1 at different concentrations up to 5 μg/ml (data not shown), indicating that the effect of α-defensin-1 on viral infection was not due to nonspecific cytotoxic effects.
FIG. 1.
Effects of α-defensin-1 on HIV-1 infection. Activated CD4+ T lymphocytes (A) at 106 per sample and HeLa-CD4 cells (B) were infected with HIV-1HxB2 Env-pseudotyped, replication-defective, luciferase-expressing viruses and then treated with α-defensin-1 at different concentrations or with CAF from the K cell line at 10 or 50% (vol/vol) for 48 h. The results (means ± standard deviations) from triplicate determinations in a single experiment are presented as histograms. Similar results were obtained in an independent experiment. Primary macrophages were infected with replication component HIV-1BaL at a multiplicity of infection of 0.01 (C) in the presence of 10% CAF or 1 μg of α-defensin-1/ml for 2 h. Virus was washed out and replaced by media with the same concentration of CAF or α-defensin-1. HIV-1 p24 was measured by ELISA. The results (means ± standard deviations) from triplicate determinations at day 7 in a single experiment are presented as histograms.
α-Defensin-1 does not inhibit HIV-1 infection at the level of viral entry.
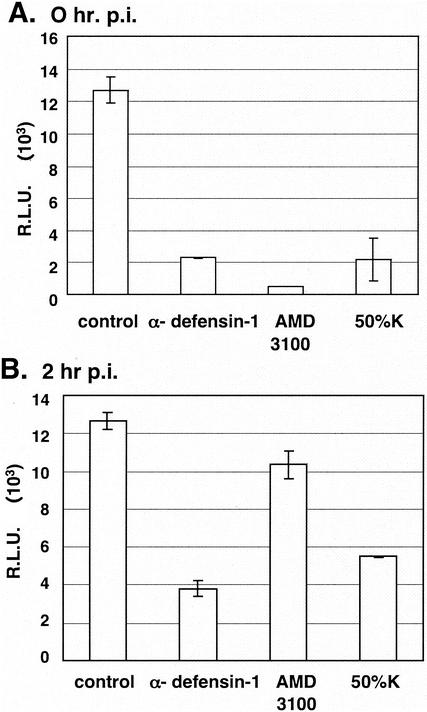
To determine whether α-defensin-1 inhibited HIV-1 infection at the level of viral entry, α-defensin-1 at 5 μg/ml was added at the same time (Fig. 2A) or 2 h following (Fig. 2B) infection of activated CD4+ T lymphocytes by HIV-1HxB2 Env-pseudotyped virus. As a control, cells were treated with a CXCR4 entry inhibitor, AMD3100 (13). As expected, viral infection was inhibited by 96% when cells were exposed to AMD3100 at 0 h p.i. (Fig. 2A, column 3) whereas no significant inhibitory effect on viral infection was observed when AMD3100 was added at 2 h p.i. (Fig. 2B, column 3). In contrast, a similar level of α-defensin-1 inhibition of viral infection was observed when it was added at 0 and 2 h p.i. (Fig. 2A and B, column 2), indicating that α-defensin-1 does not inhibit HIV-1 infection at the level of viral entry. As expected, HIV-1 infection was inhibited by CAF when cells were treated with CAF at 0 and 2 h p.i. (Fig. 2A and B, column 4).
FIG. 2.
Effects of α-defensin-1 on HIV-1 entry. Activated CD4+ T lymphocytes were treated with 5 μg of α-defensin-1/ml at the same time as (A) or 2 h after (B) infection by HIV-1HxB2 Env-pseudotyped virus. Cells were also treated with CAF (50%K) and 1 μM CXCR4 entry inhibitor AMD3100 as controls. Luciferase activity (in RLU) was measured at 48 h p.i. The results (means ± standard deviations) from triplicate determinations in a single experiment are presented as histograms. Similar results were obtained in an independent experiment.
Pretreatment of cells with α-defensin-1 blocks HIV-1 infection.
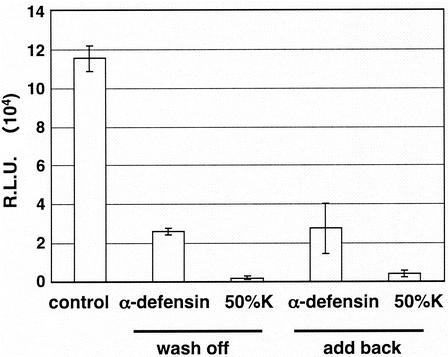
To study whether pretreatment of cells with α-defensin-1 had any effect on HIV-1 infection, HeLa-CD4 cells were treated with α-defensin-1 at 5 μg/ml or CAF at 50% for 16 h, washed twice with PBS, and placed in fresh culture medium without (Fig. 3, columns 2 and 3) or with (Fig. 3, columns 4 and 5) inhibitors during infection by HIV-1vsv Env-pseudotyped viruses. The use of this pseudotype eliminates any influence that α-defensin-1 may have on the coreceptors after 16 h of incubation. When luciferase activity was determined 48 h p.i., HIV-1vsv infection was inhibited by 78% in cells pretreated with α-defensin-1 despite washing out the compound prior to infection (Fig. 3, column 2). CAF at 50% inhibited HIV-1vsv infection by 98% (Fig. 3, column 3) after a similar washout. No additional inhibition was observed when α-defensin-1 was added back during the infection (Fig. 3, column 4). This result demonstrated that pretreatment of cells with α-defensin-1 or CAF was sufficient to block viral infection, suggesting that the effect on cells was sustained for a long period. Furthermore, direct inactivation of virions was not required for its inhibitory effect on HIV-1 infection.
FIG. 3.
Effects of α-defensin-1 pretreatment of cells on HIV-1 infection. HeLa-CD4 cells were pretreated with 5 μg of α-defensin-1/ml or CAF (50%K) for 16 h. Cells were washed and placed in fresh media without (wash off) or with (add back) α-defensin-1 or CAF prior to infection with HIV-1vsv. Luciferase activity (in RLU) was measured at 48 h p.i. The results (means ± standard deviations) from triplicate determinations in a single experiment are presented as histograms.
α-Defensin-1 inhibits HIV-1 gene expression.
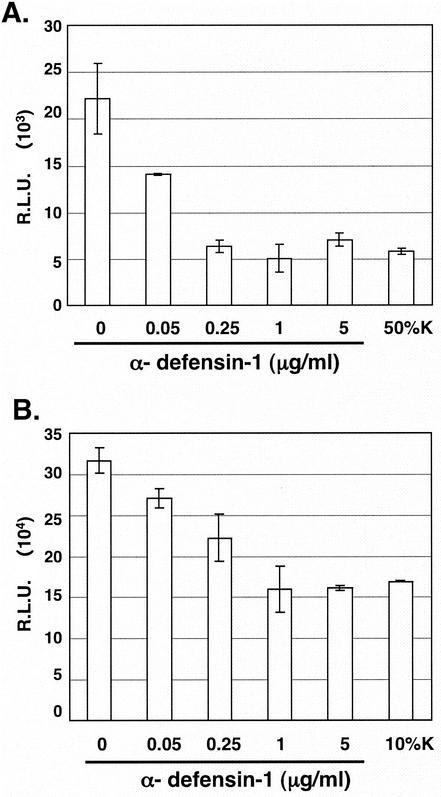
To investigate the effect of α-defensin-1 on HIV-1 gene expression, activated CD4+ T lymphocytes and HeLa-CD4 cells were infected with HIV-1vsv Env-pseudotyped viruses for 2 h and viruses were washed off with PBS. Infected cells were further incubated at 37°C for 16 h to allow viral integration to proceed prior to treatment with α-defensin-1 at different concentrations. Infected cells were also treated with CAF as a control. When luciferase activity was measured, α-defensin-1 inhibited HIV-1 gene expression in a dose-dependent manner (Fig. 4). HIV-1 gene expression was reduced by 70% when infected activated CD4+ T lymphocytes were exposed to α-defensin-1 at concentrations of 0.25 μg/ml or higher (Fig. 4A), whereas the inhibition reached 50% when infected HeLa-CD4 cells were treated with α-defensin-1 at 1 to 5 μg/ml (Fig. 4B). The degree of inhibition of HIV-1 gene expression by α-defensin-1 at high concentrations was similar to that with treatment of CAF at 10 to 50%.
FIG. 4.
Effects of α-defensin-1 on HIV-1 gene expression. Activated CD4+ T lymphocytes (A) and HeLa-CD4 cells (B) were infected with luciferase-expressing HIV-1vsv and incubated for 16 h. Infected cells were then treated with α-defensin-1 at different concentrations or with CAF (50%K or 10%K) for 24 h before luciferase activity was measured. The results (means ± standard deviations) from triplicate determinations in a single experiment are presented as histograms. Similar results were obtained from an independent experiment.
α-Defensins 1 to 3 do not mediate CAF inhibition of HIV-1 gene expression.
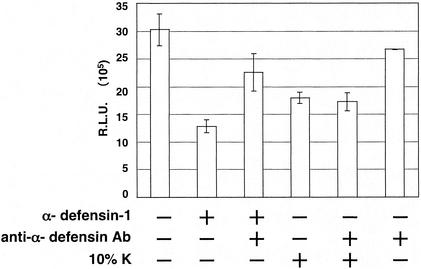
We then determined whether α-defensin-1 contributes to the inhibitory effect of CAF on HIV-1 gene expression. A neutralizing antibody against α-defensins 1 to 3 (D21) was added to CAF to see whether the inhibitory effect of CAF on viral gene expression was reversed. This antibody has been shown to eliminate anti-X4 HIV activity in conditioned media from CD8+ T lymphocytes from long-term nonprogressors (49). The addition of an α-defensin-specific antibody (D21) at 0.5 μg/ml reversed the inhibition of HIV-1 gene expression mediated by α-defensin-1 in HeLa-CD4 cells (Fig. 5, column 3). A similar result was obtained in activated CD4+ T lymphocytes when using the D21 antibody at 1 μg/ml (data not shown). Addition of antibody alone did not affect HIV-1 gene expression at the concentrations used, although higher doses of the antibody had some direct inhibitory effects in our assay (Fig. 5, column 6, and data not shown). However, no change in the inhibitory effect of CAF on HIV-1 gene expression was observed when the antibody (D21) against α-defensins 1 to 3 was added to CAF (Fig. 5, column 5), suggesting that α-defensins 1 to 3 do not mediate the inhibition of HIV-1 gene expression by CAF in this assay. Note that the D21 antibody recognizes α-defensins 1 to 3, which are highly homologous. Our results confirmed the specificity of the D21 antibody against α-defensin-1; however, this antibody can neutralize α-defensin-2 and α-defensin-3 as well.
FIG. 5.
Effects of CAF on HIV-1 gene expression in the presence of a neutralizing antibody against α-defensins 1 to 3. HeLa-CD4 cells were infected with luciferase-expressing HIV-1vsv and incubated for 16 h. Infected cells were then treated with α-defensin-1 at 5 μg/ml or CAF in the presence or absence of a neutralizing antibody (D21) against α-defensins 1 to 3 for 24 h before luciferase activity was measured. The D21 antibody at 0.5 μg/ml, when present, was incubated with α-defensin-1 or CAF for 30 min at 37°C before addition of the mixture to the cells. The results (means ± standard deviations) from triplicate determinations in a single experiment are presented as histograms. Similar results were obtained in an independent experiment.
α-Defensins 1 to 3 are not detectable in supernatants of HVS-transformed CD8+ cells that have HIV-1 inhibitory activity.
We have previously shown that the conditioned media from HVS-transformed CD8+ cell lines CAF10, K, and N#2 have potent anti-HIV activity (3, 29). To determine the presence of α-defensins in the conditioned media from cell lines CAF10, K, and N#2, an ELISA for α-defensins 1 to 3 was performed. We found that α-defensins 1 to 3 levels in the conditioned media from these cell lines were below the level of detection of 50 pg/ml (data not shown). This included the supernatant from cell line K, which was used as a control in the α-defensin studies.
α-Defensin-1 does not inhibit LTR-driven gene expression mediated by PMA or Tat.
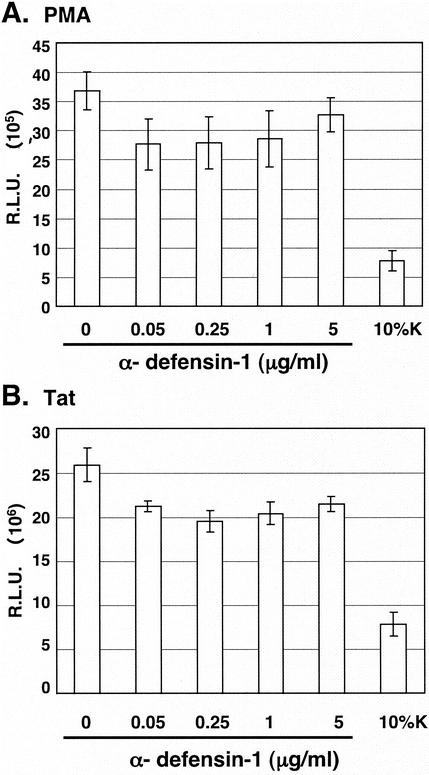
Studies from several laboratories, including ours, have found that CAF inhibits HIV gene expression mediated from activation of the LTR sequences (3, 6, 10). We first analyzed the effects of α-defensin-1 on Tat-independent PMA-mediated HIV-1 LTR activation in HeLa-CD4 cells transiently transfected with the HIV-LTR-Luc plasmid. The transfected cells were treated with or without α-defensin-1 at different concentrations for 16 h prior to PMA stimulation for 8 h and measurement of luciferase activity. In addition, transfected cells were treated with 10% CAF as a control. As expected, CAF reduced PMA-mediated LTR activation by 80% in comparison to cells treated with control medium (Fig. 6A). However, no significant reduction of PMA-mediated LTR activation was observed in cells treated with α-defensin-1 even at the concentration that inhibited HIV-1 infection (Fig. 6A).
FIG. 6.
Effect of α-defensin-1 on PMA- or Tat-mediated HIV-LTR activation. (A) HeLa-CD4 cells were transiently transfected with the HIV-LTR-Luc plasmid for 16 h before treatment with α-defensin-1 at different concentrations or CAF. After 16 h of incubation, cells were stimulated with PMA for 8 h prior to measurement of luciferase activity. (B) HeLa-CD4 cells were cotransfected with plasmids expressing HIV-LTR-Luc and Tat for 16 h prior to treatment with α-defensin-1 and CAF as described above. After 16 h of incubation, luciferase activities were measured. The results (means ± standard deviations) from triplicate determinations in a single experiment are presented as histograms. Similar results were obtained in an independent experiment.
To further investigate whether α-defensin-1 inhibited Tat-mediated LTR activation, HeLa-CD4 cells were transiently cotransfected with the plasmids expressing Tat and HIV-LTR-Luc. The transfected cells were treated with or without α-defensin-1 at different concentrations for 16 h prior to measurement of luciferase activity. As observed with PMA-mediated HIV-1 LTR activation, α-defensin-1 did not have a significant inhibitory effect on Tat-mediated HIV-1 LTR activation, whereas CAF inhibited HIV-1 LTR activation by 70% (Fig. 6B). Taken together, this result suggests that α-defensin-1 does not inhibit PMA- and Tat-mediated LTR activation and therefore is not the major component which contributes to the inhibitory effect of CAF on activated HIV-1 LTR-driven gene expression in our system.
CAF-mediated inhibition of HIV-1 LTR activation does not involve α-defensins 1 to 3.
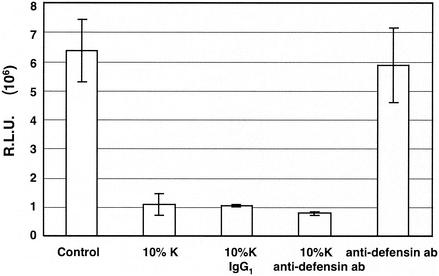
To determine whether α-defensins 1 to 3 play any role in the inhibitory effect of CAF on HIV-1 LTR activation, we tested whether the D21 antibody against α-defensins 1 to 3 could eliminate CAF-mediated inhibition of HIV-LTR activation. HeLa-CD4 cells were transiently cotransfected with the Tat-expressing and the HIV-LTR-Luc plasmids. The transfected cells were treated or not treated with CAF in the presence of control mouse IgG1 or D21 antibodies. The antibody against α-defensins (D21) had no effect on Tat-mediated HIV-1 LTR activation (Fig. 7, column 5). The D21 antibody at 0.5 μg/ml, which was sufficient to block the inhibitory effect of α-defensin-1 on HIV-1 gene expression (Fig. 5, column 3), did not abolish CAF activity on HIV-1 LTR activation (Fig. 7, column 4). As expected, the control mouse IgG1 antibody did not reverse the inhibitory effect of CAF on HIV-1 LTR activation (Fig. 7, column 3). Similar results were obtained when HIV-1 LTR activation was mediated by PMA (data not shown). This result suggests that α-defensins 1 to 3 do not account for the inhibitory effect of CAF on HIV-1 LTR activation.
FIG. 7.
Effects of CAF on HIV-1 LTR activation in the presence of a neutralizing antibody against α-defensins 1 to 3. HeLa-CD4 cells were transfected with plasmids expressing HIV-LTR-Luc and Tat for 16 h. Cells were then treated with 10% CAF in the presence or absence of 0.5 μg of control antibody (mouse IgG1)/ml or 0.5 μg of neutralizing antibody (D21) against α-defensins 1 to 3/ml for 24 h before luciferase activity was measured. The results (means ± standard deviations) from triplicate determinations in a single experiment are presented as histograms.
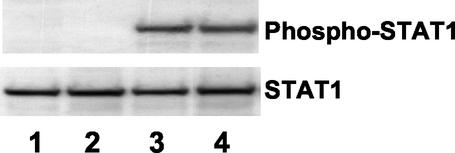
α-Defensin-1 does not induce STAT1 activation.
We have previously shown that CAF inhibits HIV-1 LTR gene expression through STAT1 activation (3). In response to stimulation, STAT proteins are activated by tyrosine phosphorylation on a specific residue (reviewed in reference 12). To determine whether α-defensin-1 induced STAT1 activation, HeLa cells were treated or not treated with α-defensin-1 at 1 μg/ml or 10% CAF at 37°C for 15 min. Cells were also treated with gamma interferon (IFN-γ) at 10 ng/ml as a positive control. WCE were prepared, and tyrosine phosphorylation of STAT1 was analyzed by immunoblotting with an antibody against phosphorylated tyrosine residue Y701 on STAT1. The same blot was then stripped and reprobed with antibody against STAT1 proteins to ensure the equal loading of each sample. In agreement with our previous observation, CAF and IFN-γ induced STAT1 activation within 15 min of stimulation (Fig. 8, lanes 3 and 4). In contrast, α-defensin-1 did not induce STAT1 tyrosine phosphorylation (Fig. 8, lane 2). In light of our previous results, this study suggests that α-defensin-1 inhibition is distinct from CAF inhibition and cannot account for the component(s) of CAF that inhibits HIV-1 LTR gene expression.
FIG. 8.
α-Defensin-1 does not induce STAT1 activation. HeLa-CD4 cells were treated for 15 min with 1 μg of α-defensin-1/ml (lane 2), 10% CAF (lane 3), or 10 ng of IFN-γ/ml (lane 4). Untreated control is shown in lane 1. WCE were prepared and analyzed by Western blot analysis with an antibody against phospho-STAT1 antibody. The blot was then stripped and reprobed with STAT1 antibody.
DISCUSSION
CD8+ T-lymphocyte antiviral factor(s) may play an important role in cell-mediated immune response for partial control of HIV-1 infection. Several studies show that CAF can inhibit HIV-1 RNA transcription, especially at the step of LTR activation (3, 6, 10, 23, 25). We have demonstrated that this inhibitory effect is mediated through STAT1 activation. A recent report indicates that α-defensins 1 to 3 contribute to CAF activity (49), although their antiviral mechanism is not clear. In this study, we examined the effects of α-defensin-1 on HIV-1 infection at different stages of infection and determined the role of α-defensins 1 to 3 in CAF-mediated inhibition of HIV-1 transcription.
We demonstrated that α-defensin-1-like CAF inhibited HIV-1 infection after viral entry and suppressed HIV-1 gene expression. Recombinant α-defensin-1 in our studies appeared to have greater inhibitory activity than that reported with synthetic α-defensin and is more in line with the activity associated with native defensins (49). However, a neutralizing antibody against α-defensins 1 to 3 failed to reverse the inhibitory effect of CAF on HIV-1 gene expression, which prompted us to further investigate the contribution of α-defensin-1 on CAF-mediated HIV-1 LTR activation. We tested the following criteria: the effects of α-defensin-1 on Tat-independent PMA-mediated and Tat-dependent HIV-1 LTR activation, the activation of STAT1 tyrosine phosphorylation, the effect of the neutralizing antibody against α-defensins 1 to 3 on the inhibition of HIV-1 LTR activation by CAF, and the concentration of α-defensins 1 to 3 in our CD8+ cell supernatants which have potent CAF activities. Our results showed that α-defensin-1 did not account for CAF-mediated inhibition of HIV-1 gene expression and LTR activation.
Our studies suggest that α-defensin-1 and CAF have distinct properties. Although they both can inhibit HIV-1 infection and gene expression, only CAF, not α-defensin-1, activated STAT1 tyrosine phosphorylation and inhibited HIV-1 LTR activation mediated by PMA or Tat, as reported by a number of groups. α-Defensins 1 to 3 were below the level of detection in our CD8+ cell supernatants, and the broadly neutralizing antibody D21 reversed the inhibitory effect on HIV-1 gene expression mediated by α-defensin-1 but not CAF (Fig. 5). In addition, the D21 antibody failed to neutralize the effect of CAF-mediated inhibition of LTR activation (Fig. 7). Our results indicate that α-defensin-1 inhibits HIV-1 infection through a STAT1-independent signaling pathway. However, it is not a component of CAF responsible for inhibition of HIV-1 transcription, particularly that mediated by the STAT1 signaling pathway.
We observed that α-defensin-1 inhibited HIV-1 gene expression in infected cells (Fig. 4) when added after viral entry, even as late as 16 h. Furthermore, this effect on cells persisted after defensins were washed out prior to challenge with virus. These data suggest that defensins work on the cell rather than the virus and interfere with steps following viral fusion and entry. Furthermore, this effect on the cell is durable. The experiment with the VSV-pseudotyped virus suggests that α-defensin-1 can inhibit HIV gene expression perhaps as late as integration and transcription; however, there was no effect on PMA- or Tat-dependent HIV-1 LTR activation in a transient transfection system (Fig. 6). Additional effects may be at the level of nuclear import and integration, as those steps are not easily and distinctly defined. If α-defensin-1 inhibits HIV-1 at the level of transcription, other HIV viral proteins such as Vpr (2, 15, 18, 19) and nucleocapsid NC (47, 48), which are positive regulators of LTR activities and present in infected cells but not in a transient transfection system, may be involved in α-defensin-1-mediated inhibition of HIV-1 gene expression.
The mechanisms by which α-defensin-1 and related compounds inhibit viruses are diverse. α-Defensin-1 inhibits and directly binds to herpes simplex virus type 1 in the absence of serum, suggesting that it may directly inactivate virions (11). Conversely, retrocyclin inhibits HIV-1 proviral DNA formation but does not cause direct inactivation of HIV-1 (8). In addition, a synthetic peptide, T22 ([Tyr-5,12, Lys-7]polyphemusin II), structurally similar to defensin, inhibits CXCR4-mediated viral entry (30, 31, 33). None of these mechanisms could completely account for the anti-HIV activity observed with α-defensin-1. Although it is generally believed that the killing (or inactivating) by defensins is a result of disruption of the microbial or cellular membrane (reviewed in reference 44), we did not find any cytotoxic effect of α-defensin-1 at concentrations up to 5 μg/ml. It is possible that α-defensin-1 affects a cell signaling event(s) that also regulates HIV-1 infection and expression. One possible signaling pathway involved in α-defensin-1-mediated inhibition of HIV-1 infection is PKC signaling since α-defensin-1 is a potent PKC inhibitor (4) and PKC signaling modulates HIV-1 infection (16).
Further studies are required to elucidate the mechanism(s) of inhibition of HIV-1 by α-defensins prior to the development of defensins as therapeutic agents. Nevertheless, we demonstrate that α-defensin-1 does not contribute to CAF-mediated inhibition of HIV-1 transcription. The identification of the factor(s) derived from CD8+ cell supernatants responsible for the full HIV-1 inhibitory profile remains a challenge.
Acknowledgments
This work was supported by NIH RO1 grant AI43698-04.
REFERENCES
- 1.Barker, E., K. N. Bossart, and J. A. Levy. 1998. Primary CD8+ cells from HIV-infected individuals can suppress productive infection of macrophages independent of beta-chemokines. Proc. Natl. Acad. Sci. USA 95:1725-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bukrinsky, M., and A. Adzhubei. 1999. Viral protein R of HIV-1. Rev. Med. Virol. 9:39-49. [DOI] [PubMed] [Google Scholar]
- 3.Chang, T. L., A. Mosoian, R. Pine, M. E. Klotman, and J. P. Moore. 2002. A soluble factor(s) secreted from CD8(+) T lymphocytes inhibits human immunodeficiency virus type 1 replication through STAT1 activation. J. Virol. 76:569-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charp, P. A., W. G. Rice, R. L. Raynor, E. Reimund, J. M. Kinkade, Jr., T. Ganz, M. E. Selsted, R. I. Lehrer, and J. F. Kuo. 1988. Inhibition of protein kinase C by defensins, antibiotic peptides from human neutrophils. Biochem. Pharmacol. 37:951-956. [DOI] [PubMed] [Google Scholar]
- 5.Chen, B. K., K. Saksela, R. Andino, and D. Baltimore. 1994. Distinct modes of human immunodeficiency virus type 1 proviral latency revealed by superinfection of nonproductively infected cell lines with recombinant luciferase-encoding viruses. J. Virol. 68:654-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, C. H., K. J. Weinhold, J. A. Bartlett, D. P. Bolognesi, and M. L. Greenberg. 1993. CD8+ T lymphocyte-mediated inhibition of HIV-1 long terminal repeat transcription: a novel antiviral mechanism. AIDS Res. Hum. Retrovir. 9:1079-1086. [DOI] [PubMed] [Google Scholar]
- 7.Cocchi, F., A. L. DeVico, A. Garzino-Demo, S. K. Arya, R. C. Gallo, and P. Lusso. 1995. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 270:1811-1815. [DOI] [PubMed] [Google Scholar]
- 8.Cole, A. M., T. Hong, L. M. Boo, T. Nguyen, C. Zhao, G. Bristol, J. A. Zack, A. J. Waring, O. O. Yang, and R. I. Lehrer. 2002. Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc. Natl. Acad. Sci. USA 99:1813-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connor, R. I., K. E. Sheridan, D. Ceradini, S. Choe, and N. R. Landau. 1997. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J. Exp. Med. 185:621-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Copeland, K. F., P. J. McKay, and K. L. Rosenthal. 1995. Suppression of activation of the human immunodeficiency virus long terminal repeat by CD8+ T cells is not lentivirus specific. AIDS Res. Hum. Retrovir. 11:1321-1326. [DOI] [PubMed] [Google Scholar]
- 11.Daher, K. A., M. E. Selsted, and R. I. Lehrer. 1986. Direct inactivation of viruses by human granulocyte defensins. J. Virol. 60:1068-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darnell, J. E., Jr. 1997. STATs and gene regulation. Science 277:1630-1635. [DOI] [PubMed] [Google Scholar]
- 13.Donzella, G. A., D. Schols, S. W. Lin, J. A. Este, K. A. Nagashima, P. J. Maddon, G. P. Allaway, T. P. Sakmar, G. Henson, E. De Clercq, and J. P. Moore. 1998. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat. Med. 4:72-77. [DOI] [PubMed] [Google Scholar]
- 14.Dragic, T., V. Litwin, G. P. Allaway, S. R. Martin, Y. Huang, K. A. Nagashima, C. Cayanan, P. J. Maddon, R. A. Koup, J. P. Moore, and W. A. Paxton. 1996. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381:667-673. [DOI] [PubMed] [Google Scholar]
- 15.Felzien, L. K., C. Woffendin, M. O. Hottiger, R. A. Subbramanian, E. A. Cohen, and G. J. Nabel. 1998. HIV transcriptional activation by the accessory protein, VPR, is mediated by the p300 co-activator. Proc. Natl. Acad. Sci. USA 95:5281-5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fields, A. P., D. P. Bednarik, A. Hess, and W. S. May. 1988. Human immunodeficiency virus induces phosphorylation of its cell surface receptor. Nature 333:278-280. [DOI] [PubMed] [Google Scholar]
- 17.Geiben-Lynn, R., N. Brown, B. D. Walker, and A. D. Luster. 2002. Purification of a modified form of bovine antithrombin III as an HIV-1 CD8+ T-cell antiviral factor. J. Biol. Chem. 277:42352-42357. [DOI] [PubMed] [Google Scholar]
- 18.Hottiger, M. O., and G. J. Nabel. 2000. Viral replication and the coactivators p300 and CBP. Trends Microbiol. 8:560-565. [DOI] [PubMed] [Google Scholar]
- 19.Kino, T., A. Gragerov, O. Slobodskaya, M. Tsopanomichalou, G. P. Chrousos, and G. N. Pavlakis. 2002. Human immunodeficiency virus type 1 (HIV-1) accessory protein Vpr induces transcription of the HIV-1 and glucocorticoid-responsive promoters by binding directly to p300/CBP coactivators. J. Virol. 76:9724-9734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lacey, S. F., K. J. Weinhold, C. H. Chen, C. McDanal, C. Oei, and M. L. Greenberg. 1998. Herpesvirus saimiri transformation of HIV type 1 suppressive CD8+ lymphocytes from an HIV type 1-infected asymptomatic individual. AIDS Res. Hum. Retrovir. 14:521-531. [DOI] [PubMed] [Google Scholar]
- 21.Lehrer, R. I., and T. Ganz. 2002. Defensins of vertebrate animals. Curr. Opin. Immunol. 14:96-102. [DOI] [PubMed] [Google Scholar]
- 22.Lehrer, R. I., A. K. Lichtenstein, and T. Ganz. 1993. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu. Rev. Immunol. 11:105-128. [DOI] [PubMed] [Google Scholar]
- 23.Leith, J. G., K. F. Copeland, P. J. McKay, D. Bienzle, C. D. Richards, and K. L. Rosenthal. 1999. T cell-derived suppressive activity: evidence of autocrine noncytolytic control of HIV type 1 transcription and replication. AIDS Res. Hum. Retrovir. 15:1553-1561. [DOI] [PubMed] [Google Scholar]
- 24.Levy, J. A., C. E. Mackewicz, and E. Barker. 1996. Controlling HIV pathogenesis: the role of the noncytotoxic anti-HIV response of CD8+ T cells. Immunol. Today 17:217-224. [DOI] [PubMed] [Google Scholar]
- 25.Mackewicz, C. E., D. J. Blackbourn, and J. A. Levy. 1995. CD8+ T cells suppress human immunodeficiency virus replication by inhibiting viral transcription. Proc. Natl. Acad. Sci. USA 92:2308-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackewicz, C. E., H. W. Ortega, and J. A. Levy. 1991. CD8+ cell anti-HIV activity correlates with the clinical state of the infected individual. J. Clin. Investig. 87:1462-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monell, C. R., and M. Strand. 1994. Structural and functional similarities between synthetic HIV gp41 peptides and defensins. Clin. Immunol. Immunopathol. 71:315-324. [DOI] [PubMed] [Google Scholar]
- 28.Moriuchi, H., M. Moriuchi, C. Combadiere, P. M. Murphy, and A. S. Fauci. 1996. CD8+ T-cell-derived soluble factor(s), but not beta-chemokines RANTES, MIP-1 alpha, and MIP-1 beta, suppress HIV-1 replication in monocyte/macrophages. Proc. Natl. Acad. Sci. USA 93:15341-15345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosoian, A., A. Teixeira, E. Caron, J. Piwoz, and M. E. Klotman. 2000. CD8+ cell lines isolated from HIV-1-infected children have potent soluble HIV-1 inhibitory activity that differs from beta-chemokines. Viral Immunol. 13:481-495. [DOI] [PubMed] [Google Scholar]
- 30.Murakami, T., T. Nakajima, Y. Koyanagi, K. Tachibana, N. Fujii, H. Tamamura, N. Yoshida, M. Waki, A. Matsumoto, O. Yoshie, T. Kishimoto, N. Yamamoto, and T. Nagasawa. 1997. A small molecule CXCR4 inhibitor that blocks T cell line-tropic HIV-1 infection. J. Exp. Med. 186:1389-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murakami, T., T. Y. Zhang, Y. Koyanagi, Y. Tanaka, J. Kim, Y. Suzuki, S. Minoguchi, H. Tamamura, M. Waki, A. Matsumoto, N. Fujii, H. Shida, J. A. Hoxie, S. C. Peiper, and N. Yamamoto. 1999. Inhibitory mechanism of the CXCR4 antagonist T22 against human immunodeficiency virus type 1 infection. J. Virol. 73:7489-7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy, C. J., B. A. Foster, M. J. Mannis, M. E. Selsted, and T. W. Reid. 1993. Defensins are mitogenic for epithelial cells and fibroblasts. J. Cell. Physiol. 155:408-413. [DOI] [PubMed] [Google Scholar]
- 33.Nakashima, H., M. Masuda, T. Murakami, Y. Koyanagi, A. Matsumoto, N. Fujii, and N. Yamamoto. 1992. Anti-human immunodeficiency virus activity of a novel synthetic peptide, T22 ([Tyr-5,12, Lys-7]polyphemusin II): a possible inhibitor of virus-cell fusion. Antimicrob. Agents Chemother. 36:1249-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakashima, H., N. Yamamoto, M. Masuda, and N. Fujii. 1993. Defensins inhibit HIV replication in vitro. AIDS 7:1129.. [DOI] [PubMed] [Google Scholar]
- 35.Prohaszka, Z., K. Nemet, P. Csermely, F. Hudecz, G. Mezo, and G. Fust. 1997. Defensins purified from human granulocytes bind C1q and activate the classical complement pathway like the transmembrane glycoprotein gp41 of HIV-1. Mol. Immunol. 34:809-816. [DOI] [PubMed] [Google Scholar]
- 36.Rubbert, A., D. Weissman, C. Combadiere, K. A. Pettrone, J. A. Daucher, P. M. Murphy, and A. S. Fauci. 1997. Multifactorial nature of noncytolytic CD8+ T cell-mediated suppression of HIV replication: beta-chemokine-dependent and -independent effects. AIDS Res. Hum. Retrovir. 13:63-69. [DOI] [PubMed] [Google Scholar]
- 37.Ruegg, C. L., and M. Strand. 1990. Inhibition of protein kinase C and anti-CD3-induced Ca2+ influx in Jurkat T cells by a synthetic peptide with sequence identity to HIV-1 gp41. J. Immunol. 144:3928-3935. [PubMed] [Google Scholar]
- 38.Ruegg, C. L., and M. Strand. 1991. A synthetic peptide with sequence identity to the transmembrane protein GP41 of HIV-1 inhibits distinct lymphocyte activation pathways dependent on protein kinase C and intracellular calcium influx. Cell. Immunol. 137:1-13. [DOI] [PubMed] [Google Scholar]
- 39.Schonwetter, B. S., E. D. Stolzenberg, and M. A. Zasloff. 1995. Epithelial antibiotics induced at sites of inflammation. Science 267:1645-1648. [DOI] [PubMed] [Google Scholar]
- 40.Tomaras, G. D., and M. L. Greenberg. 2001. CD8+ T cell mediated noncytolytic inhibition of human immunodeficiency virus type I. Front. Biosci. 6:D575-D98. [DOI] [PubMed] [Google Scholar]
- 41.Tomaras, G. D., S. F. Lacey, C. B. McDanal, G. Ferrari, K. J. Weinhold, and M. L. Greenberg. 2000. CD8+ T cell-mediated suppressive activity inhibits HIV-1 after virus entry with kinetics indicating effects on virus gene expression. Proc. Natl. Acad. Sci. USA 97:3503-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Wetering, S., S. P. Mannesse-Lazeroms, J. H. Dijkman, and P. S. Hiemstra. 1997. Effect of neutrophil serine proteinases and defensins on lung epithelial cells: modulation of cytotoxicity and IL-8 production. J. Leukoc. Biol. 62:217-226. [DOI] [PubMed] [Google Scholar]
- 43.Walker, C. M., D. J. Moody, D. P. Stites, and J. A. Levy. 1986. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science 234:1563-1566. [DOI] [PubMed] [Google Scholar]
- 44.Yang, D., A. Biragyn, L. W. Kwak, and J. J. Oppenheim. 2002. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 23:291-296. [DOI] [PubMed] [Google Scholar]
- 45.Yang, D., Q. Chen, O. Chertov, and J. J. Oppenheim. 2000. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J. Leukoc. Biol. 68:9-14. [PubMed] [Google Scholar]
- 46.Yang, D., O. Chertov, S. N. Bykovskaia, Q. Chen, M. J. Buffo, J. Shogan, M. Anderson, J. M. Schroder, J. M. Wang, O. M. Howard, and J. J. Oppenheim. 1999. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 286:525-528. [DOI] [PubMed] [Google Scholar]
- 47.Zhang, J., and C. S. Crumpacker. 2002. Human immunodeficiency virus type 1 nucleocapsid protein nuclear localization mediates early viral mRNA expression. J. Virol. 76:10444-10454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang, J. L., P. L. Sharma, and C. S. Crumpacker. 2000. Enhancement of the basal-level activity of HIV-1 long terminal repeat by HIV-1 nucleocapsid protein. Virology 268:251-263. [DOI] [PubMed] [Google Scholar]
- 49.Zhang, L., W. Yu, T. He, J. Yu, R. E. Caffrey, E. A. Dalmasso, S. Fu, T. Pham, J. Mei, J. J. Ho, W. Zhang, P. Lopez, and D. D. Ho. 2002. Contribution of human alpha-defensin 1, 2, and 3 to the anti-HIV-1 activity of CD8 antiviral factor. Science 298:995-1000. [DOI] [PubMed] [Google Scholar]